Abstract
Purpose.
Corneal stromal scarring partly involves the production of corneal myofibroblasts. The purpose of this study was to examine the effects of rapamycin (an inhibitor of the mammalian target of rapamycin [mTOR] pathway) on myofibroblast formation in vitro and in-vivo.
Methods.
Human corneal fibroblasts were grown in culture and transformed into myofibroblasts using TGF-β (2 ng/mL). The phosphorylation (activation) of the mTOR pathway was examined by immunoblotting. Cell proliferation with and without rapamycin was examined by thiazolyl blue tetrazolium bromide (MTT) assay and Ki67 staining. The expression of the myofibroblast differentiation marker smooth muscle actin (SMA) was examined by immunostaining and immunoblotting. The functional effects of rapamycin were measured using a gel contraction assay. For in vivo studies, 140 μm laser ablation was performed on rabbit corneas followed by subconjunctival rapamycin or vehicle. Corneal haze development was graded at 4 weeks, while the expression of myofibroblast markers was examined by immunostaining and immunoblotting.
Results.
The TGF-β activated the mTOR pathway with peak phosphorylation at 2 to 4 hours. Treatment of corneal fibroblasts with rapamycin reduced their proliferation by 46% compared to control. Rapamycin significantly inhibited TGF-β–induced expression of myofibroblast markers (17.2% SMA positive cells with rapamycin compared to 69.0% in control). Rapamycin also significantly inhibited TGF-β–induced collagen gel contraction. In the rabbit eyes treated with rapamycin, corneal haze development was significantly less compared to controls (0.75 ± 0.4 vs. 2.17 ± 0.7).
Conclusions.
Rapamycin appears to inhibit proliferation and differentiation of corneal myofibroblasts and, thus, may provide an effective therapeutic measure for preventing corneal scarring.
Keywords: rapamycin, corneal haze, excimer laser
Rapamycin reduces corneal scarring after photorefractive keratectomy.
Introduction
The optical clarity of the corneal stroma is dependent largely on its structural organization, particularly its highly ordered matrix.1,2 In pathologic conditions that involve a stromal wound healing response, a less ordered extracellular matrix may be produced, leading to stromal scarring, opacification, and subsequent loss of vision. Previous studies have demonstrated the key role of stromal myofibroblasts in corneal scarring and the production of a fibrotic extracellular matrix.3 Pathophysiologically, the myofibroblasts can arise from the resident stromal keratocytes and from bone marrow–derived cells.4,5
Although a number of signaling mechanisms have been studied in the pathophysiology of myofibroblast formation and scarring, clinically, there are limited treatment options for inhibiting fibrosis in the cornea. Corticosteroids can exert a moderate antifibrotic effect, but their use often is limited by their side effects. Antiproliferative chemotherapeutic agents, such as mitomycin C and 5-fluorouracil (5-FU), are used at the time of surgical procedures to minimize postoperative corneal scarring; however, their use can be associated with complications, such as reduced cell density,6 limbal stem cell deficiency,7–11 and melting,12 which precludes their regular or repeated use. While the use of mitomycin C in the central cornea for brief durations (i.e., following excimer laser ablations) appears to be relatively safe, there still is a need to identify agents with antifibrotic effects and good safety profile that can be used in the clinical setting to inhibit corneal scarring in pathologic conditions. In this study, we have examined the use of rapamycin, a clinically approved medication, as a potential antifibrotic agent for the cornea.
Rapamycin, also known as sirolimus, is a macrolide with immunosuppressive and antiproliferative properties. It is a natural product isolated from the fungus Streptomyces hygroscopicus.13 Clinically, it is used widely for immunosuppression following organ transplantation. One of the primary mechanisms of action of rapamycin is through the inhibition of mammalian target of rapamycin (mTOR). The mTOR is a serine/threonine protein kinase that has been found to affect many cellular functions, such as cell growth, proliferation, and metabolism.14 It has been reported to have antifibrotic effects in a number of organs, such as skin15 and liver.16 In the cornea it has been shown to modulate scarring, neovascularization, and inflammation.17–20 In this study, we demonstrated that TGF-β activates the mTOR pathway in corneal stromal fibroblasts, and that rapamycin can inhibit their proliferation and modulate their transformation into myofibroblasts, hence reducing corneal scar formation.
