Abstract
Purpose
While the dissemination of robotic prostatectomy and intensity-modulated radiotherapy (IMRT) may fuel increased use of prostatectomy and radiotherapy, these new technologies may also have spillover effects related to diagnostic testing for prostate cancer. Therefore, we examined the association of regional technology penetration with receipt of prostate specific antigen (PSA) testing and prostate biopsy.
Methods
In this retrospective cohort study, we included 117,857 men age 66 and older from the 5% sample of Medicare beneficiaries living in the Surveillance Epidemiology and End Results (SEER) areas from 2003 – 2007. Regional technology penetration was measured as the number of providers performing robotic prostatectomy or IMRT per population in a healthcare market (i.e., hospital referral region). We assessed the association of technology penetration with rates of PSA testing and prostate biopsy with generalized estimating equations.
Results
High technology penetration was associated with increased rates of PSA testing (442 versus 425 per 1,000 person-years, p<0.01) and similar rates of prostate biopsy (10.1 versus 9.9 per 1,000 person-years, p=0.69). The impact of technology penetration on PSA testing and prostate biopsy was much smaller than the effect of age, race, and comorbidity (e.g., PSA testing rate per 1,000 person-years: 485 versus 373 for men with only one versus 3+ co-morbid conditions, p<0.01).
Conclusions
Increased technology penetration was associated with slightly higher rates of PSA testing and no change in prostate biopsy rates. Collectively, our findings temper concerns that adoption of new technology accelerates diagnostic testing for prostate cancer.
Keywords: intensity-modulated radiotherapy, prostate biopsy, prostate cancer, PSA testing, robotic prostatectomy
Introduction
Prostate cancer is a common and expensive disease with annual spending exceeding $11 billion in the United States in 2010.1,2 Treatment for prostate cancer is associated with significant morbidity, including urinary, sexual, and gastrointestinal side effects.3 With the promise of decreased morbidity and enhanced effectiveness, new therapeutic technologies such as intensity-modulated radiotherapy (IMRT) and robotic prostatectomy have been introduced over the last decade. However, both of these technologies carry significant price tags including start-up costs approaching $3 million and, in the case of IMRT, high per episode costs.4–7
Many worry that the imperative to recoup start-up costs for these expensive technologies fosters incentives to maximize their use.8 Acquisition of both IMRT and robotic capabilities was followed quickly by rapid increases in utilization of these treatment modalities, despite limited evidence supporting their effectiveness.7,9–11 While these direct effects of new therapeutic technologies are well established, there are no studies addressing their potential spillover effects on diagnostic practice patterns for prostate cancer. For instance, marketing and local press coverage of new technologies as well as specialist to primary care physician conversations may contribute to the perception of lower morbidity of treatment, which in turn may shift decision making in favor of identifying additional disease.12–14 Specifically, as the downsides of treatment appear lessened, local physicians may consciously or subconsciously alter their thresholds to recommend PSA testing or prostate biopsy.
For these reasons, we used linked SEER-Medicare data to evaluate the impact of market-level technology dissemination on diagnostic testing for prostate cancer.
Patients and Methods
Study population
To assess diagnostic testing for prostate cancer, we used the five percent Medicare sample to identify male beneficiaries residing in the Surveillance, Epidemiology, and End Results (SEER) areas between 2003 and 2007. This sample included men from the 5% non-cancer sample and men with cancer identified with the 5% flag. These men were followed beginning at age 66 years to allow assessment of their general health status in the year prior. Our study included only those without a prior diagnosis of prostate cancer enrolled in the Medicare fee-for-service program (i.e., eligible for Parts A and B of Medicare for at least 12 months and not enrolled in a Medicare Advantage plan, n=117,857). Subjects were followed for PSA testing and prostate biopsy until they were diagnosed with prostate cancer, lost Medicare eligibility, died, or until December 31, 2007, whichever came first.
To characterize prostate cancer technology diffusion, we used SEER – Medicare to identify all patients newly diagnosed with loco-regional prostate cancer between 2003 and 2007. We included subjects 66 years of age and older in the fee-for-service program eligible for Parts A and B of Medicare for at least 12 months before and after prostate cancer diagnosis (n=61,678).
Characterizing prostate cancer technology diffusion
We assessed technology diffusion for prostate cancer treatment at the level of a health care market, as defined by the Hospital Referral Region (HRR). Briefly, HRRs are a collection of ZIP codes in which Medicare beneficiaries residing in these areas receive their tertiary medical care.15 Medicare beneficiaries were assigned to their respective HRR based on the ZIP code of their primary residence.
