Abstract
Fetal development in an obese maternal intrauterine environment has been shown to predispose the offspring for a number of metabolic disorders in later life. The observation that a large percentage of women of child-bearing age in the US are overweight/obese during pregnancy is therefore a source of concern. A high fat (HF) diet-induced obesity in female rats has been used as a model for maternal obesity. The objective of this study was to determine cellular development in brains of term fetuses of obese rats fed a HF diet from the time of weaning. Fetal brains were dissected out on gestational day 21 and processed for immunohistochemical analysis in the hypothalamic as well as extra-hypothalamic regions. The major observation of this study is that fetal development in the obese HF female rat induced several alterations in the HF fetal brain. Marked increases were observed in orexigenic signaling and a significant decrease was observed for anorexigenic signaling in the vicinity of the 3rd ventricle in HF brains. Additionally, our results indicated diminished proliferation and maturation of stem-like cells in the hypothalamus (3rd ventricular region) as well as in the cortex. The results from the present study indicate developmental alterations in the hypothalamic and extra-hypothalamic regions in the HF fetal brain suggestive of a predisposition for the development of obesity and possibly neurodevelopmental abnormalities in the offspring.
Keywords: Maternal obesity, High fat diet, Fetal brain cellular development, Hypothalamic appetite regulation
Introduction
Approximately 36% of the adults in the US are classified as obese (BMI> 30) (Ogden et al. 2012). Available evidence from epidemiological and animal studies indicates that altered nutritional experiences during early periods in life decisively impact on the development of obesity and associated metabolic diseases in later life via the phenomenon of developmental programming (Fernandez-Twinn and Ozanne 2010). In addition to the association with adult-onset metabolic disorders, childhood obesity has been associated with increased risk for mental health disorders such as depression, anxiety, and attention deficit hyperactivity disorder (Rofey et al. 2009; Waring and Lapane 2008). Dodds et al. (Dodds et al. 2011) have suggested that increased body weight gains in the pre-pregnancy and pregnancy periods in the mother pose a higher risk for the development of autism in the offspring. Hence the observation that approximately 50% of women of child-bearing age in the US are overweight (Flegal et al. 2010) suggests that the offspring of such women may be at a higher risk not only for the onset of metabolic diseases but also for neurodevelopmental diseases.
We and others have demonstrated that feeding a high fat (HF) diet to female rats resulted in the predisposition for the development of obesity and metabolic disorders in the offspring (Gupta et al. 2009; Srinivasan et al. 2006; Sullivan et al. 2011). In this study, we hypothesized that the altered obese maternal environment in rats consuming a HF diet may negatively impact on the development of the HF fetal brain circuitry including hypothalamus which controls feeding behavior as well as other brain regions involved in diverse behavioral functions and possibly neurodevelopmental abnormalities in the offspring.
Methods
The Institutional Animal Care and Use Committee at the University at Buffalo approved all animal procedures (protocol # BCH06064N). Twenty-four day-old Sprague-Dawley female rats were randomly divided into two groups and were fed ad libitum either a high fat (HF) diet (% caloric distribution: 59.5 fat, 24.4 carbohydrate, 16 protein) or a rodent laboratory chow (LC) (% caloric disrtibution:10.9 fat, 70 carbohydrate, 19 protein) until the end of the experiment. HF and LC female rats (~120 day-old) were bred with 3 month-old male rats fed LC from postnatal day 24. Pregnant HF and LC rats continued to consume their respective diets during gestation. The term fetuses were rapidly removed on embryonic day 21 from the anesthetized dam and further anesthetized (ketamine/xylazine) immediately before being perfused with 4% paraformaldehyde. The dissected brains were processed in gradient of sucrose before slicing into 25 µm thick sections.