Methods
Human Corneal Fibroblast Culture
Human corneoscleral buttons were provided generously by the Illinois Eye Bank (Bloomington, IL). Corneal fibroblasts were isolated according to a modified method described previously.21 Briefly, the central corneal button was removed with an 8.0 mm trephine, and the epithelium and endothelium were removed by gentle scraping with a scalpel blade. Central corneal buttons then were cut into small pieces measuring 1 × 2 mm2 and digested with 1 mg/mL collagenase type IA (Sigma-Aldrich, St. Louis, MO) in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) for 1 hour at 37°C with gentle shaking to isolate the stromal cells. The cells were collected by centrifugation, resuspended in the same medium, and plated at density of 1.5 × 105 cells/cm2. The next day, the media was changed to DMEM with 3% FBS and the cells were grown to 80% confluence before passage. Most experiments were done on third or fourth passage corneal fibroblasts. In some of the experiments, porcine corneal fibroblasts, isolated from fresh eyes in a similar fashion, also were used for gathering preliminary data.
Western Blot Analysis
Cells cultured on 100-mm dishes were rinsed twice with PBS and harvested in radioimmunoprecipitation assay (RIPA) buffer supplemented with protease/phosphatase inhibitors. After protein concentration measurement, equal amounts of each sample were mixed with sample buffer, denatured by heating at 95°C for 10 minutes, and subjected to electrophoresis on 4% to 20% Tris-Glycine gels (Invitrogen, Grand Island, NY). The protein bands were transferred to nitrocellulose membranes and equal loading was verified by Ponceau S staining. The membranes were incubated in 5% BSA in Tris-buffered saline (TBS) for 1 hour followed by an overnight incubation (4°C) with primary antibodies at the optimal concentration. The membranes were washed with TBS with 0.03% Tween 20 and incubated with the horseradish peroxidase (HRP)–conjugated secondary antibody for 1 hour at room temperature. Detection was performed with ECL Plus Western Blotting Detection System (Amersham, Buckinghamshire, UK) and recorded on film (HyBlot CL; Denville Scientific, Inc., Metuchen, NJ). The following antibodies were used: rabbit anti–phospho-mTOR (Ser2448), rabbit anti–phospho-S6 ribosomal protein (Ser240/244, 61H9), rabbit anti-p70 S6 kinase, rabbit anti–phospho-p70 S6 kinase (Thr389, 108D2), and rabbit anti-GAPDH, all from Cell Signaling Technology, Inc. (Danvers, MA). Also used were rabbit antifibronectin (H-300) and mouse anti–α-actin (1A4), from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunofluorescence Staining
Cells grown on cover slips were fixed in 4% paraformaldehyde for 10 minutes and then permeabilized with TBS + 0.3% Triton X-100 for 10 minutes at room temperature. After three 5-minute washes with TBS + 0.03% Triton X-100, the cells/tissues were incubated with 10% normal donkey serum in TBS containing 1% BSA (TBS-BSA) for 1 hour at room temperature to block nonspecific binding. Afterward, the cells were incubated overnight at 4°C with the corresponding primary antibodies at optimal dilutions in TBS-BSA. The primary antibodies included mouse anti-actin α-smooth muscle antibody (1A4) from Sigma-Aldrich, rabbit anti-fibronectin (H-300) from Santa Cruz Biotechnology, mouse anti-fibronectin from BD Biosciences (San Diego, CA), rabbit anti-Ki67 from Abcam (Cambridge, MA), and rabbit anit-Smad2/3 (D7G7) from Cell Signaling Technology, Inc.
After three 5-minute washes with TBS + 0.03% Triton X-100, cells were incubated with the corresponding secondary antibodies for 1 hour at room temperature. Cells were washed with TBS + 0.03% Triton X-100 and 4′6-diamidino-2-phenylindole (DAPI) or 2-phenylindole (PI) was used to counterstain the nuclei. Negative controls were stained in a similar fashion using an irrelevant antibody to exclude nonspecific staining. Paraffin-embedded rabbit corneal tissues were sectioned and stained in the same fashion after deparaffinization. The slides were visualized and photographed using a Zeiss LSM 710 microscope (Carl Zeiss, Jena, Germany).
Thiazolyl Blue Tetrazolium Bromide (MTT) Assay
The effect of rapamycin on cell proliferation was detected using a thiazolyl blue tetrazolium bromide (MTT; Sigma-Aldrich) assay.22 Human corneal fibroblasts were plated at density of 103 cells per well in 96-well plates, and treated with different concentrations of rapamycin for 24, 48, and 72 hours, and 7 days. After removal of media, 20 μL of 5 mg/mL MTT solution was replaced in wells and incubated at 37°C for 3.5 hours. The solution then was removed and replaced by 150 μL MTT solvent. Plates were placed on a plate shaker for 15 minutes to enhance solubilization and the absorbance was measured at 562 nm with a 96-well plate reader (GENios plate reader; Tecan, Salzburg, Austria).
