Abstract
Interactions between the integrin, α2β1, and extracellular matrix (ECM), particularly collagen, play a pivotal role in platelet adhesion and thrombus formation. Platelets interact with collagen in the subendothelial matrix that is exposed by vascular damage. To evaluate the potential of α2β1 inhibitors for anticancer and antithrombotic applications, we have developed a series of small molecule inhibitors of this integrin based on a prolyl-2,3-diaminopropionic acid (DAP) scaffold using solid-phase parallel synthesis. A benzene-sulfonamide substituent at the N-terminus of the dipepetide and a benzyl urea at the DAP side chain resulted in tight and highly selective inhibition of α2β1-mediated adhesion of human platelets and other cells to collagen.
Introduction
The blood vessel wall is lined by a continuous layer of endothelial cells that not only interfaces directly with the blood but also serves as a barrier that separates cells and proteins in the blood from proteins in the subendothelial extracellular connective tissue matrix (ECM). Thus, when vascular trauma disrupts the endothelium, platelets are exposed to a variety of ECM proteins, initiating the formation of a hemostatic plug under physiologic circumstances1 or thrombosis when endothelial disruption perturbs the integrity of an atherosclerotic plaque.2 Collagen, among the ECM proteins, plays a pivotal role in platelet-mediated hemostasis and thrombosis, because it is not only a ligand for platelet adhesion and an agonist for aggregation but is also the binding site for von Willebrand factor (vWF) in the ECM.3 Following vascular trauma, circulating platelets slow, tether, and roll on collagen-bound vWF, allowing the platelet collagen receptor GPVI to interact with collagen. Collagen binding to GPVI, a member of the immunoglobulin receptor superfamily, then initiates a signaling pathway that triggers the activation of a second platelet collagen receptor α2β1, as well as the platelet adhesion receptor αIIbβ3.4,5
α2β1 is a widely expressed integrin that binds to type I collagen, as well as type II-V and to laminins 1 and 5, depending on the cellular context.6 It has been implicated in hemostasis and thrombosis, as well as cancer metastasis, wound healing, and angiogenesis.7,8 αβ1 is one of nine integrins whose α-subunit has an inserted (I)-domain that is responsible for binding of the integrin to its natural ligand. Importantly, α2β1 has multiple conformational states that can be regulated by intracellular signaling pathways.4,9-11
Although α2β1 was the first collagen receptor to be identified on platelets,12,13 the relative roles of α2β1 and GPVI in mediating platelet response to collagen has been extensively debated in recent years. Using mouse models, Nieswandt et al. reported that α2β1 is essential for platelet adhesion on type I collagen solubilized by pepsin but may be dispensable when platelets adhere to physiologically relevant fibrillar collagen under low and high shear conditions. For example, they observed only a slight delay in ex vivo aggregation of β1-null mouse platelets stimulated by fibrillar collagen.4,5,14,15 On the other hand, overexpression of α2β1 in humans due to polymorphisms in the α2 gene has been associated with stroke and nonfatal myocardial infarction.16 Moreover, additional experiments suggest that both α2β1 and GPVI play a significant role in hemostasis and thrombosis in vivo.17 For example, Kahn et al. observed that pharmacologic inhibition of human platelets and of α2-deficient mouse platelets resulted in reduced adhesion on fibrillar collagen under flow conditions. Also in vivo α2-deficient mice displayed delayed thrombotic responses in the tail-bleeding model.18,19
Given that α2β1 functions in platelets after they first adhere to collagen and that patients with α2β1 deficiency only exhibit a mild bleeding diathesis,12,20-22 α2β1 appears to be a good target for the development of safer and more effective small-molecule antithrombotic agents, especially in light of the increase in the overall incidence of cardiovascular disease.23 Previously, benzenesulfonyl-Pro-Phe dipeptide derivatives were developed as inhibitors of the closely related integrin α4β1 (Figure 1a).24,25 By varying the Phe derivative, it was possible to obtain high potency and selectivity for α4β1. In related studies, inhibitors of the integrin αIIbβ3 have been developed with 2,3-diaminopropionic acid (DAP) occupying a roughly equivalent position as the Phe derivative in the α4β1 inhibitors (Figure 1b).26,27
Figure 1.
Chemical structure of antagonists of α4β1 and RIIbβ3 integrins.
Here, we combine the prolyl-sulfonylamide fragment with derivatives of DAP to develop selective inhibitors of α2β1. These compounds are potent inhibitors of the adhesion of platelets and transfected cells to immobilized soluble collagen type I. However, they do not inhibit the binding of the isolated α2 I-domain to immobilized Type I collagen. This behavior suggests that they inhibit adhesion via binding to the β1 I-like domain.
Results and Discussion
Chemistry
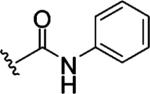
The prolyl-diaminopropionic acid derivatives were synthesized by the solid-phase route described in Scheme 1. Fmoc-DAP(Alloc)-OH was attached to bromomethyl Wang resin using CsI as the catalyst.28 Following deprotection of the Fmoc group with 20% PIP in DMF, Fmoc-Pro was appended under standard peptide coupling conditions. Deprotection of the Fmoc group followed by the sulfonamide formation gave intermediate 3 for the modification of the 3-position of the DAP fragment. The allyloxycarbonyl (Alloc) group of DAP was removed using Pd(PPh3)4 and PhSiH3 in degassed CH2Cl2 under argon gas. Acylation or urea formation of the released amine with concomitant cleavage from the resin generated the desired α2β1 inhibitors. The side chain of the DAP fragment was varied in a similar manner. The nitro-substituted compound 28 was treated with SnCl2•2H2O to generate the amine which was modified for compounds 30-32.
Scheme 1.
Inhibition of Human Platelet Adhesion
A series of compounds were tested to determine their ability to inhibit the adhesion of washed human platelets to immobilized soluble collagen type I (Tables 1 and 2). Because the expression level of α2β1 on platelets can vary among normal individuals by a factor of 4 and affect α2β1-mediated platelet function,16,29 the inhibitory activities for each compound in the platelet adhesion assay reported in Table 1 and 2 were evaluated using platelets from a single individual. Parallel experiments with platelets from other individuals showed a very similar trend in affinities relative to a standard compound (9), but the midpoints of the curves were systematically shifted toward higher concentrations by as much as a factor of 3 to 4.
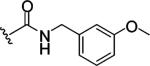
Table 1.
In Vitro Human Platelet Adhesion Inhibitory Activity