We assessed regional prostate cancer technology penetration by measuring functional capacity, i.e., the per capita rates of physicians delivering robotic surgery or IMRT. Using explicit Healthcare Common Procedure Coding System (HCPCS) codes (see Table 1), we identified all patients who underwent robotic prostatectomy and IMRT.7,16 We then assigned each patient to a treating physician. The treating surgeon was identified using Unique Physician Identifier and National Provider Identifier Numbers, which are submitted with Medicare claims. The treating radiation oncologist was assigned in a similar manner as the provider who performed the clinical planning and simulation.17 We then assigned each physician to an HRR based on the provider ZIP code available in the physician claims.
Table 1.
Healthcare Common Procedure Coding System (HCPCS) codes used in the analyses.
| Procedure | HCPCS code |
|---|---|
| Robotic prostatectomy* | 55866 |
| IMRT | G0174, 77418, 0073T |
| PSA testing | 84153, G0103 |
| Prostate biopsy | 55700 |
HCPCS code 55866 specifies laparoscopic radical prostatectomy, the overwhelming majority of which are known to be robotic prostatectomies.29
Finally, we characterized regional technology penetration separately for robotic prostatectomy and IMRT for each year by calculating provider densities. The numerator was the number of physicians providing robotic prostatectomy or IMRT treatments in each HRR in a given year. The denominator was the number of male Medicare beneficiaries residing within the HRR based on population estimates for the ZIP Code Tabulation Areas.18 We then sorted HRRs into three equal groups (tertiles).
We validated our claims based measure of robotic prostatectomy providers by independently abstracting the number of providers offering this procedure in each HRR from the sole manufacturer's (Intuitive Surgical) historical webpages from 2004–2007. Historical websites were retrieved from web.archive.org using the search URL http://www.davinciprostatectomy.com/hospitals.html on 09/27/2012. We found strong correlation between our claims based measure and the data abstracted from the historical webpages (r=0.81, p<0.001).
Outcome
Our primary outcome was the use of a diagnostic test to detect prostate cancer, either a PSA test or a prostate biopsy. For these measures, the numerators were whether or not at least one test was performed in each person-year; and the denominator was the time each subject was under observation in that year. We identified PSAs using explicit HCPCS codes in claims from the physician/carrier and outpatient files (see Table 1). To avoid counting PSA tests conducted in response to a suspicious test result, tests performed within 90 days of a prior test were excluded.19 Prostate biopsies were identified using claims from the physician/carrier file (Table 1).20
Statistical analyses
Subjects were categorized by age, race, comorbidity,21 education, income, and urban residence (Table 2). Regional characteristics were obtained from the Health Resources and Services Administration’s Area Resource File (number of hospital beds, number of urologists, and number of radiation oncologists per 100,000 men; Medicare managed care penetration) and categorized into tertiles based on the cohort. We described bivariate associations of demographic and regional characteristics with PSA testing and prostate biopsy by calculating the number of tests performed per person-year for each of the demographic or regional strata. To describe variation in PSA and prostate biopsy testing rates across HRRs, we calculated testing rates for each HRR and then described the median and range of these rates across all HRRs.
Table 2.
Rates of PSA testing or prostate biopsy. Numerator is the number of annual PSA tests or prostate biopsies, denominator is time in years subjects were under observation (subjects had to be alive, without prostate cancer, and eligible for both Part A and B at the beginning of a year to be included).
