Abstract
Purpose.
Ligands for aryl hydrocarbon receptor (AHR), such as dioxins, are highly toxic. One such ligand, TCDD, was found to exert potent immunosuppressive capacities in mice developing pathogenic autoimmune processes, including EAU, but its toxicity makes it unusable for humans. A recently identified endogenous AHR ligand, ITE, is also immunosuppressive, but is nontoxic and could therefore be useful for therapy in humans. Here, we tested ITE for its capacity to inhibit EAU and related immune responses.
Methods.
EAU was induced in B10.A mice by immunization with interphotoreceptor retinoid-binding protein (IRBP; 40 μg) in CFA. Treatment with ITE was by daily intraperitoneal injection of 0.2 mg. Disease severity was assessed by both fundoscopy and histological examination. Draining lymph node cells were tested for proliferation by thymidine uptake and for cytokine production and release by ELISA. In addition, the intracellular expression of cytokines and Foxp3 was determined by flow cytometry. Serum antibodies were measured by ELISA.
Results.
Treatment with ITE efficiently inhibited the development of EAU in mice, as well as the cellular immune responses against IRBP and PPD. ITE treatment inhibited the expansion of both Th1 and Th17 subpopulations, as well as their release of the signature cytokines, IFN-gamma and IL-17. The treatment moderately increased, however, the proportion of Foxp3 expressing T-regulatory cells. Antibody production was not affected by the treatment.
Conclusions.
ITE, an endogenous AHR ligand, efficiently inhibits EAU development and related cellular immune responses. Being nontoxic, ITE may be considered for treatment of pathogenic immunity in humans.
Keywords: aryl hydrocarbon receptor ligand, experimental autoimmune uveitis, T-cell mediated immunity
Development of EAU and T-cell–mediated immune responses are inhibited by ITE, a novel endogenous nontoxic ligand for aryl hydrocarbon receptor.
Introduction
The family of T-helper (Th) lymphocytes consists of several subpopulations of cells that selectively produce certain “signature” cytokines, which are involved in mediation, or suppression of immune responses.1–3 Of particular importance to this study are the effector populations of Th1, that produce IFN-γ and Th17, that make IL-17 and IL-22, as well as the populations of regulatory T-cells (Treg), that secrete TGF-β and IL-10 and are responsible for the homeostasis of the immune system.4,5 The transcription factors responsible for Th1, Th17, and Tregs are T-bet, RORγt, and Foxp3, respectively.1–5
Accumulating data of recent years have revealed the important function of the ligand-activated transcription factor aryl hydrocarbon receptor (AHR) in mediating cellular events in response to halogenated aromatic hydrocarbons, as well as nonhalogenated polycyclic aromatic hydrocarbons.6–8 The latter group of compounds includes the well-studied and highly toxic 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a member of the dioxin family of environmental toxic compounds. The physiological ligands of AHR have not been identified yet and its biological activities have been mainly investigated by using high-affinity ligands. A study by Veldhoen et al.9 showed that 6-formylindolo [3,2-b]carbazole (FICZ) enhances the generation of Th17 cells and, consequently, increases the severity of experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis.10,11 A more extended study, by Quintana et al.,12 confirmed the enhancement of EAE by FICZ, but also demonstrated an opposing effect by TCDD, namely, reduction in EAE development, apparently due to reduction in Th17 generation, along with concomitant increase in the development of Tregs. Both Th17 and Treg cells express AHR and require TGF-β for their generation, and the balance between them determines to a large extent the outcome of response to immunopathogenic stimuli.11,12 Similar to its inhibitory effect on EAE,12 TCDD was also found by Zhang et al.13 to effectively inhibit the development of experimental autoimmune uveitis (EAU), the animal model for several uveitic conditions in humans.14,15
In view of its high toxicity, TCDD cannot be considered for use in humans. The identification of an endogenous AHR ligand in mammalian lung16 made it possible, therefore, to test a nontoxic compound for its capacity to inhibit the development of EAE. The compound, 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), was found to efficiently suppress EAE in mice, apparently by enhancing Treg activity and reducing the Th17 cell function.17
In the present study, we examined the capacity of ITE to inhibit the development of EAU and related immune responses. We found that treatment with ITE effectively suppresses the development of EAU, as well as the cellular immune response against the uveitogenic molecule, IRBP.
