Abstract
The clinical efficacy of anti-angiogenic therapies has been difficult to predict, and biomarkers that can predict responsiveness are sorely needed in this era of personalized medicine. CVX-060 is an angiopoietin-2 (Ang2) targeting therapeutic, consisting of two peptides that bind Ang2 with high affinity and specificity, covalently fused to a scaffold antibody. In order to optimize the use of this compound in the clinic the construction of a predictive model is described, based on the efficacy of CVX-060 in 13 cell line and 2 patient-derived xenograft models. Pretreatment size tumors from each of the models were profiled for the levels of 27 protein markers of angiogenesis, SNP haplotype in 5 angiogenesis genes, and somatic mutation status for 11 genes implicated in tumor growth and/or vascularization. CVX-060 efficacy was determined as tumor growth inhibition (TGI%) at termination of each study. A predictive statistical model was constructed based on the correlation of these efficacy data with the marker profiles, and the model was subsequently tested by prospective analysis in 11 additional models. The results reveal a range of CVX-060 efficacy in xenograft models of diverse tissue types (0-64% TGI, median = 27%) and define a subset of 3 proteins (Ang1, EGF, Emmprin), the levels of which may be predictive of TGI by Ang2 blockade. The direction of the associations is such that better efficacy correlates with high levels of target and low levels of compensatory/antagonizing molecules. This effort has revealed a set of candidate predictive markers for CVX-060 efficacy that will be further evaluated in ongoing clinical trials.
Introduction
The process of angiogenesis in neoplastic development can be carried out by a large number of angiogenic activators including VEGF (vascular endothelial growth factor), MMPs (matrix metaloproteases), PIGF (placental growth factor), FGFs (fibroblast growth factors), HGF (hepatocyte growth factor), PDGFs (platelet-derived growth factors), and Ang family proteins (angiopoietins). Compounds targeting these factors (or their cognate receptors) have shown anti-angiogenic effect and often significant and pan-tumor inhibition in preclinical models. In 2003 the first anti-angiogenic agent obtained FDA approval (bevacizumab) and several other agents have followed suit in the past decade. In general this class of agents has shown substantial benefit in a wide variety of cancers yet has been plagued by three primary issues: acquired resistance, rebound of tumor growth upon withdrawal of compound, and lack of biomarkers to predict or track response [1]. The latter point is of special note as not all patients in any given indication show response and the potential toxicities with particularly VEGF-targeting agents highlights the need to prospectively identify patients that are unlikely to benefit from these therapies. Despite over a decade of intense preclinical and clinical evaluation no single marker/method that consistently predicts responsiveness for an anti-angiogenic agent has been identified [2-4].
In contrast to the depth of preclinical knowledge, clinical experience, FDA-approved compounds, and reported biomarker work on the VEGF pathway, much less is known about targeting the angiopoietin pathway. VEGF and angiopoietins are thought to act at different times and with different roles in the angiogenic process; VEGF directs vascular sprouting, while angiopoietins facilitate vascular maturation and remodeling [5-8]. Angiopoietins are released by tumor cells and endothelial cells (TCs and ECs, respectively) and are thought to act primarily on the ECs via interactions with the Tie2 receptor and integrins [9-12]. In the context of stimulation of the Tie2 receptor on ECs Ang1 is thought to be the primary agonist [13] leading to pro-maturation signaling via EC survival [14,15] and recruitment of pericytes to seal the vessels [16,17]. Ang2 on the other hand is believed to be a much weaker agonist of Tie2, and as a competitor with Ang1 functionally causes a reduction in Tie2 agonism, leading to pro-remodeling signaling [18-21]. The process of destabilizing vasculature to allow for remodeling, via loss of pericytes and ECs, is believed to be an initial step in formation of new blood vessels. Therapeutic inhibition of Ang2 signaling should theoretically lead to stronger Ang1 signaling and hence vascular stabilization. Indeed the compound CVX-060 (PF-04856884), an Ang2-specific trap, induces significant reductions in microvessel density and tumor growth in several preclinical models [22].