| Characteristics | Person years |
PSA testing rate per 1,000 person-years |
Prostate biopsy rate per 1,000 person-years |
|---|---|---|---|
| Age, years (%)* | |||
| 66–69 | 177,866 | 405 | 13 |
| 70–74 | 104,056 | 462 | 13 |
| 75–79 | 80,640 | 437 | 10 |
| 80–84 | 50,922 | 369 | 7 |
| 85+ | 29,041 | 261 | 3 |
| Race/ethnicity (%)* | |||
| White | 368,586 | 421 | 11 |
| Black | 27,509 | 330 | 17 |
| Hispanic | 12,023 | 314 | 11 |
| Asian | 19,673 | 409 | 12 |
| Other/unknown | 14,734 | 379 | 10 |
| Comorbidity (%)* | |||
| 0 | 291,681 | 403 | 12 |
| 1 | 85,008 | 458 | 10 |
| 2 | 36,569 | 418 | 9 |
| 3+ | 29,267 | 343 | 6 |
| Lived in census tract in which 25% or more of adults had a college education** | |||
| No | 239,796 | 383 | 11 |
| Yes | 188,901 | 448 | 12 |
| Median annual household income of census tract* | |||
| Low (≤ $38,543) | 141,960 | 366 | 10 |
| Intermediate | 143,588 | 413 | 11 |
| High (≥ $54,091) | 143,149 | 455 | 12 |
| Residing in rural area* | |||
| No | 374,758 | 419 | 11 |
| Yes | 67,767 | 364 | 10 |
| Year*** | |||
| 2003 | 89,247 | 407 | 12 |
| 2004 | 89,319 | 399 | 11 |
| 2005 | 88,277 | 406 | 10 |
| 2006 | 87,641 | 413 | 11 |
| 2007 | 88,041 | 428 | 11 |
| Number of urologists per 100,000 men 65 and over* | |||
| Low (≤ 55) | 148,518 | 404 | 10 |
| Intermediate | 167,491 | 414 | 11 |
| High (≥ 88) | 126,516 | 414 | 12 |
| Number of radiation oncologists per 100,000 men 65 and over* | |||
| Low (≤ 23) | 154,970 | 397 | 10 |
| Intermediate | 171,871 | 430 | 11 |
| High (≥ 38) | 115,684 | 400 | 12 |
| Number of hospital beds per 100,000 men 65 and over** | |||
| Low(≤ 4,797) | 151,368 | 418 | 11 |
| Intermediate | 172,627 | 414 | 11 |
| High (≥ 6,861) | 118,530 | 397 | 12 |
| Medicare managed care penetration** | |||
| Low (≤ 5.2%) | 149,293 | 400 | 11 |
| Intermediate | 152,538 | 420 | 12 |
| High (≥ 21%) | 140,694 | 411 | 10 |
| Surgical technology*** | |||
| Low (0 providers per 100,000) | 76,929 | 383 | 10 |
| Intermediate | 227,835 | 414 | 11 |
| High (≥ 3 providers per 100,000) | 137,761 | 420 | 11 |
| IMRT technology** | |||
| Low (≤ 8 providers per 100,000) | 122,980 | 394 | 11 |
| Intermediate | 219,740 | 416 | 11 |
| High (≥ 13.5 providers per 100,000) | 99,805 | 418 | 12 |
IMRT=Intensity-modulated radiotherapy
P-values from bivariate generalized estimating equation models:
p<0.001 for association with rates of PSA testing and biopsy
p<0.001 for association with rates of PSA testing, p<0.05 for association with biopsy
p<0.001 for association with rates of PSA testing, not significant for association with biopsy
We performed bivariate and multivariable analyses with the person-year as our unit of analysis. The dependent variable was whether or not at least one PSA test was performed in a person-year. To account for the longitudinal nature of our data, we fit generalized estimating equations with a log link, using the natural logarithm of the time under observation as an offset.22 These models accounted for the fact that the likelihood of PSA testing from one year to another is more similar within the same subject than between different subjects. They also allowed us to account for the nested structure of our data (i.e., subjects nested within HRRs). We then examined the association of regional technology penetration with PSA testing rates by adding the continuous measures of robotic prostatectomy and IMRT provider density to the models along with an interaction term. Technology penetration was allowed to vary from year to year within a given HRR. Multivariable models were adjusted for subject- and market-level covariates (neighborhood socioeconomic status,23 number of hospital beds, number of urologists, and number of radiation oncologists per 100,000 men; Medicare managed care penetration, HRR-level provider volume), and for time in years since the beginning of the study. Because the effect of technology penetration on diagnostic testing could change over time, we also allowed for an interaction between technology penetration and time. From these models, we calculated adjusted PSA testing rates for the average HRR with low (i.e., robotic prostatectomy and IMRT penetration in the lowest tertile) and with high technology penetration (i.e., robotic prostatectomy and IMRT penetration in the highest tertile). The association of prostate biopsy rates with regional technology penetration was examined using similar models.
Sensitivity analyses
We performed sensitivity analyses excluding HRRs crossing SEER boundaries into areas from which SEER data was not available (n=21). We also estimated models not adjusting for number of urologists and radiation oncologists in a market, because adjusting for these covariates may be overly aggressive. We performed additional analyses only including PSA tests ordered by urologists or only including men less likely to benefit from prostate cancer diagnosis and treatment (i.e., those aged 75 and older with three or more comorbidities). The changes made in these sensitivity analyses did not materially affect the association between technology penetration and diagnostic testing for prostate cancer, so only results from the primary analyses are presented.
