Abstract
Objective
To examine and compare brain activation patterns of premenopausal women with normal sexual function and those with hypoactive sexual desire disorder (HSDD) during viewing of validated sexually explicit film clips.
Design
Cross-sectional pilot study.
Setting
University-based clinical research center.
Patient(s)
Premenopausal women.
Intervention(s)
None.
Main Outcome Measure(s)
Areas of brain activation during viewing of sexually explicit film clips.
Result(s)
Women with normal sexual function showed significantly greater activation of the right thalamus, left insula, left precentral gyrus, and left parahippocampal gyrus in comparison with women with HSDD, who exhibited greater activation of the right medial frontal gyrus and left precuneus regions.
Conclusion(s)
Women with HSDD may have alterations in activation of limbic and cortical structures responsible for acquiring, encoding, and retrieving memory, the processing and memory of emotional reactions, and areas responsible for heightened attention to one’s own physical state.
Keywords: Brain activation patterns, female sexual dysfunction, functional neuroimaging, hypoactive sexual desire disorder (HSDD), sexual stimuli
Female sexual dysfunction (FSD) is an important problem that has a significant impact on quality of life for many women. The most prevalent subtype of FSD is hypoactive sexual desire disorder (HSDD), which is defined by the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) as the persistent or recurrent deficiency (or absence) of sexual fantasies and desire for sexual activity that causes marked distress or interpersonal difficulty (1). Estimates of its prevalence vary from 12% to 19%, depending on the methods used for diagnosis (2).
The etiology of HSDD has not been completely elucidated, but it is thought to be multifactorial, with neuroendocrine, psychiatric, and behavioral components playing a role. Previous studies have focused primarily on behavioral and biologic aspects of the disorder, with use of validated behavioral questionnaires and hormonal assays being the predominant methods of assessment. However, exclusive use of these methods has not yet yielded a satisfactory explanation for the cause and development of female sexual dysfunction disorders (3).
An exciting area of research that is now being applied to the study of female sexual function and dysfunction is functional neuroimaging. Functional magnetic resonance imaging (fMRI) is a neuroimaging technique that measures oxygen metabolism and brain blood flow, which are correlated with neuronal activity evoked by cognitive/mental processes (4–7). The potential role of fMRI in the study of female sexual response and FSD has only recently been realized and will undoubtedly add an invaluable contribution to our understanding of female sexual functioning (8).
Previous neuroimaging studies have investigated the brain regions activated while women viewed sexually explicit videos (9–12). Results from these studies, as well as our current investigation, provide evidence that limbic, somatosensory, visual, and association cortices are active while women with normal sexual function view videos of heterosexual couples engaging in sexual activity. More specifically, the brain areas previously reported to be activated in the processing of sexually explicit stimuli include the occipitotemporal, anterior cingulate, medial prefrontal, orbitofrontal, precentral, insular cortex, hypothalamus, thalamus, and amygdala (13).
Only one study to date has directly compared brain activation patterns in women with normal sexual function with women diagnosed with HSDD. Arnow et al. (9) demonstrated that women with normal sexual function showed brain activations in multiple regions, most prominently in the middle occipital gyrus, anterior cingulate cortex, bilateral thalamus, caudate nucleus, left globus pallidus, and cerebellum while women with HSDD showed activation of the left inferior parietal lobule, postcentral gyrus, and precuneus. When comparing the two groups, women with normal sexual function showed greater activation of the bilateral entorhinal cortex while women with HSDD exhibited greater activation of the medial frontal gyrus, right inferior frontal gyrus, and bilateral putamen. The medial frontal gyrus activation has been associated with self-monitoring (14); thus, it is possible that women with HSDD allocate more attention to monitoring their response, which may be inhibitory to sexual functioning.
Clearly, there is a need for further neuroimaging studies of women with HSDD. Our study determined whether there are differences in brain activation patterns upon viewing sexually explicit film clips by women with acquired HSDD compared with those with normal sexual function. We hypothesized that women with HSDD will exhibit brain activation patterns that are different than those that are seen in women with normal sexual function.
MATERIALS AND METHODS
Participants
A total of 16 healthy premenopausal women were recruited to participate in the study. The participants were recruited by Internet announcements, and they were screened by telephone to determine initial eligibility. The inclusion criteria for women with normal sexual function were that they had to be premenopausal, right-handed, strictly heterosexual, in a stable relationship of at least 1 year duration (with an expectation of remaining in the same relationship for at least 1 more year), and have had experience viewing sexually explicit images. Exclusion criteria were a known history of mental illness, a history of drug or alcohol abuse, diagnosis of a female sexual function disorder, use of other medication or herbal preparations for the purpose of improving sexual performance, a history of sexual offenses, an abnormality in vision that would impair viewing the images, and implants that would preclude fMRI procedures (such as pacemakers). For women with HSDD, the inclusion and exclusion criteria were the same except that they had to meet the criteria for the diagnosis of HSDD, as diagnosed by clinical interview and validated questionnaires.