CVX-060 has shown promising efficacy and limited toxicity in a phase 1 trial (NCT00879684), and is now being tested in a phase 2 RCC trial (NCT01441414) [23,24]. As no information exists regarding predictive biomarkers for angiopoietin therapy we performed a systematic study to identify biomarkers correlating with CVX-060 efficacy in xenograft (XG) models. Here we describe the design of a promising campaign to identify biomarkers for anti-angiogenic agents in preclinical models and report a multi-protein signature that correlates with tumor growth inhibition (TGI) by CVX-060 across a wide variety of tumor types. An initial set of XGmodels were used to build a predictive model, and then a second set of XG models were used for an independent and blinded prospective evaluation of the model. The results indicated that higher Ang2 and lower Ang1 may correlate with better CVX-060 response, consistent with known angiopoietin biology. In addition lower EGF/EGFR and Emmprin may correlate with better CVX-060 response. Using only Ang1, EGF, and Emmprin as a multi-protein signature yielded a predictive model with considerable accuracy in the prospective evaluation.
Materials & Methods
Tumor xenograft studies
Cell lines (ATCC, Manassas, VA; or TGEN, Scottsdale, AZ) were cultured as suggested by their commercial source to subconfluence, harvested with trypsin, and mixed 1:1 with Matrigel (BD Bioscience) immediately before implantation in the upper right flank of young adult female Nu-Foxn1 nu mice (Charles River Laboratories, Wilmington, MA; or Harlan, Indianapolis, IN) (except SKOV3 which was implanted in NOD.Cg-Prkdc scid Il2rgtm1Wjl/SzJ (NSG) mice). Patient-derived XG (PDX) models (OVX243 and OVX276) were passaged in vivo to achieve sufficient material for dosing studies, and were used for the CVX-060 efficacy study at 5-6 passages after collection from human tumor. Tumor explants were implanted in the upper right flank of young adult female Nu-Foxn1 nu mice. Tumor volume was measured twice weekly using the formula: Volume = (Length x Width2) x π/6. Subcutaneously inoculated tumors staged to the desired volume (average about 350 mm3) were randomized and dosed accordingly. Ten animals were used per group: Group 1 = vehicle (PBS), Group 2 = CVX-060 (produced at Pfizer, [22]) at 10 mg/kg, Group 3 = no treatment. Group 3 was allowed to grow to average of 500 mm3 before collecting for “pretreatment size” profiling. Group 1 and 2 were dosed intraperitoneally in a volume of 0.2 mL per mouse once per week (i.p. QW) until vehicle group mean reached ~2000 mm3 at which time all groups were terminated. Tumor growth inhibition (TGI) was determined as %TGI = (1-treatment growth/control growth) x 100 at termination.
Ethics statement
Tumor tissues for PDX studies were obtained from patients in Hebei Medical University Fourth Hospital through collaboration with Beijing Keluoen Translational Medicine Institute with approval by the Institutional Review Boards of the hospital and the written informed consent from patients. All animal procedures conducted in China at Crown Bioscience SPF facility were in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Crown Bioscience (Crown Bioscience IACUC Committee). All other animal experiments were conducted under the institutional guidelines of CovX/Pfizer, TGEN Drug Development, Jackson Laboratory, or Crown Bioscience’s Institutional Animal Care and Use Committee. CovX is an AALAC-accredited unit (#001442).
Tumor collection and sample preparation
Pretreatment size tumors were collected at ~500 mm3, immediately bisected and flash frozen and stored at -80°C until analysis. For nucleic acid extraction tumors were homogenized in FastPrep microhomogenizers with lysing matrix D (MP Biomedicals, Pasadena, CA) using 1 mL trizol/chloroform method (Invitrogen, Chicago IL), followed by isolation with DNeasy spin columns (Qiagen, Valencia, CA). Nucleic acid concentration was determined by UV, followed by dilution to equivalent levels and storage at -80°C until analysis. For protein extraction tumors were homogenized in FastPrep microhomogenizers with lysing matrix D using 1x protease/phosphatase inhibitor cocktail (HALT, Thermo Scientific, Waltham, MA), followed by addition of lysis buffer (Cell Signaling Technology, Beverly, MA) and incubation at 4°C for 1 hr. Lysates were clarified by centrifugation (14000 rpm, 10 min, 4°C) and supernatants were used directly for ELISAs.
Somatic mutation analysis
Mutation status for genes of interest was determined via comparison with published data where possible, and via mass spectrometry analysis of 238 somatic mutations across common oncogenes in genomic DNA from The OncoCarta Panel v1.0 (Sequenom, San Diego, CA) for confirmation.