We performed all analyses using Stata version 12SE and SAS version 9.3. All tests were 2-tailed; and we considered p<0.05 as statistically significant. The University of Michigan Medical School Institutional Review Board exempted this study from review in accordance with the Code of Federal Regulations Title 45, subpart A, section 46.101, paragraph b, subparagraph 4.
Results
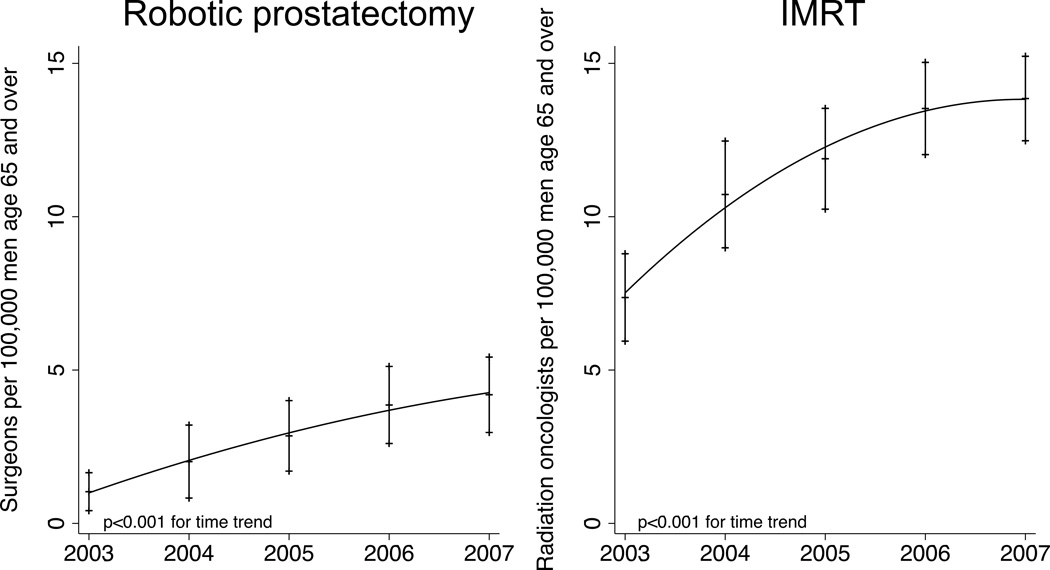
Regional technology penetration for prostate cancer changed significantly over the study period for both robotic prostatectomy and IMRT (Figure 1, p<0.001). Typical low technology areas had a mean of 0 robotic prostatectomy and 5.7 IMRT providers compared with high technology areas that had a mean of 7.0 robotic prostatectomy and 17.6 IMRT providers per 100,000 male Medicare beneficiaries.
Figure 1.
Regional technology penetration for robotic prostatectomy and intensitymodulated radiotherapy (IMRT) across 69 Hospital Referral Regions in SEER-Medicare from 2003–2007.
PSA testing and biopsy rates varied widely across HRRs, with a median of 391 (range 318 – 515) PSA tests per 1,000 person-years and 10.3 (range 3.7 – 19.3) prostate biopsies per 1,000 person-years. In bivariate analyses, older, more infirm, and more socioeconomically disadvantaged subjects had lower rates of PSA testing and prostate biopsy (Table 2). Regional technology penetration was associated with higher rates of PSA testing (Table 2). High IMRT penetration was associated with a small but statistically significant increase in prostate biopsy rates in bivariate analysis (Table 2). The effect of IMRT penetration was more pronounced in regions with high levels of surgical technology and the effect of robotic prostatectomy penetration was more pronounced in regions with high levels of IMRT technology (Table 3).
Table 3.