Materials and Methods
Mice
Eight- to 12-week-old female B10.A mice were obtained from the National Cancer Institute Animal facility (Division of Cancer Treatment; Frederick, MD). The animals were housed in a pathogen-free facility and all experiments were performed in compliance with the ARVO Statement for The Use of Animals in Ophthalmic and Vision Research, under protocols approved by the Animal Care and Use Committee of the National Eye Institute, National Institutes of Health.
Reagents
Bovine whole IRBP was prepared as described by Pepperberg et al.18 Purified pertussis toxin (PTX) was purchased from List Biological Laboratories (Campbell, CA). Tuberculin purified protein derivative (PPD) was from Parke-Davis (Morris Plains, NJ). Complete Freund's adjuvant (CFA) was prepared by adding Mycobacterium tuberculosis H37RA (Difco, Detroit, MI) into incomplete Freund's adjuvant (Difco), to a concentration of 2.5 mg/mL. ITE (Tocris Bioscience, Bristol, UK) was prepared for use by solubilizing in dimethyl sulfoxide (DMSO) to 100 mM, followed by suspending the agent in PBS to a fine suspension by extended vortexing, or sonication.
Induction and Scoring of EAU
Mice were immunized by injection of 0.2 mL emulsion containing 40 μg bovine whole IRBP in CFA, administered by subcutaneous injections into the tail base and the two thighs. PTX, 0.2 μg, was injected intraperitoneally, concurrently with the immunization. On day 12 postimmunization, the mouse eyes were examined for inflammatory changes by fundoscopy, as detailed by Xu et al.19 The fundoscopy focused on vascular dilation, white focal vascular lesions, white linear vascular lesions, retinal hemorrhage, and retinal detachment. According to the severity of these changes, the EAU clinical scores were graded on a scale of 0 to 3. Following euthanization on day 14 postimmunization, the eyes were collected and ocular inflammation was assessed histologically, using a scale of 0 to 4, in half-point increments, as detailed elsewhere.20
The kinetics of EAU development in control and ITE-treated mice was analyzed by fundoscopy, at different time points following immunization, as indicated.
Treatment With ITE
Daily treatment started on day 0 and consisted of 200 μg of ITE suspended in 0.2 mL PBS, given intraperitoneally. Control mice were similarly treated with 0.2 mL of the vehicle, PBS containing 3.6% DMSO.
Lymphocyte Proliferation Responses
Draining lymph nodes (LN) were collected from immunized mice on day 14 postimmunization and pooled within each group. LN cells were cultured as detailed elsewhere.21 Briefly, the cells were cultured in quadruplicate in flat-bottomed 96-well plates, at 4 × 105 cells in 0.2 mL of RPMI-1640 medium, supplemented with 2% serum replacement HL-1 (Lonza, Walkersville, MD), 2-mercaptoethanol (50 μM), and antibiotics. The stimulants included IRBP at several concentrations and PPD, at 5 μg/mL. Following incubation for 72 hours, the cultures were pulsed with [3H]-thymidine (0.5 μCi/10 μL/well) for an additional 16 hours and the incorporated thymidine was assessed by liquid scintillation spectrometry. Data are presented as mean delta counts per minute (Δcpm).
Cytokine Production Assays
LN cells were cultured in 24-well plates at 5 × 106 cells/well in 1 mL medium detailed above and were stimulated with whole IRBP at 10 μg/mL. Supernatants were collected following incubation for 48 hours and their levels of IFN-γ, IL-17, IL-10, and granulocyte macrophage colony-stimulating factor (GM-CSF) were determined by ELISA kits (R&D Systems, Minneapolis, MN), according to the protocols recommended by the manufacturer.
