Abstract
Study Objective
To study the effect of intravenous (IV) sedation on blood pressure (BP), heart rate (HR), and respiratory rates (RR) to determine if IV sedatives differ with respect to their effect on BP, HR, and RR.
Design
Prospective, randomized, single-blinded, placebo-controlled study.
Setting
Monitored patient care room at a clinical research center.
Subjects
60 healthy ASA physical status 1 volunteers.
Interventions
Subjects were randomized to receive, in increasing doses, one of three IV sedatives: propofol, midazolam, or dexmedetomidine; or saline control.
Measurements
Blood pressure (systolic, diastolic), HR, and RR were recorded.
Main Results
A significant dose-dependent BP reduction occurred with dexmedetomidine and, to a lesser degree, with propofol; and there was good agreement of predicted versus measured drug concentrations for all sedatives. Blood pressure and HR of participants who received midazolam did not change.
Conclusions
When administered in sedative doses, dexmedetomidine and, to a lesser extent, midazolam, reduces BP in a dose-dependent fashion. Dexmedetomidine also reduces HR. Midazolam does not affect BP or HR.
Keywords: Dexmedetomidine, hemodynamics, midazolam, propofol, sedation, intravenous
1. Introduction
Despite their common use, no prospective studies have examined the cardiovascular effects of midazolam, propofol and dexmedetomidine while controlling for measured drug plasma concentrations. Drug plasma quantification is of critical importance because the effect of intravenous (IV) anesthetics is subject to marked pharmacokinetic and pharmacodynamic variability across individuals [1]. These factors may explain disagreements among recent comparative studies in the clinical setting, which were based on administered rather than measured doses [2–5].
To better characterize the hemodynamic side effects of commonly used IV sedatives in a more controlled setting, we prospectively studied healthy volunteers who were randomly assigned to receive increasing doses of midazolam, propofol, dexmedetomidine, or saline control. Our primary study goal was to test whether IV sedatives differed with respect to their effect on blood pressure (BP), heart rate (HR), and respiratory rate (RR). Our secondary objective was to quantify the strength of agreement of plasma drug concentrations predicted by target-controlled infusion (TCI) and lipid chromatography mass spectroscopy (LC/MS) measurements. This information has significant impact on the clinical care of patients receiving IV anesthesia.
2. Materials and methods
2.1 Study design
After University of Alabama Hospital Institutional Review Board approval, 60 healthy, ASA physical status 1 human volunteers were randomized to receive one of three IV sedatives: propofol, midazolam, or dexmedetomidine; or saline control. Subjects’ age ranged from 21 to 55 years, height from 152 to 197 cm, weight from 46 to 117 kg, with a gender distribution of 25 men and 35 women. Informed consent for the study, a targeted physical examination, and a medical history to determine study eligibility were obtained during a screening visit. Only healthy volunteers (ASA physical status 1) were enrolled. Subjects with any preexisting medical condition were excluded. Other exclusion criteria were obesity (body mass index > 30 kg/m2); pregnancy; age less than 18 years; positive smoking, alcohol, or recreational drug history; and the use of any medications on a regular basis. Volunteers participated on a single occasion and were paid for their involvement.
On the day of the study, we placed an 18-gauge IV catheter for infusion of the IV sedative in one arm and another 18-gauge IV catheter in the antecubital vein of the contralateral arm. All subjects were monitored using electrocardiography, pulse oximetry, and non-invasive blood pressure.