Snp analysis
Single nucleotide polymorphism (SNP) status for the locations of interest was determined via qPCR of genomic DNA using specific primer/probes to the exact SNP locations (rs699947, rs833061, rs1570360, rs2010463, rs3025039, rs3814055, rs11549467, rs4073; Applied Biosystems, Carlsbad, CA). SNP haplotype was determined by observing relative Ct for each nucleotide possibility.
ELISA
Tumor lysate was analyzed for specific protein maker levels using ELISA kits predesigned and validated for each marker (R&D, Minneapolis, MN). Total protein concentration in the lysates was determined via BCA method (Thermo Scientific). Marker concentration in each individual sample was then normalized to the total protein concentration in the same sample for a ng marker/g total protein value. Values shown for each XG model represent the median concentration from 3-10 tumors per model for 88% of the analyses, the remaining 12% of the data employed less than 3 tumors per model/marker.
Data Analysis
Replicates of xenograft TGI and protein profiling experiments were summarized using median values and are available in Table S1. Comparison between TGI and linear baseline protein expression values was performed using the Pearson linear correlation coefficient implemented in R. The model for prediction of TGI values was constructed using BRB-ArrayTools v4.2.1 [25]. The linear protein expression values were then transformed into log2 values. To develop the classifier on the training data, we applied the quantitative trait prediction workflow in BRB-ArrayTools, using Least Angle Regression (LAR) [26] with default parameters: 0 % error threshold, no inclusion of 2 way interactions, and 10-fold cross-validation. The full LAR model with all parameters is available in Figure S1.
Results
CVX-060 induced TGI in “training set” xenograft models
In order to evaluate the commonality of CVX-060 efficacy a panel of 15 cell line and patient derived XG models were dosed with vehicle or CVX-060 at 10 mg/kg QW. To potentially limit variability between indications we focused on models from 3 tumor types; ovarian cancer, renal cell carcinoma, and colorectal cancer. Tumor growth curves and calculated tumor growth inhibition at termination revealed a wide range of CVX-060 responses (0-64% TGI, median = 27%), with no preference for tumor type (Figure S2 and Table 1). A cohort of pretreatment size tumors (500 mm3) were collected from each study for profiling in order to identify predictive biomarkers of CVX-060 affect. Correlations of somatic mutations, single nucleotide polymorphisms (SNPs), or protein level with CVX-060 induced TGI were then evaluated.
Table 1. CVX-060 induced tumor growth inhibition in “training set” xenograft models.
| model | type | median CVX-060 TGI% |
|---|---|---|
| OVCAR5 | ovarian | 0 |
| ES2 | ovarian | 11 |
| HeyC2 | ovarian | 21 |
| IGROV1 | ovarian | 23 |
| A2780 | ovarian | 32 |
| OVX276 | ovarian* | 38 |
| SKOV3 | ovarian | 49 |
| OV90 | ovarian | 51 |
| OVX243 | ovarian* | 61 |
| A498 | RCC | 22 |
| Caki1 | RCC | 26 |
| G401 | RCC | 32 |
| SN12CCP | RCC | 45 |
| HT29 | CRC | 27 |
| Colo205 | CRC | 64 |
patient-derived xenograft
Lack of correlation between TGI and common somatic mutations or angiogenesis-related SNPs
Somatic mutations in the VHL, SetD2, and VEGFR2 genes have been observed in human tumors and implicated in response to hypoxia and potentially anti-angiogenic compounds [27-34]. Numerous other more commonly mutated genes are also used to segregate patients and predict sensitivity to marketed drugs (BRAF, KRAS, EGFR, etc.) [35,36] and may play a role in general response to anti-angiogenic compounds. We therefore profiled for mutation status in a set of 11 genes and looked for enrichment of any of these mutations in the highly responsive or less responsive XG models. Mutation status did not correlate with TGI for any of the 11 genes evaluated (Table S2).
Several VEGF gene SNP haplotypes have been reported to correlate with particular anti-angiogenic response in retrospective analysis of clinical trials [37,38]. Additional SNPs in NR1/2, Hif1a, and IL8 have similarly been implicated in anti-angiogenic response in clinic [38]. We therefore profiled for SNP status at 8 discrete regions suggested from literature and looked for enrichment of any of these SNP haplotypes in the highly responsive or less responsive XG models. SNP status did not correlate with CVX-060 TGI for any of the 8 SNPs evaluated (Table S3). A similar analysis was performed with TGI values from a VEGF-targeting therapeutic which suggested correlation between TGI and several of these SNP haplotypes (data not shown), indicating potential for SNP predictive power in XGs.