Intracellular Expression of Cytokines
LN cells were collected on days 7 and 14 postimmunization and intracellular staining for IFN-γ, IL-17, and IL-10 was carried out as detailed elsewhere.22 Briefly, cultured cells were stimulated with 20 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich, St. Louis, MO) and 1mM ionomycin (Sigma-Aldrich) in the presence of GolgiStop (BD Bioscience, San Jose, CA) for 4 hours. After staining of surface markers (CD3 and CD4), cells were fixed, permeabilized by permeabilization/fixation buffer (BD Bioscience), and incubated with the cytokine-specific antibodies for 30 minutes according to the protocols recommended by the manufacturers (eBioscience, San Diego, CA). After staining, the cells were analyzed (FACSCalibur; BD Biosciences) and data were processed by Flojo (Tree Star, Ashland, OR).
Intracellular Expression of Foxp3
Spleen cells were used to test the expression of Foxp3, using the method described elsewhere.22 Briefly, isolated splenocytes were fixed and permeabilized at 4°C before intracellular staining with allophycoryanin-conjugated anti-Foxp3 antibody, following the protocols recommended by the manufacturer (eBioscience). After staining, the cells were analyzed (FACSCalibur) and data were processed by Flojo.
Serum Antibody Levels
Conventional ELISA was used to determine the levels of antibody against IRBP in the mouse sera, collected on day 14 postimmunization.
Statistical Analysis
Statistical analysis was performed using Prism software (GraphPad, San Diego, CA). P values less than 0.05 were considered significant.
Results
ITE Treatment Suppresses EAU Development
To examine the capacity of ITE to inhibit the induction of EAU, we treated groups of B10.A mice with the compound, as detailed in the Materials and Methods section, during the 14 days of disease development. Control groups were similarly treated with the vehicle. EAU development was monitored by clinical examination on day 12 and the mouse eyes were collected on day 14 for histological examination. Clinical and histological data of repeated experiments are summarized in Figures 1A and 1B, respectively, and show that treatment with ITE significantly inhibited EAU development, as determined by the two criteria. Representative eye sections of mice treated by ITE and their controls are shown in Figure 1C. Remarkably more severe changes are seen in the eye of the untreated control mouse, scored at “2.5,” than in the eye of the ITE-treated mouse, scored at “0.5.” The changes are detailed in the legend to Figure 1.
Figure 1.
Treatment with ITE-suppressed EAU development. (a) A summary of clinical scores, assessed on day 12 postimmunization and expressed as mean ± SEM for n = 20 mice per group. (b) A summary of histopathological scores of mouse eyes collected on day 14 postimmunization and presented as mean ± SEM for n = 25 mice per group. (c) Histological sections of representative eyes of each group, showing the reduced severity in the eye of the ITE-treated mouse. The severe changes in the control eye typically include retinal detachment with exudate accumulation in the subretinal space and infiltrating inflammatory cells in the retina and vitreous. Only mild changes are seen, on the other hand, in the eye of the ITE-treated mouse and mainly include inflammatory cell infiltration at the optic nerve head. The sections were stained with hematoxylin and eosin. Original magnification ×25 (top); ×100 (middle/bottom). (d) Kinetics of the inhibitory effect of ITE on the development of clinical EAU, as assessed by fundoscopy, at the indicated time points, in two separate experiments. Inhibition of ocular inflammation is observed throughout the tested time period. **P < 0.001.
To examine the effect of ITE on the kinetics of EAU development, we monitored by fundoscopy the disease levels at different time points following immunization in ITE-treated mice and their controls. Figure 1D records the results of two experiments and shows that the treatment delayed disease onset and inhibited the development of ocular inflammatory changes throughout the tested period.
ITE Treatment Suppresses Cellular Immune Response Against the Uveitogenic Antigen
EAU is mediated by cellular immune response against the immunopathogenic antigen, IRBP.14,15 To determine the effect of ITE on the development of cellular immune response, we collected the draining LNs of mice of the two groups and tested their cells for proliferative response against several concentrations of IRBP. Data of a representative experiment are shown in Figure 2A and the data of 6 to 8 experiments are summarized in Figure 2B. Lymph node cells from the control mice responded considerably more vigorously than cells from the ITE-treated mice when stimulated by the different tested IRBP concentrations.
Figure 2.
Treatment with ITE reduced the antigen-specific T-cell proliferative responses. Draining LN cells were collected on day 14 postimmunization and their proliferative response was determined by the thymidine incorporation assay as detailed in the Materials and Methods section. (a) Data of a representative experiment, expressed as means ± SEM response of quadruplicate wells (n = 5 mice per group). (b) Combined data of six to eight repeated experiments, presented as the ratio between the levels of thymidine incorporated in cultures of ITE-treated group/levels in cultures of the corresponding controls. *P < 0.05; **P < 0.005.