2.2 Intravenous sedation
Subjects were randomized to one of the treatment groups on the day prior to the study. Randomization was achieved with a schedule created with SAS, version 9.2 software (SAS Institute, Cary, NC, USA). Subjects received 4 dose levels ranging from very mild (anxiolytic) to mild sedation. Drug doses were based on previous published data [7–9] and pharmacokinetic simulation using Stanpump software [6]. Predicted dexmedetomidine plasma concentrations were 0.1, 0.2, 0.4, and 0.8 ng/mL; predicted midazolam concentrations were 10, 20, 40, and 80 ng/mL; and predicted propofol concentrations were 0.1, 0.2, 0.4, and 0.8 µg/mL. Intravenous sedatives were infused with a Graseby 3400 infusion pump (Smiths Medical MD, Inc., St. Paul, MN, USA) controlled by Stanpump software, which is freely available at opentci.org [6]. The effect-site concentrations were based on a three-compartment pharmacokinetic model adjusted by height, weight, age, and gender, and correspond approximately to fixed doses of 12.5 to 50 µg/kg/min propofol, 0.1 to 0.5 µg/kg/hr dexmedetomidine, and 3.0 to 5.0 mg/hr midazolam. This computer-assisted infusion was approved by the United States Food and Drug Administration (IDE # G060183). Pharmacokinetics for propofol were published by Marsh et al. [7], and pharmacokinetic parameters for the administration of dexmedetomidine and midazolam were published by Dyck et al. [8] and Greenblatt et al. [9], respectively. Each level was maintained for 20 minutes, followed immediately by the next dose level. Dose increases were not administered if subjects could not readily manipulate the mechanical slider to rate their sedation or if they could not be quickly aroused with tactile stimulation.
2.3 Sedation rating
To assure a graded progression of sedation, we also used two sedation scales. At the end of the 20-minute sedation level, subjects rated their alertness/sedation using a mechanical slide algometer with the end points labeled “very alert” and “very sedated”. The Observer Assessment of Alertness and Sedation (OAAS) scale [10] was obtained and recorded by a study nurse who was blinded to subjects’ group assignment.
2.4 Plasma drug analysis
Blood samples were obtained in two 5-mL aliquots at the end of each sedation level. Immediately after the blood draw, samples were spun at 1,800 ×g for 10 minutes using a refrigerated centrifuge, then separated and stored as plasma at −75 °C until analyzed.
2.5 Propofol
The protein precipitation method was used to extract propofol from the human serum samples. Fifty µL of plasma was mixed with 200 µL of 1% (v/v) acetic acid in methanol and 50 µL of thymol (3000 nM) as per internal standard, then vortexed and centrifuged at 1,800 ×g for 10 min. The supernatant was taken for LC-MS/MS analysis. A highly sensitive and specific atmospheric pressure chemical ionization (APCI) in negative ion mode liquid chromatography-tandem mass spectrometry method was used to detect serum concentrations of propofol. Chromatography was carried out on a 100 × 2.1 mm internal diameter (ID) Xtera C18 column (Waters Corp., Milford, MA, USA) using an isocratic mobile phase that consisted of methanol and 0.05% aqueous ammonium hydroxide solution (98:2) at a flow rate of 1.0 mL/min. LC/MS/MS analyses was performed using a system consisting of a model SIL-HT refrigerated Shimadzu autosampler (Shimadzu Scientific Instruments, Inc., Columbia, MD, USA) and an API 4000 (Applied Biosystems/MDS Sciex, Concord, Ontario, Canada) mass spectrometer. Multiple reaction ion mass spectrometry (MRM) analysis was conducted by monitoring the precursor ion to product ion transitions from m/z 177/161 (Propofol) and m/z 149/133 (thymol). The LC-MS system was controlled by BioAnalyst 1.4.2 software (AB Sciex, Framingham, MA, USA). Standard calibration curves were prepared over a linear range of 50–10,000 nM at 8 concentrations: 50, 100, 250, 500, 1,000, 5,000, and 10,000 nM. The standards were prepared in serum containing no propofol. Given the sensitivity and quality control measures, we could quantify propofol levels on 41 plasma samples.