PSA testing rates (number of annual tests per 1,000 person-years) according to technology penetration.
| IMRT technology penetration |
Surgical technology penetration | Total | ||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| Low | 390 | 380 | 424 | 394 |
| Intermediate | 378 | 430 | 393 | 416 |
| High | 379 | 408 | 449 | 418 |
| Total | 383 | 414 | 420 | 411 |
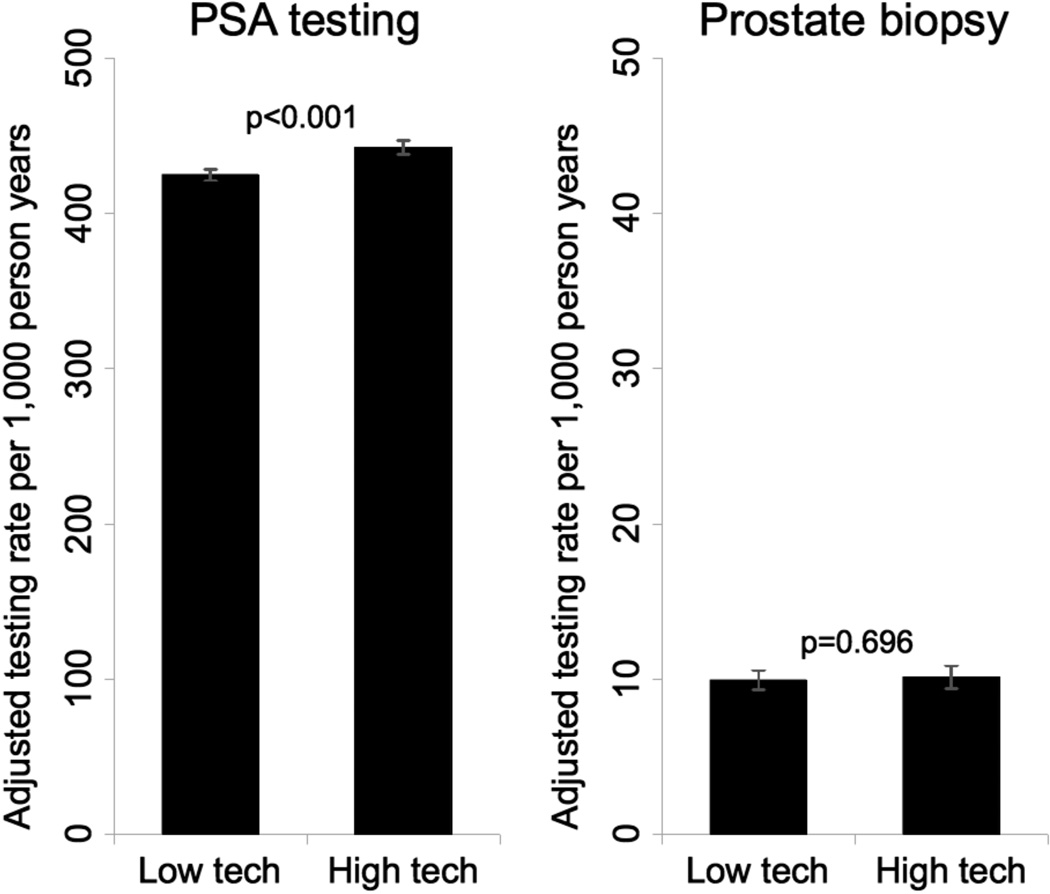
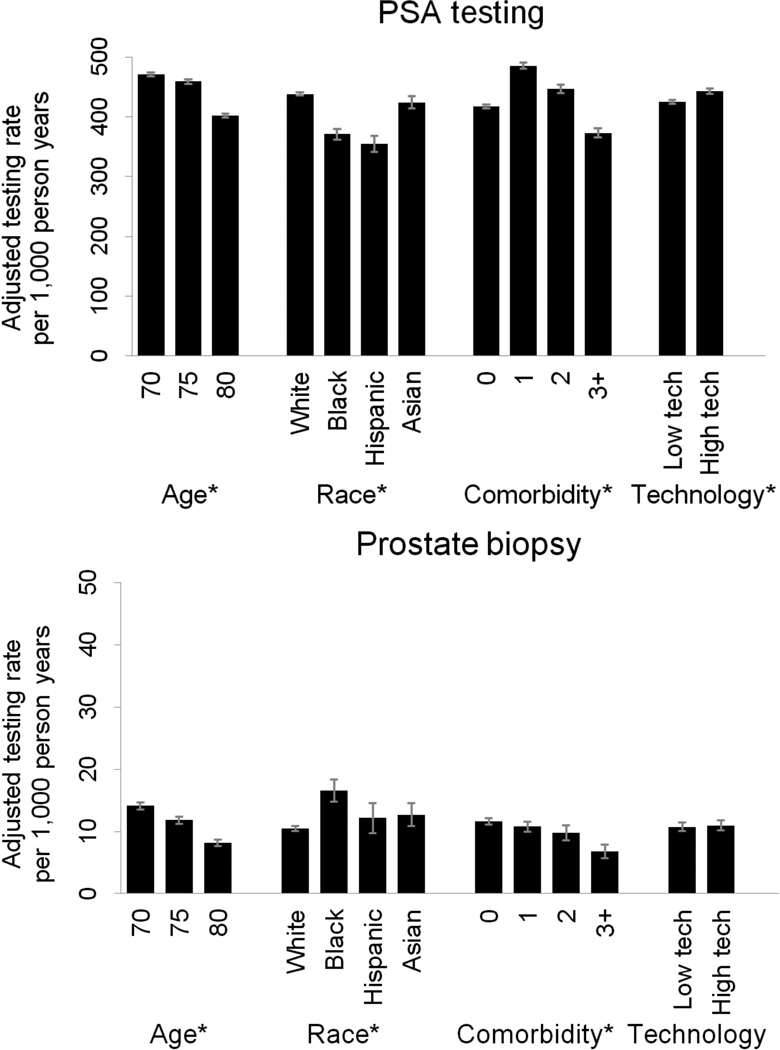
We accounted for this interaction between robotic prostatectomy and IMRT technology penetration in adjusted multivariable generalized estimating equations. Based on these models, living in an HRR with high technology penetration was associated with a small increase in the rates of PSA testing (from 425 to 442 per 1,000 person-years, p<0.001), while rates of prostate biopsy did not differ significantly (9.9 vs. 10.1 per 1,000 person-years, p=0.696, Figure 2). The impact of technology penetration on PSA testing and prostate biopsy was much smaller than the effect of age, race, and comorbidity (Figure 3).
Figure 2.
Adjusted rates of PSA testing and prostate biopsy by regional technology penetration. Models were adjusted for socioeconomic status; rural vs. urban residence; number of hospital beds, number of urologists, and number of radiation oncologists per 100,000 men aged 65 and older; Medicare managed care penetration; market-level provider volume; and time in years since the beginning of the study. Error bars represent 95% confidence intervals.
Figure 3.
Effect of regional technology penetration on PSA testing and prostate biopsy compared to effect of age, race, and comorbidity. Models were adjusted for socioeconomic status; rural vs. urban residence; number of hospital beds, number of urologists, and number of radiation oncologists per 100,000 men aged 65 and older; Medicare managed care penetration; market-level provider volume; and time in years since the beginning of the study. Error bars represent 95% confidence intervals. * denotes p<0.001.