ITE Treatment Reduces the Proportions of Cells Expressing IFN-γ, IL-17, and IL-10 in Draining LNs
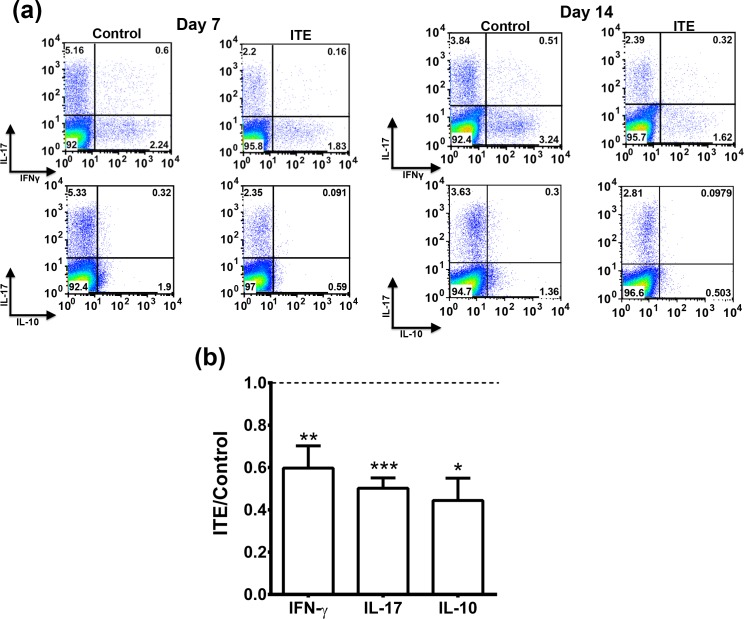
To further learn about the effect of ITE treatment on the immune system, we analyzed lymphocytes of the draining LNs of mice of the two groups for their intracellular expression of the “signature cytokines” of Th1 and Th17 (i.e., IFN-γ and IL-17, respectively). Flow cytometric data of representative experiments are recorded in Figure 3A, and data collected in two to seven repeated experiments are summarized in Figure 3B. In all repeated experiments, the proportions of cells expressing IFN-γ or IL-17 were lower in the ITE-treated mice than in their controls. Similar effects were seen on days 7 and 14 postimmunization.
Figure 3.
ITE treatment reduced the proportions of cells expressing IFN-γ, IL-17, or IL-10. Draining LN cells collected on days 7 or 14 postimmunization were tested by flow cytometry for intracellular expression of the three cytokines. (a) Representative experiments; (b) Data of combined two to seven experiments, presented as the ratio between the percentage of cells expressing the tested cytokine among LN cells from the ITE-treated mice and the percentage in the corresponding control mice. *P < 0.05; **P < 0.005; ***P < 0.0001.
We also determined the proportions of cells expressing IL-10, a cytokine made by various subpopulations of LN cells. The proportions of cells producing IL-10 were remarkably lower than those of cells expressing IFN-γ or IL-17 (Fig. 3A). IL-10 has immunosuppressive activity, but, unexpectedly, the proportions of cells expressing this cytokine were lower in LNs of ITE-treated mice than in their controls in all experiments.
ITE Treatment Reduces the Secretion of Inflammatory Cytokines by Cultured Draining LN Cells
To further analyze the immunosuppressive effect of ITE treatment on the different Th subpopulations, we collected the supernatants of the cultured LN cells and measured by ELISA the levels of IL-17, IFN-γ, and IL-10. Data of representative experiments with LN cells collected on days 7 and 14 postimmunization are shown in Figure 4A and data of this and four to six additional experiments are summarized in Figure 4B. In accord with the patterns of intracellular expression of the three cytokines by lymphocytes of the two groups, the levels of the three cytokines were lower in cultures of cells of the ITE-treated mice than in their controls. In addition to the mentioned three cytokines, we also measured the levels of GM-CSF, a cytokine that was recently reported to play a major role in the immunopathogenic process of EAE.23,24 As shown in Figures 4A and 4B, LN cells from ITE-treated mice secreted lower levels of GM-CSF than did their controls.