Gel Contraction Assay
Fibroblast-embedded collagen gels were prepared according to a modified method described previously.23 Briefly, 24-well plates were precoated with 0.2% BSA for 1 hour. Rapamycin- or dimethyl sulfoxide (DMSO, control)–treated fibroblasts were resuspended in DMEM (106/mL) and added to a neutralized collagen solution (2.3 mL of cells were added to mixture of type I collagen from a rat tail tendon (600 μL), and 36 μL of 0.1 mol/L NaOH and 69 μL 10 × PBS), yielding a final concentration of 150,000 cells/mL and 1 mg/mL collagen. The gel solutions were added to 24 well plates (600 μL per well) and incubated in 37°C for 1 hour for polymerization. After 24 hours of treatment with TGF-β (2 ng/mL; R&D Systems, Minneapolis, MN), the gels were detached and photographed. The degree of contraction was quantified by measuring the surface area using ImageJ software.24
Excimer Laser-Induced Corneal Scarring Model
We performed 140 μm ablation in 6.0 mm optic zone size with the NIDEK EC-5000 excimer laser (NIDEK Co., Gamagori, Japan) on the right eye of 12 female New Zealand White (NZW) rabbits (20 weeks old, 3–4 kg). All procedures complied with the ARVO Statement for Use of Animals in Ophthalmic and Vision Research.
Anesthesia was achieved by subcutaneous injection of ketamine (30 mg/kg) and xylazine (5 mg/kg). In addition, topical proparacaine 1% (Alcon Surgical, Inc., Ft. Worth, TX) was applied to each eye just before surgery. Six eyes received 1 mg subconjunctival rapamycin (LC Laboratories, Woburn, MA) immediately and at 2 weeks following the surgery. Six eyes received vehicle in the same fashion. Corneas were followed by slit-lamp microscopy and the degree of haze formation was graded by two masked observers at 4 weeks.25 At 1 month postsurgery, the animals were euthanized and their corneas were either embedded in paraffin for immunostaining or underwent protein extraction for Western blotting.
Results
TGF-β Activates the mTOR Pathway in Corneal Fibroblasts In Vitro
Corneal fibroblasts in culture were serum starved for 24 hours, then treated with TGF-β (2 ng/mL). The TGF-β was found to activate the mTOR pathway in a time-dependant manner with a peak phosphorylation (activation) of mTOR and its downstream effector P70S6K between 2 and 4 hours (Fig. 1). The ribosomal protein S6, which regulates translation downstream to P70S6K, was activated (phosphorylated) similarly, which persisted up to 24 hours, consistent with the fact that TGF-β activates pathways that partly increase protein translation.
Figure 1.
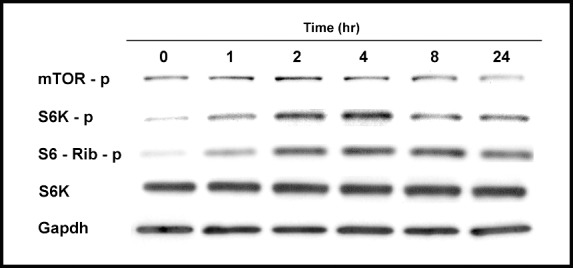
The TGF-β activates the mTOR pathway in a time-dependent manner. Corneal fibroblasts were treated with TGF-β (2 ng/mL), and investigated for expression of activated mTOR and its downstream effectors.
Rapamycin Inhibits Corneal Myofibroblast Differentiation In Vitro
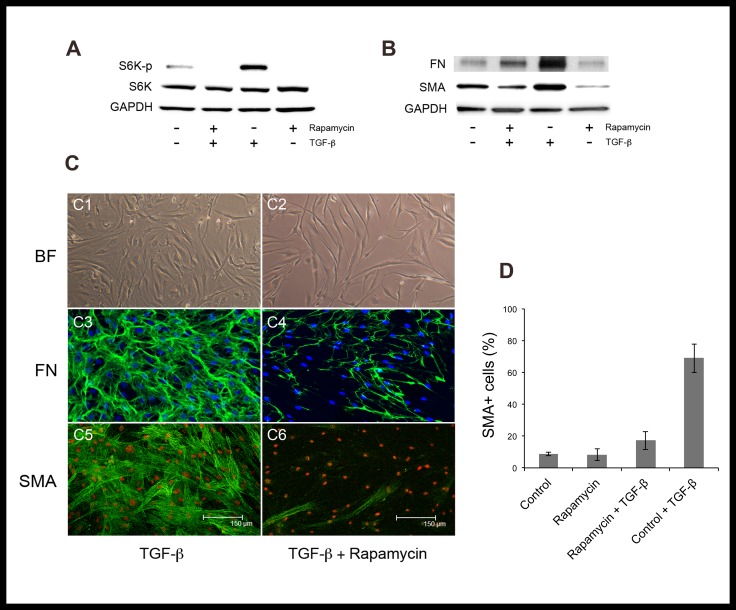
Human corneal fibroblasts were cultured in high density, low serum (3% FBS) conditions to minimize spontaneous myofibroblast transformation before being treated with TGF-β. Treatment with TGF-β effectively induced myofibroblast differentiation, as evident by the expression of α-smooth muscle actin (α-SMA) and fibronectin (Fig. 2). Pretreatment with rapamycin (1 μg/mL) for 48 hours significantly inhibited the formation of myofibroblasts, and thus, maintained the cells in a fibroblast phenotype. The percentage of α-SMA expressing cells after TGF-β was 17.2 ± 5.7 in rapamycin-treated cells compared to 69.0 ± 9.0 in control (P < 0.001).