Immunocytochemistry
Representative coronal sections (5 sections/animal/3–4 fetuses per group each from a different mother) were immunostained and analyzed (Stachowiak et al. 2013). Briefly, free floating sections were incubated in 10% normal goat serum (Immunogen; Grand Island, NY), followed by overnight incubation with variety of primary antibodies: rabbit anti-NPY (1:500 dilution; ABCAM, Cambridge, MA), rabbit anti-agouty-related protein AgRP (1:200); LS BIOSCIENCES, Inc, mouse anti-insulin receptor antibody (1:800 dilution; ABCAM, Cambridge, MA), rabbit anti-α-MSH (1:20,000 dilution; ABCAM, Cambridge, MA), rat anti-Nestin antibody (1:500 dilution; Pharmigen, Franklin Lakes, NJ), mouse anti-βIII Tubulin monoclonal antibody (1:1000 dilution; Sigma-Aldrich, St. Louis, MO), and TOPRO reagent (Invitrogen, Grand Island, NY) for labeling double strand DNA. After multiple rinses in phosphate-buffered saline, sections were incubated for 2–3 h with a dilution of the appropriate secondary antibodies. The following antibodies were used: Alexa Fluor 488 goat anti-mouse (1:2000), Alexa Fluor 594 goat anti- rat (1:1500); Molecular Probes, Carlsbad, CA; Cy3-conjugated goat anti-rabbit (1:2000); Jackson Immunoresearch, West Grove, PA). Immunofluorescence staining was analyzed using Zeiss Axioimager Z1 microscope. Representative sections are shown.
Statistical analysis
The difference between numbers of immunostained cells on brain sections (4 sections/stain) from HF and LC rats were evaluated using Students’ t test. P < 0.05 was considered significant.
Results and Discussion
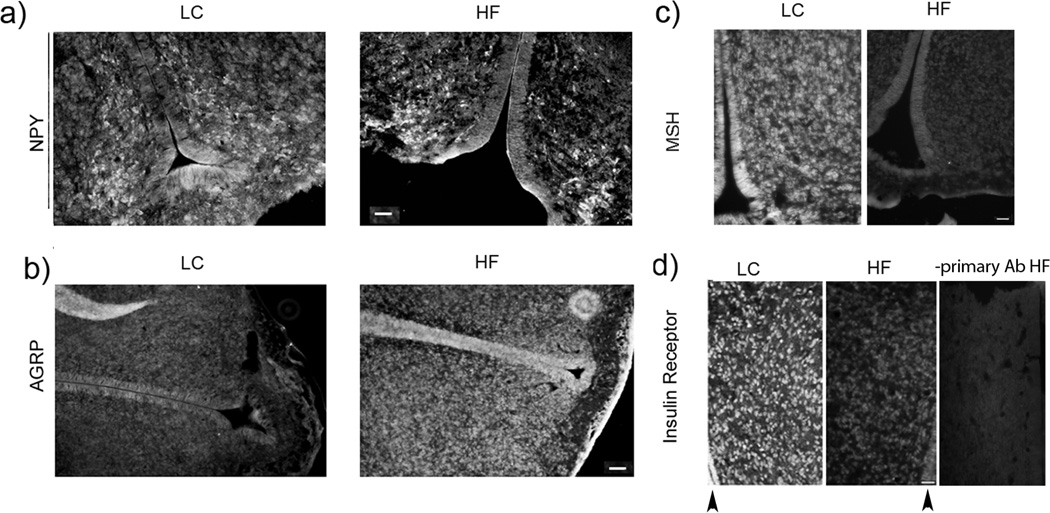
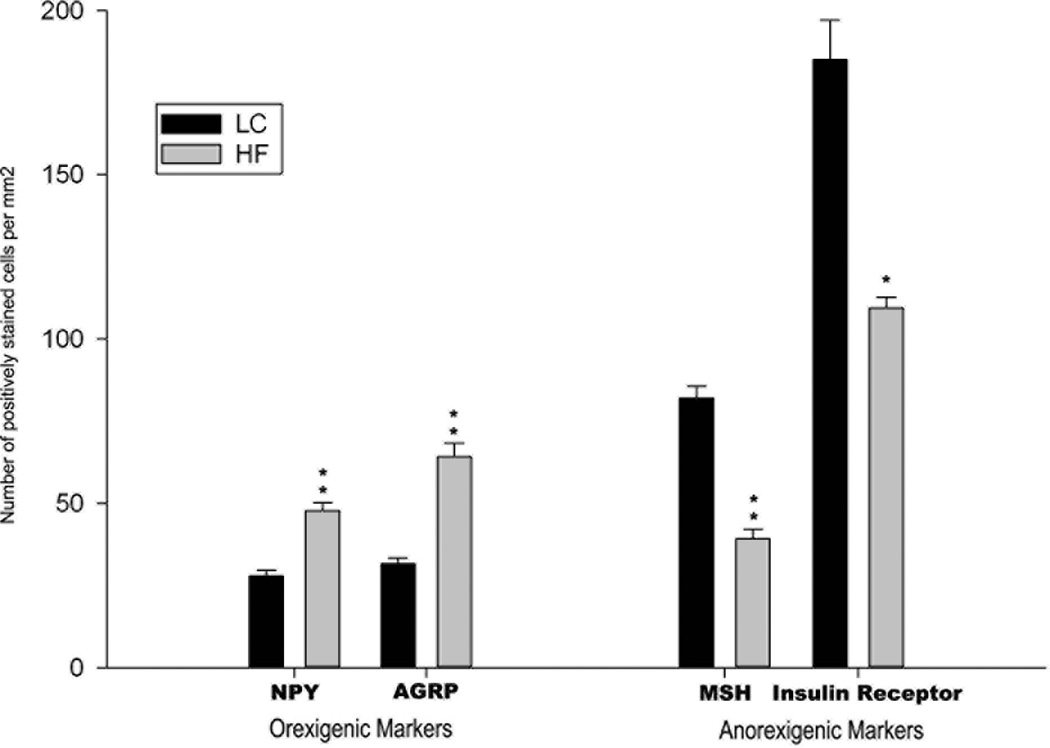
Earlier we reported that female rats weaned onto a HF diet were significantly heavier than age-matched LC females on postnatal day 100 (prepregnancy period) (body weights, means ± SEM; HF: 372±13 g vs LC: 314±6 g; n=8) and were also heavier on gestational day 21 (HF: 538±8 g vs LC: 467±4 g; n=8) (Gupta et al. 2009). The average term fetal body weights were similar for both the groups (HF: 5.2±0.2 g vs LC: 5.0±0.3 g; n=6–8 litters). This study indicated that maternal obesity