| |||||
|---|---|---|---|---|---|
| Compound | R1 | IC50(μM) | Compound | R1 | IC50(μM) |
| 5 |

|
0.660 | 12 |
|
0.296 |
| 6 |

|
0.367 | 13 |
|
0.023 |
| 7 |

|
>10 | 14 |
|
0.107 |
| 8 |
|
0.185 | 15 |

|
0.467 |
| 9 |

|
0.015 | 16 |
|
0.160 |
| 10 |

|
0.357 | 17 |

|
0.108 |
| 11 |

|
0.185 | |||
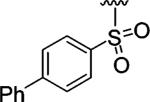
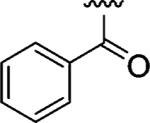
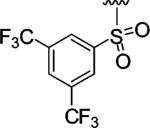
Table 2.
In Vitro Human Platelet Adhesion Inhibitory Activity

| |||||
|---|---|---|---|---|---|
| Compound | R2 | IC50 (μM) | Compound | R2 | IC50 (μM) |
| 9 |

|
0.015 | 25 |
|
0.287 |
| 18 |
|
>10 | 26 |

|
0.036 |
| 19 |

|
0.029 | 27 |

|
0.070 |
| 20 |
|
0.039 | 28 |

|
0.105 |
| 21 |

|
0.035 | 29 |

|
0.144 |
| 22 |

|
0.017 | 30 |

|
0.131 |
| 23 |
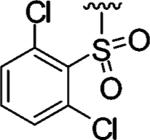
|
>10 | 31 |
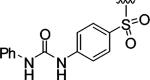
|
0.151 |
| 24 |