Figure 2.
Rapamycin inhibits TGF-β–induced activation of mTOR pathway and corneal myofibroblast differentiation in vitro. Pretreatment of human corneal fibroblasts with rapamycin for 48 hours inhibited the activation (phosphorylation) of S6K at 6 hours after TGF-β (A). Treatment with rapamycin inhibited the expression of myofibroblast differentiation marker SMA and the production of fibronectin after 7 days of TGF-β (B). Bright field microscopy, fibronectin, and α-SMA staining of control (C1, C3, C5) versus rapamycin-treated (C2, C4, C6) cells after stimulation with TGF-β for 7 days. Pretreatment with rapamycin significantly reduced the percentage of α-SMA–positive cells (P < 0.001, [D]).
Rapamycin Inhibits Corneal Fibroblast Proliferation
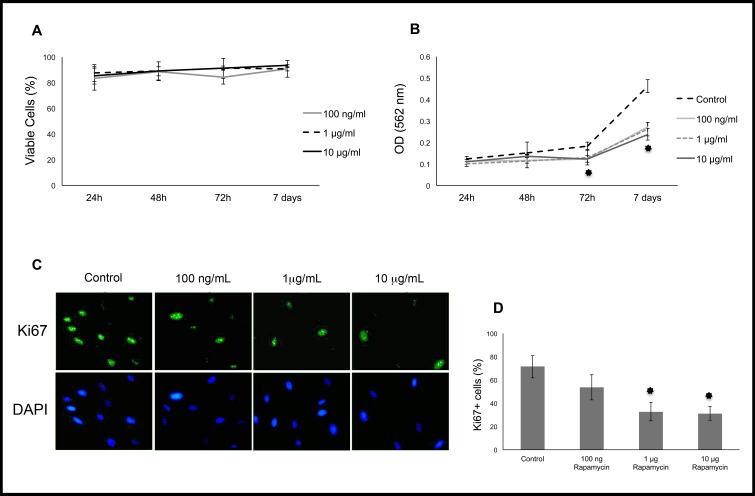
Rapamycin also is known to have antiproliferative effects.14 Therefore, rapamycin was evaluated in proliferating corneal fibroblasts cultured in DMEM with 3% FBS. After 24, 48, and 72 hours of rapamycin treatment, proliferation was measured using the proliferation marker Ki67 and an MTT assay. Results showed that proliferation was inhibited by nearly 50%, particularly at higher concentrations of rapamycin (Fig. 3). To rule out toxicity due to rapamycin, a trypan blue exclusion test was used, demonstrating 80% to 90% cell viability when exposed to rapamycin up to 10 μg/mL for 72 hours.
Figure 3.
Trypan blue exclusion assay showed no toxicity from rapamycin at concentrations up to 10 μg/mL (A). The MTT assay indicates lower absorbance after day 3 in rapamycin-treated cells (B). Immunostaining for the proliferation marker Ki67 in cells treated with rapamycin versus control. The percentage of Ki67-positive nuclei was significantly lower at 1 and 10 μg/mL rapamycin (P < 0.001, [C, D]). *P < 0.001.
