Abstract
Background
Physical inactivity contributes to atherosclerotic processes, which manifest as increased arterial stiffness. Arterial stiffness is associated with myocardial demand and coronary perfusion and is a risk factor for stroke and other adverse cardiac outcomes. Poststroke mobility limitations often lead to physical inactivity and sedentary behaviors. This exploratory study aimed to identify functional correlates, reflective of daily physical activity levels, with arterial stiffness in community-dwelling individuals >1 year poststroke.
Methods
Carotid–femoral pulse wave velocity (cfPWV) was measured in 35 participants (65% men; mean ± SD age 66.9 ± 6.9 years; median time poststroke 3.7 years). Multivariable regression analyses examined the relationships between cfPWV and factors associated with daily physical activity: aerobic capacity (VO2 peak), gait speed, and balance ability (Berg Balance Scale). Age and the use of antihypertensive medications, known to be associated with pulse wave velocity, were also included in the model.
Results
Mean cfPWV was 11.2 ± 2.4 m/s. VO2 peak and age were correlated with cfPWV (r = −0.45 [P = .006] and r = 0.46 [P = .004], respectively). In the multivariable regression analyses, age and the use of antihypertensive medication accounted for 20.4% of the variance of cfPWV, and the addition of VO2 peak explained an additional 4.5% of the variance (R2 = 0.249).
Conclusions
We found that arterial stiffness is elevated in community-dwelling, ambulatory individuals with stroke relative to healthy people. Multivariable regression analysis suggests that aerobic capacity (VO2 peak) may contribute to the variance of cfPWV after accounting for the effects of age and medication use. Whether intense risk modification and augmented physical activity will improve arterial stiffness in this population remains to be determined.
Keywords: Arterial stiffness, physical activity, stroke
Adverse cardiovascular events, including stroke, often result from chronic atherosclerosis. Although the rate of atherosclerotic progression is highly variable, older adults typically have structural changes to the vascular system on examination, including lipid accumulation, plaque formation, and calcification of the arterial walls.1
Atherosclerotic burden is reflected by increased arterial stiffness,2 whereby aortic pressure is augmented, resulting in increased arterial wall stress and left ventricular afterload and reduced coronary perfusion pressure.3 Arterial stiffness is elevated in at-risk populations, including older adults and individuals with coronary artery disease, hypertension, dyslipidemia, and diabetes.4 Increased stiffness is associated with elevated cardiovascular risk.5 It is an independent risk factor for all-cause and cardiovascular mortality and other adverse cardiac outcomes,6–8 and an independent predictor of coronary heart disease and stroke.9 Among older adults, elevated arterial stiffness was associated with the presence of lacunar infarcts and white matter hyperintensities, which are markers of silent cerebrovascular disease.10
Carotid–femoral pulse wave velocity (cfPWV), the criterion standard method for measuring arterial stiffness, reflects the pulse wave propagation model within the arterial tree and can be measured noninvasively using mechanotransducers.11 It is calculated as D(m)/Δt (sec), where Δt is the transit time of pulse waves between the carotid and femoral arteries (D).11 A higher PWV indicates greater stiffness.
Among individuals with stroke, the degree to which arterial stiffening has occurred is an important consideration, particularly because of its association with increased cardiovascular risk. Arterial stiffness is acutely elevated after ischemic stroke12 and is associated with older age, diabetes, hypertension,13 and the metabolic syndrome.14 This acute increase in arterial stiffness may be caused by a complex interaction of multiple factors, including the occurrence of inflammatory processes and oxidative stress and the presence of endothelial dysfunction.12
Whether arterial stiffness remains elevated in the later poststroke stages is also important, because the risk for recurrent events remains high; the 5-year rate for recurrent stroke is 26% and at 10 years is nearly 40%.15 Cardiovascular risk factors are poorly managed in the later stages after stroke,16 and comorbid cardiovascular conditions, such as hypertension and heart disease, are highly prevalent.17 In addition, mobility limitations that persist after the initial neuromotor sequelae from stroke often lead to long-term inactivity and sedentary behaviors. Whether mobility limitations and the resultant low levels of physical activity contribute to atherosclerotic processes (as evidenced by elevated arterial stiffness) in the long-term after stroke has not been previously examined. Given that physical inactivity is a modifiable cardiovascular risk factor, this exploratory study aimed to identify functional correlates, reflective of daily physical activity levels, with arterial stiffness in community-dwelling individuals at least 1 year poststroke.