Figure 4.
Treatment with ITE suppressed the secretion of inflammatory cytokines by LN cells. Cells of draining LNs, collected on day 14 postimmunization, were incubated with IRBP, at 1 μg/mL. The 48-hour culture supernatants were tested by ELISA for the four indicated cytokines. (a) Data of representative experiments, presented as actual levels measured in the culture supernatants. (b) Data of combined four to six experiments, presented as the ratio between the cytokine levels in cultures of ITE-treated mice and the levels in cultures of the corresponding controls. *P < 0.05; **P < 0.005; ***P < 0.0001.
ITE Treatment Moderately Increases the Proportion of Foxp3+ Cells
Treg cells typically express the transcription factor Foxp34,5 and are identified by the expression of this factor. To determine the proportion of CD4 cells expressing Foxp3 in our experimental mice, we used the flow cytometric assay, as described in Materials and Methods. We tested spleen and LN cells from mice on days 7 or 14 postimmunization and the data of representative experiments are depicted in Figure 5A. Data of repeated experiments are combined in Figure 5B. The percentage of Foxp3+ cells was moderately higher in the ITE-treated mice than in their controls. It is of note that in both mouse groups the percentage of Foxp3+ was higher on day 14 than on day 7 postimmunization.
Figure 5.
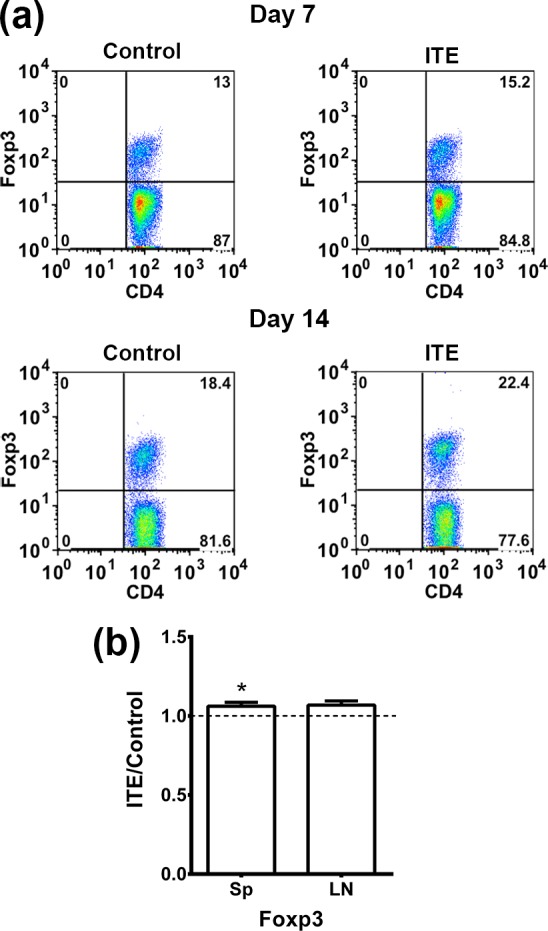
Treatment with ITE moderately increased the percentage of Foxp3+ T- cells. Spleen and draining LN cells were collected on days 7 and 14 postimmunization from mice of the two groups and intracellular expression of Foxp3 was measured using flow cytometry of CD3+CD4+ gated cells. (a) Data from a representative experiment with spleen cells of the two groups. (b) Data of combined experiments, expressed as the ratio between the values in cultures from ITE-treated and cultures of untreated controls. Only spleen cells were examined in eight experiments, whereas both spleen and LNs were tested in three experiments. Sp, spleen. *P < 0.05.
Treatment With ITE Does Not Affect Antibody Production
To measure the levels of antibodies against the immunizing antigen, IRBP, we employed the ELISA. The responses of pooled sera of mice of the two groups are shown in Figure 6. Essentially no difference was detected between sera of the two groups, suggesting that ITE treatment had little or no effect on the activity of B cells.
Figure 6.