Discussion
We found that PSA testing and prostate biopsy rates varied widely across health care markets (i.e., HRRs). Market-level technology penetration for prostate cancer treatment, measured as providers performing robotic prostatectomy and IMRT per capita, had the strongest effect on PSA testing in markets with both high robotic prostatectomy and high IMRT technology penetration. However, after adjusting for covariates in multivariable analyses, technology penetration had only minimal impact on PSA testing and no significant effect on prostate biopsy rates (Figure 2). The impact of technology penetration on diagnostic testing for prostate cancer was much smaller than the effect of immutable patient factors, such as age, race, and comorbidity (Figure 3).
This is the first study to broadly examine the impact of new technology on prostate cancer-related services. Several studies found evidence that dissemination of robotic prostatectomy was associated with increased use of radical prostatectomy itself.9–11 For example, overall prostatectomy volume increased by a mean of 12 cases per year after a hospital acquired a robot while it decreased by 1 case per year for hospitals that did not acquire a robot between 2000 and 2009.11 While these data provide evidence that dissemination of new technology impacts use of the technologies themselves, there could also be spillover effects related to diagnostic testing for prostate cancer. For example, pressure to recoup the high investment costs, marketing, and direct conversations among physicians12–14 may indirectly influence physician practices regarding PSA testing and prostate biopsies.
