Abstract
Background
TcSMUG L products were recently identified as novel mucin-type glycoconjugates restricted to the surface of insect-dwelling epimastigote forms of Trypanosoma cruzi, the etiological agent of Chagas disease. The remarkable conservation of their predicted mature N-terminal region, which is exposed to the extracellular milieu, suggests that TcSMUG L products may be involved in structural and/or functional aspects of the interaction with the insect vector.
Methodology and Principal Findings
Here, we investigated the putative roles of TcSMUG L mucins in both in vivo development and ex vivo attachment of epimastigotes to the luminal surface of the digestive tract of Rhodnius prolixus. Our results indicate that the exogenous addition of TcSMUG L N-terminal peptide, but not control T. cruzi mucin peptides, to the infected bloodmeal inhibited the development of parasites in R. prolixus in a dose-dependent manner. Pre-incubation of insect midguts with the TcSMUG L peptide impaired the ex vivo attachment of epimastigotes to the luminal surface epithelium, likely by competing out TcSMUG L binding sites on the luminal surface of the posterior midgut, as revealed by fluorescence microscopy.
Conclusion and Significance
Together, these observations indicate that TcSMUG L mucins are a determinant of both adhesion of T. cruzi epimastigotes to the posterior midgut epithelial cells of the triatomine, and the infection of the insect vector, R. prolixus.
Author Summary
Chagas disease, the major tropical human disease in much of Latin America, affects approximately 11 million people. There are 300,000 new cases of Chagas disease and approximately 21,000 deaths, annually. Triatomine vectors, including Rhodnius prolixus, are able to transmit the protozoan Trypanosoma cruzi, the etiological agent of disease. To develop within insects, the flagellates undergo morphological changes, modulating surface molecules to enable interactions with insect tissues such as the perimicrovilar membranes in the midgut which is an essential step for their development and successful transmission to a vertebrate host. The surface of T. cruzi is covered in glycosyl phosphatidylinositol (GPI)-anchored mucin molecules that determine parasite protection and establishment of a persistent infection in vertebrates. A particular kind of mucin, termed TcSMUG L, is only present at surface of the insect-dwelling stages of protozoan and, according to our results, it is involved in the interaction between T. cruzi and its invertebrate host, determining both the ex vivo adhesion to the insect midgut cells and the in vivo development in the vector. Collectively, our work adds new insight into the relevance of mucin-type glycoconjugates in the infection of insect vectors and points to them as promising targets to develop transmission-blocking strategies for this disease.
Introduction
Described by its discoverer, Carlos Chagas [1], [2], as “one of the most injurious tropical illnesses, specially to children in contaminated areas, either in determining a chronic sickly condition in which people become unable to perform vital activities or as an important factor of human degeneration,” Chagas disease remains a major tropical human disease in much of Latin America, affecting approximately 11 million people. There are 300,000 new cases of Chagas disease each year, with approximately 21,000 deaths annually [3]. Various triatomine vectors, including Rhodnius, Triatoma and Pastrongylus, are able to acquire and transmit Trypanosoma cruzi, the etiological agent of Chagas disease [4], [5]. During their development within insects, parasites undergo profound morphological changes, modulating surface molecules to enable interactions with specific insect tissues that are essential for their survival, development and successful transmission to a vertebrate host [6], [7]. T. cruzi-insect vector interactions begin when the insect feeds on the blood of an infected vertebrate host. Once ingested, most of the bloodstream trypomastigotes differentiate into non-infective epimastigote forms. In the posterior midgut, they repeatedly divide by binary fission and adhere to perimicrovillar membranes (PMM) secreted by the underlying midgut epithelial cells [8]–[11]. In the rectum, a proportion of epimastigotes attaches to the rectal cuticle through hydrophobic interactions and transforms into non-replicative infective metacyclic trypomastigotes, which are released together with insect feces and urine during blood feeding [12]–[14].
The entire surface of T. cruzi is covered in glycosylphosphatidylinositol (GPI)-anchored mucin molecules that determine parasite protection and establishment of a persistent infection in vertebrate hosts [15]. T. cruzi mucins comprise a large gene family that can be split into two major groups, termed T. cruzi mucin gene family (TcMUC) and T. cruzi small mucin-like gene family (TcSMUG), based on sequence comparisons [16]. TcMUC codes for more than 1,000 polymorphic products, which are largely co-expressed on the surface of the mammal-dwelling stages [16]–[18]. In addition to their putative immune modulatory role [17], [19], one particular TcMUC product termed TSSA (trypomastigote small surface antigen) was recently shown to be involved in trypomastigote adhesion to non-macrophagic cells [20]. The second mucin group, TcSMUG, displays significantly less diversity and codes for very small open reading frames. Upon processing of the signal peptide and GPI-anchoring signal, the average predicted molecular mass for the mature apo-mucins would be ∼7 kDa, with Thr representing as much as 50% of the residues. The hydroxyl groups of some of these Thr residues are further derivatized with short O-linked oligosaccharide chains in the Golgi/post-Golgi compartments, which increases the molecular mass of the mature mucins to 35–50 kDa, depending on both the particular TcSMUG product and the parasite isolate [21], [22]. TcSMUG is composed of two subgroups of genes, named L and S, which display >80% identity on average. Mass spectrometry analyses identified TcSMUG S products as the backbone for the 35/50 kDa mucins (known as Gp35/50 mucins) expressed on the surface of insect-dwelling stages [22]. Upon transmission to the mammalian host, Gp35/50 mucins on the surface of metacyclic trypomastigotes bind to non-macrophagic cells in a receptor-mediated manner and induce a bidirectional Ca2+ response, which likely contributes to host-cell invasion [15]. Recent data indicated that TcSMUG L products, though not revealed in the T. cruzi proteomic data sets published so far, constitute a novel mucin-type glycoconjugate restricted to epimastigote forms [22]–[26]. In addition to displaying substantial structural homologies and a common evolutionary origin, comparative analyses highlighted certain differences between TcSMUG L and TcSMUG S products [26]. First, TcSMUG L products, unlike those of TcSMUG S, are not acceptors of sialic acid residues, likely due to the absence of terminal β-Gal residues in the proper configuration. Secondly, and at variance with TcSMUG S products that are expressed at fairly similar levels on every T. cruzi stock, TcSMUG L expression seems quite variable among different parasite isolates. Finally, the remarkable conservation of TcSMUG L deduced products within the predicted mature N-terminal peptide, which does not undergo O-glycosylation, suggest that they are under positive selection against diversification [26]. Because of these features, it has been speculated that structural and/or functional constraints rather than immunological issues limit TcSMUG diversification.
In the present work, we investigated the role of TcSMUG L mucins in the attachment of T. cruzi epimastigotes from the Dm28c stock to the midgut epithelium of R. prolixus and the consequent development of the protozoan in the insect vector.