Study Design
The study was approved by the Human Investigation Committee at the Wayne State University School of Medicine. If a participant met the study criteria based on the initial telephone screen, she was invited to the Clinical Research Center at the Department of Obstetrics and Gynecology. Informed consent was obtained before initiating study-related activities. The participant completed a series of questionnaires, including the Sexual Function Questionnaire (SFQ) (15), the Female Sexual Distress Scale (FSDS) (16), and a demographics form that included questions about age, medical history, level of education, race, ethnicity, relationship status, and sexual orientation. A urine pregnancy test was administered to rule out pregnancy.
The diagnosis of acquired HSDD was defined by DSM-IV criteria and confirmed by a structured clinical interview. Depression was ruled out using the patients’ reported medical history and questions asked during the structured clinical interview. Additionally, scores from the Sexual Function Questionnaire (SFQ) and the Female Sexual Distress Scale (FSDS) (with a scores of ≥23 for the desire domain of the SFQ and of ≤15 for the FSDS, respectively) were also used to assist in the diagnosis of HSDD.
Upon completion of these activities, the participant was taken to the fMRI suite at Harper University Hospital. The participant viewed sexually explicit video clips that were previously validated to be arousing in a population of premenopausal women (17). During functional scanning, she viewed sexually explicit scenes that were projected from a laptop computer in the magnetic resonance control room through an Avotec Silent Vision projection system, which projected images onto a screen attached inside the bore of the scanner. A mirror situated no more than six inches in front of the participant’s face enabled her to view the videos being projected on the screen.
A standard fMRI block design was used with 12 alternating 1-minute blocks of a blue control screen, neutral video, and sexually explicit video for 32 minutes (blue, sexual, blue, neutral, blue, and sexual, etc.). Sexually explicit images consisted of male and female heterosexual intercourse and other sexual activity. Control images consisted of male and female interactions in a nonsexual manner. In between each sexually explicit and active control film clip, participants viewed a 1-minute interlude of a blank blue screen to allow arousal to return to baseline. This additional control procedure was included to minimize “carryover” of arousal into the active control condition. At the completion of the study, participants were compensated $100.00 for their time and inconvenience.
Data Acquisition
Functional MRI was performed on a 3T Siemens Verio scanner. Head movement was limited by using individually molded foam head holders. Prior to fMRI, a high-resolution T1-weighted structural scan was acquired to rule out structural abnormalities and for subsequent anatomic localization. Functional MRI to acquire BOLD contrast used echo planar imaging (EPI) (EPI gradient refocused images; Field of view = 64 × 64; TR = 3,000 ms; TE = 39 ms; voxel size = 4 × 4 × 5). Slices were oriented axially or near axially along the AC-PC line. The first four volumes from each run were discarded to allow for T1 equilibration effects.
Data Analysis and Main Outcome Measures
The data were analyzed using Statistical Parametric Mapping 2 (SPM2; Department of Cognitive Neurology, London; www.fil.ion.ucl.ac.uk/spm). Realignment, normalization, and smoothing were performed using standard SPM2 algorithms. All analyses were performed on brains that have been spatially normalized to the Montreal Neurological Institute (MNI) high-resolution T1 template and subsequently converted to Talairach space using the icbm2tal application from the BrainMap Web site (18). The Talairach client was used to label data (19, 20). Images were resampled into this space with 2-mm isotropic voxels and smoothed with a Gaussian kernel of 6 mm full-width at half-maximum height to minimize noise and residual differences in gyral anatomy.
The general linear model was applied from which statistical inferences were based on the theory of random Gaussian fields, and changes relative to the experimental conditions were modeled by convolution of boxcar epochs with the canonical hemodynamic response function (HRF) to approximate the activation patterns (21). After normalization, subject-specific parameter estimates pertaining to each regressor was calculated at each voxel, and contrast images (statistical parametric mapping, SPMs) were calculated by applying appropriate linear contrasts to the parameter estimates. This first-level analysis (individual subject) was performed on individual subject data in which regional brain activation during the erotic video was contrasted with the active (nonsexual) control video to localize brain systems responsive to sexual arousal.
Once these neural systems were identified, group comparisons were performed. The individual subject contrast images were compared using a one-sample t-test across the subject group in a second-level, random effects (e.g., mixed effects model) analysis to allow for inferences to the general population. Subsequently, between-group differences in each contrast of interest were examined using two-sample t-tests and analysis of covariance (ANCOVA) models to correct for age. From voxel-wise comparisons, activation foci within a priori regions were considered statistically significant with a height threshold of P<.001 uncorrected, with an extent threshold of at least five contiguous voxels.