Rapamycin Prevents Collagen Gel Contraction
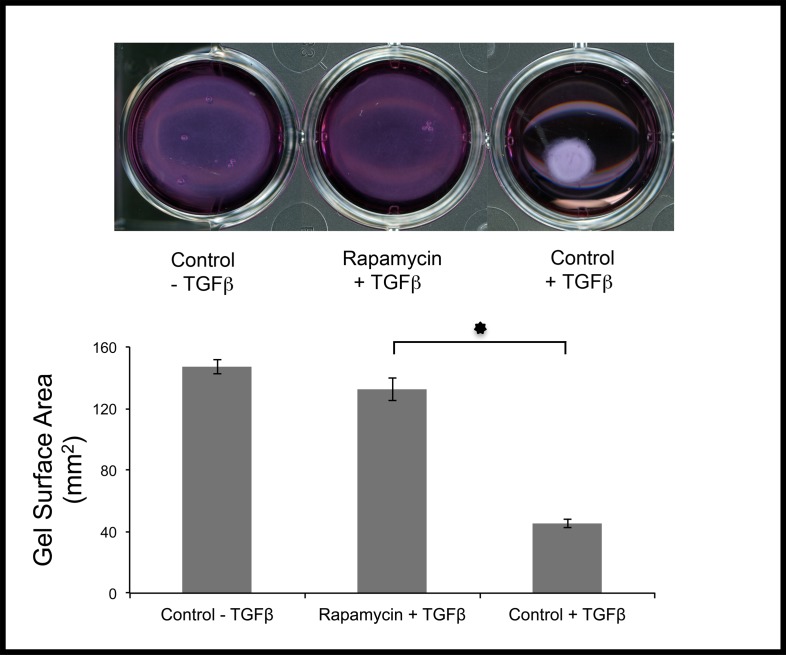
To measure the functional effects of rapamycin on fibroblasts, a gel contraction assay was used. This assay is based on the contractile properties of cells grown inside a matrix. Corneal fibroblasts were pretreated with rapamycin or DMSO (control) for 2 days, then embedded in collagen gels followed by exposure to TGF-β for 24 hours. Treatment with rapamycin dramatically inhibited the contraction of collagen gels, consistent with its ability to prevent myofibroblast formation (Fig. 4).
Figure 4.
Rapamycin inhibited cell-mediated contraction of collagen gels. Human corneal fibroblasts pretreated with rapamycin or DMSO were embedded in collagen gels, then exposed to TGF-β for 24 hours. Gel contraction was significantly blocked by rapamycin (*P < 0.001).
Rapamycin Does Not Affect Nuclear Translocation of Smad2/3 Induced by TGF-β
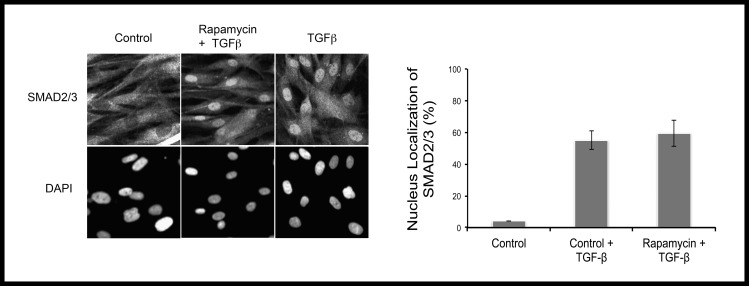
The canonical TGF-β signaling, involving the activation of Smads, has been studied extensively in corneal myofibroblast transformation.26,27 To examine the effect of rapamycin on the canonical TGF-β signaling, corneal fibroblasts were serum starved for 24 hours and then stimulated with TGF-β (2 ng/mL) for 45 minutes. The activation (nuclear translocation) of Smad2/3 was evaluated by immunofluorescence staining. As shown in Figure 5, Smad2/3 was translocated into the nucleus upon TGF-β treatment, which was unaffected by rapamycin. This suggested that the effects of rapamycin are partly mediated through noncanonical pathways of TGF-β signaling.
Figure 5.
Rapamycin did not affect nuclear translocation of Smad2/3. Rapamycin pretreated and control fibroblasts were subjected to immunostaining for Smad2/3 after 45 minutes of stimulation with TGF-β (2 ng/mL). As shown in the figure, there is no effect of rapamycin on nuclear translocation of SMAD2/3 (P = not significant).