Materials and Methods
Insects and Parasites
R. prolixus (Hemiptera: Reduviidae) were obtained from a longstanding colony reared in the laboratory at 28°C and 60–70% relative humidity [27] where they were fed on chickens weekly and raised as previously described [28]. For the in vivo experiments, the insects were fasted for approximately 15 days and were then fed with infected heat-inactivated citrated human blood using an artificial apparatus similar to that described previously [29]. The T. cruzi Dm28c clone, classified in the TcI phylogenetic group [30], was maintained in Novy-MacNeal-Nicolle media (NNN) and brain heart infusion media (BHI- DIFCO) supplemented with bovine serum albumin (BSA) and hemin. For the in vivo and ex vivo experiments, epimastigotes were collected during the exponential growth phase, washed three times in 0.15 M NaCl, 0.01 M phosphate-buffer, pH 7.2 (PBS) and used immediately [11], [31].
Ethics Statement
R. prolixus were fed and raised according to the Ethical Principles in Animal Experimentation approved by the Ethics Committee in Animal Experimentation (CEUA/FIOCRUZ) under the approved protocol number P-54/10-4/LW12/11. The experiments performed with citrated human blood using an artificial apparatus were conducted according to the Ethical Principles in Animal Experimentation approved by the Ethics Committee in Animal Experimentation (CEUA/FIOCRUZ) under the approved protocol number L-0061/08. All blood donors provided informed written consent. Both protocols are from CONCEA/MCT (http://www.cobea.org.br/), which is associated with the American Association for Animal Science (AAAS), the Federation of European Laboratory Animal Science Associations (FELASA), the International Council for Animal Science (ICLAS) and the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Mucin Purification
Epimastigotes (109) were delipidated using a water/chloroform/butan-1-ol treatment and further extracted with butan-1-ol at 4°C as described previously [32]. Briefly, the soluble fraction was evaporated under an N2 stream, and the insoluble material was re-extracted with 66% butan-1-ol in water. The butan-1-ol phase (F1) contained mainly lipids, phospholipids and glycoinositolphosphates (GIPLs), whereas the aqueous phase (F2) is enriched in mucins [32]. Both phases were further extracted with 9% butan-1-ol in water. Delipidated parasite pellets were also extracted with 9% butan-1-ol in water and the mucin-rich aqueous (F3) and butan-1-ol (F4) phases were stored. The final parasite pellets were resuspended in denaturing loading buffer containing 6 M urea and 100 µg/ml DNAse I (SIGMA).
Concanavalin A (ConA)-Fractionation and Phosphatidilinositol-Specific Phospholipase C (PI-PLC) Treatment
In order to enrich in glycoconjugates, pellets containing 108 parasites were homogenized in ConA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP40, 0.1% Na deoxycholate, 1 mM PMSF, 50 µM TLCK, 1 mM DTT) and fractionated in batch using 200 µl of ConA-sepharose (GE Healthcare) [26]. Elution was carried out with 300 µl of ConA buffer with 0.5 M α methylmannoside (Sigma, St. Louis, MO). Parasite total lysates were treated with PI-PLC and submitted to Triton X-114 partition as described [26], to ascertain the presence of GPI anchor.
Gel Electrophoresis and Western Blots
Gel electrophoresis was performed under denaturing conditions in 15% SDS-PAGE. For Western blots using total proteins, lysates corresponding to ∼107 parasites prepared as described [26] were loaded in each lane, transferred to PVDF membranes (GE Healthcare), reacted with the appropriate antiserum followed by HRP-conjugated secondary Abs (Sigma) and developed using chemiluminescence (Pierce). Antibodies to TcSMUG L were affinity-purified and used as described by [26]. Rabbit antiserum to glutamate dehydrogenase from T. cruzi (TcGDH) was used at 1∶3,000 dilution [33].
Peptides
Peptides used in this study were synthesized bearing an acetyl group on their N-termini and a C-terminal Cys residue (GenScript). Sequences were derived from the predicted N-terminal region of mature TcSMUG L (AVFKAAGGDPKKNTTC), TcSMUG S (VEAGEGQDQTC) and TSSA (TPPSGTENKPATGEAPSQPGAC) products. When indicated, peptides were synthesized with a biotin group instead of the acetyl group on their N-termini. Although bioinformatics methods indicate that the sequences EEGQYDAAVFAVFKAAGGDPKKNTT and EEGQYDAAVFVEAGEGQDQT constitute the predicted mature N-termini for TcSMUG L and S products, respectively [26], mass spectrometry-based data using purified epimastigote total mucins [34], strongly suggested a further trimming of the EEGQYDAAVF sequence in vivo.
Ex Vivo Interaction between R. prolixus Posterior Midgut Cells and T. cruzi Epimastigotes
After washing in PBS, epimastigotes were suspended in fresh BHI to a density of 2.5×107 cells/ml. Samples of an interaction medium composed of 200 µl of this parasite suspension together with posterior midguts, freshly dissected and washed only in PBS, from insects collected 10 days after a non-infectious blood meal, were placed in Eppendorf microtubes [10] and incubated for 30 min at 25°C (non-treated control group). Under these conditions, epimastigotes adhered to the luminal surface of midgut epithelium cells [11]. For the experimental groups, the midguts were previously incubated (30 min, 25°C) in PBS supplemented with TcSMUG S (negative control), TcSMUG L or TSSA peptides at different concentrations. The treated-posterior midguts were then washed in fresh PBS and immediately added to the BHI interaction medium containing parasites. After incubation (30 min, 25°C), all midgut preparations were spread onto glass slides to count the number of attached parasites. A Zeiss microscope with reticulated ocular, equipped with a video microscopy camera, was used for counting parasites attached to 100 randomly chosen epithelial cells in 10 different fields of each midgut preparation. For each experimental group, 10 insect midguts were used [35], [36].
In Vivo Infection Assays
Fifth-instar nymphs of regularly fed R. prolixus, which had been starved for 7 days after the last ecdysis, were fed on artificial bloodmeal apparatus with a mixture of heat-inactivated citrated human blood and epimastigotes (2×105 parasites/ml) as previously described [37]. TcSMUG S (negative control), TSSA or TcSMUG L peptide was added to the infected blood meal to a final concentration of 30 µg/ml just before feeding. At days 7, 14 or 21, the entire digestive tracts consisting of anterior midgut (stomach), posterior midgut and rectum of 10 insects were dissected and homogenized in a small volume of PBS. Afterwards, additional PBS was added to fill the homogenates to 1 ml [38], [39]. The number of parasites in each homogenate was determined using a Neubauer hemocytometer [40], [41]. Each experiment was repeated at least three times.
Light Microscopy
Posterior midgut compartments obtained by dissection were fixed for 2 h at room temperature in 2.5% glutaraldehyde diluted in 0.1 M cacodylate buffer, pH 7.2, and washed twice in the same buffer. Post-fixation was performed in the dark for 2 h in 1% osmium tetroxide diluted in 0.1 M cacodylate buffer, pH 7.2, followed by dehydration with continuous acetone series (70%, 90% and 100%, respectively). Samples were then embedded in epoxy resin and polymerized at 60°C for three days. Thick plastic sections were stained with toluidine blue and observed under an Axioplan MC 100 spot microscope [10].