Therefore, we examined whether regional technology penetration for prostate cancer treatments had effects on diagnostic testing for prostate cancer. We found no effect on prostate biopsy rates and a statistically significant but clinically small effect on rates of PSA testing. For each one thousand patients followed for a year, only 17 additional PSA tests were performed in markets with high technology penetration. Our findings, to some degree, should allay concerns that these new technologies may spur the utilization of services aimed at identifying new cases of prostate cancer. Compared to technology penetration, patient factors such as age, race, and comorbidity were much more important predictors of PSA testing and prostate biopsy, which is consistent with previous studies examining factors associated with PSA testing.24
Our study has several limitations. First, we could have incompletely ascertained the number of providers practicing in the 21 HRRs crossing SEER boundaries into areas from which SEER data was not available. Therefore, we performed sensitivity analyses excluding these fractional HRRs, with results that were consistent with our main findings. Second, given the limitations inherent to using Medicare data, our results may not reflect the effects of technology penetration on younger patients or those enrolled in Medicare Managed Care plans. However, the majority of prostate cancer diagnoses occur in the elderly25 and men in this age group have the least to gain from early detection. Therefore, it is especially important to evaluate factors associated with PSA testing and prostate biopsy in this population. As such, our findings are generalizable to the largest incidence population at highest risk for overuse of PSA testing and prostate biopsy. Third, given the observational nature of our data, we cannot exclude unmeasured confounding. To mitigate this problem, we controlled for a wide range of potential confounders, such as demographic and market factors, as well as socioeconomic class.
Conclusions
Our study has important implications for patients, payers, and policymakers. For patients, our findings provide insight regarding the degree to which the availability of new technology might influence the use of related healthcare services. For instance, when laparoscopic donor nephrectomy was introduced, there was a coincident significant increase in the number of individuals volunteering as living kidney donors,26 likely because it increased the palatability of donation. We hypothesized that aggressive direct-to-consumer marketing of new technologies may increase the palatability of undergoing treatment for prostate cancer in a similar manner, which in turn could have upstream effects on PSA testing and prostate biopsy. However, our data suggest no clinically important association between dissemination of new technology and the use of these healthcare services, thus allaying concerns that dissemination of robotic prostatectomy and IMRT may drive diagnostic testing for prostate cancer. For payers and policymakers, our findings are of immediate interest as they consider coverage decisions for other new technologies such as proton beam therapy or histotripsy.27,28 While our work begins to elucidate the broader effects of technology dissemination on prostate cancer care, we will need future work evaluating the impact of new technology on the decision of whether or not to treat as well as on quality of care. This will allow us to gain a more complete understanding of how technology dissemination affects decision making along the prostate cancer care continuum.
Acknowledgments
Funding: FRS: T32 DK07782 from the NIH/NIDDK and Postdoctoral Fellowship PF-12-118-01-CPPB from the American Cancer Society. BLJ: Postdoctoral Fellowship 121805-PF-12-008-01-CPHPS from the American Cancer Society. BKH and VBS: R01 CA168691 from NIH/NCI.
Abbreviations
- HCPCS
Healthcare Common Procedure Coding System
- HRR
Hospital Referral Region
- IMRT
intensity-modulated radiotherapy
- PSA
prostate specific antigen
- SEER
Surveillance Epidemiology and End Results
References
- 1.American Cancer Society. [accessed March 18, 2013];Cancer Facts & Figures. 2013 Available at: http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2013.
- 2.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J. Natl. Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor KL, Luta G, Miller AB, et al. Long-term disease-specific functioning among prostate cancer survivors and noncancer controls in the prostate, lung, colorectal, and ovarian cancer screening trial. J. Clin. Oncol. 2012;30:2768–2775. doi: 10.1200/JCO.2011.41.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel HRH, Linares A, Joseph JV. Robotic and laparoscopic surgery: Cost and training. Surgical Oncology. 2009;18:242–246. doi: 10.1016/j.suronc.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Joseph JV, Leonhardt A, Patel HRH. The cost of radical prostatectomy: retrospective comparison of open, laparoscopic, and robot-assisted approaches. Journal of Robotic Surgery. 2008;2:21–24. doi: 10.1007/s11701-007-0052-8. [DOI] [PubMed] [Google Scholar]