Correlation between subset of pretreatment marker levels and CVX-060 effect
Levels of specific proteins in serum or tumor are commonly used as predictive or pharmacodynamic markers in clinic. A meta-analysis of literature on preclinical VEGF-therapy resistance mechanisms and correlates of clinical VEGF-therapy induced OS/PFS yielded a list of 22 markers of potential general relevance to anti-angiogenic compounds [2-4,39-43]. Additionally a differential gene expression analysis previously performed on Colo205XG tumors acutely treated with vehicle or CVX-060 identified additional pharmacodynamic markers potentially involved in CVX-060 response (data not shown). A list of all 27 markers evaluated is shown in Table 2.
Table 2. Pearson’s correlation of marker concentration as quantified by ELISA vs. Median CVX-060 TGI%.
| Protein | Pearson's R | p-value |
|---|---|---|
| EGFR | -0.55 | 0.07 |
| EMMPRIN | -0.50 | 0.06 |
| Angpt1 | -0.44 | 0.10 |
| EGF | -0.40 | 0.14 |
| cMET | -0.28 | 0.31 |
| FGF2 | -0.27 | 0.35 |
| Angplt4 | -0.26 | 0.35 |
| Axl | -0.22 | 0.43 |
| GCSF | -0.20 | 0.47 |
| MMP7 | -0.17 | 0.59 |
| THBS1 | -0.15 | 0.58 |
| MCP1 | -0.15 | 0.62 |
| PIGF | -0.14 | 0.63 |
| PDGFRb | -0.13 | 0.63 |
| huSDF1a | -0.13 | 0.69 |
| PDGFRa | -0.03 | 0.92 |
| VEGF | -0.03 | 0.93 |
| HGF | -0.01 | 0.98 |
| PROK1 | 0.03 | 0.92 |
| TGFa | 0.09 | 0.75 |
| IL8 | 0.11 | 0.73 |
| Hif1a | 0.18 | 0.59 |
| pmTOR | 0.19 | 0.53 |
| msSDF1a | 0.25 | 0.43 |
| PDGFbb | 0.31 | 0.35 |
| Angpt2 | 0.41 | 0.13 |
| Hif2a | 0.42 | 0.26 |
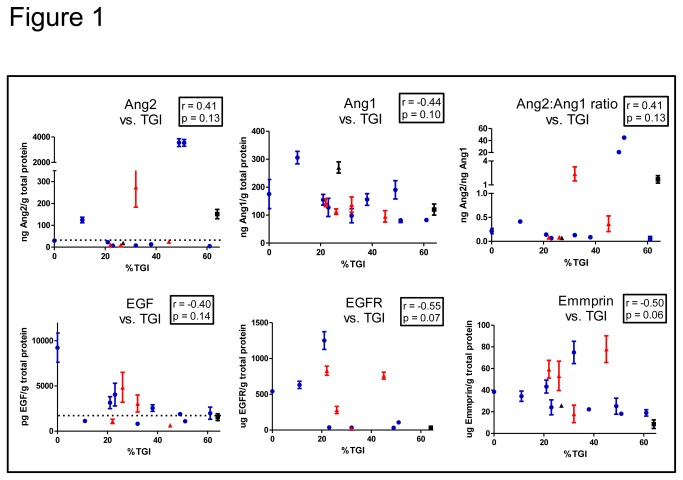
Pretreatment tumors from the “training set” XG models were subjected to ELISA to quantify the levels of each of the 27 protein markers of interest. Alignment of median marker level of each vs. median CVX-060 TGI revealed that higher Ang2 tracked with higher TGI (Pearson R = 0.41, n.s.), as predicted given this is the target of CVX-060. Similarly lower Ang1 (Pearson R = -0.44, n.s.), and hence higher Ang2:1 ratio (Pearson R = 0.41, n.s.), tracked with higher TGI. Finally lower EGF, EGFR, and Emmprin each tracked with higher TGI (Figure 1). These findings did not appear to be restricted to any one tumor type (e.g. ovarian, RCC, or CRC) as representative models from each type could be found along the curve in each graph.