Methods
This study was a secondary cross-sectional analysis of a subset of data from a larger randomized, controlled trial examining the effects of exercise on cardiovascular function among individuals with stroke.18 For this analysis, data were available for 35 of the 48 participants from the main trial.
Ethics Statement
This study was approved by the University of British Columbia Clinical Research Ethics Board in May 2010. Informed written consent was obtained from all participants.
Participants
Individuals were eligible for the main trial if they were at least 1 year poststroke, living in the community, and able to walk 5 meters independently. Exclusion criteria included stroke of noncardiogenic origin (e.g., aneurysm, tumor, or infection), participation in stroke rehabilitation services, and the presence of uncontrolled arrhythmias, a pacemaker, or musculoskeletal or other issues that would preclude participation in the main trial intervention.
Assessments
Participant demographics were recorded, including age, sex, details of stroke (i.e., time poststroke, type, and location), and relevant medical history. Participants were then assessed for stroke severity and lower limb impairment using the National Institutes of Health Stroke Scale,19 where higher scores indicate greater severity (maximum score 42), and the Chedoke–McMaster Stroke Assessment,20 where the Leg and Foot Impairment inventories were combined for a maximum score of 14 (higher scores indicate greater motor recovery).
Assessment of Arterial Stiffness
Central arterial stiffness was measured noninvasively using 2 mechanotransducers (Complior; Artech Medical, Pantin, France) placed over the carotid and femoral arteries of the participant’s nonparetic side to determine cfPWV. Participants were requested to abstain from caffeine and tobacco 4 hours before the measurement and to abstain from alcohol consumption 12 hours before the measurement. Measurements were taken after 10 minutes of supine rest. Ten consistent and reproducible waveforms that met the software’s quality indices were obtained to determine cfPWV.
Potential Correlates with Arterial Stiffness
We sought to determine what dimensions of physical activity were independently correlated with arterial stiffness. Given the sample size of 35 participants with arterial stiffness data, 3 to 4 variables could be accommodated into a regression model.21 The following variables, known to be associated with level of physical activity, were explored as potential correlates with cfPWV.
Aerobic Capacity
Aerobic capacity, a measure of cardiorespiratory fitness, is reflective of daily physical activity levels. It is associated with free-living physical activity after stroke,22–24 and low levels of physical activity are associated with the risk of cardiovascular events, mediated by elevated levels of inflammatory biomarkers, high blood pressure, impaired lipid profile, and a high body mass index.25 Conversely, increased physical activity, particularly exercise that is aerobic in nature, is effective in attenuating other atherosclerotic risk factors, such as hypertension, insulin resistance and glucose intolerance, dyslipidemia, and obesity.26
Aerobic capacity was determined using a maximal exercise ramp protocol27 on a leg cycle ergometer (Excalibur; Lode Medical Technology, Groningen, the Netherlands) and metabolic cart for measurement of breath-to-breath gas exchange (ParvoMedics, Sandy, UT). The American College of Sports Medicine guidelines for test termination were followed.28 The protocol was adjusted to use 10- or 15-watt increments to maintain a test time between 8 and 10 minutes. VO2 peak, the criterion standard for cardiorespiratory fitness, was the primary outcome determined as the highest value achieved from the test.
Walking Ability
Gait speed is the most widely used and accepted measure of walking ability. The average of 2 trials of self-selected gait speed was measured over a 9-meter distance, where the middle 5 meters were timed. Gait aids were permitted. Gait speed is associated with home and community walking activity29 and participation.30
Balance Ability
The Berg Balance Scale (BBS) is the most commonly used clinical measure of functional balance. It is evaluated by performing tasks, such as standing with eyes closed, turning and looking, and stepping up and down on a step.31 The maximum score is 56, and higher scores indicate better balance function. The BBS has high test–retest reliability and concurrent validity with measures of functional independence with the stroke population.32 Poor balance may contribute to decline in cardiorespiratory fitness through balance-related inactivity.33
Analysis
Participant characteristics were described using mean ± SD (min-max) for continuous variables and the median (10th and 90th percentile) if the distribution was skewed. Categorical variables were described using frequency (percentage).
To examine the correlates with arterial stiffness, we first conducted bivariate analyses between cfPWV and each candidate variable (i.e., VO2 peak, self-selected gait speed, and BBS score), as well as age and blood pressure medication use. Scatterplots were visually inspected for outliers and to confirm the linearity of associations.