- 6.Pollack A. Hospitals Look to Nuclear Tool to Fight Cancer. [accessed September 12, 2011];The New York Times. 2007 Available at: http://www.nytimes.com/2007/12/26/business/26proton.html?scp=2&sq=nuclear+tool&st=nyt,
- 7.Jacobs BL, Zhang Y, Skolarus TA, et al. Growth Of High-Cost Intensity-Modulated Radiotherapy For Prostate Cancer Raises Concerns About Overuse. Health Affairs. 2012;31:750–759. doi: 10.1377/hlthaff.2011.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zietman A, Goitein M, Tepper JE. Technology evolution: is it survival of the fittest? J. Clin. Oncol. 2010;28:4275–4279. doi: 10.1200/JCO.2010.29.4645. [DOI] [PubMed] [Google Scholar]
- 9.Makarov DV, Yu JB, Desai RA, et al. The Association Between Diffusion of the Surgical Robot and Radical Prostatectomy Rates. Med. Care. 2011;49:333–339. doi: 10.1097/MLR.0b013e318202adb9. [DOI] [PubMed] [Google Scholar]
- 10.Neuner JM, See WA, Pezzin LE, et al. The association of robotic surgical technology and hospital prostatectomy volumes. Cancer. 2012;118:371–377. doi: 10.1002/cncr.26271. [DOI] [PubMed] [Google Scholar]
- 11.Stitzenberg KB, Wong Y, Nielsen ME, et al. Trends in radical prostatectomy: centralization, robotics, and access to urologic cancer care. Cancer. 2012;118:54–62. doi: 10.1002/cncr.26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alkhateeb S, Lawrentschuk N. Consumerism and its impact on robotic-assisted radical prostatectomy. BJU International. 2011;108:1874–1878. doi: 10.1111/j.1464-410X.2011.10117.x. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg PL, Ghavamian R. Searching Robotic Prostatectomy Online: What Information Is Available? Urology. 2011;77:941–945. doi: 10.1016/j.urology.2010.07.505. [DOI] [PubMed] [Google Scholar]
- 14.Richstone L, Kavoussi LR. Barriers to the diffusion of advanced surgical techniques. Cancer. 2008;112:1646–1649. doi: 10.1002/cncr.23369. [DOI] [PubMed] [Google Scholar]
- 15.The Dartmouth Institute for Health Policy & Clinical Practice. [accessed April 7, 2012];Glossary - Dartmouth Atlas of Health Care. 2012 Available at: http://www.dartmouthatlas.org/tools/glossary.aspx.
- 16.Hu JC, Gu X, Lipsitz SR, et al. Comparative Effectiveness of Minimally Invasive vs Open Radical Prostatectomy. JAMA. 2009;302:1557–1564. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 17.Pollack CE, Weissman G, Bekelman J, et al. Physician social networks and variation in prostate cancer treatment in three cities. Health Serv Res. 2012;47:380–403. doi: 10.1111/j.1475-6773.2011.01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Census Bureau. [accessed February 6, 2009];ZIP Code Tabulation Areas. Available at: http://www.census.gov/geo/ZCTA/zcta.html,
- 19.Etzioni R, Berry KM, Legler JM, et al. Prostate-specific antigen testing in black and white men: an analysis of Medicare claims from 1991–1998. Urology. 2002;59:251–255. doi: 10.1016/s0090-4295(01)01516-3. [DOI] [PubMed] [Google Scholar]
- 20.Welch HG, Fisher ES, Gottlieb DJ, et al. Detection of Prostate Cancer via Biopsy in the Medicare-SEER Population During the PSA Era. Journal of the National Cancer Institute. 2007;99:1395–1400. doi: 10.1093/jnci/djm119. [DOI] [PubMed] [Google Scholar]
- 21.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 22.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. 2nd ed. Stata Press; 2008. Second Edition. [Google Scholar]
- 23.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N. Engl. J. Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 24.Drazer MW, Huo D, Schonberg MA, et al. Population-based patterns and predictors of prostate-specific antigen screening among older men in the United States. J. Clin. Oncol. 2011;29:1736–1743. doi: 10.1200/JCO.2010.31.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009. Bethesda, MD: National Cancer Institute; 2012. [accessed November 27, 2012]. Available at: http://seer.cancer.gov/csr/1975_2009_pops09, [Google Scholar]
- 26.Axelrod DA, McCullough KP, Brewer ED, et al. Kidney and pancreas transplantation in the United States, 1999–2008: the changing face of living donation. Am. J. Transplant. 2010;10:987–1002. doi: 10.1111/j.1600-6143.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- 27.Brada M, Pijls-Johannesma M, De Ruysscher D. Proton therapy in clinical practice: current clinical evidence. J. Clin. Oncol. 2007;25:965–970. doi: 10.1200/JCO.2006.10.0131. [DOI] [PubMed] [Google Scholar]
- 28.Schade GR, Keller J, Ives K, et al. Histotripsy Focal Ablation of Implanted Prostate Tumor in an ACE-1 Canine Cancer Model. J. Urol. 2012;188:1957–1964. doi: 10.1016/j.juro.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DI. Robotic Prostatectomy: What We Have Learned and Where We Are Going. Yonsei Med J. 2009;50:177–181. doi: 10.3349/ymj.2009.50.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]