RESULTS
Subject Characteristics
Six women had normal sexual function, and 10 women had symptoms consistent with HSDD. Women with normal sexual function were statistically significantly younger, having a mean age of 29 (±4.4) years versus 37 (±7.4) years in women with HSDD (P≤.05). Three women with normal sexual function and two women with HSDD reported current use of hormonal contraception. As expected, the mean scores for the SFQ desire domain was higher for women with normal sexual function: 21.2 (±2.4) versus 12.6 (±2.8) in the HSDD group (P≤.001). Accordingly, women with normal sexual function had lower distress as measured by the FSDS, with mean scores of 3.0 (±5.6) versus 25.3 (±7.7) in the HSDD group (P≤.001) (Supplemental Table 1, available online).
Brain Activation During Viewing of Sexually Explicit Video Clips
For women with normal sexual function, the most significant activation was found at the lingual gyrus, inferior parietal lobule, thalamus, superior temporal gyrus, and insula on the right and at the culmen of the vermis, parahippocampal gyrus, claustrum, hippocampus, and precentral gyrus on the left. Bilateral activation of the insula and claustrum was also seen (Fig. 1 and Supplemental Table 2). In women diagnosed with hypoactive sexual desire disorder (HSDD), significant activation was found at the right middle temporal and fusiform gyri; on the left, activation was found at the caudate body, declive, precuneus, anterior cingulate gyrus, amygdala, and middle occipital gyrus. There was also bilateral activation of the thalamus, medial frontal gyrus, and culmen (Fig. 2 and Supplemental Table 3).
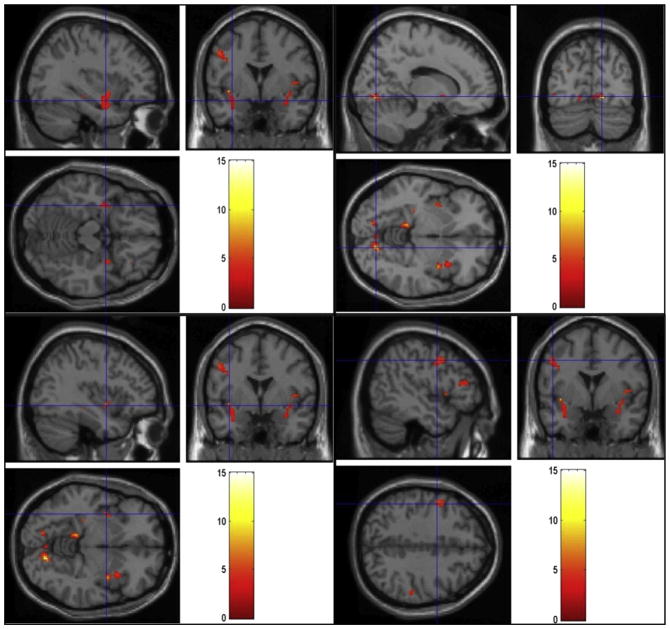
FIGURE 1.
Regions of activation during viewing of sexual videos in the normal group. Cross-hairs reflect local maxima in MNI space (x, y, z coordinates). Left parahippocampal gyrus (top left), right lingual gyrus (top right), bilateral claustrum/insula (bottom left), and left precentral gyrus (bottom right).
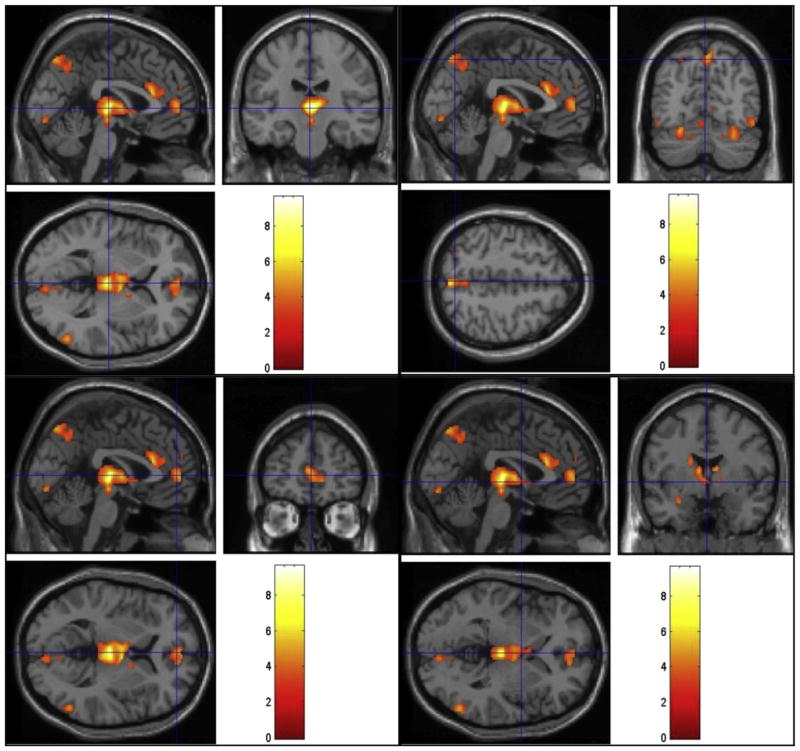
FIGURE 2.