Fluorescence Microscopy and Histochemical Studies
Dissected posterior midgut fragments were fixed for 1 h at room temperature in 4% p-formaldehyde diluted in 0.1 M cacodylate buffer, pH 7.2. Afterwards, samples were washed in PBS containing 1% of BSA, pH 7.2 (PBS-BSA) and incubated for 30 min in 50 mM ammonium chloride solution followed by another washing step in PBS-BSA at room temperature. Tissues were then incubated with biotin-labeled TcSMUG S, TSSA or TcSMUG L peptide diluted in PBS-BSA for 1 h at room temperature and washed again in PBS-BSA before incubation with FITC-labeled-Avidin conjugate (SIGMA) (1∶100) for 1 h and washed in distilled water in the dark for 10 min [42]. For the control groups, the incubation with biotin-labeled peptides was omitted. Finally, the tissues were spread onto glass slides for visualization using an emission filter of 488 nm and observed under an Axioplan MC 100 spot microscope coupled to an Axiovision system computer [43].
Data Analysis
Results were analyzed using ANOVA and Tukey's tests [44] using Stats Direct Statistical Software, version 2.2.7 (StatsDirect Ltd., Sale, Cheshire, UK). Differences between treated- and control-groups were considered non-statistically significant when p>0.05. Probability values are specified in the text.
Results
TcSMUG L Products Are Expressed as Mucin-Like Molecules in Dm28c Epimastigotes
Previous results indicate that the expression level of TcSMUG L-encoded products is quite variable among epimastigotes from different T. cruzi isolates [26]. Therefore, as a first step toward the validation of our R. prolixus infection model, we undertook preliminary characterization of TcSMUG L products in the DM28c stock. Western blotting assays carried out over total epimastigote lysates and probed with affinity-purified antibodies directed against an N-terminus-derived TcSMUG L peptide revealed a major ∼35 kDa band, thus in the range of fully processed TcSMUG L products described in other parasite stocks [26] (Fig. 1A). As controls, we used analogous fractions from epimastigotes from Adriana and CL Brener stocks, which showed the greatest differences in terms of TcSMUG L expression [26]. The results were normalized by re-probing the membrane with antiserum directed against TcGDH. Densitometric analyses indicated that TcSMUG L expression levels from the DM28c stock were roughly equivalent (86%) to that of CL Brener. These products were removed from the parasite surface following PI-PLC treatment [26], a molecular signature of GPI-anchored molecules (not shown), and were specifically retained following ConA chromatography (Fig. 1B), indicating they bear terminal α-D-mannosyl and/or α-D-glucosyl residues, as described for other stocks [26]. To analyze whether TcSMUG L products behaved as mucin-type proteins, i.e., underwent extensive O-glycosylation, we purified total mucins from Dm28c epimastigotes following a standard butan-1-ol extraction protocol [32] and probed these fractions by Western blot. As shown in Fig. 1C, TcSMUG L products were mostly detected in the F3 fraction, which was highly enriched in gp35/50, as verified by mAb 2B10 and 10D8 reactivity (not shown). The presence of high-molecular weight aggregates in purified TcSMUG L products has been described for other T. cruzi mucin-type glycoconjugates [22], [26]. A minor fraction was also revealed in the pellet, which might be ascribed to incomplete extraction. Together, these results strongly suggest that Dm28c epimastigotes express high levels of fully processed TcSMUG L product on their surface.
Figure 1. Western blots of TcSMUG L products from T. cruzi.
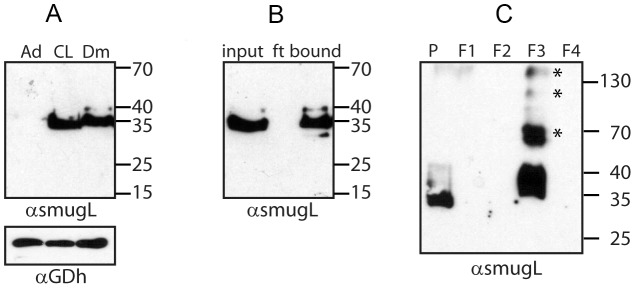
A) Extracts of epimastigotes from different parasite stocks (Ad, Adriana; CL, CL Brener; Dm, Dm28c) were probed with either anti-TcSMUG L antibodies or anti-glutamate dehydrogenase (GDH) antiserum. B) ConA-fractionated extracts of Dm28c epimastigotes were probed with anti-TcSMUG L antiserum. ft, flow-through. C) Butan-1-ol extraction analysis of Dm28c delipidated epimastigotes. Fractions, named according to [19], were probed with affinity-purified anti-TcSMUG L antibodies. Molecular mass markers (in kDa) are indicated at right. *Denotes aggregates.
TcSMUG L Products Are Involved in Epimastigote Ex Vivo Attachment to R. prolixus Posterior Midgut Epithelium
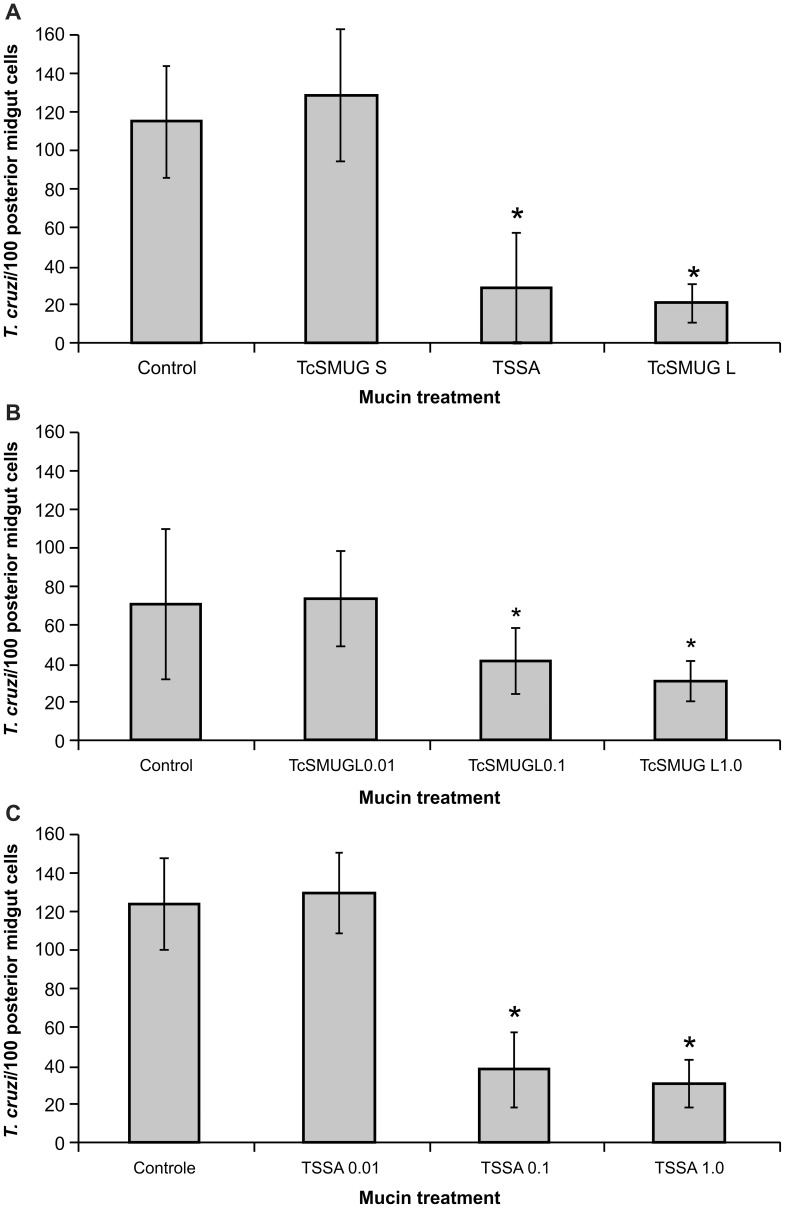