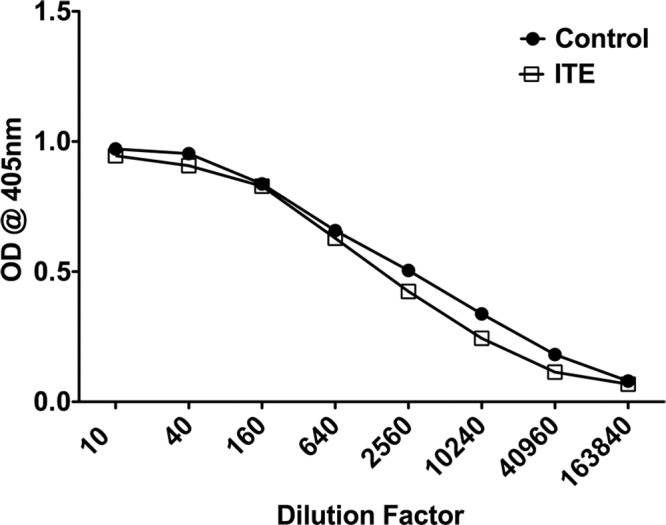
Treatment with ITE had little to no effect on the production of serum antibody levels against IRBP. Sera were collected on day 14 postimmunization and antibody levels against IRBP were measured by ELISA. Each point represents the mean optical density at 405 nm for two replicate experiments.
Discussion
Data recorded here show for the first time that treatment of mice with ITE, an endogenous nontoxic AHR ligand, suppresses the development of EAU, an animal model for various uveitic conditions in humans,14,15 as well as the cellular immune response against the uveitogenic antigen, IRBP. The inhibition of EAU was demonstrated by both the clinical (fundoscopy) and histological examinations; compilation of repeated experiments showed that the suppression was significant as determined by both parameters (Fig. 1). Our data are in line with those of Quintana et al.,17 showing that treatment of mice with ITE effectively inhibited the development of EAE, an animal disease that serves as a model for multiple sclerosis.10,11 Our findings are also in accord with the observation that EAU development is inhibited by another AHR ligand, TCDD, a compound that is highly toxic and, therefore, cannot be used in humans.13 Fundoscopy analysis showed that ITE treatment inhibited the development of EAU at all tested time points (Fig. 1D).
Treatment with ITE also inhibited the cellular immune responses against the uveitogenic antigen, IRBP, and the CFA component, PPD, as measured in vitro. The inhibition was demonstrated by reduced proliferation (Fig. 2) and release of IFN-γ and IL-17 by cultured LN cells when exposed to the immunizing antigens (Fig. 4). Furthermore, we also analyzed the cytokine production by intracellular flow cytometry, an assay that allowed us to demonstrate the inhibited immune response by the reduced proportions of cells expressing IFN-γ and IL-17, the signature cytokines of Th1 and Th17, respectively (Fig. 3). The effect of AHR engagement by agents such as ITE on the immune response is attributed to a large extent to its capacity to reduce activation of Stat1.12,25 Reduced Stat1 decreases the differentiation of Th17 cells, but favors the activation of Foxp3 Treg cells. Our finding, showing a moderately increased proportion of Foxp3+ cells in ITE-treated mice, is in accord with these assumptions. The enhanced Treg cell activity in ITE-treated mice is assumed to interfere with IFN-γ production and, consequently, to reduce Th1 cell development.13
Treatment with ITE also inhibited the production of IL-10 in our culture system. This observation differs from those showing increased IL-10 production in mice treated with ITE,17 or TCDD13 during the development of EAE or EAU, respectively. The difference between our study and the other two could be attributed to the use of different mouse strains, namely, B10.A in our study and C57Bl/6 in the other two studies. It is conceivable, therefore, that different cell populations, with different responses to AHR, are responsible for the production of the bulk of IL-10 in the two lines of mice.
The immunosuppressive effects of ITE in the EAU system resemble those of another AHR ligand, TCDD.13 Unlike TCDD, however, ITE is nontoxic, a crucial feature that makes it possible to consider ITE for use as an immunosuppressant agent in humans affected by immune-mediated eye disease. ITE was also found to inhibit the development of EAE17 and early studies are in progress to test the therapeutic effects of this agent in patients with multiple sclerosis.