Multivariable regression analyses were performed to determine the influence of daily physical activity levels on arterial stiffness. Because age and the use of antihypertensive medications are known to influence pulse wave velocity,11 these variables were included in the model. Each candidate variable (i.e., VO2 peak, gait speed, and BBS score) was then entered individually to the model to determine the additional variance of cfPWV that was explained. Tolerance values, variance inflation factors, and residuals were examined.
Statistical Package for the Social Sciences software (version 17.0; SPSS, Inc., Chicago IL) was used for all analyses.
Results
Of the original 48 participants in the main trial, we were unable to obtain pulse wave signal values for 11 patients. VO2 peak data were unavailable for 2 additional participants (because of an issue with the metabolic cart; in addition, 1 patient was unable to pedal on the cycle ergometer, and the exercise test was therefore not performed). Data from 35 participants were available for analysis. In general, participant characteristics between those with and without full datasets were similar (for characteristics in Table 1), except that there were relatively fewer men (only 38%).
Table 1.
Participant characteristics
| Characteristic | n | Value |
|---|---|---|
| Age (y), mean ± SD (min-max) | 35 | 66.9 ± 6.9 (51–80) |
| Sex, n (%) | 35 | |
| Men | 24 (65) | |
| Women | 13 (35) | |
| Time poststroke (y), median (10th, 90th percentile) | 35 | 3.7 (1.4, 9.7) |
| Stroke type, n (%) | 35 | |
| Lacunar | 4 (11) | |
| Ischemic | 13 (37) | |
| Hemorrhagic | 13 (37) | |
| Unknown | 5 (15) | |
| Stroke location, n (%) | 35 | |
| Cortical | 9 (26) | |
| Subcortical | 15 (43) | |
| Brainstem | 4 (11) | |
| Unknown | 7 (20) | |
| Hemisphere affected, n (%) | 35 | |
| Right | 16 (46) | |
| Left | 16 (46) | |
| Bilateral | 2 (6) | |
| Unknown | 1 (3) | |
| Antihypertensive and lipid-lowering agents, n (%) | ||
| None | 5 (14) | |
| Beta-adrenergic antagonists | 11 (31) | |
| Angiotensin-converting enzyme inhibitors | 15 (43) | |
| Angiotensin receptor blockers | 8 (23) | |
| Calcium channel blockers | 13 (37) | |
| Statins | 21 (60) | |
| Fibrates | 2 (6) | |
| Diabetes, n (%) | 35 | |
| None | 28 (80) | |
| Type 2 | 7 (20) | |
| Smoking history, n (%) | 35 | |
| Never smoked | 14 (40) | |
| Formerly smoked | 20 (57) | |
| Currently smoking | 1 (3) | |
| Resting blood pressure | ||
| Systolic (mm Hg), mean ± SD (min-max) | 35 | 122.3 ± 12.1 (90–144) |
| Diastolic (mm Hg), mean ± SD (min-max) | 35 | 66.9 ± 6.6 (45–81) |
| Body mass index (kg/m2), mean ± SD (min-max) | 35 | 27.2 ± 4.5 (17–35) |
| NIHSS score, median (10th, 90th percentile) | 35 | 1.0 (0, 4.8) |
| CMSA Impairment Inventory, median (10th, 90th percentile) | 35 | |
| Leg scores | 6 (5, 7) | |
| Foot scores | 6 (2, 7) | |
| Gait speed, m/s, mean ± SD (min-max) | 35 | 0.94 ± 0.39 (0.10–1.69) |
| Berg Balance Scale score, median (10th, 90th percentile) | 35 | 51 (39, 56) |
| VO2 peak, mL/kg/min, mean ± SD (min-max) | 35 | 17.8 ± 6.3 (9.4–35.1) |
| Carotid–femoral pulse wave velocity, m/s, mean ± SD (min-max) | 35 | 11.2 ± 2.4 (5.6–16.3) |
Abbreviations: CMSA, Chedoke–McMaster Stroke Assessment; NIHSS, National Institutes of Health Stroke Scale; SD, standard deviation; VO2 peak, aerobic capacity.