To assess whether TcSMUG L products can act as direct ligands for possible receptors in insect epithelial midgut cells, we tested the effect of pre-treatment of dissected midguts with a peptide spanning the TcSMUG L mature N-terminus. As controls, we assayed in parallel the effect of the corresponding peptide derived from TcSMUG S and TSSA, a member of the TcMUC family of mucins. As a first set of experiments, in posterior R. prolixus midgut preparations obtained from a control (non-treated) group, 114.8±28.2 epimastigotes were found attached per 100 midgut cells (Fig. 2A). Similar adhesion rates (128.8±34.7/100 midgut cells) were obtained when midguts were first incubated with 1 µg/ml of a control TcSMUG S peptide (p>0·05) (Fig. 2A). In contrast, attachment of only 28.5±28.4 and 20.8±10.06 epimastigotes per 100 cells of the midgut epithelium were recorded when the flagellates were pre-incubated with 1 µg/ml of either TcSMUG L or a control TcMUC-derived (TSSA) peptide (p<0·0001), respectively (Fig. 2A). A dose-dependent effect on the ex vivo attachment of epimastigotes was verified for the latter molecules, indicating that the presence of either synthetic peptide blocked a potential ligand-receptor interaction involved in epimastigote attachment (Fig. 2B). As shown in Fig. 2B, incubation with 0.01 µg/ml of the TcSMUG L peptide did not affect flagellate adhesion rates when compared with the control group, whereas incubation with 0.1 µg/ml or 1 µg/ml of the TcSMUG L peptide reduced T. cruzi attachment to 40.8 ±16.78 and 30.8 ±10.42 (p<0·01) epimastigotes per 100 midgut cells, respectively. Similarly, midgut incubation with 0.01 µg/ml of the TSSA peptide resulted in 128.6±20.87epimastigotes attached per 100 midgut cells and did not affect flagellate adhesion rates when compared with the control group (123.2±23.74 epimastigotes/100 midgut cells), whereas incubation with 0.1 µg/ml or 1 µg/ml of the same peptide reduced T. cruzi attachment to 37.6 ±19.65 and 30.6 ±12.4 (p<0·001) epimastigotes per 100 midgut cells, respectively (Fig. 2C). Therefore, our results showed that the pre-incubation of R. prolixus midguts with the TcSMUG L or TSSA peptide promote significant alteration of the epimastigote-midgut interaction rate.
Figure 2. Effect of surface mucins on ex vivo T. cruzi attachment to the midgut epithelium of Rhodnius prolixus.
Midguts obtained from male fifth-instar nymphs 10 days after the bloodmeal were previously incubated for 30 min in PBS supplemented with the indicated mucin peptides and added with BHI interaction medium containing flagellates (2.5×107/ml). Pre-incubation with mucin peptides was omitted in control (non-treated) group. Adhered epimastigotes were counted per 100 epithelial cells in 10 different fields of each midgut preparation. (A) Pre-incubation in 1 µg/ml of TcSMUG S, TSSA or TcSMUG L. (B) Pre-incubation in 0.01, 0.1 or 1.0 µg/ml of TcSMUG L. (C) Pre-incubation in 0.01, 0.1 or 1.0 µg/ml of TSSA. Each group represents mean ± S.D. of parasites attached in 10 midguts. Asterisk represents experimental groups with statistical significance compared to the control. Trypanosoma cruzi small mucin S (TcSMUG S), Trypanosoma cruzi small mucin L (TcSMUG L) and trypomastigote small surface antigen (TSSA).
TcSMUG L Products Are Involved in T. cruzi In Vivo Development in the Insect Vector
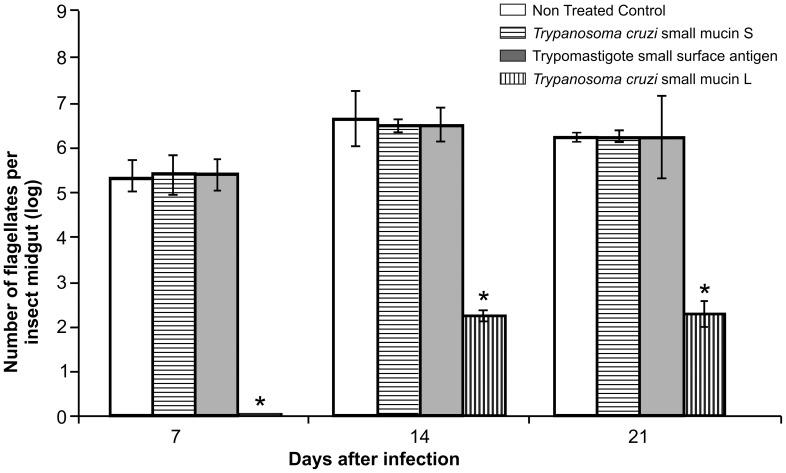
Upon ingestion of approximately 2×105 Dm28c epimastigotes/ml of blood, fifth-instar nymphs of R. prolixus became heavily infected with T. cruzi (Fig. 3). In the control group, the infection levels varied from 3.33±0.35×105 flagellates/ml of digestive tract homogenate 7 days after infection to 2.06±0.10×106 flagellates/ml of digestive tract 21 days post-infection. Similar infection levels were observed throughout the time frame of the experiment in insect groups fed with blood supplemented with either TcSMUG S or TSSA peptide (p>0·05). In contrast, nymphs fed with blood supplemented with TcSMUG L peptide showed significantly reduced infection levels. Direct counts revealed 2.3±0.12×102 (p<0·0001) and 2.3±0.27×102 (p<0·0001) flagellates/ml of digestive tract homogenate 14 and 21 days post-infection, respectively, representing a ∼4-log difference from controls. Even more compelling, no parasites were observed 7 days post-infection in TcSMUG L peptide-treated insects. Together, these results suggest that soluble TcSMUG L peptide significantly inhibits the normal development of Dm28c parasites in R. prolixus, likely by interfering between the interaction of endogenous TcSMUG L products displayed on the surface of epimastigotes and triatomid midgut receptors.
Figure 3. Effect of surface mucins on T. cruzi in vivo development in the digestive tract of Rhodnius prolixus.
Insects were fed on citrated, complement-inactivated human blood containing 2×105 flagellates/ml. Each mucin peptide was added to the bloodmeal at a concentration of 30 µg/ml and insects dissected as days 7, 14 or 21 post feeding. Each point represents mean±S.D of flagellates/ml in the whole gut of 10 insects. Asterisk represents experimental groups with statistical significance compared to the control.