Regions of activation during viewing of sexual videos in the hypoactive sexual desire disorder group. Cross-hairs reflect local maxima in MNI space (x, y, z coordinates). Bilateral thalamus (top left), left precuneus (top right), bilateral medial frontal gyrus (bottom left), and left amygdala (bottom right, outside of the cross-hairs in coronal view).
Determination of Group Differences in Brain Activation
Women with normal sexual function showed significantly greater activation of the right thalamus and the left insula, middle occipital gyrus, precentral gyrus, inferior frontal gyrus, precuneus, parahippocampal gyrus, and supramarginal gyrus in comparison with women with HSDD (Fig. 3 and Supplemental Table 4). In contrast, women with HSDD showed greater activation of the right medial frontal gyrus and left precuneus (Fig. 4 and Supplemental Table 5).
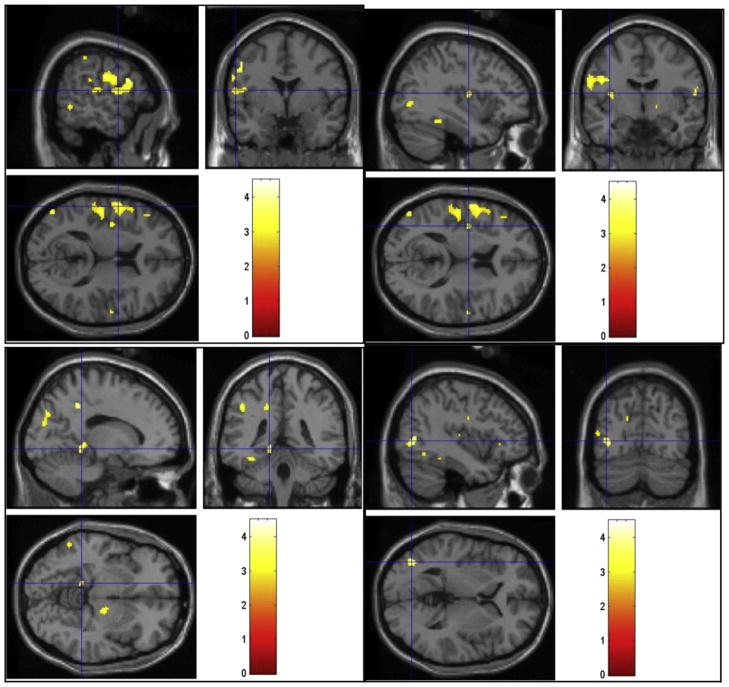
FIGURE 3.
Regions of activation that were greater in the normal group than the hypoactive sexual desire disorder group during viewing of sexual videos. Cross-hairs reflect local maxima in MNI space (x, y, z coordinates). Left precentral gyrus (top left), left insula (top right), left parahippocampal gyrus (bottom left), and left middle occipital gyrus (bottom right).
FIGURE 4.
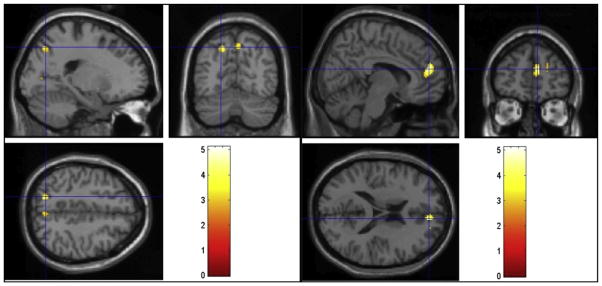
Regions of activation that were greater in the hypoactive sexual desire disorder group than the normal group during viewing of sexual videos. Cross-hairs reflect local maxima in MNI space (x, y, z coordinates). Left precuneus (left) and right medial frontal gyrus (right).
DISCUSSION
Our findings illustrate brain activation patterns of women with normal sexual function and those with HSDD during viewing of sexually explicit videos; furthermore, they show that there are several striking differences in activation between the two groups, some of which may help explain the underlying neural mechanisms involved in the female sexual response.