2.6 Midazolam
Solvent precipitation was used to extract midazolam from human serum sample. Two hundred µL of plasma was mixed with 800 µL of 1% (v/v) acetic acid in MeOH and 200 µL of triazolam (600 nM) as internal standard, then vortexed and centrifuged at 1,800 ×g for 10 minutes. The supernatant was taken for LC-MS/MS analysis. APCI-positive ion mode for LC/MS/MS was used to detect serum concentrations of dexmedetomidine. Chromatography was carried out on a 100 × 2.0 mm ID Phenomenex Phenyl-Hexyl column using a mobile phase that consisted of a gradient of 10% to 100% acetonitrile in 0.1% formic acid over 5 minutes, with a flow rate of 0.3 mL/min. The LC/MS analyses were performed as described for propofol. The MRM analysis was conducted by monitoring the precursor ion to product ion transitions from m/z 326/291 (midazolam) and m/z 343/239 (triazolam) and controlled the LC-MS/MS system using the BioAnalyst 1.4.2 software. Standard calibration curve was prepared over a linear range of 10 to 5,000 nM at 8 different concentrations: 10, 50, 100, 250, 500, 1,000, 2,500, and 5000 nM. Given the sensitivity and quality control measures, we could quantify midazolam levels on 48 plasma samples.
2.8 Dexmedetomidine
We used the liquid-liquid extraction method for dexmedetomidine from human plasma samples. We mixed 200 (one of each plasma sample with 2.0 mL of ethyl acetate after adding 100 µL of triazolam (100 nM) as an internal standard, then vortexed and, under a stream of nitrogen to dryness, reconstituted the sample in 80% aqueous methanol equal to the original volume of plasma and transferred it to the autosampler for LC/MS analysis. A highly sensitive and specific atmospheric pressure chemical ionization (APCI) in the positive ion mode liquid chromatography-tandem mass spectrometry method was used to quantify plasma concentrations. Chromatography was carried out on a 100 × 2.0 mm ID Phenomenex Phenyl-Hexyl column using mobile phase consisted of a gradient of 10% to 100% acetonitrile in 0.1% HCOOH over 5 minutes with a flow rate of 0.3 mL/min. The LC/MS/MS analyses were performed as described for propofol. We conducted the MRM analysis by monitoring the precursor ion to product ion transitions from m/z 201/95 (dexmedetomidine) and m/z 343/239 (triazolam), controlled by BioAnalyst 1.4.2 software. The standard calibration curves were prepared over a linear range of 10 to 5,000 nM at 8 concentrations: 10, 50, 100, 250, 500, 1000, 2500, and 5000 nM. Given the sensitivity and quality control measures, we could quantify dexmedetomidine levels on 44 plasma samples.
2.9 Statistical analysis
To address the primary study goal -- to test whether IV sedatives differed with respect to their effect on vital signs -- we performed separate two-factor repeated-measures analyses of variance (rm ANOVA) for each of the vital sign measures [systolic blood pressure (SBP), diastolic blood pressure (DBP), HR, and RR].
To determine the agreement of predicted versus measured drug concentrations, we used linear regression analysis. The strengths of agreement were expressed as adjusted R2. All analyses were performed with SAS version 9.2 software. A significance level of α = 0.05 was considered significant.
3. Results
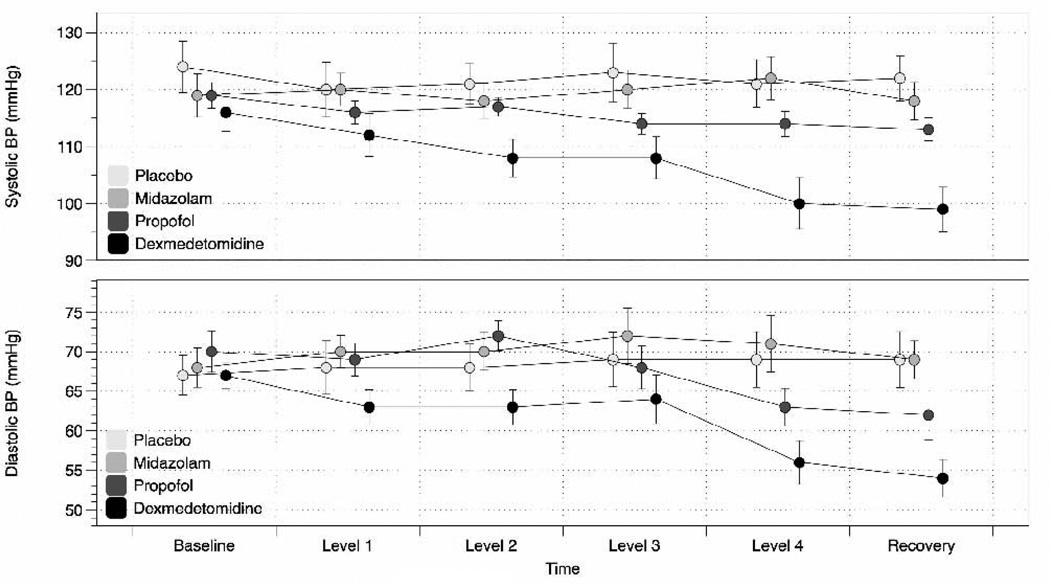
The drug-by-time interaction was significant for SBP and DBP (P < 0.0001) but not on RR or HR. Statistical details are shown in Table 1. We observed a significant dose-dependent BP reduction with dexmedetomidine and, to a lesser degree, with propofol. Mean BP values over time are shown in Fig. 2. Oxygenation as measured by pulse oximetry remained > 95% for all study participants.