Participant Characteristics
Characteristics for the 35 participants are presented in Table 1. Participants sustained strokes of mild to moderate severity as evidenced by the National Institutes of Health Stroke Scale and Chedoke–McMaster Stroke Assessment leg and foot scores. Twenty-two (63%) participants did not require gait aids for ambulation, 11 (32%) used a cane, and 2 (6%) used a walker. Participants had gait speeds that were 72.2% ± 29.3% of age-matched healthy individuals,34 and aerobic capacity was 56.4% ± 18.3% of normative values.28
Correlates with Arterial Stiffness
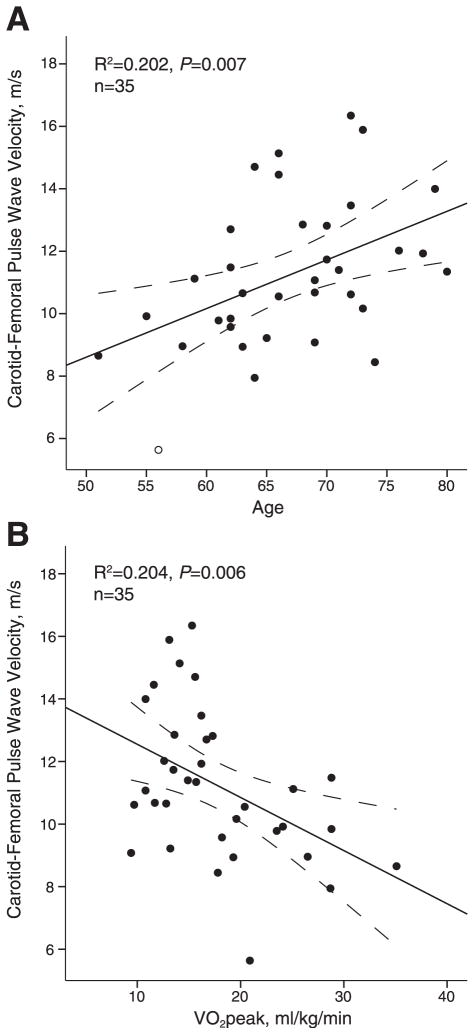
The bivariate analyses revealed an inverse association between cfPWV and VO2 peak (r = −0.45; P = .006) and a positive association between cfPWV and age (r = 0.46; P = .004; Table 2). There were no relationships between cfPWV and balance or walking ability or antihypertension medication use. Scatterplots depicting the relationships between age and cfPWV and VO2 peak and cfPWV are shown in Figure 1.
Table 2.
Correlations between carotid–femoral pulse wave velocity and measures reflective of daily physical activity, age, and use of antihypertension medications
| n | r (95% CI) | P value | |
|---|---|---|---|
| VO2 peak | 35 | −0.45 (0.14–0.68) | .006 |
| Gait speed | 35 | 0.08 (−0.26–0.41) | .63 |
| Berg Balance Scale | 35 | 0.12 (−0.25–0.42) | .49 |
| Age | 35 | 0.46 (0.16–0.69) | .004 |
| Antihypertensive medication use (yes/no) | 35 | 0.17 (−0.18–0.47) | .34 |
Abbreviations: CI, confidence interval; VO2 peak, aerobic capacity.
Figure 1.
Scatterplots for carotid–femoral pulse wave velocity with (A) age and (B) aerobic capacity (VO2 peak). Linear regression and 95% confidence intervals (dashed lines) are shown.
Results from the multivariable regression analyses are presented in Table 3. In model 1, age and antihypertensive medication use alone accounted for 20.2% of the variance of cfPWV. In model 2, VO2 peak alone explained 20.4% of the variance of cfPWV. In models 3 to 5, each candidate variable (i.e., VO2 peak, gait speed, and BBS score) was entered individually in the model, along with age and antihypertension medication use. Relative to model 1, the addition of gait speed (model 4) or BBS score (model 5) resulted in small increases in R2 values (1.4% and 0.6%, respectively), whereas the addition of VO2 peak (model 3) explained an additional 4.5% of the variance of cfPWV (R2 = 0.249).
Table 3.