induced by feeding a HF diet to rats resulted in increased levels of serum insulin and leptin in term HF fetuses (Srinivasan et al. 2006). In order to determine if a predisposition for adult-onset obesity in the HF offspring was programmed during fetal development in the HF intrauterine environment, immunoreactivity of orexigenic and anorexigenic neuropeptides/proteins was examined in the hypothalamic region of term fetal HF brains. Increases in the immunoreactive NPY and AgRP cells indicative of an increased orexigenic drive were observed in the term HF fetal brains compared to LC fetal brains (Fig. 1a,1b and 1e). A suppressed anorexigenic signaling in the hypothalamus of HF fetal brains was indicated by the reduced number of α-MSH immunoreactive cells (Fig. 1c). These results corroborate other studies indicating that maternal HF diet consumption programs the hypothalamic pathways that regulate feeding in the offspring (Gupta et al. 2009). A recent study showed that prenatal exposure to HF diet alter the population of fetal hypothalamic neurons containing NPY ((Poon et al. 2012).
Fig. 1.
Histological analysis of the diencephalic region of term fetal MF and HF rats brain sections. Immunostaining of (a) NPY, (b) AGRP, (c) α-MSH and (d) insulin receptor (arrowheads indicate 3rd ventricle). Bar size: a- and b - 100 µm; c and d- 50 µm. (e) bars show average numbers of immunostained cells/mm2 counted on 4 brain sections from the corresponding brain areas. Difference between HF and MF sections were evaluated using Students’ t test: *P < 0.05; ** P<0.001.
Since term HF fetuses are hyperinsulinemic (Srinivasan et al. 2006), we examined possible changes in the levels of brain insulin receptor (IR). Immunostaining revealed strong IR immunoreactivity in the diencephalon parenchyma and walls of the 3rd ventricle in the brains of LC fetuses. The intensity of IR immunostaining (Fig. 1d) and the number of IR immunoreactive cells (Fig. 1e) were diminished in HF fetuses. These results indicated that in HF fetal brains the expression of IR is markedly reduced in the germinal zone of the 3rd ventricle and its surrounding.