Table 1.
Type 3 tests of fixed effects
| Fixed effects | Numerator DF |
Denominator DF | F value | Pr > F |
|---|---|---|---|---|
| Drug | 3 | 5 | 3.84 | 0.0144 |
| Time | 5 | 280 | 7.04 | < 0.0001 |
| Drug × Time | 15 | 280 | 3.31 | < 0.0001 |
Statistical Information about explanatory variables. P < 0.05 is considered significant.
DF=degrees of freedom, F=Fisher statistic (from Fisher-Snedecor distribution), Pr=probability, Pr > F=the right-tail probability assigned to the observed F statistic.
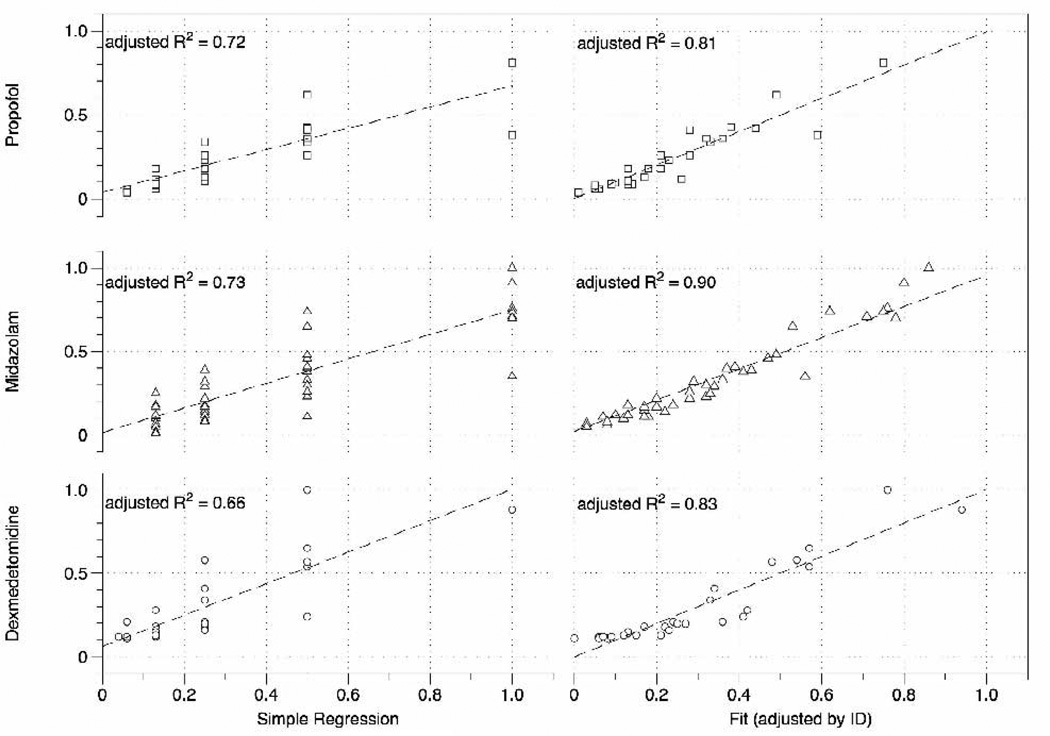
Fig. 2.