Regression models to examine correlates with carotid–femoral pulse wave velocity*
| Correlates with cfPWV | Age and antihypertensive medications only
|
VO2 peak only
|
Age and antihypertensive medications, plus
|
||
|---|---|---|---|---|---|
| VO2 peak
|
Gait speed
|
BBS score
|
|||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| Age, y | 0.16 ± 0.06 (.01) | 0.10 ± 0.08 (.18) | 0.16 ± 0.06 (.01) | 0.16 ± 0.06 (.02) | |
| Antihypertensive medications | −0.28 ± 0.96 (.77) | −0.42 ± 0.95 (.66) | −0.14 ± 0.98 (.89) | −0.21 ± 0.98 (.83) | |
| VO2 peak | −0.17 ± 0.06 (.006) | −0.11 ± 0.08 (.19) | |||
| Gait speed | 0.73 ± 0.01 (.47) | ||||
| BBS score | 0.02 ± 0.05 (.66) | ||||
| Overall model R2 | 0.204 | 0.204 | 0.249 | 0.218 | 0.210 |
| Overall model P value | 0.03 | 0.006 | 0.03 | 0.05 | 0.06 |
| R2 change from model 1 | — | — | 0.045 | 0.014 | 0.006 |
| P value for additional variable† | — | — | 0.16 | 0.44 | 0.63 |
Abbreviations: BBS, Berg Balance Scale; cfPWV, carotid–femoral pulse wave velocity; VO2 peak, aerobic capacity.
Nonstandardized β ± standard error (P).
Based on likelihood ratio test of nested models.
Discussion
Arterial stiffness can provide important information regarding the progression of atherosclerosis and can be measured noninvasively in individuals with stroke. Ours is the first study to measure arterial stiffness (cfPWV) among community-dwelling individuals with stroke and to examine its association with measures reflective of daily physical activity levels.
The 11.2 m/s cfPWV measured in our sample was higher than reported reference values. In a meta-analysis of 11 studies, cfPWV collected from the Complior device (which was used in the current study) was 8.86 m/s among adults 23 to 72 years of age who did not have cardiovascular disease or risk factors.35 For older adults (60–69 years of age), a reference value of cfPWV as 10.3 m/s was derived from an algorithm to convert data obtained from various instrumentation (including the Complior device).36 Therefore, among our participants of older adults with stroke, the degree to which cfPWV is elevated relative to nonstroke cohorts is greater than the 0.5-m/s difference that is considered to be clinically meaningful37 and further highlights the multiple issues in the stroke population that affect cardiovascular health and increases their risk for recurrent events.
That age is associated with cfPWV among individuals in the later stroke stages is consistent with previous reports involving healthy adults38,39 and those in the acute stroke phase.12,13 Age is an established cardiovascular risk factor, and atherosclerotic disease progression is associated with increasing age. Age-associated changes to the vascular system include the overproduction of collagen, lipid accumulation, plaque formation, and arterial wall calcification, resulting in repetitive arterial wall stress and elastin fiber fracture, which contributes to increased arterial stiffness.1,2
In the multivariable regression analyses, the addition of each measure of daily physical activity levels into the models that included age and antihypertension medication use provided additional explanations for the variance of cfPWV. While gait speed and balance ability resulted in small increases in R2, the variable that contributed to the greatest change was VO2 peak, accounting for an additional 4.5% of the variability observed in cfPWV (Table 3; model 3). While this increase was not statistically significant, the overall model remained significant, suggesting that aerobic capacity, an objective measure of daily physical activity,40 may contribute to poststroke arterial stiffness among individuals with the same age and blood pressure medication use. However, this observation would need to be confirmed in a larger study. Diminished exercise capacity reflects low levels of daily physical activity engaged by the participants, which may provide an explanation for its contribution to the model.
The scatterplot between cfPWV and VO2 peak (Fig 1B) generated some interesting observations, notably the differences in the distribution of cfPWV values between participants in the higher versus lower ranges of VO2 peak. While individuals with VO2 peak values >20 mL/kg/min consistently had low arterial stiffness, there was a broad range of cfPWV values among individuals with more compromised fitness (<20 mL/kg/min). It is possible that a threshold for aerobic fitness may exist before improvements in arterial stiffness are observed. Study participants at the low end of the fitness spectrum (VO2 peak values <20 mL/kg/min) were likely to be the most sedentary yet potentially have the most to gain, even with modest levels of exercise. Indeed, it has been previously shown that the greatest improvements in cardiovascular health outcomes were observed among individuals with the most compromised fitness levels, including a reduced risk for cardiovascular-related mortality41 and cardiovascular disease42 and lowered arterial stiffness.43
While physical inactivity is a known risk factor for atherosclerosis, only a few cross-sectional studies have reported the inverse relationship between arterial stiffness and measures of physical activity or physical fitness,44–46 and none focused on individuals with stroke. Augmented physical activity has the potential to improve arterial stiffness, even in the presence of aging. Tanaka et al43 found that sedentary and recreationally active men had lower central arterial compliance compared to endurance-trained men regardless of age group, suggesting that age-associated increases in arterial stiffness may be mitigated with regular aerobic exercise. Exercise training has also been shown to improve arterial stiffness among individuals with coronary artery disease,47 older adults with multiple risk factors,48 and those undergoing hemodialysis.49 Only one study has examined the effects of exercise on arterial function in individuals with stroke. Takatori et al50 concluded that intensive strengthening may have some benefit to vascular stiffness on the paretic side, but analogous findings were not observed on the nonparetic side.