Rapamycin Prevents Corneal Scarring In Vivo
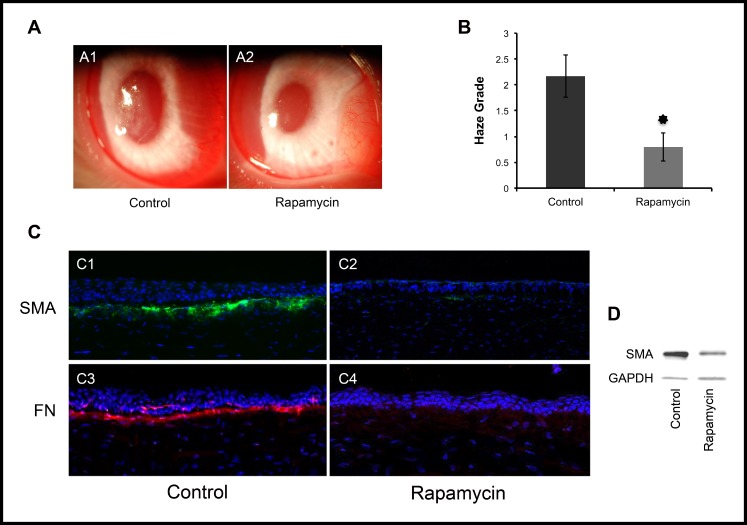
Finally, to determine the in vivo effects of rapamycin, we used a well-established excimer laser–induced corneal scarring model.28 The model involves ablating the anterior corneal stroma with excimer laser, which induces a stromal wound healing response marked by proliferation and development of myofibroblasts, ultimately resulting in stromal haze formation. A total of 12 NZW rabbits underwent excimer laser ablation, then were divided into two equal groups of six that received either rapamycin (1 mg) or vehicle by subconjunctival injection immediately after the laser procedure and 2 weeks later. As shown in Figure 6, rapamycin treatment significantly inhibited corneal haze formation (0.8 ± 0.27 vs. 2.16 ± 0.4, P < 0.001). Histologically, rapamycin-treated corneas demonstrated reduced numbers of SMA-positive myofibroblasts and lower levels of fibronectin in the anterior corneal stroma compared to control corneas. These results demonstrated that rapamycin can inhibit effectively myofibroblast formation and scar formation in vivo.
Figure 6.
Rapamycin reduces scar formation after excimer laser keratectomy in a rabbit model. Corneal haze was graded on a scale of 0 to 4 (0, complete clarity; 1/2, minimal haze with careful oblique illumination; 1, mild haze not interfering with visibility of fine iris details; 2, mild obscuration of iris details; 3, moderate obscuration of iris and lens; and 4, complete obscuration of the anterior chamber and iris).28 The average haze grade was significantly less with rapamycin compared to control (0.8 ± 0.27 vs. 2.16 ± 0.4, P <0.001, [A, B]). Immunostaining (C), and Western blotting (D) for SMA and fibronectin show lower expression of SMA (C1, C2) and fibronectin (C3, C4) in rapamycin-treated cornea versus control. *P < 0.001.
Discussion
In this study, we demonstrated that the mTOR pathway is activated by TGF-β in corneal fibroblasts and treatment with rapamycin, an inhibitor of mTOR, reduces corneal scarring, partly by limiting fibroblast proliferation and inhibiting myofibroblast formation. The mTOR is a kinase, which regulates numerous functions, including cell growth, proliferation, metabolism, and autophagy.14 Therefore, the effects of rapamycin on corneal scarring likely are mediated through multiple mechanisms. Recently, another group evaluated the use of rapamycin alone or combined with bevacizumab in a rabbit model of photorefractive keratectomy.17 Rapamycin was applied topically on the stromal bed just once. Their results demonstrated a modest beneficial effect from rapamycin, which they concluded was due partly to a reduction in keratocyte apoptosis. In our study we did not specifically examine apoptosis; however, our in vitro results indicated that rapamycin also can inhibit myofibroblast formation directly. In vivo, rapamycin could be inhibiting proliferation and myofibroblast differentiation, either from keratocyte-derived precursors and/or bone marrow–derived precursors. The inhibitory effect of rapamycin on myofibroblast formation was shown previously in another study using a rat alkali injury model, where they demonstrated that rapamycin inhibited the expression of SMA in corneal fibroblasts in vitro.18
One of the most well known pathways upstream to mTOR is the phosphoinositol-3-kinase (PI3K) pathway.28 In our study, rapamycin did not appear to affect the nuclear translocation of Smad, suggesting that its effects do not directly involve the canonical (Smad-dependent) TGF-β pathway. The PI3K pathway actually has been recognized as an important modulator of TGF-β signaling in regulating pathologic fibrosis in the cornea.29 Therefore, it is likely that the effects of rapamycin on corneal myofibroblast formation are mediated partly through modulation of the PI3K/mTOR signaling axis.
The mTOR is actually a subunit in two different complexes, namely mTORC1 and mTORC2. Rapamycin mainly targets mTORC1; however, long-term exposure to rapamycin also inhibits mTORC2.30 Although, we did not specifically examine which mTOR subtype is involved in modulating myofibroblast formation, based on the concentrations of rapamycin and the duration of exposure needed to see an effect, we believe that inhibition of mTORC1 and mTORC2 is necessary. In particular, mTORC1 can be inhibited by low nanomolar concentrations with brief durations, while mTORC2 inhibition requires high nanomolar to low micromolar concentrations for at least 24 hours – similar to what was used in our study.31 Further studies are needed to define precisely the role of each subtype in the corneal stromal wound healing and scar formation.