|
0.050 | 32 |

|
0.236 |
The first modification was conducted in the side chain of the DAP fragment to determine the preferred interaction for good α2β1 binding (Table 1). Benzamide compound 5 showed moderate activity, which was enhanced by an ethyl linker between the amide and the hydrophobic aromatic ring. It is clearly seen that the carbonyl group is essential for α2β1 binding. Changing the carbonyl group (6) to a sulfonyl group (7) resulted in more than a 25-fold decrease in potency.
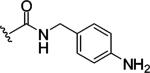
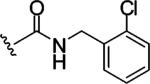
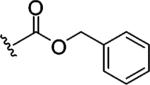
Replacing one of the CH2 groups with oxygen or NH to provide urethane 8 and urea 9 showed an improvement in potency. The 10-fold increase in potency of urea 9 over the urethane 8 suggests that the hydrogen bonding donor NH group of the urea might play a critical role in enhancing binding potency, although the rigidity and electronic properties are also affected. Aliphatic substitution within the urea (10 and 11) or conversion of the benzyl urea to a phenyl urea (12) resulted in loss of potency. We also investigated the effect of substituents on the phenyl ring of the benzyl urea group. Substitution at the para position (13) with an amino group had little effect on potency. However, data from chloro- and methoxy-subsitituted compounds 14-17 resulted in more than a 10-fold decrease in potency over unsubstituted compound 9.
Encouraged by these results, we next determined the effect of varying the N-terminal benzenesulfonyl group, while holding the urea side chain constant (Table 2). It is clearly seen that the sulfonyl group is essential for α2β1 binding. Replacing the sulfonyl group (9) with a carbonyl group (18) caused a greater than 600-fold decrease in potency. Therefore, further analogues focused on the sulfonylated Pro-DAP. Generally, the activity of the compounds was very sensitive to the position (ortho, meta, and para) of the substituents. The 3,5-disubstituted compounds (19-22) were well tolerated, regardless of the properties of substituents (electron-donating and electron-withdrawing groups). This series displayed similar levels of potency to the unsubstituted lead compound 9. However, 2,6-dichlorobenzenesulfonyl compound 23 was more than 600-fold less active than 3,5-dichlorobenzenesulfonyl compound 22. It is likely that the two substituents at the 2 and 6 positions cause an unfavorable orientation of the phenyl ring relative to the pyrrolidine ring of the Pro residue. In contrast to the planar 2-naphthalene sulfonyl compound 24, staggered biphenyl sulfonyl compound 25 showed a 5-fold decrease in potency, suggesting the importance of the spatial hydrophobic functionality for potency. Although the substitutions at meta and para positions were well tolerated (19-22, 24), an attempt to improve the activity of 9 by introducing various substituents at the para position (26-32) did not significantly improve potency.
Selectivity of the Inhibitors
Integrins are heterodimers composed of R and β subunits. There are eighteen different integrin α subunits and eight different β subunits that combine to form twenty-four structurally and functionally diverse receptors. To confirm that our compounds are specific α2β1 inhibitors, we examined the specificity of five selected potent α2β1 inhibitors against three closely related integrins, α1β1, α4β1, and α5β1 (Table 3).30 Because our goal was to determine the specificity of the compounds, we selected from the compounds with the highest potency (Tables 1 and 2), selecting only one compound, 24, with moderate potency as a control. To evaluate α1β1-mediated adhesion to collagen IV, we used K562 cells transfected with the α1 integrin subunit. None of the tested compounds inhibited K562 cell adhesion to collagen VI at 1 μM concentration, and only parial effects were observed at 3 μM concetnrations of the compounds. Similarly, the compounds had no effect on α5β1-mediated K562 adhesion to fibronectin.
Table 3.
Potency and Selectivity of Selected Inhibitors against Related Integrins
| IC50 (μM) |
|||||||
|---|---|---|---|---|---|---|---|
| integrin | cell line | ligand | 9 | 13 | 19 | 22 | 24 |