Whether intense risk modification and increased levels of physical activity poststroke will reduce arterial stiffness remains to be determined. Our participants represented individuals with relatively mild severity of stroke (all were capable of ambulation and living in the community), yet all subjects had compromised aerobic fitness levels and elevated arterial stiffness. Given the presence of cardiovascular comorbidities17 and elevated risk for recurrent events in this population,15 regular physical activity may attenuate the risk of adverse cardiovascular outcomes. Of note, a cross-sectional study of physically active individuals with spinal cord injury and age-matched recreationally active healthy controls found no differences in arterial stiffness between the 2 groups,51 suggesting that physical activity may preserve arterial function, even among individuals with mobility limitations.51 Exercise-related improvements in arterial stiffness also appear to be reversible with detraining,49 underscoring the need for training programs to be ongoing in nature for at-risk populations, such as stroke.
Study Limitations
Because of the cross-sectional nature of this study, we are only able to evaluate independent correlates with cfPWV at a single point in time, and therefore we cannot infer the causality of higher aerobic capacity on reduced arterial stiffness. That we were only able to obtain pulse wave signal for approximately 80% of participants from the main trial also limits the use of this measure. In addition, the small sample size limited our ability to determine the independent contribution of aerobic fitness to arterial stiffness. Future work may provide additional evidence of the negative health implications of reduced fitness among individuals with stroke who also present with age-related changes to the vascular system. Finally, it has been suggested that arterial stiffness may be more relevant for primary prevention,52 and indeed, no study has yet examined the predictive value of arterial stiffness in the occurrence of secondary events. Longitudinal studies that examine relationships between arterial stiffness and recurrent stroke and other cardiovascular variables may be the focus of future work.
In conclusion, among community-dwelling individuals with stroke, arterial stiffness measured by cfPWV is higher relative to values reported for older adults. Multivariable regression analysis suggests that aerobic fitness (VO2 peak) may contribute to the variance of cfPWV, after accounting for the effects of age and medication use. Whether intense risk modification and augmented physical activity will improve arterial stiffness remains to be determined, but given the elevated risk of cardiovascular events, including recurrent stroke, providing opportunities to engage in regular exercise in this population is an important consideration.
Acknowledgments
Supported in part by the Vancouver Foundation/Carl and Elsie Halterman Research Fund and the Canadian Institutes of Health Research (CIHR; MOP-111183). Dr. Tang is supported by the CIHR (MFE-98550) and the Michael Smith Foundation for Health Research (MSFHR; ST-PDF-03003[11-1]CLIN), Dr. Eng is supported by the CIHR (MSH-63617) and the MSFHR, Dr. Madden is supported by the CIHR Institute of Aging (MOP-20R43383), Dr. Krassioukov is supported by the Heart and Stroke Foundation of British Columbia and Yukon, the Christopher and Dana Reeve Foundation, and the Rick Hansen Institute.
