Abstract
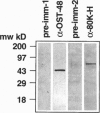
Advanced glycation endproducts (AGEs) are derivatives of nonenzymatic reactions between sugars and protein or lipids, and together with AGE-specific receptors are involved in numerous pathogenic processes associated with aging and hyperglycemia. Two of the known AGE-binding proteins isolated from rat liver membranes, p60 and p90, have been partially sequenced. We now report that the N-terminal sequence of p60 exhibits 95% identity to OST-48, a 48-kDa member of the oligosaccharyltransferase complex found in microsomal membranes, while sequence analysis of p90 revealed 73% and 85% identity to the N-terminal and internal sequences, respectively, of human 80K-H, a 80- to 87-kDa protein substrate for protein kinase C. AGE-ligand and Western analyses of purified oligosaccharyltransferase complex, enriched rough endoplasmic reticulum, smooth endoplasmic reticulum, and plasma membranes from rat liver or RAW 264.7 macrophages yielded a single protein of approximately 50 kDa recognized by both anti-p60 and anti-OST-48 antibodies, and also exhibited AGE-specific binding. Immunoprecipitated OST-48 from rat rough endoplasmic reticulum fractions exhibited both AGE binding and immunoreactivity to an anti-p60 antibody. Immune IgG raised to recombinant OST-48 and 80K-H inhibited binding of AGE-bovine serum albumin to cell membranes in a dose-dependent manner. Immunostaining and flow cytometry demonstrated the surface expression of OST-48 and 80K-H on numerous cell types and tissues, including mononuclear, endothelial, renal, and brain neuronal and glial cells. We conclude that the AGE receptor components p60 and p90 are identical to OST-48, and 80K-H, respectively, and that they together contribute to the processing of AGEs from extra- and intracellular compartments and in the cellular responses associated with these pathogenic substances.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Boguski M. S., Gish W., Wootton J. C. Issues in searching molecular sequence databases. Nat Genet. 1994 Feb;6(2):119–129. doi: 10.1038/ng0294-119. [DOI] [PubMed] [Google Scholar]
- Araki N., Higashi T., Mori T., Shibayama R., Kawabe Y., Kodama T., Takahashi K., Shichiri M., Horiuchi S. Macrophage scavenger receptor mediates the endocytic uptake and degradation of advanced glycation end products of the Maillard reaction. Eur J Biochem. 1995 Jun 1;230(2):408–415. doi: 10.1111/j.1432-1033.1995.0408h.x. [DOI] [PubMed] [Google Scholar]
- Banerjee D., Redman C. M. Biosynthesis of high density lipoprotein by chicken liver: nature of nascent intracellular high density lipoprotein. J Cell Biol. 1983 Mar;96(3):651–660. doi: 10.1083/jcb.96.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F. G., Striker L. J., Elliot S. J., Andreani D., Striker G. E. Synthesis and release of insulinlike growth factor I by mesangial cells in culture. Am J Physiol. 1988 Dec;255(6 Pt 2):F1214–F1219. doi: 10.1152/ajprenal.1988.255.6.F1214. [DOI] [PubMed] [Google Scholar]
- Doi T., Vlassara H., Kirstein M., Yamada Y., Striker G. E., Striker L. J. Receptor-specific increase in extracellular matrix production in mouse mesangial cells by advanced glycosylation end products is mediated via platelet-derived growth factor. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2873–2877. doi: 10.1073/pnas.89.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M., Shimizu N. Purification of two distinct proteins of approximate Mr 80,000 from human epithelial cells and identification as proper substrates for protein kinase C. Biochem J. 1990 Sep 15;270(3):583–589. doi: 10.1042/bj2700583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori O., Brett J., Slattery T., Cao R., Zhang J., Chen J. X., Nagashima M., Lundh E. R., Vijay S., Nitecki D. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995 Oct 27;270(43):25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Wall D. A., Ma A. Isolation of rat hepatocyte plasma membranes. I. Presence of the three major domains. J Cell Biol. 1983 Jan;96(1):217–229. doi: 10.1083/jcb.96.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. R., Nilsson T., Peterson P. A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990 Oct;9(10):3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUWABARA T., COGAN D. G. Studies of retinal vascular patterns. I. Normal architecture. Arch Ophthalmol. 1960 Dec;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]
- Kelleher D. J., Kreibich G., Gilmore R. Oligosaccharyltransferase activity is associated with a protein complex composed of ribophorins I and II and a 48 kd protein. Cell. 1992 Apr 3;69(1):55–65. doi: 10.1016/0092-8674(92)90118-v. [DOI] [PubMed] [Google Scholar]
- Kirstein M., Aston C., Hintz R., Vlassara H. Receptor-specific induction of insulin-like growth factor I in human monocytes by advanced glycosylation end product-modified proteins. J Clin Invest. 1992 Aug;90(2):439–446. doi: 10.1172/JCI115879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein M., Brett J., Radoff S., Ogawa S., Stern D., Vlassara H. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9010–9014. doi: 10.1073/pnas.87.22.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn R. R., Cerami A., Monnier V. M. Collagen aging in vitro by nonenzymatic glycosylation and browning. Diabetes. 1984 Jan;33(1):57–59. doi: 10.2337/diab.33.1.57. [DOI] [PubMed] [Google Scholar]
- Kumar V., Heinemann F. S., Ozols J. Purification and characterization of avian oligosaccharyltransferase. Complete amino acid sequence of the 50-kDa subunit. J Biol Chem. 1994 May 6;269(18):13451–13457. [PubMed] [Google Scholar]