Agreement of predicted versus measured drug concentrations using a simple regression model (left panel) and the model adjusted by individual intercepts. Adjusted R2 =adjusted coefficient of determination, ID=internal diameter.
We noted a BP reduction in the dexmedetomidine group starting at very mild levels of sedation at which most subjects appear somnolent but remain awake (level 2). The reduction in BP was most significant at level 4. At that level, participants were somnolent but arousable using verbal stimulation. Consistent with its pharmacological profile, the BP reduction continued into recovery 20 minutes after the infusion was discontinued. We also noted a mild reduction in BP in the propofol group. The BP reduction was significant at levels 2 and 3 (SPB) and at levels 2 and 4 (DBP). By contrast, subjects in the midazolam group and, as expected, in the saline control group, maintained their BP throughout the study.
The results of our correlation analysis, predicted (by the Stanpump infusion program Stanpump) versus measured drug concentration, showed an overall good agreement (Fig. 2). Linear regression analysis resulted in adjusted R-squared values (R2adj) ranging from 0.66 (dexmedetomidine) to 0.73 (midazolam). When adjusted by individual intercepts, the agreement of predicted versus measured drug ratio was very good for all drugs; R2adj (dexmedetomidine) = 0.83, R2adj (midazolam) = 0.90, R2adj (propofol) = 0.81. In simple terms, the latter model tells us how well the infusion software predicts a dose increase when accounting for individual patient characteristics.
4. Discussion
Intravenous sedatives are commonly used in a variety of clinical or outpatient settings [12–16]. The most common clinical applications for IV sedation are to facilitate medical procedures such as endoscopies, to treat mechanically ventilated patients in intensive care units (ICUs), and to facilitate procedural interventions in children. At higher doses, propofol and, in special cases, dexmedetomidine, are also used to induce and maintain general anesthesia. Many effects of IV anesthetics have been described in the literature. However, almost all of these reports are based on a fixed (standard infusion) or predicted (computer-assisted infusion) dose and relatively few studies simultaneously evaluate sedative anesthetics in a side-by-side comparison. Thus, it is no surprise that information in the existing literature on drug side effects is conflicting; for example, phase III trials on dexmedetomidine in the ICU patients show that 54% of patients receiving dexmedetomidine as a continuous infusion developed hypotension compared with 30% of controls, while 13% developed hypertension versus 24% of controls*. The apparent dichotomy of effects, the observation of both hypotension and hypertension, and the high incidence of BP abnormalities in the control group of mechanically ventilated ICU patients makes it somewhat difficult to apply this information to other patient groups, or to make comparisons with the effects of other sedatives when given at comparable doses.
The difficulty in evaluating drugs in the context of a clinical procedure also may be shown using the more recent example of procedural sedation in children undergoing MRI procedures.
In a direct comparison of propofol to dexmedetomidine, Heard et al. [3] found higher SBP with dexmedetomidine. In a similar study, Koroglu et al. [5] detected no BP change, while Mason et al. [17] observed hypotension associated with the administration of IV dexmedetomidine during pediatric MRI procedures. While the exact hemodynamic profile of the commonly used IV sedatives propofol, midazolam, and dexmedetomidine remains controversial, its characterization continues to be highly important for clinical practice.
We designed this study to account for several potential confounders; our selection of healthy human volunteers eliminated potential coexisting diseases and the effect of a clinical procedure on vital signs. Our design, a prospective, randomized, placebo-controlled analysis. Thus, we could differentiate even subtle differences in the cardiopulmonary effects of IV sedative drugs.
Our key finding, when comparing the three sedative drugs, was a sustained dosedependent reduction of SBP and DBP with dexmedetomidine and, to a lesser degree, propofol, as shown in Fig. 1. Meanwhile, midazolam did not affect BP. All three agents were effective at producing a desired level of sedation, as indicated independently by sedation self-rating and observer assessment of sedation.