Light Microscopy and Histochemical Localization of TcSMUG L Recognition Sites in the Posterior Midgut of R. prolixus
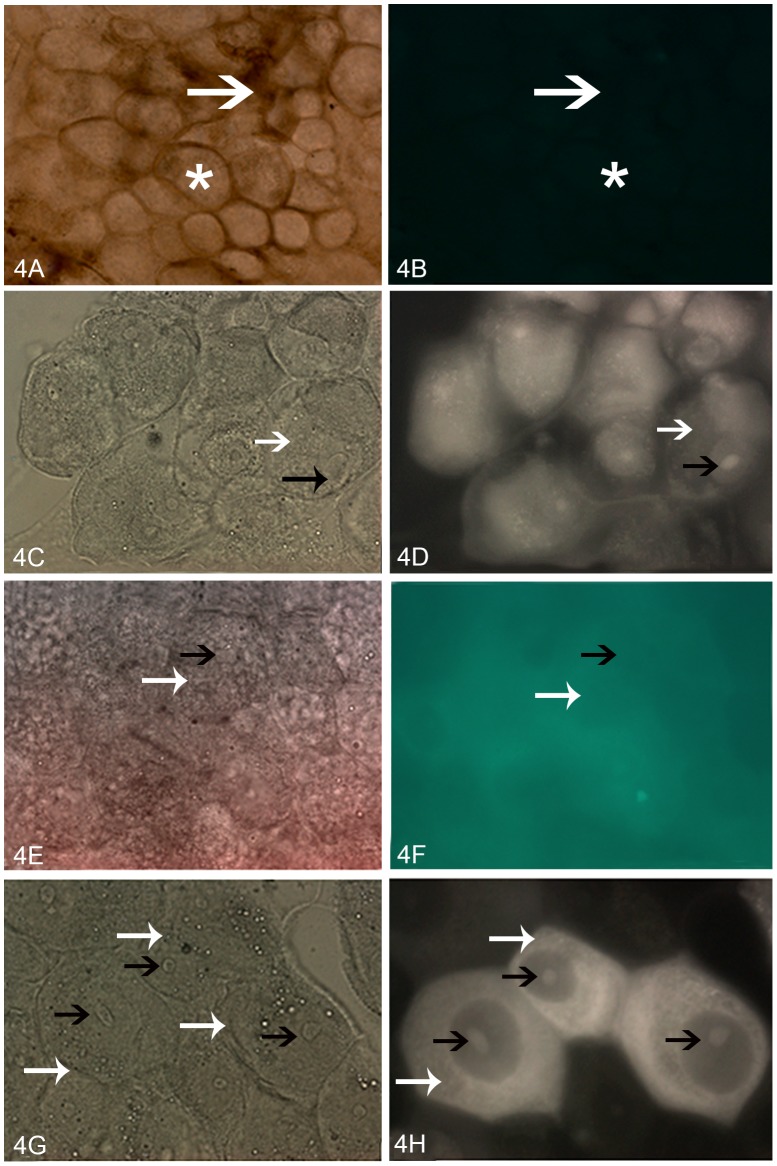
Light microscopy of R. prolixus midgut showed a single columnar epithelium composed by posterior midgut cells. Toluidine-stained granules were observed in the apical and medial region, where a round nucleus was located. As previously described [10], these epithelial cells were closely joined at their medial and basal regions, whereas a brush border associated with the PMM was observed at the luminal surface of their apical regions (Fig. S1). No significant labeling was obtained after incubation of R. prolixus posterior midgut surface with Avidin-FITC conjugate alone (Fig. 4A, B) or after previous incubation with biotin-labeled TcSMUG S peptide followed by the Avidin-FITC conjugate (Fig. 4E, F). However, in line with previous results, fluorescence of specific binding sites was observed on the surface of luminal posterior midgut cells after pre-incubation with biotin-labeled TcSMUG L (Fig. 4C, D) or TSSA (Fig. 4G, H) peptide under the same conditions. Unexpectedly, the samples pre-incubated with TSSA also showed some intracellular staining, particularly in the nucleolus, which may be attributed to partial permeabilization of the cells during fixation.
Figure 4. Photomicrographs of posterior midgut epithelial cells of fifth-instar R. prolixus incubated with biotin-labeled peptides.
(A) Light microscopy showing single-globe columnar epithelial cells(white star) and PMM (white arrow). (B) Fluorescence microscopy showing that no demarcation was observed after incubation with avidin-FITC-labeled conjugate alone. Light and fluorescence microscopy, respectively, of samples incubated with biotin-labeled TcSMUG L (C and D), biotin-labeled TcSMUG S (E and F), and biotin-labeled TSSA (G and H). Fluorescence of the surface and nucleolus of the midgut cells is indicated by white and black arrows (respectively). 400×.
Discussion
During its life cycle, T. cruzi adheres to specific host molecules/cell types as essential steps for parasite survival. Depending on the parasite developmental stage and the nature of the involved molecules, these interactions trigger a variety of events such as bidirectional cell signaling, host cell internalization, parasite replication or transformation to infective stages [45], [46]. Within the triatomid vector, different lines of research have established that molecules able to inhibit parasite attachment to insect tissues ex vivo also often efficiently block the in vivo development of T. cruzi [35]. For instance, purified GIPLs were shown to bind to the luminal surface of the posterior midgut. Accordingly, their exogenous addition dramatically impaired both ex vivo attachment of epimastigotes to this organ and the flagellate multiplication in the insect digestive tract, which prevented the successful colonization of the vector [11]. Similar effects were described for different carbohydrate-binding proteins (CBPs) of the epimastigote surface with a strong affinity for higher glycan oligomers and sulfated glycosaminoglycans (S-GAGs) present in the posterior midgut of R. prolixus [36], [47], [48]. The net negative charge of both S-GAGs and specific carbohydrates may act as a first, non-specific step prior to T. cruzi adhesion to specific receptors in the luminal midgut PMM [35]. In addition, an antiserum raised against R. prolixus PMM and midgut tissue interfered with midgut structural organization and slowed the development of T. cruzi in the insect vector [49].
The entire surface, including the cell body and the flagellum, of various T. cruzi developmental forms is covered with mucins that play a key role in parasite protection [50]–[52], infectivity, and development [15]. T. cruzi mucins are anchored to the outer leaflet of the plasma membrane through a GPI motif and undergo extensive glycosylation in their central Thr-rich domain. These features confer strong hydrophilic characteristics and an extended (“rod-like”) structural conformation [53], which is often used to elevate an outermost peptide above the parasite glycocalix. This N-terminal peptide, which is not predicted to be O-glycosylated, is thus ideally suited to participate in cell-to-cell interaction phenomena [54].