| α1β1a | K562b | collagen IV | 29% | 22% | 26% | 32% | 30% |
| α2β1 | platelet | collagen I | 0.015 | 0.023 | 0.029 | 0.017 | 0.050 |
| α2β1 | K562b | collagen I | 0.138 | 0.102 | 0.089 | 0.083 | 0.177 |
| α4β1 | Jurkat | VCAM | 2.128 | 1.966 | 0.770 | 0.637 | 4.334 |
| α5β1 | K562 | fibronectin | > 10 | > 10 | > 10 | > 10 | > 10 |
% inhibition at 3 μM.
Transfected K562 cell.
A number of groups have recently reported a series of structurally similar α4β1 antagonists.24,25 Therefore, we examined the specificity of our compounds for α2β1 versus α4β1, employing Jurkat cells, which use α4β1 to specifically adhere to VCAM-1. We were encouraged to find that all five compounds exhibited a high degree of specificity for α2β1 versus α4β1 (Table 4). The absolute degree of specificity depends on whether one compares the results of α4β1-mediated Jurkat cell adhesion to α2β1-mediated platelet adhesion or K562 cell adhesion data; however, in both cases, the compounds showed considerable specificity for the desired target. Unsubstituted benzenesulfonyl compounds 9 and 13 showed high selectivity for α2β1 over α4β1, as did 2-naphthalenesulfonyl compound 24. It is noteworthy that 3,6-disubstituted benzene-sulfonyl compounds 19 and 22 exhibited reduced selectivity over α4β1. This modification had previously been shown to increase potency for α4β1.25 Interestingly, we determined that a previously reported α4β1 antagonist (3,5-dichlorobenzenesulfonyl-Pro-Tyr)25 showed no selectivity under this condition (the IC50's of α4β1 antagonist to α2β1 and α4β1 were 0.336 and 0.356 μM, respectively).
Table 4.
Selectivity of Selected Inhibitors for α2β1 versus α4β1
| 9 | 13 | 19 | 22 | 24 | |
|---|---|---|---|---|---|
| IC50 (platelets, μM) | 0.01 5 | 0.02 3 | 0.02 9 | 0.01 7 | 0.05 0 |
| IC50 (α4β1)/IC50 (α2β1) | 142 | 85 | 27 | 38 | 87 |
| Jurkat versus platelets | |||||
| IC50 (α4β1)/IC50 (α2β1) | 15 | 19 | 8.6 | 7.7 | 24 |
| Jurkat versus tranfected K562 cells |
We also examined α2β1-mediated adhesion using K562 cells transfected with α2. Untransfected cells do not adhere to type I collagen under our experimental conditions; thus, the transfected cells adhere to immobilized type I collagen in an α2β1-dependent manner. Comparison of adhesion with these cells to adhesion with platelets provides a measure of the sensitivity of the assay to the type of cell employed. The first four compounds listed in Table 4 are all approximately equipotent within the errors of the assays (up to a factor of 2), but the fifth compound (naphthyl-substituted sulfonamide 24) is 2- to 3-fold less potent using both cell types. Thus, there is reasonable agreement concerning the ability of the assay to discriminate between compounds with different affinities (although more low-affinity compounds would need to be tested to further support this statement). However, all compounds in the human platelet adhesion assay were approximately 1 order of magnitude more potent than in the assay with transfected K562 cells. Presumably, the different potency of antagonists against α2β1 in platelets versus K562 cells reflects differences in levels of expression as well as the degree of integrin activation.31
We also examined the specificity of the most potent compound 9 using ADP stimulated platelet aggregation, an effect that is ultimately mediated by αIIbβ3. These studies showed that the inhibitor did not affect platelet aggregation stimulated by addition of ADP agonist in the presence of the compound (Supplementary Figure 1). These result shows that the compounds do not affect signaling through this pathway, and that the compounds do not inhibit the function of activate αIIbβ3.
Possible Location of the Binding Site of the Inhibitor