In women with normal sexual function, there were multiple areas that were activated during viewing of sexually explicit film clips, including cortical areas of the limbic system (the bilateral insula, left hippocampus, and left parahippocampal gyrus), which have all been shown to be activated in previous studies of women with normal sexual function (22–24). The insula’s activation during the viewing of sexual videos (12, 22, 25, 26), electrical stimulation of the clitoris (27), and orgasm in women (28, 29) may be due to its regulatory role of autonomic nervous system response during sexual arousal (12) or more generally to its role in integrating viscerosensory information (30). A related possibility is that the insula integrates the states of physical and emotional pleasure one experiences during sexual arousal (31).
The activation of the hippocampus may represent its critical role in learning and memory (32) and its importance for relating sensory stimuli to past memories (33). Memories of past experiences, both positive and negative, can influence sexual response (34). It is interesting that women with a history of sexual abuse and posttraumatic stress disorder (PTSD) have been found to have a smaller hippocampus and do not exhibit activation of this region during memory tasks compared with controls (35).
There was significant activation of the cerebellum, comparable with patterns seen in previous studies. Although the cerebellum is primarily thought to be involved with movement coordination and motor learning, it may also be important for cognitive, emotional, and motivational processes (36, 37). Cerebellar activation has been noted when subjects are exposed to emotionally laden stimuli (38) as well as excerpts from erotic films (39, 40). The cerebellar vermis has been implicated as a key region that is involved with sexual arousal, specifically the induction of penile erection in men (41).
In women with HSDD, a different pattern of brain activation emerged, with significant activation seen in cortical structures such as the bilateral medial frontal gyrus and left anterior cingulate gyrus as well as limbic structures including the left amygdala and bilateral thalamus. The medial frontal gyrus has been implicated in attention and reflection about one’s emotions and mental states (42, 43). Arnow et al. (9) also identified activation of this region in women with HSDD and proposed that these women allocated more attention to monitoring and evaluating their response to the sexual stimuli presented. Such self-monitoring has been observed to be associated with sexual dysfunction (44).
The anterior cingulate gyrus plays a role in the evaluation of motivational/emotional information and the initiation of goal-directed behavior (45) and has been found to be activated by visually evoked sexual arousal (12, 22). In a review of the function of the anterior cingulate cortex, it was noted that 16 out of 19 neuroimaging studies of sexual response showed activation of this region and that it was the most frequently activated brain structure; however, recent studies have suggested a much broader range of function for this cortical area, including homeostasis, pain, desire, addiction, and cognition (46) as well as its involvement in disorders such as depression (47), obsessive-compulsive disorder (48), apathy, and posttraumatic stress disorder (49). Given its extensive involvement, it is difficult to know whether activation of this area in women with HSDD reflected the evaluation of emotional information or some other function; furthermore, this differentiation may depend on structural and functional heterogeneity of this region.
The amygdala has a central role in emotional processing, learning, and memory. Amygdalar activation has been demonstrated in response to sexual stimuli (12, 39, 50, 51). A strong positive correlation between emotional arousal and amygdala activity have been reported for both appetitive and aversive stimuli (52, 53). The amygdala is also known to play an important role in fear conditioning and is implicated in the pathophysiology of anxiety disorders (54). Whether amygdalar activation in women with HSDD represents anxiety, fear, or another negative emotional response is an intriguing question that remains unanswered.
For comparing brain activations patterns between women with normal sexual function and those with HSDD, ANCOVA was used to correct for the age difference we observed between the two groups. Women with normal sexual function showed significantly greater activation of the right thalamus, left insula, left precentral gyrus, and left parahippocampal gyrus. The thalamus is a multifunctional brain region that has traditionally been considered to be a “relay station” that handles sensory and motor inputs to and from the ipsilateral cerebral hemisphere. However, in addition to sensory and motor integration, it plays a role in arousal, consciousness, affective behavior, and memory as well as the processing of emotionally laden stimuli (55). Thalamic activation has been demonstrated in numerous studies of men viewing sexually explicit film clips (12, 56, 57) and some of women (10, 22), suggesting its integral role in the sexual response.
The parahippocampal gyrus is implicated in memory encoding and retrieval and is activated in healthy women with normal sexual response (24, 58). The entorhinal cortex, which serves as the main interface between the hippocampus and neocortex, has been found to be more significantly activated in women with no history of sexual dysfunction (9). Activation of this area may correlate with the superior ability of women with normal sexual function, compared with HSDD women, to establish and retrieve emotional memories related to sexual events. It is interesting that the parahippocampal gyrus has been found to be more significantly activated in surgically postmenopausal women after estrogen/testosterone treatment compared with baseline, suggesting that sex steroids can enhance the limbic system response to visual sexual stimuli (23).