The results presented here strongly suggest that the N-terminal peptide of TcSMUG L products is required for efficient interaction between the parasite and the insect midgut and the subsequent growth of the flagellate in the invertebrate host. As shown, addition of the exogenous peptide led to a significant reduction in ex vivo adhesion to the insect midgut, and also inhibition of in vivo development within vectors. Due to its small molecular size, this effect is unlikely to be caused by steric effects, where the TcSMUG L peptide would prevent access of parasite recognition molecules to specific sites in the insect gut cells. Quite the opposite, we favor the hypothesis that the exogenous TcSMUG L peptide exerts its inhibitory effect by outcompeting the parasite binding sites in the triatomine luminal surface of the midgut epithelium. This idea is further supported by histochemical data showing intense labeling of the surface of luminal posterior midgut cells after pre-incubation with biotin-labeled TcSMUG L peptide. Therefore, it is likely that TcSMUG L products act as surface adhesion molecules, promoting epimastigote adhesion and colonization through recognition of specific receptor(s) on insect cells. In this framework, a distinct expression profile verified for TcSMUG L products [26] could contribute to the biological heterogeneity found between different isolates of T. cruzi in terms of triatomid infectivity. Moreover, drastic reduction in TcSMUG L expression upon differentiation to metacyclic trypomastigotes suggests a developmental regulation program that could help to explain why these latter forms are detached from the midgut surface [26].
One unexpected and puzzling finding was that the exogenous TSSA-derived peptide showed adhesion properties to insect midgut cells, as well as ex vivo inhibition on epimastigote attachment. It is worth mentioning that TSSA belongs to the TcMUC group of genes, which is expressed during the mammalian-dwelling stages of the protozoan [20], [21], [54]. In particular, TSSA expression is restricted to the surface of blood trypomastigotes, the parasite stage ingested by the vector during an infective blood meal, and amastigote-to-bloodstream trypomastigote intermediate forms. From a structural staindpoint, and despite showing similar bias in amino acid composition (with Cys, Phe, Trp and Tyr amino acids -all residues that could perturb the physicochemical properties of T. cruzi mucins- being underrepresented or absent), there are no obvious similarities in the primary sequences of the TSSA and TcSMUG L peptides that could explain their similar binding properties. Indeed, the labeling pattern obtained for TSSA in posterior midgut sections is different than that obtained for the TcSMUG L peptide, suggesting they recognize different receptor(s) on the surface of insect cells, although more studies would be required to address this point. Importantly, and in strict correlation with its expression profiling, the interaction between TSSA and insect midgut cells seems to have no biological relevance, as it had no effect on parasite in vivo development.
Although little is known about the mechanisms leading to the remodeling of the surface coat when the flagellate moves from the mammal into the insect vector, it is reasonable to suppose that TSSA is shed during this process. Free in the insect stomach, TSSA may reach the posterior midgut and be recognized by PMM receptors for mucins or other glycoconjugates. Transfer of antigenic epitopes from T. cruzi to the PMM of Triatoma infestans has been previously described [55]. In spite of this, TSSA does not seem to participate in the protozoan development of R. prolixus, which is compatible with its lack of expression in insect-dwelling stages of T. cruzi.
Altogether, these findings establish that TcSMUG L products are involved in the interaction between T. cruzi and its invertebrate host. Indeed, our results demonstrate that these products are involved in successful adhesion to the epithelial cells of insect vectors both ex vivo and in vivo, although the exact molecular mechanism, and particularly the putative receptor on the surface of the insect cells, should be further explored. Most importantly, a severe reduction in flagellate population in the digestive tract of R. prolixus was observed when triatomines were infected with epimastigotes of T. cruzi and simultaneously orally treated with the TcSMUG L peptide. Collectively, our work adds new insight into the relevance of mucin-type glycoconjugates in the infection of insect vectors and points to them as promising targets to develop transmission-blocking strategies for this disease.
Supporting Information
Light microscopy of toluidin blue-stained posterior midgut cells of R. prolixus 10 days after feeding. Oblique (a) and transverse (b) sections of the apical region of columnar epithelial cells, with brush border associated with perimicrovillar membranes (thick black arrow), round nuclei (thin black arrow) and the posterior midgut lumen (L). 400×.
(TIF)
Acknowledgments
The authors thank Rodrigo Mexas, Heloisa Maria Nogueira Diniz and Genilton José Vieira (Image Production and Treatment Sector/FIOCRUZ) for help with figures and Beatriz Ferreira and Giovana Moraes (Sample Procedure Sector/UENF) for help with light and fluorescence microscopy. We also thank Dr. J. Cazzulo (IIB-INTECH) for the anti-TcGDH antiserum and Dr. N. Yoshida (UNSP, Brazil) for kindly providing mAbs 2B10 and 10D8.
Funding Statement
This investigation received financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Oswaldo Cruz (Papes), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (Faperj) and Instituto Nacional de Entomologia Molecular (INEM-CNPq), to ESG and PA, and the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and Fundación Bunge y Born to CAB. ESG and PA are Research Fellows of the CNPq. SB holds a fellowship from the ANPCyT; GEC and IMD hold fellowships from CONICET and CAB is a career investigator from CONICET. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chagas C (1909) Nova Tripanossomíase humana. Estudos Sobre a Morfologia e o ciclo Evolutivo do Schizotrypanum cruzi n. gen, n. sp., Agente Etiológico de Nova Entidade Mórbida do Homem. Mem Inst Oswaldo Cruz 1: 159–218 Technical Report Series n° 975. [Google Scholar]
- 2. Chagas C (1911) Nova Entidade Mórbida do Homem: Resumo Geral de Estudos Etiológicos e Clínicos. Mem Inst Oswaldo Cruz 3: 219–275. [Google Scholar]