The affinity state of α2β1 can be regulated by inside-out signaling pathways that affect the position of β1 C7 helix.32 Unlike the metal ion-dependent adhesion site (MIDAS) on the α2 I-domain which is involved in direct binding of collagen, the MIDAS on the β1 I-like domain provides an intrinsic ligand binding site to activate the I domain.10 To define the binding site and mode of action, all compounds were evaluated in a recombinant human α2 I-domain/soluble collagen I enzyme-linked immunosorbant assay (ELISA).33 These studies showed that the present inhibitors did not inhibit I-domain binding to immobilized type I collagen, although a number of unrelated compounds have been shown to inhibit binding to this site. This behavior is consistent with binding to the β1 I-like domain MIDAS located near a key regulatory interface with the α2 I-domain. Given the specificity of these compounds for α2β1 over other β1 integrins, it would appear that this site requires the presence of both integrin subunits.
Conclusions
In conclusion, we have identified a series of prolyl-2,3-diaminopropionic acid (DAP) derivatives that bind tightly to integrin α2β1. Solid-phase parallel synthesis was used to optimize the different portions of the compound and obtain potent antagonists. The SAR on this series of diamino-propionic acid derivatives shows that the following features were sufficient to provide high potency and selectivity: (a) a urea group with a distal benzylic group linked by a proper spacer was critical for α2β1 binding; (b) the sulfonyl group at the N-terminal residue was necessary for tight binding; (c) the potency was extremely sensitive to the position of substituents on the aryl sulfonyl moiety (ortho Vs meta and para position).
The goal of this work is to develop selective small molecule inhibitors of α2β1 to allow pharmacological investigations of the role of this integrin in various disease processes. Our compounds are of sufficient potency for this purpose and are selective for α2β1 over α1β1, α4β1, α5β1, and αIIbβ3. In contrast, a known α4β1 antagonist showed no selectivity under our assay conditions. Currently, we are using these α2β1 inhibitors to study the mechanism of α2β1 mediated-thrombus formation in vitro and in vivo. Further development of these compounds might ultimately lead to clinically useful agents for treatment of thrombosis and/or cancer.
Experimental Section
General
Unless otherwise indicated, all reactions were run under argon gas. Anhydrous solvents were obtained via passage through an activated alumina column34 and from commercial suppliers. 1H and 13C NMR spectra were recorded on an AM-500 or DRX-500 spectrometer. Chemical shifts are reported relative to internal DMSO-d6 (δ 2.50 for 1H and δ 39.52 for 13C). High-resolution mass spectra were accomplished using an Autospec high resolution double focusing electrospray ionization/chemical ionization spectrometer with either DEC 11/73 or OPUS software data system. Preparative HPLC was performed on a Varian HPLC system, using a GRACEVYDAC C-18 column, 250 × 22 mm, 100 Å, and a flow rate of 10 mL/min; λ ) 254 nm; mobile phase A, 0.1% TFA in H2O, and mobile phase B, 0.1% TFA in CH3CN. The purified fractions were lyophilized. Compound purities were determined by analytical RP-HPLC using a GRACEVYDAC C-18 column eluted at a rate of 1 mL/min with a gradient of solvent B varying at no faster than 1%/min. All compounds had a purity of 95% or greater based on the integrated peak area (detection at 210 nm).
General Procedure for the Preparation of Inhibitors 5–32
The 4-(bromomethyl)phenoxymethyl polystyrene resin was swelled in DMF (15 mL/g resin). Fmoc-DAP(Alloc)-OH (1.5 equiv), CsI (1.0 equiv), and DIEA (2 equiv) were added, and the reaction was stirred at 25 °C for 18 h. The resin was filtered and washed repeatedly with DMF and MeOH. After deprotecting the Fmoc group by treatment of 20% PIP in DMF, the resin was washed with DMF. This resin was then suspended with DMF and stirred with Fmoc-Pro-OH or proline analogue (3 equiv), HATU (3 equiv), HOAT (3 equiv), and DIEA (6 equiv) for 3 h. The resin was filtered and washed with DMF. After deprotecting the Fmoc group by treatment of 20% PIP in DMF, the resin was washed with DMF. This resin was then suspended with CH2Cl2 and stirred with benzenesulfonyl chloride derivatives (3 equiv) and DIEA (6 equiv) for 18 h. The resin was filtered, washed with CH2Cl2 and DMF, and dried overnight. To a peptide resin washed with oxygen-free CH2Cl2 in the presence of argon was added a solution of PhSiH3 (25 equiv), and the resin was stirred for 2 min. Subsequently, Pd-(PPh3)4 (0.5 equiv) was added under argon. The reaction was stirred for 2 h under argon. Then, the resin was washed repeatedly with CH2Cl2 and DMF. This resin was then suspended with DMF and stirred with isocyanate derivatives (3 equiv) for 18 h. The resin was filtered, washed with DMF and CH2Cl2, and dried.