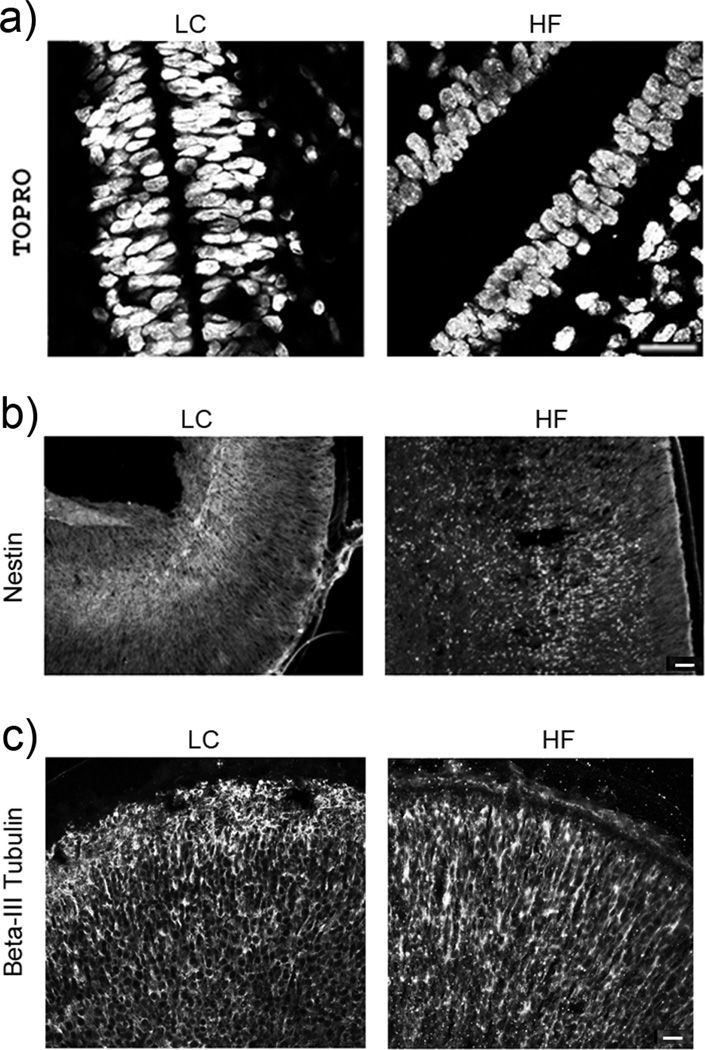
Given the role of IR signaling in the stem cell biology, we analyzed the effects of the HF maternal environment on the stratification of cells in the ventral region of the 3rd ventricle by staining with TOPRO. Significant differences were observed in the TOPRO labeling between the HF and LC fetal brains. (Fig. 2a). In LC fetal brains, nuclei of the cells surrounding 3rd ventricle formed 3–5 layers of cells, which were elongated and tightly organized (Fig. 2a). In HF fetal brains, the nuclear layers were fewer (2–3 layers of cells) and the nuclei were smaller, rounded, and more uniform in size. In the offspring of obese mothers fed a HF diet during gestation only, increased proliferation of neuronal and neuroepithelial cells of the embryonic 3rd ventricle and increased migration of these cells to hypothalamic regions were observed (Chang et al. 2008).
Fig. 2.
Histological analysis of the area of the 3rd ventricle and brain cortex. Immunostaining of (a) TOPRO stained cell nuclei in the area of the 3rd ventricle (neurogenic area), (b) Nestin-expressing neural progenitor cells in brain cortex and (c) β-III Tubulin-expressing immature neurons in brain cortex. Bar size: a - 50 µm; b and c - 100 µm.
The germinal layer of the 3rd ventricle is a source of the neural stem/progenitor cells that migrate during development into brain parenchyma and differentiate into various types of neurons, astrocytes and oligodendroglia. This process is strictly time regulated so distant parts of brain can become functional link of the neuronal circuit. The layered structure of the mature cerebral cortex is created in an inside-out order. Immunostaining for nestin, a neural progenitor marker, indicated that changes in cellular development are not limited to the hypothalamic and thalamic regions but could also be detected in the telencephalic structures in HF fetal brains. Nestin expressing cells were markedly increased not only in the hypothalamus, but also in the brain cortex of the HF fetal rats indicating broader spectrum of developmental changes. In the LC fetal brains immature nestin+ cells were found only in the deeper layer of dorsal and lateral cortices. In contrast, in HF fetal brains nestin+ cells were present throughout the layers of cortices (Fig. 2b), suggesting impaired layering and maturation of cortical neurons. Scattered distribution of Nestin+ cells through the entire region of cortices in the HF fetal brain indicates a delayed cellular organization which could possibly disrupt intercellular connectivity. The organization of neuronal connections into vertical columns allows cortical columns to act as the functional units so any disruption of circuitry may have functional consequences.
We also observed changes in distribution of newly differentiated, βIII-Tubulin expressing neurons (Fig. 2c). In LC fetal brains such neurons were uniformly distributed in all cortical layers, emphasizing optimal stratification of the region. In contrast, in the HF fetal brains the βIII-Tubulin expressing neurons formed tight columnar structures. Thick bundles of processes run from deepest layers toward most outer layers of cortex but connectivity of distant molecular layer I is less prominent than in controls.