References
- 1.Cohn JN. Arterial stiffness, vascular disease, and risk of cardiovascular events. Circulation. 2006;113:601–603. doi: 10.1161/CIRCULATIONAHA.105.600866. [DOI] [PubMed] [Google Scholar]
- 2.Barodka VM, Joshi BL, Berkowitz DE, et al. Review article: Implications of vascular aging. Anesth Analg. 2011;112:1048–1060. doi: 10.1213/ANE.0b013e3182147e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols WW, Edwards DG. Arterial elastance and wave reflection augmentation of systolic blood pressure: Deleterious effects and implications for therapy. J Cardiovasc Pharmacol Ther. 2001;6:5–21. doi: 10.1177/107424840100600102. [DOI] [PubMed] [Google Scholar]
- 4.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543–551. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Nurnberger J, Keflioglu-Scheiber A, Opazo Saez AM, et al. Augmentation index is associated with cardiovascular risk. J Hypertens. 2002;20:2407–2414. doi: 10.1097/00004872-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Meaume S, Benetos A, Henry OF, et al. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 7.Chirinos JA, Zambrano JP, Chakko S, et al. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005;45:980–985. doi: 10.1161/01.HYP.0000165025.16381.44. [DOI] [PubMed] [Google Scholar]
- 8.Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 9.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 10.Hatanaka R, Obara T, Watabe D, et al. Association of arterial stiffness with silent cerebrovascular lesions: The Ohasama study. Cerebrovasc Dis. 2011;31:329–337. doi: 10.1159/000322599. [DOI] [PubMed] [Google Scholar]
- 11.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 12.Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Arterial stiffness indexes in acute ischemic stroke: Relationship with stroke subtype. Atherosclerosis. 2010;211:187–194. doi: 10.1016/j.atherosclerosis.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 13.De Silva D, Woon FP, Chen C, et al. Profile and associations of central pulse wave velocity and central pulse pressure among ischemic stroke patients. Eur J Neurol. 2008;15:196–198. doi: 10.1111/j.1468-1331.2007.02024.x. [DOI] [PubMed] [Google Scholar]
- 14.De Silva DA, Woon FP, Gan HY, et al. Arterial stiffness, metabolic syndrome and inflammation amongst Asian ischaemic stroke patients. Eur J Neurol. 2008;15:872–875. doi: 10.1111/j.1468-1331.2008.02208.x. [DOI] [PubMed] [Google Scholar]
- 15.Mohan KM, Wolfe CD, Rudd AG, et al. Risk and cumulative risk of stroke recurrence: A systematic review and meta-analysis. Stroke. 2011;42:1489–1494. doi: 10.1161/STROKEAHA.110.602615. [DOI] [PubMed] [Google Scholar]
- 16.Kopunek SP, Michael KM, Shaughnessy M, et al. Cardiovascular risk in survivors of stroke. Am J Prev Med. 2007;32:408–412. doi: 10.1016/j.amepre.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth EJ. Heart disease in patients with stroke: Incidence, impact, and implications for rehabilitation. Part 1: Classification and prevalence. Arch Phys Med Rehabil. 1993;74:752–760. doi: 10.1016/0003-9993(93)90038-c. [DOI] [PubMed] [Google Scholar]
- 18.Tang A, Eng JJ, Krassioukov AV, et al. Post-stroke exercise: Effects on cardiovascular risk, fitness and function. Arch Phys Med Rehabil. 2012;93:e11. [Google Scholar]
- 19.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 20.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 21.Vittinghoff E, Glidden DV, Shiboski SC, et al. Regression methods in biostatistics. New York: Springer; 2005. [Google Scholar]
- 22.Baert I, Feys H, Daly D, Troosters T, et al. Are patients 1 year post-stroke active enough to improve their physical health? Disabil Rehabil. 2012;34:574–580. doi: 10.3109/09638288.2011.613513. [DOI] [PubMed] [Google Scholar]
- 23.Resnick B, Michael K, Shaughnessy M, et al. Inflated perceptions of physical activity after stroke: Pairing self-report with physiologic measures. J Phy Act Health. 2008;5:308–318. doi: 10.1123/jpah.5.2.308. [DOI] [PubMed] [Google Scholar]
- 24.Katoh J, Murakami M, Hirayama M, et al. Correlation of pedometric measurement of daily physical activity with exercise endurance by oxygen uptake kinetics in ambulatory stroke patients. J Phys Ther Sci. 2002;14:77–80. [Google Scholar]
- 25.Mora S, Cook N, Buring JE, et al. Physical activity and reduced risk of cardiovascular events: Potential mediating mechanisms. Circulation. 2007:116. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: A statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 27.Pang MYC, Eng JJ, Dawson AS, et al. A community-based fitness and mobility exercise (FAME) program for older adults with chronic stroke: A randomized, controlled trial. J Am Geriatr Soc. 2005;53:1667–1674. doi: 10.1111/j.1532-5415.2005.53521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 29.Fulk GD, Reynolds C, Mondal S, et al. Predicting home and community walking activity in people with stroke. Arch Phys Med Rehabil. 2010;91:1582–1586. doi: 10.1016/j.apmr.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38:2096–2100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 31.Berg K, Wood-Dauphinee SL, Williams JI, et al. Measuring balance in the elderly: Validation of an instrument. Can J Public Health. 1992;83(Suppl):S7–S11. [PubMed] [Google Scholar]