Compounds 18–32 were prepared through a similar manner. The nitro-substituted compound 28 in DMF was treated with SnCl2•2H2O (20 equiv, 2 M) and stirred at 25 °C for 20 h to generate the amine. After filtration and washing, the resin in CH2Cl2 was treated with R3Cl (2 equiv) or isocyanate (2 equiv) and DIEA (3 equiv) to obtain compounds 30–32. The final compounds were cleaved from the resin by treatment of 100% TFA.
Human Platelet Adhesion Assay
Flat bottom microtiter plates (96-well) (Immulon 2, Dynatech Laboratories, Chantilly, VA) were coated with soluble type I collagen dissolved in 50 mM NaHCO3 buffer, pH 8.0, containing 150 mM NaCl as previously described.35 Unoccupied protein binding sites on the wells were blocked with 5 mg/mL bovine serum albumin dissolved in the same buffer. Human platelets were isolated from blood anticoagulated with 0.1 volume 3.8% sodium citrate by gel-filtration using GFP buffer (4 mM HEPES buffer, pH 7.4, containing 135 mM NaCl, 2.7 mM KCl, 5.6 mM glucose, 3.3 mM NaH2PO4, 0.35 mg/mL bovine serum albumin, and 2 mM MgCl2). Aliquots (100 μL) of the gel-filtered platelet suspension containing 1.25 × 108 platelets/mL were added to the protein-coated wells in the absence or presence of an inhibitor. Following incubation for 30 min at 37 °C without agitation, the plates were washed with the Tris-buffered NaCl, containing 2 mM MgCl2, pH 7.4, and the number of adherent platelets measured using the colorimetric assay reported by Bellavite et al.36 Briefly, 150 μL of a 0.1 M citrate buffer, pH 5.4, containing 5 mM p-nitrophenyl phosphate and 0.1% Triton X-100 was added to the wells after washing. After incubation for 60 min at 25 °C in the absence of ambient light, color was developed by the addition of 100 μL of 2 N NaOH and the absorbance at 405 nm was read using an EL800 Universal Microplate Reader (Bio-Tek Instruments, Inc., Winooski, Vermont).
Cell Adhesion Assay for Specificity of Inhibitors 30
The ligands (3 μg/mL of collagen IV for α1β1 or 3 μg/mL of collagen I for α2β1) were immobilized on 96-well flat microtiter plates (100 μL for each well) in PBS buffer solution overnight at 4 °C. In the case of VCAM (3 μg/mL, for α4β1) and fibronectin (10 μg/mL, for α5β1), 20 mM acetic acid was used instead of PBS buffer solution. In the case of α1β1 and α2β1, wells were blocked with 1% BSA in HBSS buffer solution without Ca2+ containing Mg2+ for 1 h. In the case of α4β1 and α5β1, 1% BSA in HyQ HBSS buffer solution containing Ca2+ and Mg2+ was used. Cells in the same buffer solution without BSA were labeled with incubation of 12.5 μM CMFDA at 37 °C for 30 min. After centrifugation and washing with buffer solution containing 1% BSA, cells were resuspended in the same buffer solution (1 × 106 cells/mL) and incubated in the presence of different concentrations of inhibitors at 25 °C for 15 min. Cells were added to the wells (100 μL/well) and incubated at 37 °C for 30 min. Unbound cells were washed out, and bound cells were lysed by the addition of 0.5% Triton X-100. The plates were read using a Cytofluor 2350 fluorescence plate reader (Millipore, Bedford, MA) with 485 nm (excitation) and 530 nm (emission).
Supplementary Material
Acknowledgment
We thank Seth Snyder for helpful discussions and Paul Billings for performing platelet aggregation studies. This work was supported by National Institutes of Health (grant no. EB002048).
Footnotes
Supporting Information Available: Analytical data for new compounds and platelet aggregation. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ruggeri ZM. Platelets in atherothrombosis. Nature Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Badimon L, Badimon JJ, Chesebro HJ. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N. Engl. J. Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 3.Santoro SA. Platelet surface collagen receptor polymorphisms: variable receptor expression and thrombotic/hemorrhagic risk. Blood. 1999;93:3575–3577. [PubMed] [Google Scholar]