In this study, we used 1 mg subconjunctival injection of rapamycin, partly based on previous clinical studies.32–34 Ideally, a topical formulation of rapamycin that gets into the stroma would circumvent the need for subconjunctival delivery; however, given the extreme insolubility of rapamycin in aqueous solutions, developing such a formulation has been challenging.35 On the other hand, highly insoluble drugs potentially can provide a depot with slow release of the drug after local injection. Likewise, such drugs are ideal for sustained release implants. Previously, a rapamycin implant was shown to be effective in preventing corneal graft rejection in a rabbit model.19 With improvements in drug delivery, we anticipate topical formulations of rapamycin to be available for clinical use in the future.
In summary, rapamycin, an inhibitor of the mTOR pathway, appears to be a promising agent for clinically preventing corneal scarring. Its effects appear to be mediated partly by limiting the proliferation of corneal fibroblasts and their transformation into myofibroblasts. An ocular formulation of rapamycin currently is in clinical trials for posterior segment disease,32–34 which could make it potentially available for future anterior segment applications.
Acknowledgments
The authors thank Ruth Zelkha, MS, for her generous technical assistance in imaging, and the Illinois and Michigan Eye Banks (Ann Arbor, MI) for providing human corneal tissue.
Supported by the National Eye Institute of the National Institutes of Health with Career Development Grant K08EY017561-A1 (ARD) and Core Grant EY01792, and a Career Developmental Award (ARD), an unrestricted departmental grant from Research to Prevent Blindness, and a grant from ASCRS Foundation.
Disclosure: B.Y. Milani, None; F.Y. Milani, None; D. Park, None; A. Namavari, None; J. Shah, None; H. Amirjamshidi, None; H. Ying, None; A.R. Djalilian, None
References
- 1. Birk DE, Trelstad RL. Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J Cell Biol. 1984; 99: 2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freegard TJ. The physical basis of transparency of the normal cornea. Eye (Lond). 1997; 11 (Pt 4): 465–471 [DOI] [PubMed] [Google Scholar]
- 3. Wilson SE. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp Eye Res. 2012; 99: 78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson SE, Mohan RR, Netto M, et al. RANKL, OPG, and M-CSF expression in stromal cells during corneal wound healing. Invest Ophthalmol Vis Sci. 2004; 45: 2201–2211 [DOI] [PubMed] [Google Scholar]
- 5. Barbosa FL, Chaurasia SS, Cutler A, et al. Corneal myofibroblast generation from bone marrow-derived cells. Exp Eye Res. 2010; 91: 92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Netto MV, Mohan RR, Sinha S, Sharma A, Gupta PC, Wilson SE. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, cellular proliferation, haze, and long-term keratocyte density in rabbits. J Refract Surg. 2006; 22: 562–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lichtinger A, Pe'er J, Frucht-Pery J, Solomon A. Limbal stem cell deficiency after topical mitomycin C therapy for primary acquired melanosis with atypia. Ophthalmology. 2010; 117: 431–437 [DOI] [PubMed] [Google Scholar]
- 8. Dudney BW, Malecha MA. Limbal stem cell deficiency following topical mitomycin C treatment of conjunctival corneal intraepithelial neoplasia. Am J Ophthalmol. 2004; 137: 950–951 [DOI] [PubMed] [Google Scholar]
- 9. Sauder G, Jonas JB. Limbal stem cell deficiency after subconjunctival mitomycin C injection for trabeculectomy. Am J Ophthalmol. 2006; 141: 1129–1130 [DOI] [PubMed] [Google Scholar]
- 10. Ditta LC, Shildkrot Y, Wilson MW. Outcomes in 15 patients with conjunctival melanoma treated with adjuvant topical mitomycin C: complications and recurrences. Ophthalmology. 2011; 118: 1754–1759 [DOI] [PubMed] [Google Scholar]
- 11. Salomão DR, Mathers WD, Sutphin JE, Cuevas K, Folberg R. Cytologic changes in the conjunctiva mimicking malignancy after topical mitomycin C chemotherapy. Ophthalmology. 1999; 106: 1756–1760 [DOI] [PubMed] [Google Scholar]
- 12. Dougherty PJ, Hardten DR, Lindstrom RL. Corneoscleral melt after pterygium surgery using a single intraoperative application of mitomycin-C. Cornea. 1996; 15: 537–540 [PubMed] [Google Scholar]
- 13. Vézina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo). 1975; 28: 721–726 [DOI] [PubMed] [Google Scholar]