- 3.WHO-World Health Organization, (2012). Research Priorities for Chagas Disease, Human African Trypanosomiasis and Leishhmaniasis. Technical Report of the TDR Disease Reference Group on Chagas Disease, Human African Trypanosomiasis and Leishmaniasis. Technical Report Series n° 975. Available: http://www.who.int/tdr/publications/research_priorities/en/index.html. Accessed 06 April 2013. [PubMed]
- 4. Garcia ES, Ratcliffe NA, Whitten MM, Gonzalez MS, Azambuja P (2007) Exploring the Role of Insect Host Factors in the Dynamics of Trypanosoma cruzi-Rhodnius prolixus Interactions. J Insect Phys 53: 11–21. [DOI] [PubMed] [Google Scholar]
- 5.Schaub GA (2009) Interactions of Trypanosomatids and Triatomines. In: Simpson SJ, Casas J, editors. Advances in Insect Physiology. Burlington. pp. 177–242. [Google Scholar]
- 6. Garcia ES, Genta FA, Azambuja P, Schaub GA (2010) Interactions Between Intestinal Compounds of Triatomines and Trypanosoma cruzi . Trends in Parasitology 26: 499–505. [DOI] [PubMed] [Google Scholar]
- 7. Castro PC, Moraes CS, Gonzalez MS, Ratcliffe NA, Azambuja P, et al. (2012) Trypanossoma cruzi Immune Responses Modulation Decreases Microbiota in Rhodnius prolixus Gut and is Crucial For Parasite Survival and Development. PLoS One 7 (5) e36591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez MS, Nogueira NFS, Feder D, de Souza W, Azambuja P, et al. (1998) Role of The Head in the Ultrastructural Midgut Organization in Rhodnius prolixus: Evidence From Head Transplantation Experiments and Ecdysone Therapy. J Insect Phys 44: 553–560. [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez MS, Nogueira NFS, Mello CB, de Souza W, Schaub GA, et al. (1999) Influence of Brain on the Midgut Arrangement and Trypanosoma cruzi Development in the Vector, Rhodnius prolixus . Exp Parasitol 92: 100–108. [DOI] [PubMed] [Google Scholar]
- 10. Alves CR, Albuquerque-Cunha JM, Mello CB, Garcia ES, Nogueira NFS, et al. (2007) Trypanosoma cruzi: Attachment to Perimicrovillar Membrane Glycoproteins of Rhodnius prolixus . Exp Parasitol 116: 44–52. [DOI] [PubMed] [Google Scholar]
- 11. Nogueira NF, Gonzalez MS, Gomes JE, de Souza W, Garcia ES, et al. (2007) Trypanosoma cruzi: Involvement of Glycoinositolphospholipids in the Attachment to the Luminal Midgut Surface of Rhodnius prolixus . Exp Parasitol 116 (2) 120–8. [DOI] [PubMed] [Google Scholar]
- 12. Garcia ES, Azambuja P (1991) Development and Interactions of Trypanosoma cruzi Within the Insect Vector. Parasitol Today 7 (9) 240–244. [DOI] [PubMed] [Google Scholar]
- 13. Kleffmann T, Schmidt J, Schaub GA (1998) Attachment of Trypanosoma cruzi Epimastigotes to Hydrophobic Substrates and Use of this Property to Separate Stages and Promote Metacyclogenesis. J Eukaryotic Microbiol 45: 548–555. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt J, Kleffmann T, Schaub GA (1998) Hydrophobic Attachment of Trypanosoma cruzi to a Superficial Layer of the Rectal Cuticle in the Bug Triatoma infestans . Parasitol Res 84: 527–536. [DOI] [PubMed] [Google Scholar]
- 15. Yoshida N (2006) Molecular Basis of Mammalian Cell Invasion by Trypanosoma cruzi . An Acad Bras Cienc 78 (1) 87–111. [DOI] [PubMed] [Google Scholar]
- 16. Campo VA, Di Noia JM, Buscaglia CA, Agüero F, Sánchez DO, et al. (2004) Differential Accumulation of Mutations Localized in Particular Domains of the Mucin Genes Expressed in the Vertebrate Host Stage of Trypanosoma cruzi . Mol Biochem Parasitol 133: 81–91. [DOI] [PubMed] [Google Scholar]
- 17. Buscaglia CA, Campo VA (2004) The Surface Coat of the Mammal-Dwelling Infective Trypomastigote Stage of Trypanosoma cruzi is Formed by Highly Diverse Immunogenic Mucins. J Biol Chem 279 (16) 15860–15869. [DOI] [PubMed] [Google Scholar]
- 18. Campo VA, Buscaglia CA, Di Noia JM, Frasch AC (2006) Immunocharacterization of the Mucin-Type Proteins From the Intracellular Stage of Trypanosoma cruzi . Microbes Infect 8 (2) 401–409. [DOI] [PubMed] [Google Scholar]
- 19. Almeida IC, Gazzinelli RT (2001) Proinflammatory Activity of Glycosylphosphatidylinositol Anchors Derived from Trypanosoma cruzi: Structural and Functional Analyses. J Leuk Biol 70: 467–477. [PubMed] [Google Scholar]
- 20. Canepa GE, Degese MS, Budu A, Garcia CRS, Buscaglia CA (2012a) Involvement of TSSA (Trypomastigote Small Surface Antigen) in Trypanosoma cruzi Invasion of Mammalian Cells. Biochem J 444: 211–218. [DOI] [PubMed] [Google Scholar]
- 21. Canepa GE, Mesías CA, Yu H, Chen X, Buscaglia CA (2012b) Structural Features Affecting Trafficking, Processing, and Secretion of Trypanosoma cruzi Mucins. The J Biol Chem 287: 26365–26376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakayasu ES, Yashunsky DV, Nohara LL, Torrecillas AC, Nikolaev AV, et al. (2009) GPIomics: Global Analysis of Glycosylphosphatidylinositol-anchored Molecules of Trypanosoma cruzi . Mol Syst Biol 5: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paba J, Santana JM, Teixeira AR, Fontes W, Sousa MV, et al. (2004) Proteomic Analysis of the Human Pathogen Trypanosoma cruzi . Proteomics 4 (4) 1052–1059. [DOI] [PubMed] [Google Scholar]
- 24. Atwood JA 3rd, Weatherly DB, Minning TA, Bundy B, Cavola C, et al. (2005) The Trypanosoma cruzi Proteome. Science 309 (5733) 473–476. [DOI] [PubMed] [Google Scholar]
- 25. Ferella M, Nilsson D, Darban H, Rodrigues C, Bontempi EJ, et al. (2008) Proteomics in Trypanosoma cruzi localization of novel proteins to various organelles. Proteomics 8 (13) 2735–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Urban I, Boiani Santurio L, Chidichimo A, Yu H, Chen X, et al. (2011) Molecular Diversity of the Trypanosoma cruzi TcSMUG Family of Mucin Genes and Proteins. Biochem J 438: 303–313. [DOI] [PubMed] [Google Scholar]
- 27.Azambuja P, Garcia ES (1997) Care and Maintence of Triatomine Colonies. In: Crampton JM, Beard CB, Louis C, editors. Molecular Biology of Insect Disease Vectors: a Methods Manual. Chapman and Hall, London. pp. 56–64. [Google Scholar]
- 28. Perlowagora-Szumlewicz A, Moreira CJC (1994) In vivo Differentiation of Trypanosoma cruzi. Experimental Evidence of the Influence of the Vector Species on Metacyclogenesis. Mem Inst Oswaldo Cruz 89: 603–618. [DOI] [PubMed] [Google Scholar]
- 29. Garcia ES, Vieira E, Lima Gomes JEP, Gonçalves AM (1984a) Molecular Biology of the Interaction Trypanosoma cruzi/invertebrate Host. Mem Inst Oswaldo Cruz 79: 33–37. [Google Scholar]
- 30. Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, et al. (2009) A New Consensus for Trypanosoma cruzi Intraspecific Nomenclature: Second Revision Meeting Recommends TcI to TcVI. Mem Inst Oswaldo Cruz 104 (7) 1051–1054. [DOI] [PubMed] [Google Scholar]
- 31.Garcia ES, Azambuja P (1997) Infection of Triatomines with Trypanosoma cruzi. In: Crampton JM, Beard CB, Louid C, editors. Molecular Biology of Insect Disease Vectors: A Methods Manual. Chapman and Hall, London. pp 146–155. [Google Scholar]