- 4.Nieswandt B, Brakebusch C, Bergmeier W, Schulte V, Bouvard D, Mokhatari-Nejad R, Lindhout T, Heemskerk JWM, Zirngibl H, Fassler R. Glycoprotein VI but not α2β1 integrin is essential for platelet interaction with collagen. EMBO J. 2001;20:2120–2130. doi: 10.1093/emboj/20.9.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieswandt B, Watson SP. Platelet-collagen interaction: GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 6.Holtkotter O, Nieswandt B, Smyth N, Muller W, Hafner M, Schulte V, Krieg T, Eckes B. Integrin α2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J. Biol. Chem. 2002;277:10789–10794. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- 7.Sweeney SM, DiLullo G, Slater SJ, Martinez J, Iozzo RV, Lauer-Fields JL, Fields GB, Antonio JDS. Angiogenesis in collagen I requires α2β1 ligation of a GFP*GER sequence and possibly p38 MAPK activation and focal adhesion disassembly. J. Biol. Chem. 2003;278:30516–30524. doi: 10.1074/jbc.M304237200. [DOI] [PubMed] [Google Scholar]
- 8.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat. ReV. Mol. Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 9.Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat. ReV. Drug DiscoVery. 2003;2:703–715. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- 10.Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC. Structural basis of collagen recognition by integrin α2β1. Cell. 2000;101:47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 11.White DJ, Puranen S, Johnson MS, Heino J. The collagen receptor subfamily of the integrins. Int. J. Biochem. Cell Biol. 2004;36:1405–1410. doi: 10.1016/j.biocel.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwenhuis HK, Akkerman JWN, Houdijk WPM, Sixma JJ. Human blood platelets showing no response to collagen fail to express surface glycoprotein Ia. Nature. 1985;318:470–472. doi: 10.1038/318470a0. [DOI] [PubMed] [Google Scholar]
- 13.Santoro SA. Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell. 1986;46:913–920. doi: 10.1016/0092-8674(86)90073-5. [DOI] [PubMed] [Google Scholar]
- 14.Savage B, Ginsberg MH, Ruggeri ZM. Influence of fibrillar collagen structure on the mechanisms of platelet thrombus formation under flow. Blood. 1999;94:2704–2715. [PubMed] [Google Scholar]
- 15.Kuijpers MJE, Schulte V, Bergmeier W, Lindhout T, Brakebusch C, Offermanns S, Fassler R, Heemskerk JWM, Nieswandt B. Complementary roles of platelet glycoprotein VI and integrin α2β1 in collagen-induced thrombus formation in flowing whole blood ex vivo. FASEB J. 2003;17:685–687. doi: 10.1096/fj.02-0381fje. [DOI] [PubMed] [Google Scholar]
- 16.Kritzik M, Savage B, Nugent DJ, Santoso S, Ruggeri ZM, Kunicki TJ. Nucleotide polymorphisms in the α2 gene define multiple alleles that are associated with differences in platelet α2β1 density. Blood. 1998;92:2382–2388. [PubMed] [Google Scholar]
- 17.He L, Pappan LK, Grenache DG, Li Z, Tollefsen DM, Santoro SA, Zutter MM. The contribution of the α2β1 integrin to vascular thrombosis in vivo. Blood. 2003;102:3652–3657. doi: 10.1182/blood-2003-04-1323. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Kahn ML. Reciprocal signaling by integrin and nonintegrin receptors during collagen activation of platelets. Mol. Cell. Biol. 2003;23:4764–4777. doi: 10.1128/MCB.23.14.4764-4777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarratt KL, Chen H, Zutter MM, Santoro SA, Hammer D. a., Kahn ML. GP VI and α2β1 play independent critical roles during platelet adhesion and aggregate formation to collagen under flow. Blood. 2005;106:1268–1277. doi: 10.1182/blood-2004-11-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieuwenhuis HK, Sakariassen KS, Houdijk WPM, Nievelstein PFEM, Sixma JJ. Deficiency of platelet membrane glycoprotein Ia associated with a decreased platelet adhesion to subendothelium. Blood. 1986;68:692–695. [PubMed] [Google Scholar]
- 21.Kehrel B, Balleisen L, Kokott R, Mesters R, Stenzinger W, Clemetson KJ, Loo J. v. d. Deficiency of intact thrombospondin and membrane glycoprotein Ia in platelets with defective collagen-induced aggregation and spontaneous loss of disorder. Blood. 1988;71:1074–1078. [PubMed] [Google Scholar]