- 32.Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: A systematic review. Phys Ther. 2008;88:559–566. doi: 10.2522/ptj.20070205. [DOI] [PubMed] [Google Scholar]
- 33.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: The role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005;86:1552–1556. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 34.Bohannon RW, Williams Andrews A. Normal walking speed: A descriptive meta-analysis. Physiotherapy. 2011;97:182–189. doi: 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Khoshdel AR, Thakkinstian A, Carney SL, et al. Estimation of an age-specific reference interval for pulse wave velocity: A meta-analysis. J Hypertens. 2006;24:1231–1237. doi: 10.1097/01.hjh.0000234098.85497.31. [DOI] [PubMed] [Google Scholar]
- 36.Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papaioannou TG, Protogerou AD, Nasothimiou EG, et al. Assessment of differences between repeated pulse wave velocity measurements in terms of ‘bias’ in the extrapolated cardiovascular risk and the classification of aortic stiffness: Is a single PWV measurement enough? J Hum Hypertens. 2012;26:594–602. doi: 10.1038/jhh.2011.76. [DOI] [PubMed] [Google Scholar]
- 38.Abhayaratna WP, Barnes ME, O’Rourke MF, et al. Relation of arterial stiffness to left ventricular diastolic function and cardiovascular risk prediction in patients ≥65 years of age. Am J Cardiol. 2006;98:1387–1392. doi: 10.1016/j.amjcard.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 39.Amar J, Ruidavets JB, Chamontin B, et al. Arterial stiffness and cardiovascular risk factors in a population-based study. J Hypertens. 2001;19:381–387. doi: 10.1097/00004872-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Blair SN, Kohl HW, Paffenbarger RS, et al. Physical fitness and all-cause mortality – A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 41.Warburton DER, Whitney Nicol C, Bredin S. Health benefits of physical activity: The evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams PT. Physical fitness and activity as separate heart disease risk factors: A meta-analysis. Med Sci Sports Exerc. 2001;33:754–761. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka H, Dinenno FA, Monahan KD, et al. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 44.Vaitkevicius P, Fleg J, Engel J, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 45.Binder J, Bailey KR, Seward JB, et al. Aortic augmentation index is inversely associated with cardiorespiratory fitness in men without known coronary heart disease. Am J Hypertens. 2006;19:1019–1024. doi: 10.1016/j.amjhyper.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Arena R, Arrowood JA, Fei DY, et al. Maximal aerobic capacity and the oxygen uptake efficiency slope as predictors of large artery stiffness in apparently healthy subjects. J Cardiopulm Rehabil Prev. 2009;29:248–254. doi: 10.1097/HCR.0b013e3181a3338c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edwards DG, Schofield RS, Magyari PM, et al. Effect of exercise training on central aortic pressure wave reflection in coronary artery disease. Am J Hypertens. 2004;17:540–543. doi: 10.1016/j.amjhyper.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Madden KM, Lockhart C, Cuff D, et al. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care. 2009;32:1531–1535. doi: 10.2337/dc09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mustata S, Chan C, Lai V, et al. Impact of an exercise program on arterial stiffness and insulin resistance in hemodialysis patients. J Am Soc Nephrol. 2004;15:2713–2718. doi: 10.1097/01.ASN.0000140256.21892.89. [DOI] [PubMed] [Google Scholar]
- 50.Takatori K, Matsumoto D, Okada Y, et al. Effect of intensive rehabilitation on physical function and arterial function in community-dwelling chronic stroke survivors. Top Stroke Rehabil. 2012;19:377–383. doi: 10.1310/tsr1905-377. [DOI] [PubMed] [Google Scholar]
- 51.Jae SY, Heffernan KS, Lee M, et al. Arterial structure and function in physically active persons with spinal cord injury. J Rehabil Med. 2008;40:535–538. doi: 10.2340/16501977-0212. [DOI] [PubMed] [Google Scholar]
- 52.Zoungas S, Asmar RP. Arterial stiffness and cardiovascular outcome. Clin Exp Pharmacol Physiol. 2007;34:647–651. doi: 10.1111/j.1440-1681.2007.04654.x. [DOI] [PubMed] [Google Scholar]