- Lee S. C., Liu W., Brosnan C. F., Dickson D. W. Characterization of primary human fetal dissociated central nervous system cultures with an emphasis on microglia. Lab Invest. 1992 Oct;67(4):465–476. [PubMed] [Google Scholar]
- Li Y. M., Arkins S., McCusker R. H., Jr, Donovan S. M., Liu Q., Jayaraman S., Dantzer R., Kelley K. W. Macrophages synthesize and secrete a 25-kilodalton protein that binds insulin-like growth factor-I. J Immunol. 1996 Jan 1;156(1):64–72. [PubMed] [Google Scholar]
- Li Y. M., Brunke D. L., Dantzer R., Kelley K. W. Pituitary epithelial cell implants reverse the accumulation of CD4-CD8- lymphocytes in thymus glands of aged rats. Endocrinology. 1992 May;130(5):2703–2709. doi: 10.1210/endo.130.5.1572290. [DOI] [PubMed] [Google Scholar]
- Li Y. M., Tan A. X., Vlassara H. Antibacterial activity of lysozyme and lactoferrin is inhibited by binding of advanced glycation-modified proteins to a conserved motif. Nat Med. 1995 Oct;1(10):1057–1061. doi: 10.1038/nm1095-1057. [DOI] [PubMed] [Google Scholar]
- Makita Z., Radoff S., Rayfield E. J., Yang Z., Skolnik E., Delaney V., Friedman E. A., Cerami A., Vlassara H. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991 Sep 19;325(12):836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- Makita Z., Vlassara H., Cerami A., Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992 Mar 15;267(8):5133–5138. [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981 Jan 30;211(4481):491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Stevens V. J., Cerami A. Nonenzymatic glycosylation, sulfhydryl oxidation, and aggregation of lens proteins in experimental sugar cataracts. J Exp Med. 1979 Nov 1;150(5):1098–1107. doi: 10.1084/jem.150.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Edman J. C., Standring D. N., Fried V. A., Smith M. C., Roth R. A., Rutter W. J. Insulin-like growth factor II receptor as a multifunctional binding protein. Nature. 1987 Sep 24;329(6137):301–307. doi: 10.1038/329301a0. [DOI] [PubMed] [Google Scholar]
- Neeper M., Schmidt A. M., Brett J., Yan S. D., Wang F., Pan Y. C., Elliston K., Stern D., Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992 Jul 25;267(21):14998–15004. [PubMed] [Google Scholar]
- Radoff S., Cerami A., Vlassara H. Isolation of surface binding protein specific for advanced glycosylation end products from mouse macrophage-derived cell line RAW 264.7. Diabetes. 1990 Dec;39(12):1510–1518. doi: 10.2337/diab.39.12.1510. [DOI] [PubMed] [Google Scholar]
- Radoff S., Vlassara H., Cerami A. Characterization of a solubilized cell surface binding protein on macrophages specific for proteins modified nonenzymatically by advanced glycosylated end products. Arch Biochem Biophys. 1988 Jun;263(2):418–423. doi: 10.1016/0003-9861(88)90654-6. [DOI] [PubMed] [Google Scholar]
- Sakai K., Hirai M., Minoshima S., Kudoh J., Fukuyama R., Shimizu N. Isolation of cDNAs encoding a substrate for protein kinase C: nucleotide sequence and chromosomal mapping of the gene for a human 80K protein. Genomics. 1989 Aug;5(2):309–315. doi: 10.1016/0888-7543(89)90063-3. [DOI] [PubMed] [Google Scholar]
- Schmidt A. M., Vianna M., Gerlach M., Brett J., Ryan J., Kao J., Esposito C., Hegarty H., Hurley W., Clauss M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992 Jul 25;267(21):14987–14997. [PubMed] [Google Scholar]
- Silberstein S., Kelleher D. J., Gilmore R. The 48-kDa subunit of the mammalian oligosaccharyltransferase complex is homologous to the essential yeast protein WBP1. J Biol Chem. 1992 Nov 25;267(33):23658–23663. [PubMed] [Google Scholar]
- Skolnik E. Y., Yang Z., Makita Z., Radoff S., Kirstein M., Vlassara H. Human and rat mesangial cell receptors for glucose-modified proteins: potential role in kidney tissue remodelling and diabetic nephropathy. J Exp Med. 1991 Oct 1;174(4):931–939. doi: 10.1084/jem.174.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. High-affinity-receptor-mediated uptake and degradation of glucose-modified proteins: a potential mechanism for the removal of senescent macromolecules. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5588–5592. doi: 10.1073/pnas.82.17.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. Novel macrophage receptor for glucose-modified proteins is distinct from previously described scavenger receptors. J Exp Med. 1986 Oct 1;164(4):1301–1309. doi: 10.1084/jem.164.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Manogue K. R., Dinarello C. A., Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988 Jun 10;240(4858):1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Bucala R., Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994 Feb;70(2):138–151. [PubMed] [Google Scholar]
- Vlassara H., Li Y. M., Imani F., Wojciechowicz D., Yang Z., Liu F. T., Cerami A. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): a new member of the AGE-receptor complex. Mol Med. 1995 Sep;1(6):634–646. [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Moldawer L., Chan B. Macrophage/monocyte receptor for nonenzymatically glycosylated protein is upregulated by cachectin/tumor necrosis factor. J Clin Invest. 1989 Dec;84(6):1813–1820. doi: 10.1172/JCI114366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S. D., Schmidt A. M., Anderson G. M., Zhang J., Brett J., Zou Y. S., Pinsky D., Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994 Apr 1;269(13):9889–9897. [PubMed] [Google Scholar]
- Yang Z., Makita Z., Horii Y., Brunelle S., Cerami A., Sehajpal P., Suthanthiran M., Vlassara H. Two novel rat liver membrane proteins that bind advanced glycosylation endproducts: relationship to macrophage receptor for glucose-modified proteins. J Exp Med. 1991 Sep 1;174(3):515–524. doi: 10.1084/jem.174.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]