- 22.Handa M, Watanabe K, Kawai Y, Kamata T, Koyama T, Nagai H, Ikeda Y. Platelet unresponsiveness to collagen: involvement of glycoprotein Ia-IIa (α2β1 integrin) deficiency associated with a myeloproliferative disorder. Thrombos. Haemostas. 1995;73:521–528. [PubMed] [Google Scholar]
- 23.Jackson SP, Schoenwaelder SM. Antiplatelet therapy: in search of the ‘magic bullet’. Nat. ReV. Drug DiscoVery. 2003;2:1–15. doi: 10.1038/nrd1198. [DOI] [PubMed] [Google Scholar]
- 24.Hagmann WK, Durettea PL, Lanzaa T, Kevina NJ, Laszloa S. E. d., Kopkaa IE, Younga D, Magriotisa PA, Lia B, Lina LS, Yanga G, Kameneckaa T, Changa LL, Wilsona J, MacCossa M, Millsa SG, Riperb GV, McCauleyb E, Eggerb LA, Kidambib U, Lyonsc K, Vincentc S, Stearnsc R, Collettic A, Tefferac J, Tonga S, Fenyk-Melodyd J, Owensa K, Levorsea D, Kime P, Schmidtb JA, Mumford RA. The discovery of sulfonylated dipeptides as potent VLA-4 antagonists. Bioorg. Med. Chem. Lett. 2001;11:2709–2713. doi: 10.1016/s0960-894x(01)00544-3. [DOI] [PubMed] [Google Scholar]
- 25.Chang LL, Truonga Q, Mumfordb RA, Eggerb LA, Kidambib U, Lyonsc K, McCauleyb E, Riperb GV, Vincentc S, Schmidtb JA, MacCossa M, Hagmanna WK. The discovery of small molecule carbamates as potent dual α4β1/α4β7 integrin antagonists. Bioorg. Med. Chem. Lett. 2002;12:159–163. doi: 10.1016/s0960-894x(01)00710-7. [DOI] [PubMed] [Google Scholar]
- 26.Xue C-B, Rafalski M, Roderick J, Eyermann CJ, Mousa S, Olson RE, DeGrado WF. Design, synthesis and in vitro activities of a series of benzimidaxole/benzoxazole glycoprotein IIb/IIIa inhibitors. Bioorg. Med. Chem. Lett. 1996;6:339–344. [Google Scholar]
- 27.Xue C-B, Wityak J, Sielecki TM, Pinto DJ, Batt DG, Cain GA, Sworin M, Rockwell AL, Roderick JJ, Wang S, Orwat MJ, Frietze WE, Bostrom LL, Liu J, Higley CA, Rankin FW, Tobin AE, Emmett G, George K. Lalka, Sze JY, Meo SVD, Mousa SA, Thoolen MJ, Adrienne L. Racanelli, Hausner EA, Reilly TM, DeGrado WF, Wexler RR, Olson RE. Discovery of an orally active series of isoxazoline glycoprotein IIb/IIIa antagonists. J. Med. Chem. 1997;40:2064–2084. doi: 10.1021/jm960799i. [DOI] [PubMed] [Google Scholar]
- 28.Corbett JW, Graciani NR, Mousa SA, DeGrado WF. Solid-phase synthesis of a selective αvβ3 integrin antagonist library. Bioorg. Med. Chem. Lett. 1997;7:1371–1376. [Google Scholar]
- 29.Kunicki TJ, Orchekowski R, Annis D, Honda Y. Variability of integrin alpha 2 beta 1 activity on human platelets. Blood. 1993;82:2693–2703. [PubMed] [Google Scholar]
- 30.Marcinkiewicz C, Lobb RR, Marcinkiewicz MM, Daniel JL, Smith JB, Dangelmaier C, Weinreb PH, Beacham DA, Niewiarowski S. Isolation and characterization of EMS16, a C-lectin type protein from Echis multisquamatus venom, a potent and selective inhibitor of the α2β1 integrin. Biochemistry. 2000;39:9859–9867. doi: 10.1021/bi000428a. [DOI] [PubMed] [Google Scholar]
- 31.Walle G. R. V. d., Vanhoorelbeke K, Majer Z, Illyes E, Baert J, Pareyn I, Deckmyn H. Two functional active conformations of the integrin α2β1, depending on activation condition and cell type. J. Biol. Chem. 2005;280:36873–36882. doi: 10.1074/jbc.M508148200. [DOI] [PubMed] [Google Scholar]
- 32.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 33.Tuckwell D, Calderwood DA, Green LJ, Humphries MJ. Integrin α2 I-domain is a binding site for collagens. J. Cell Sci. 1995;108:1629–1637. doi: 10.1242/jcs.108.4.1629. [DOI] [PubMed] [Google Scholar]
- 34.Pangborn AB, Giardello MA, Grubbs RH, Rosen RK, Timmers FJ. Safe and Convenient Procedure for Solvent Purification. Organometallics. 1996;15:1518–1520. [Google Scholar]
- 35.Bennett JS, Chan C, Vilaire G, Mousa SA, DeGrado WF. Agonist-activated αvβ3 on plateletes and lymphocytes binds to the matrix protein osteopontin. J. Biol. Chem. 1997;272:8137–8140. doi: 10.1074/jbc.272.13.8137. [DOI] [PubMed] [Google Scholar]
- 36.Bellavite P, Andrioli G, Guzzo P, Arigliano P, Chirumbolo S, Manzato F, Santonastaso C. A colorimetric method for the measurement of platelet adhesion in microtiter plates. Anal. Biochem. 1994;216:444–450. doi: 10.1006/abio.1994.1066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