In the comparison between the two groups, women with HSDD demonstrated significant activation of the left precuneus as well as continued to exhibit greater activation of the right medial frontal gyrus (as previously discussed). Activation of the precuneus is associated with the recall of episodic memory (59), including past episodes related to the self (60). The precuneus is also involved in source memory, or the awareness of an event with regard to time and place, which allows an individual’s current knowledge to be associated with past experiences (61). It is possible that the increased activation of the precuneus in women with HSDD represents greater effort in the attempt to retrieve past memories within a particular context.
Together, these findings confirm previous findings of brain activations that have been observed in women with normal sexual function while watching sexually explicit video clips. In addition, they support the possible role of memory encoding/retrieval and self-monitoring in women’s sexual response and suggest that women with HSDD may have alterations in activation of the structures that are responsible for these tasks.
Our study has several limitations; we did not control for menstrual phase, which has previously been shown to influence brain activation patterns during viewing of sexually explicit stimuli (10, 62) and male faces (63). We also did not control for hormonal contraceptive use. But recent data suggest that hormonal influences on brain activation patterns may not be of much significance when the erotic stimulus is direct and explicit, as it was in our study (64). We also did not obtain hormonal or peripheral data (such as androgen levels or vaginal pulse amplitude by vaginal photoplethysmography); these parameters have not been shown to reliably differentiate between premenopausal women with normal sexual function and those with HSDD (9, 65). Our study involved a small sample of healthy women, which while appropriate for this study may limit the generalizability of our findings; however, we find it reassuring that our results replicate previous findings in functional neuroimaging studies of sexual function in women. Moreover, although we tried to minimize carryover of arousal to our neutral video clips by including 1-minute viewing of a blank screen, the time for arousal-mediated body or brain events to return to baseline is unknown. As a result, our control condition may have been associated with lingering sexual arousal or thoughts that could have served to minimize or even eliminate some differences between conditions.
Even though we corrected for age in the comparative analysis between the two groups, it is possible that age may have still exerted an effect on the findings. Although it is unlikely that such effects would be hormonally related (all patients were premenopausal), it is possible that sexual desire and arousal could be influenced by life stage. It is possible that the women with HSDD, who were older, had life experiences and greater responsibilities such as caring for children and/or older parents as well as career demands that would predispose them to sexual dysfunction. Although the participants were screened for relationship and social issues that could account for their sexual problems, they are sometimes not realized by the patient and not reported.
CONCLUSION
In conclusion, we have identified regions of brain activation in women with normal sexual function and those with HSDD during viewing of sexually explicit videos and have shown that there are differences in activation between these two groups, specifically in areas responsible for memory encoding/retrieval and self-monitoring. These tasks may play an important role in the female sexual response and may be altered in women with HSDD. Identification of these areas could potentially serve as therapeutic targets for the treatment of HSDD.
Supplementary Material
Acknowledgments
Supported by a grant from the National Institutes of Health, National Institute of Child Health and Human Development (Grant HD-01254) (to T.L.W.), and a grant from SAMSA: Primary & Behavioral Health Care Integration (PPHF-2012) (to M.T.)
Footnotes
T.L.W. has nothing to disclose. N.T.N. has nothing to disclose. R.B. has nothing to disclose. M.T. has nothing to disclose. M.P.D. has received grants from NIH/NICHD, BioSante, and Boeringer Ingleheim, has received payment for lectures from Boeringer Ingeleheim, and is a board member of the American Society for Reproductive Medicine.
References
- 1.American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Hayes RD, Dennerstein L, Bennett CM, Fairley CK. What is the “true” prevalence of female sexual dysfunctions and does the way we assess these conditions have an impact? J Sex Med. 2008;5:777–87. doi: 10.1111/j.1743-6109.2007.00768.x. [DOI] [PubMed] [Google Scholar]
- 3.Simon JA. Low sexual desire—is it all in her head? Pathophysiology, diagnosis, and treatment of hypoactive sexual desire disorder. Postgrad Med. 2010;122:128–36. doi: 10.3810/pgm.2010.11.2230. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 5.Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA. 1992;89:5675–9. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA. 1986;83:1140–4. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89:5951–5. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodard TL, Diamond MP. Contribution of imaging to our understanding of sexual function and dysfunction. Adv Psychosom Med. 2008;29:150–68. doi: 10.1159/000126629. [DOI] [PubMed] [Google Scholar]