Fig. 1.
Systolic and diastolic blood pressures (BPs) at baseline, during sedation and recovery. The x-axis = dose of the sedative drug, ranging from very mild (level 1) to moderate sedation (level 4).
Finally, we determined the precision to which drug plasma concentrations may be predicted with a computer-assisted infusion based on limited population pharmacokinetics. This infusion algorithm (TCI) has the potential to facilitate administration of IV drugs and, in the case of propofol, has been used in clinical practice for several years in European markets. In the U.S., however, TCI is limited to research applications in part because there is not enough information validating TCI. This finding is not surprising since state-of-the-art drug assays, as we established for all three IV sedatives tested, are methodologically challenging, time-consuming, and expensive. The latter is particularly true for propofol. Several studies evaluated both biological effects and pharmacological models to quantify the effects of propofol [18–20], but only one group of investigators actually described the propofol laboratory analysis [21].
The correlation of predicted versus measured plasma concentrations are shown in Fig. 2. We determine that there is good correlation of all drugs, as indicated by the adjusted R-squared. If we adjust for individual baseline variations, the dose-dependent linear agreement improves to > 0.80 for all three drugs. The improvement, moving from the simple regression of the slope-adjusted model, is greatest for dexmedetomidine, indicating that one may expect the largest individual pharmacokinetic variation with dexmedetomidine versus both midazolam and propofol.
In summary, our randomized, placebo-controlled study in healthy volunteers provides concise information about the cardiopulmonary characteristics of the three most commonly used IV sedatives in a direct comparison. This information is useful in guiding clinical practice and further research on the cognitive effects of IV anesthetic drugs. The knowledge gained also helps us to better understand the hemodynamic characteristics of intravenous sedatives.
Table 2.
Post hoc tests
| Systolic blood pressure | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Level 1 | Level 2 | Level 3 | Level 4 | Recovery | |||||||
| Placebo | A | A | A | A | A | A | ||||||
| Midazolam | A | A | A | A | A | A | ||||||
| Propofol | A | A | A | B | A | B | A | A | ||||
| Dexmedetomidine | A | A | B | B | B | B | ||||||
| Diastolic blood pressure | ||||||||||||
| Baseline | Level 1 | Level 2 | Level 3 | Level 4 | Recovery | |||||||
| Placebo | A | A | A | A | A | A | ||||||
| Midazolam | A | A | B | A | A | A | A | |||||
| Propofol | A | A | B | A | B | A | A | B | A | |||
| Dexmedetomidine | A | B | B | A | B | B | ||||||
Least significant difference test to compare the effect of treatment on systolic and diastolic blood pressure at baseline, each sedation level and recovery. A and B = those study groups that differ significantly (P < 0.05); drugs not connected by the same letter are significantly different.
Acknowledgments
Supported by the National Institute of Health grant K23RR021874 and the University of Alabama Center for Clinical and Translational Science grant UL1RR025777.
The mass spectrometer was purchased with an award to S.B. from the University of Alabama at Birmingham Health Services Foundation General Endowment Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Precedex®: Complete prescribing information (revised 10/2008). Hospira, Inc., Lake Forest, IL 60045 USA.