Deficit in neural development can lead to cognitive, motor, and intellectual disability, as well as neurological disorders such as autism, Rett syndrome, and mental retardation. For instance, in sensory areas, these verticallyintegrated columns actually have an inhibitory effect on adjacent columns (lateral inhibition) which is believed to increase resolution of sensory information needed (Palmer et al. 2012). Furthermore, perinatal exposure to a high fat diet has been shown to result in altered development of key pathways that regulate mood and behavior (Kiyohara and Yoshimasu 2009) and in increased anxiety and deficits in spatial learning ability in the offspring (Bilbo and Tsang 2010).
The mechanisms that underlie the predisposition of the offspring of obese mothers to metabolic aberrations and possibly neurodevelopmental disorders are not clearly understood. During the early developmental periods, insulin and leptin function as growth factors for the central nervous system. Since HF term fetuses are both hyperinsulinemic and hyperleptinemic (Gupta et al. 2009), it is possible that the altered levels of these hormones induce changes in the development of the brain. The results from the present study indicate developmental alterations in the hypothalamic and extra-hypothalamic regions in the HF fetal brain suggestive of a predisposition for the development of obesity and possibly neurodevelopmental abnormalities in the offspring.
Acknowledgements
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-61518 (MSP).
Footnotes
The authors declare that they have no conflict of interest.
Contributor Information
Ewa K. Stachowiak, Department of Pathology and Anatomic Sciences, School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY 14221, USA
Malathi Srinivasan, Department of Biochemistry, School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY 14221, USA.
Michal K. Stachowiak, Department of Pathology and Anatomic Sciences, School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY 14221, USA
Mulchand S. Patel, Department of Biochemistry, School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY 14221, USA
References
- Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24(6):2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28(46):12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. Journal of autism and developmental disorders. 2011;41(7):891–902. doi: 10.1007/s10803-010-1114-8. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Ozanne SE. Early life nutrition and metabolic programming. Annals of the New York Academy of Sciences. 2010;1212:78–96. doi: 10.1111/j.1749-6632.2010.05798.x. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Gupta A, Srinivasan M, Thamadilok S, Patel MS. Hypothalamic alterations in fetuses of high fat diet-fed obese female rats. The Journal of endocrinology. 2009;200(3):293–300. doi: 10.1677/JOE-08-0429. 10.1677/JOE-08-0429. [DOI] [PubMed] [Google Scholar]
- Kiyohara C, Yoshimasu K. Molecular epidemiology of major depressive disorder. Environmental health and preventive medicine. 2009;14(2):71–87. doi: 10.1007/s12199-008-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;(82):1–8. [PubMed] [Google Scholar]
- Palmer L, Murayama M, Larkum M. Inhibitory Regulation of Dendritic Activity in vivo. Front Neural Circuits. 2012;6:26. doi: 10.3389/fncir.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K, Barson JR, Fagan SE, Leibowitz SF. Developmental changes in embryonic hypothalamic neurons during prenatal fat exposure. Am J Physiol Endocrinol Metab. 2012;303(3):E432–E441. doi: 10.1152/ajpendo.00238.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofey DL, Kolko RP, Iosif AM, Silk JS, Bost JE, Feng W, Szigethy EM, Noll RB, Ryan ND, Dahl RE. A longitudinal study of childhood depression and anxiety in relation to weight gain. Child Psychiatry Hum Dev. 2009;40(4):517–526. doi: 10.1007/s10578-009-0141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab. 2006;291(4):E792–E799. doi: 10.1152/ajpendo.00078.2006. [DOI] [PubMed] [Google Scholar]
- Stachowiak EK, Oommen S, Vasu VT, Srinivasan M, Stachowiak M, Gohil K, Patel MS. Maternal obesity affects gene expression and cellular development in fetal brains. Nutritional neuroscience. 2013;16(3):96–103. doi: 10.1179/1476830512Y.0000000035. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Smith MS, Grove KL. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology. 2011;93(1):1–8. doi: 10.1159/000322038. 10.1159/000322038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics. 2008;122(1):e1–e6. doi: 10.1542/peds.2007-1955. 10.1542/peds.2007-1955. [DOI] [PubMed] [Google Scholar]