- 9.Arnow BA, Millheiser L, Garrett A, Lake Polan M, Glover GH, Hill KR, et al. Women with hypoactive sexual desire disorder compared to normal females: a functional magnetic resonance imaging study. Neuroscience. 2009;158:484–502. doi: 10.1016/j.neuroscience.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 10.Gizewski ER, Krause E, Karama S, Baars A, Senf W, Forsting M. There are differences in cerebral activation between females in distinct menstrual phases during viewing of erotic stimuli: a fMRI study. Exp Brain Res. 2006;174:101–8. doi: 10.1007/s00221-006-0429-3. [DOI] [PubMed] [Google Scholar]
- 11.Jeong GW, Park K, Youn G, Kang HK, Kim HJ, Seo JJ, et al. Assessment of cerebrocortical regions associated with sexual arousal in premenopausal and menopausal women by using BOLD-based functional MRI. J Sex Med. 2005;2:645–51. doi: 10.1111/j.1743-6109.2005.00134.x. [DOI] [PubMed] [Google Scholar]
- 12.Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park KJ. The role of functional MRI in neural assessment of female sexual dysfunction. Current Sexual Health Reports. 2007;4:33–40. [Google Scholar]
- 14.Castelli F, Happe F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–25. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- 15.Quirk FH, Heiman JR, Rosen RC, Laan E, Smith MD, Boolell M. Development of a sexual function questionnaire for clinical trials of female sexual dysfunction. J Womens Health Gend Based Med. 2002;11:277–89. doi: 10.1089/152460902753668475. [DOI] [PubMed] [Google Scholar]
- 16.Derogatis LR, Rosen R, Leiblum S, Burnett A, Heiman J. The Female Sexual Distress Scale (FSDS): initial validation of a standardized scale for assessment of sexually related personal distress in women. J Sex Marital Ther. 2002;28:317–30. doi: 10.1080/00926230290001448. [DOI] [PubMed] [Google Scholar]
- 17.Woodard TL, Collins K, Perez M, Balon R, Tancer ME, Kruger M, et al. What kind of erotic film clips should we use in female sex research? An exploratory study. J Sex Med. 2008;5:146–54. doi: 10.1111/j.1743-6109.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- 18.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–26. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friston KJHA, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:189–210. [Google Scholar]
- 22.Park K, Kang HK, Seo JJ, Kim HJ, Ryu SB, Jeong GW. Blood-oxygenation-level-dependent functional magnetic resonance imaging for evaluating cerebral regions of female sexual arousal response. Urology. 2001;57:1189–94. doi: 10.1016/s0090-4295(01)00992-x. [DOI] [PubMed] [Google Scholar]
- 23.Archer JS, Love-Geffen TE, Herbst-Damm KL, Swinney DA, Chang JR. Effect of estradiol versus estradiol and testosterone on brain-activation patterns in postmenopausal women. Menopause. 2006;13:528–37. doi: 10.1097/01.gme.0000188737.46746.cd. [DOI] [PubMed] [Google Scholar]
- 24.Kim HJ, Eun SJ, Cho SH, Seo JJ, Kang HK, Park K. Qualitative and quantitative measurement of brain activity associated with visual sexual arousal in males and females: 3. 0 tesla functional MR imaging. J Korean Radiol Soc. 2004;51:179–90. [Google Scholar]
- 25.Redoute J, Stoléru S, Grégoire MC, Costes N, Cinotti L, Lavenne F, et al. Brain processing of visual sexual stimuli in human males. Hum Brain Mapp. 2000;11:162–77. doi: 10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoleru S, Grégoire MC, Gérard D, Decety J, Lafarge E, Cinotti L, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav. 1999;28:1–21. doi: 10.1023/a:1018733420467. [DOI] [PubMed] [Google Scholar]
- 27.Michels L, Mehnert U, Boy S, Schurch B, Kollias S. The somatosensory representation of the human clitoris: an fMRI study. Neuroimage. 2010;49:177–84. doi: 10.1016/j.neuroimage.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Komisaruk BR, Whipple B. Functional MRI of the brain during orgasm in women. Annu Rev Sex Res. 2005;16:62–86. [PubMed] [Google Scholar]
- 29.Komisaruk BR, Whipple B, Crawford A, Liu W-C, Kalnin A, Mosier K. Brain activation during vaginocervical self-stimulation and orgasm in women with complete spinal cord injury: fMRI evidence of mediation by the vagus nerves. Brain Res. 2004;1024:77–88. doi: 10.1016/j.brainres.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 30.Critchley HD, Rotshtein P, Nagai Y, O’Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage. 2005;24:751–62. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Komisaruk B, Beyer-Flores C, Whipple B. The science of orgasm. Baltimore: Johns Hopkins University Press; 2006. [Google Scholar]
- 32.Zola-Morgan S, Squire LR. The neuropsychology of memory: parallel findings in humans and nonhuman primates. Ann NY Acad Sci. 1990;608:434–50. doi: 10.1111/j.1749-6632.1990.tb48905.x. [DOI] [PubMed] [Google Scholar]