- 14. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011; 12: 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoshizaki A, Yanaba K, Yoshizaki A, et al. Treatment with rapamycin prevents fibrosis in tight-skin and bleomycin-induced mouse models of systemic sclerosis. Arthritis Rheum. 2010; 62: 2476–2487 [DOI] [PubMed] [Google Scholar]
- 16. Zhu J, Wu J, Frizell E, et al. Rapamycin inhibits hepatic stellate cell proliferation in vitro and limits fibrogenesis in an in vivo model of liver fibrosis. Gastroenterology. 1999; 117: 1198–1204 [DOI] [PubMed] [Google Scholar]
- 17. Lee KS, Ko DA, Kim ES, Kim MJ, Tchah H, Kim JY. Bevacizumab and rapamycin can decrease corneal opacity and apoptotic keratocyte number following photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2012; 53: 7645–7653 [DOI] [PubMed] [Google Scholar]
- 18. Shin YJ, Hyon JY, Choi WS, et al. Chemical injury-induced corneal opacity and neovascularization reduced by rapamycin via TGF- β1/ERK pathways regulation. Invest Ophthalmol Vis Sci. 2013; 54: 4452–4458 [DOI] [PubMed] [Google Scholar]
- 19. Shi W, Gao H, Xie L, Wang S. Sustained intraocular rapamycin delivery effectively prevents high-risk corneal allograft rejection and neovascularization in rabbits. Invest Ophthalmol Vis Sci. 2006; 47: 3339–3344 [DOI] [PubMed] [Google Scholar]
- 20. Zapata G, Racca L, Tau J, Berra A. Topical use of rapamycin in herpetic stromal keratitis. Ocul Immunol Inflamm. 2012; 20: 354–359 [DOI] [PubMed] [Google Scholar]
- 21. Shafiq MA, Gemeinhart RA, Yue BY, Djalilian AR. Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma. Tissue Eng Part C Methods. 2012; 18: 340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65: 55–63 [DOI] [PubMed] [Google Scholar]
- 23. Zhu X, Li L, Zou L, et al. A novel aptamer targeting TGF-β receptor II inhibits transdifferentiation of human tenon's fibroblasts into myofibroblast. Invest Ophthalmol Vis Sci. 2012; 53: 6897–6903 [DOI] [PubMed] [Google Scholar]
- 24. Rasband WS. ImageJ. Bethesda, MD: U. S. National Institutes of Health; 1997-2012. Available at http://imagej.nih.gov/ij/ [Google Scholar]
- 25. Fantes FE, Hanna KD, Waring GO 3rd, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990; 108: 665–675 [DOI] [PubMed] [Google Scholar]
- 26. You L, Kruse FE. Differential effect of activin A and BMP-7 on myofibroblast differentiation and the role of the Smad signaling pathway. Invest Ophthalmol Vis Sci. 2002; 43: 72–81 [PubMed] [Google Scholar]
- 27. Tandon A, Tovey JC, Sharma A, Gupta R, Mohan RR. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr Mol Med. 2010; 10: 565–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharma A, Mehan MM, Sinha S, Cowden JW, Mohan RR. Trichostatin a inhibits corneal haze in vitro and in vivo. Invest Ophthalmol Vis Sci. 2009; 50: 2695–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dalle Pezze P, Sonntag AG, Thien A, et al. A dynamic network model of mTOR signaling reveals TSC-independent mTORC2 regulation. Sci Signal. 2012; 5: ra25 [DOI] [PubMed] [Google Scholar]
- 30. He J, Bazan HE. Epidermal growth factor synergism with TGF-beta1 via PI-3 kinase activity in corneal keratocyte differentiation. Invest Ophthalmol Vis Sci. 2008; 49: 2936–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006; 22: 159–168 [DOI] [PubMed] [Google Scholar]
- 32. Foster DA, Toschi A. Targeting mTOR with rapamycin: one dose does not fit all. Cell Cycle. 2009; 8: 1026–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dugel PU, Blumenkranz MS, Haller JA, et al. A randomized, dose-escalation study of subconjunctival and intravitreal injections of sirolimus in patients with diabetic macular edema. Ophthalmology. 2012; 119: 124–131 [DOI] [PubMed] [Google Scholar]
- 34. Sen HN, Larson TA, Meleth AD, Smith WM, Nussenblatt RB. Subconjunctival sirolimus for the treatment of chronic active anterior uveitis: results of a pilot trial. Am J Ophthalmol. 2012; 153: 1038–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen QD, Ibrahim MA, Watters A, et al. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: primary 6-month results of the SAVE Study. J Ophthalmic Inflamm Infect. 2013; 3: 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simamora P, Alvarez JM, Yalkowsky SH. Solubilization of rapamycin. Int J Pharm. 2001; 213: 25–29 [DOI] [PubMed] [Google Scholar]