Acknowledgments
The authors thank the Flow Cytometry and Histology Cores, National Eye Institute, National Institutes of Health, for excellent technical support.
Supported by the Intramural Research Program of the National Eye Institute, National Institutes of Health.
Disclosure: L.F. Nugent, None; G. Shi, None; B.P. Vistica, None; O. Ogbeifun, None; S.J.H. Hinshaw, None; I. Gery, None
References
- 1. Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010; 238: 247–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balasubramani A, Mukasa R, Hatton RD, Weaver CT. Regulation of the Ifng locus in the context of T-lineage specification and plasticity. Immunol Rev. 2010; 238: 216–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakayamada S, Takahashi H, Kanno Y, O'Shea JJ. Helper T cell diversity and plasticity. Curr Opin Immunol. 2012; 24: 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003; 299: 1057–1061 [PubMed] [Google Scholar]
- 5. Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009; 30: 636–645 [DOI] [PubMed] [Google Scholar]
- 6. Korn T. How T cells take developmental decisions by using the aryl hydrocarbon receptor to sense the environment. Proc Natl Acad Sci U S A. 2010; 107: 20597–20598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Veldhoen M, Duarte JH. The aryl hydrocarbon receptor: fine-tuning the immune-response. Curr Opin Immunol. 2010; 22: 747–752 [DOI] [PubMed] [Google Scholar]
- 8. Gandhi R, Kumar D, Burns EJ, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 2010; 11: 846–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008; 453: 106–109 [DOI] [PubMed] [Google Scholar]
- 10. Constantinescu CS, Farooqi N, O'Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 2011; 164: 1079–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pierson E, Simmons SB, Castelli L, Goverman JM. Mechanisms regulating regional localization of inflammation during CNS autoimmunity. Immunol Rev. 2012; 248: 205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quintana FJ, Basso AS, Iglesias AH, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008; 453: 65–71 [DOI] [PubMed] [Google Scholar]
- 13. Zhang L, Ma J, Takeuchi M, et al. Suppression of experimental autoimmune uveoretinitis by inducing differentiation of regulatory T cells via activation of aryl hydrocarbon receptor. Invest Ophthalmol Vis Sci. 2010; 51: 2109–2117 [DOI] [PubMed] [Google Scholar]
- 14. Nussenblatt RB, Gery I. Experimental autoimmune uveitis and its relationship to clinical ocular inflammatory disease. J Autoimmun. 1996; 9: 575–585 [DOI] [PubMed] [Google Scholar]
- 15. Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010; 120: 3073–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song J, Clagett-Dame M, Peterson RE, et al. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci U S A. 2002; 99: 14694–14699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quintana FJ, Murugaiyan G, Farez MF, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010; 107: 20768–20773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pepperberg DR, Okajima TL, Ripps H, Chader GJ, Wiggert B. Functional properties of interphotoreceptor retinoid-binding protein. Photochem Photobiol. 1991; 54: 1057–1060 [DOI] [PubMed] [Google Scholar]
- 19. Xu H, Koch P, Chen M, Lau A, Reid DM, Forrester JV. A clinical grading system for retinal inflammation in the chronic model of experimental autoimmune uveoretinitis using digital fundus images. Exp Eye Res. 2008; 87: 319–326 [DOI] [PubMed] [Google Scholar]
- 20. Hikita ST, Vistica BP, Jones HR, et al. Osteopontin is proinflammatory in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2006; 47: 4435–4443 [DOI] [PubMed] [Google Scholar]
- 21. Fujimoto C, Klinman DM, Shi G, et al. A suppressive oligodeoxynucleotide inhibits ocular inflammation. Clin Exp Immunol. 2009; 156: 528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi G, Lovaas JD, Tan C, et al. Cell-cell interaction with APC, not IL-23, is required for naive CD4 cells to acquire pathogenicity during Th17 lineage commitment. J Immunol. 2012; 189: 1220–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Codarri L, Gyulveszi G, Tosevski V, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011; 12: 560–567 [DOI] [PubMed] [Google Scholar]
- 24. El-Behi M, Ciric B, Dai H, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011; 12: 568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A. 2008; 105: 9721–9726 [DOI] [PMC free article] [PubMed] [Google Scholar]