- 33.LeDoux JE. Emotional memory systems in the brain. Behav Brain Res. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- 34.Basson R. Women’s sexual dysfunction: revised and expanded definitions. CMAJ. 2005;172:1327–33. doi: 10.1503/cmaj.1020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–32. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 36.Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–2. doi: 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- 37.Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20:236–60. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- 38.Turner BM, Paradiso S, Marvel CL, Pierson R, Boles Ponto LL, Hichwa RD, et al. The cerebellum and emotional experience. Neuropsychologia. 2007;45:1331–41. doi: 10.1016/j.neuropsychologia.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 41.Tsujimura A, Miyagawa Y, Fujita K, Matsuoka Y, Takahashi T, Takao T, et al. Brain processing of audiovisual sexual stimuli inducing penile erection: a positron emission tomography study. J Urol. 2006;176:679–83. doi: 10.1016/j.juro.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 42.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 44.Masters WHJV. Human sexual inadequacy. New York: Little, Brown; 1970. [Google Scholar]
- 45.Miyagawa Y, Tsujimura A, Fujita K, Matsuoka Y, Takahashi T, Takao T, et al. Differential brain processing of audiovisual sexual stimuli in men: comparative positron emission tomography study of the initiation and maintenance of penile erection during sexual arousal. Neuroimage. 2007;36:830–42. doi: 10.1016/j.neuroimage.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 46.Weston CS. Another major function of the anterior cingulate cortex: the representation of requirements. Neurosci Biobehav Rev. 2012;36:90–110. doi: 10.1016/j.neubiorev.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Ebert D, Ebmeier KP. The role of the cingulate gyrus in depression: from functional anatomy to neurochemistry. Biol Psychiatry. 1996;39:1044–50. doi: 10.1016/0006-3223(95)00320-7. [DOI] [PubMed] [Google Scholar]
- 48.Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- 49.Yucel M, Wood SJ, Fornito A, Riffkin J, Velakoulis D, Pantelis C. Anterior cingulate dysfunction: implications for psychiatric disorders? J Psychiatry Neurosci. 2003;28:350–4. [PMC free article] [PubMed] [Google Scholar]
- 50.Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004;7:411–6. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- 51.Ferretti A, Caulo M, Del Gratta C, Di Matteo R, Merla A, Montorsi F, et al. Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage. 2005;26:1086–96. doi: 10.1016/j.neuroimage.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 52.Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JDE. Neural correlates of the automatic processing of threat facial signals. J Neurosci. 2003;23:5627–33. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann NY Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 55.Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997;154:926–33. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- 56.Park K, Seo JJ, Kang HK, Ryu SB, Kim HJ, Jeong GW. A new potential of blood oxygenation level dependent (BOLD) functional MRI for evaluating cerebral centers of penile erection. Int J Impot Res. 2001;13:73–81. doi: 10.1038/sj.ijir.3900649. [DOI] [PubMed] [Google Scholar]
- 57.Sundaram T, Jeong GW, Kim TH, Kim GW, Baek HS, Kang HK. Time-course analysis of the neuroanatomical correlates of sexual arousal evoked by erotic video stimuli in healthy males. Korean J Radiol. 2010;11:278–85. doi: 10.3348/kjr.2010.11.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang JC, Park K, Eun SJ, Lee MS, Yoon JS, Shin IS, et al. Assessment of cerebrocortical areas associated with sexual arousal in depressive women using functional MR imaging. J Sex Med. 2008;5:602–9. doi: 10.1111/j.1743-6109.2007.00737.x. [DOI] [PubMed] [Google Scholar]
- 59.Lundstrom BN, Petersson KM, Andersson J, Johansson M, Fransson P, Ingvar M. Isolating the retrieval of imagined pictures during episodic memory: activation of the left precuneus and left prefrontal cortex. Neuroimage. 2003;20:1934–43. doi: 10.1016/j.neuroimage.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 60.Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, et al. Parietal cortex and representation of the mental Self. Proc Natl Acad Sci USA. 2004;101:6827–32. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage. 2005;27:824–34. doi: 10.1016/j.neuroimage.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 62.Zhu X, Wang X, Parkinson C, Cai C, Gao S, Hu P. Brain activation evoked by erotic films varies with different menstrual phases: an fMRI study. Behav Brain Res. 2010;206:279–85. doi: 10.1016/j.bbr.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 63.Rupp HA, James TW, Ketterson ED, Sengelaub DR, Janssen E, Heiman JR. Neural activation in the orbitofrontal cortex in response to male faces increases during the follicular phase. Horm Behav. 2009;56:66–72. doi: 10.1016/j.yhbeh.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abler B, Kumpfmuller D, Gron G, Walter M, Stingl J, Seeringer A. Neural correlates of erotic stimulation under different levels of female sexual hormones. PloS one. 2013;8:e54447. doi: 10.1371/journal.pone.0054447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davis SR, Davison SL, Donath S, Bell RJ. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91–6. doi: 10.1001/jama.294.1.91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.