References
- 1.DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;(5):1–20. [PubMed] [Google Scholar]
- 2.Chang C, Uchiyama A, Ma L, Mashimo T, Fujino Y. A comparison of the effects on respiratory carbon dioxide response, arterial blood pressure, and heart rate of dexmedetomidine, propofol, and midazolam in sevoflurane-anesthetized rabbits. Anesth Analg. 2009;109:84–89. doi: 10.1213/ane.0b013e3181a2ad5f. [DOI] [PubMed] [Google Scholar]
- 3.Heard C, Burrows F, Johnson K, Joshi P, Houck J, Lerman J. A comparison of dexmedetomidine-midazolam with propofol for maintenance of anesthesia in children undergoing magnetic resonance imaging. Anesth Analg. 2008;107:1832–1839. doi: 10.1213/ane.0b013e31818874ee. [DOI] [PubMed] [Google Scholar]
- 4.Phan H, Nahata MC. Clinical uses of dexmedetomidine in pediatric patients. Paediatr Drugs. 2008;10:49–69. doi: 10.2165/00148581-200810010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Koroglu A, Demirbilek S, Teksan H, Sagir O, But AK, Ersoy MO. Sedative, haemodynamic and respiratory effects of dexmedetomidine in children undergoing magnetic resonance imaging examination: preliminary results. Br J Anaesth. 2005;94:821–824. doi: 10.1093/bja/aei119. [DOI] [PubMed] [Google Scholar]
- 6.Shafer S. Stanpump, Version 1998 Edition, Open TCI. TCI program; 1998. pp, (Available online at opentci.org) [Google Scholar]
- 7.Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991;67:41–48. doi: 10.1093/bja/67.1.41. [DOI] [PubMed] [Google Scholar]
- 8.Dyck JB, Maze M, Haack C, Azarnoff DL, Vuorilehto L, Shafer SL. Computer-controlled infusion of intravenous dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993;78:821–828. doi: 10.1097/00000542-199305000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Greenblatt DJ, Abernethy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI. Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology. 1984;61:27–35. [PubMed] [Google Scholar]
- 10.Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–251. [PubMed] [Google Scholar]
- 11.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr AC. 1974;19:716–723. [Google Scholar]
- 12.Mizrak A, Koruk S, Ganidagli S, Bulut M, Oner U. Premedication with dexmedetomidine and midazolam attenuates agitation after electroconvulsive therapy. J Anesth. 2009;23:6–10. doi: 10.1007/s00540-008-0695-2. [DOI] [PubMed] [Google Scholar]
- 13.Ruokonen E, Parviainen I, Jakob SM, et al. "Dexmedetomidine for Continuous Sedation" Investigators. Dexmedetomidine versus propofol/midazolam for long-term sedation during mechanical ventilation. Intensive Care Med. 2009;35:282–290. doi: 10.1007/s00134-008-1296-0. [DOI] [PubMed] [Google Scholar]
- 14.Munro HM, Tirotta CF, Felix DE, et al. Initial experience with dexmedetomidine for diagnostic and interventional cardiac catheterization in children. Paediatr Anaesth. 2007;17:109–112. doi: 10.1111/j.1460-9592.2006.02031.x. [DOI] [PubMed] [Google Scholar]
- 15.O'Daniel TG, Shanahan PT. Dexmedetomidine: a new alpha-agonist anesthetic agent for facial rejuvenation surgery. Aesthet Surg J. 2006;26:35–40. doi: 10.1016/j.asj.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Bekker AY, Basile J, Gold M, et al. Dexmedetomidine for awake carotid endarterectomy: efficacy, hemodynamic profile, and side effects. J Neurosurg Anesthesiol. 2004;16:126–135. doi: 10.1097/00008506-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Mason KP, Zurakowski D, Zgleszewski SE, et al. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth. 2008;18:403–411. doi: 10.1111/j.1460-9592.2008.02468.x. [DOI] [PubMed] [Google Scholar]
- 18.Barakat AR, Sutcliffe N, Schwab M. Effect site concentration during propofol TCI sedation: a comparison of sedation score with two pharmacokinetic models. Anaesthesia. 2007;62:661–666. doi: 10.1111/j.1365-2044.2007.05059.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu SH, Wei W, Ding GN, Ke JD, Hong FX, Tian M. Relationship between depth of anesthesia and effect-site concentration of propofol during induction with the target-controlled infusion technique in elderly patients. Chin Med J (Engl) 2009;122:935–940. [PubMed] [Google Scholar]
- 20.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–847. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Bajpai L, Varshney M, Seubert CN, et al. Mass spectral fragmentation of the intravenous anesthetic propofol and structurally related phenols. J Am Soc Mass Spectrom. 2005;16:814–824. doi: 10.1016/j.jasms.2005.02.009. [DOI] [PubMed] [Google Scholar]