- 32. Almeida IC, Ferguson MA, Schenkman S, Travassos LR (1994) Lytic Anti-alpha-galactosyl Antibodies from Patients with Chronic Chagas' Disease Recognize Novel O-linked Oligosaccharides on Mucin-like Glycosyl-phosphatidylinositol-anchored Glycoproteins of Trypanosoma cruzi . Biochem J 304 (3) 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barderi P, Campetella O, Frasch AC, Santone JA, Hellman U, et al. (1998) The NADP+-linked Glutamate Dehydrogenase from Trypanosoma cruzi: Sequence, Genomic Organization and Expression. Biochem J 330 (2) 951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pollevick GD, Di Noia JM, Salto ML, Lima C (2000) Trypanosoma cruzi surface mucins with exposed variant epitopes. J Biol Chem 275: 27671–27680. [DOI] [PubMed] [Google Scholar]
- 35. Gonzalez MS, Silva LCF, Albuquerque-Cunha JM, Nogueira NFS, Mattos DP, et al. (2011) Involvement of Sulfated Glycosaminoglycans on the Development and Attachment of Trypanosoma cruzi to the Luminal Midgut Surface in the Vector, Rhodnius prolixus . Parasitology 138: 1–8. [DOI] [PubMed] [Google Scholar]
- 36. Oliveira-Jr FOR, Alves CR, Souza-Silva F, Calvet CM, Côrtes LMC, et al. (2012) Trypanosoma cruzi Heparin-binding Proteins Mediate the Adherence of Epimastigotes to the Midgut Epithelial Cells of Rhodnius prolixus . Parasitol 139 (6) 735–43. [DOI] [PubMed] [Google Scholar]
- 37. Cortez MR, Provençano A, Silva CE, Mello CB, Zimmermann LT, et al. (2012) Trypanosoma cruzi: Effects of Azadirachtin and Ecdysone on the Dynamic Development in Rhodnius prolixus Larvae. Experimental Parasitology 131: 363–371. [DOI] [PubMed] [Google Scholar]
- 38. Schaub GA (1989) Trypanosoma cruzi: Quantitative Studies of Development of Two Strains in Small Intestine and Rectum of the Vector Triatoma infestans . Exp Parasitol 68: 260–273. [DOI] [PubMed] [Google Scholar]
- 39. Cortez MGR, Gonzalez MS, Cabral MMO, Garcia ES, Azambuja P (2002) Dynamic Development of Trypanosoma cruzi in Rhodnius prolixus: Role of Decapitation and Ecdysone Therapy. Parasitol Res 88: 697–703. [DOI] [PubMed] [Google Scholar]
- 40. Gonzalez MS, Garcia ES (1992) Effect of Azadirachtin on the Development of Trypanosoma cruzi in Different Species of Triatomine Insect Vectors: Long-term and Comparative Studies. J Invert Pathol 60: 201–205. [DOI] [PubMed] [Google Scholar]
- 41. Gonzalez MS, Nogueira NFS, Mello CB, de Souza W, Schaub GA, et al. (1999) Influence of Brain on the Midgut Arrangement and Trypanosoma cruzi Development in the Vector, Rhodnius prolixus . Exp Parasitol 92: 100–108. [DOI] [PubMed] [Google Scholar]
- 42. Hsu SM, Raine L, Fange RH (1981) A Comparative Study of the Peroxidase - Antiperoxidase Method and Avidin Biotin Complex Method for Studying Polypeptide Hormones with Radioimmunoassay Antibodies. American. J Clinical and Pathol 75: 734–41. [DOI] [PubMed] [Google Scholar]
- 43. Albuquerque-Cunha JM, Gonzalez MS, Garcia ES, Mello CB, Azambuja P, et al. (2009) Cytochemical Characterization of Microvillar and Perimicrovillar Membranes in the Posterior Midgut Epithelium of Rhodnius prolixus . Arthropod Struc and Develop 38: 31–44. [DOI] [PubMed] [Google Scholar]
- 44.Armitage P, Berry G, Mathews JNS (2002) Comparison of Several Groups and Experimental Design. In: Armitage P, Berry G, Matthews JNS editors. Statistical Methods in Medical Research. Blackwell Science Publishing, Oxford. pp. 236–256. [Google Scholar]
- 45. Burleigh BA, Woolsey AM (2002) Cell Signaling and Trypanosoma cruzi Invasion. Cel Microbiol 4: 701–711. [DOI] [PubMed] [Google Scholar]
- 46. Tan H, Andrews NW (2002) Don't Bother to Knock – The Cell Invasion Strategy of Trypanosoma cruzi . Trends in Parasitol 18: 427–428. [DOI] [PubMed] [Google Scholar]
- 47. Bonay P, Molina R, Fresno M (2001) Binding Specificity of Mannosespecific Carbohydrate-Binding Protein from the Cell Surface of Trypanosoma cruzi . Glycobiol 11: 719–729. [DOI] [PubMed] [Google Scholar]
- 48. Bourguignon SC, Mello CB, Santos DO, Gonzalez MS, Souto-Pádron T (2006) Biological Aspects of the Trypanosoma cruzi (Dm 28c clone) Intermediate form, Between Epimastigote and Trypomastigote Obtained in Modified Liver Infusion Tryptose (LIT) medium. Acta Tropica 98: 103–109. [DOI] [PubMed] [Google Scholar]
- 49. Gonzalez MS, Hamedi A, Albuquerque-Cunha JM, Nogueira NFS, de Souza W, et al. (2006) Antiserum Against Perimicrovillar Membranes and Midgut Tissue Reduces the Development of Trypanosoma cruzi in the Insect Vector, Rhodnius prolixus . Exp Parasitol 114: 297–304. [DOI] [PubMed] [Google Scholar]
- 50. Mortara RA, Silva DA, Araguth S, Blanco MF, Yoshida NSA (1992) Polymorphism of The 35-kilodalton and 50-kilodalton Surface Glycoconjugates of Trypanosoma-cruzi Metacyclic Trypomastigotes. Infection and Immunity 60 (11) 4673–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schenkman S, Ferguson MA, Heise N, de Almeida ML, Mortara RA, et al. (1993) Mucin-like Glycoproteins Linked to the Membrane by Glycosylphosphatidylinositol Anchor are the Major Acceptors of Sialic Acid in a Reaction Catalyzed by Trans-sialidase in Metacyclic Forms of Trypanosoma cruzi . Mol Biochem Parasitol 59: 293–303. [DOI] [PubMed] [Google Scholar]
- 52. Pereira-Chioccola VL, Acosta-Serrano A, Correia de Almeida I, Ferguson MA, Souto-Padron T, Rodrigues MM, et al. (2000) Mucin-like Molecules Form a Negatively Charged Coat that Protects Trypanosoma cruzi trypomastigotes from Killing by Human anti-α-galactosyl Antibodies. J Cell Sci 113: 1299–1307. [DOI] [PubMed] [Google Scholar]
- 53. Buscaglia CA, Campo VA, Frasch AC, Di Noia MJ (2006) Trypanosoma cruzi Surface Mucins: Host-Dependent Coat Diversity. Nature Rev Microbiol 4: 229–236. [DOI] [PubMed] [Google Scholar]
- 54. Di Noia JM, Buscaglia CA, De Marchi CR, Almeida IC, Frasch AC (2002) Trypanosoma cruzi Small Surface Molecule Provides the First Immunological Evidence that Chagas' Disease is Due to a Single Parasite Lineage. J Exp Med 195: 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gutierrez LS, Burgos MH, Brengio SDR (1991) Antibodies from Chagas Patients to the Gut Epithelial Cell Surface of Triatoma infestans . Micr Electr Biol Cel 15 (2) 145–158. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Light microscopy of toluidin blue-stained posterior midgut cells of R. prolixus 10 days after feeding. Oblique (a) and transverse (b) sections of the apical region of columnar epithelial cells, with brush border associated with perimicrovillar membranes (thick black arrow), round nuclei (thin black arrow) and the posterior midgut lumen (L). 400×.
(TIF)