Figure 1. Correlation between pretreatment marker levels and CVX-060 effect.
Representative examples from Table 2 are graphically shown to illustrate the relationship between marker level (mean +/- SEM) and median CVX-060 TGI (%) in training set models. Each dot represents a single XG or PDX model, color coded by tumor type: blue = ovarian, red = RCC, black = CRC. Dashed line indicates lower limit of quantification. r = Pearson’s coefficient, p = p-value.
Pearson’s R values and p-values of all measured proteins are listed in Table 2. Despite trending with response, none of the markers used independently were significantly associated with response at the significance level of 5% (p<0.05). However due to small sample size this might be expected or a multi-protein signature may be more useful. Setting a lower significance bar (p< 0.2) indicated that amongst all the markers evaluated the levels of Ang2, Ang1, EGF, EGFR, and Emmprin each had the greatest correlation with CVX-060 TGI. These markers were prospectively used to derive a new predictive model described in the next section.
Generation and testing of predictive marker model
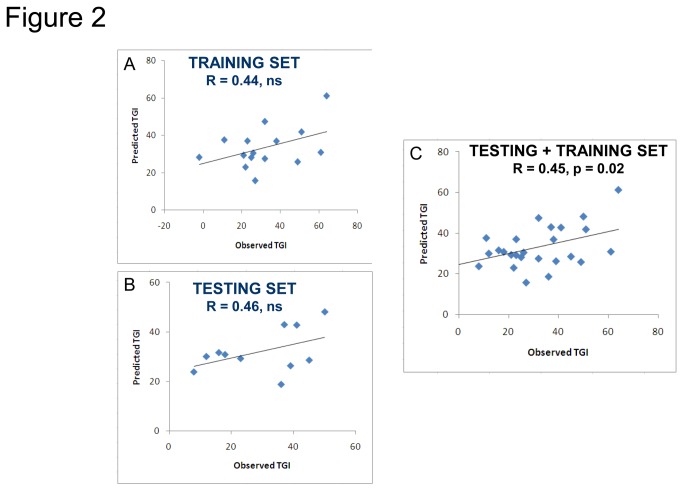
We have used Least Angle Regression (LAR) available in BRR-ArrayTools to develop a new multiprotein signature of response to CVX-060 in vivo. LAR is useful in predicting quantitative traits when the number of independent variable (genes or protein) is larger than the number of analyzed samples [25,26]. A model was constructed to identify the minimal number of markers necessary for predictive power using the 15 XG models from the training set and the 5 TGI-correlated proteins specified above. This exercise yielded a set of 3 markers: Ang1, EGF, and Emmprin. The LAR model composed of 3 markers was used to generate a predictive model that in the training set predicted TGI values correlated with observed TGI values (R = 0.44, n.s.) (Figure 2A).
Figure 2. A three-protein model for prediction of TGI.
Least Angle Regression (LAR) method identified 3 markers (Ang1, EGF, & Emmprin) sufficient to build a model to predict CVX-060 TGI. Model performance in the training set (A) and testing set (B) of xenograft lines is shown. Statistical significance was not achieved until combining both sets (C). Each dot represents the model predicted TGI% vs. median observed TGI% of a single xenograft line.
The ultimate test of any predictive biomarker/s is a prospective evaluation in new models. Towards this end we chose 11 additional XG models as a “testing set” (based on gene expression analysis identifying these models as possessing extreme levels of the key markers) and measured protein expression of the markers of interest in pretreatment size tumors. Protein levels of Ang1, EGF, and Emmprin were then used to predict TGIs, followed by running efficacy studies with CVX-060 in each model.
A comparison of predicted TGI values from the 3-protein regression model with the observed TGI values in the testing set XGs yielded comparable correlation to that seen in the training set XGs (R = 0.44, n.s., Figure 2B). While the correlation between predicted and observed TGIs was suggestive but not statistically significant in both sets, the combination of both sets yielded a significant correlation between predicted and observed values (R = 0.45, p = 0.02, Figure 2C). A comparison of predicted and observed TGIs indicated that 6/11 models were correctly predicted within 15% TGI (15% being used as a cutoff based on common experimental variability with TGI quantification) (Table 3). Taken together the prospective analysis indicated that the 3-protein set of Ang1, EGF, and Emmprin may have utility in predicting response to CVX-060.
Table 3. Prospective testing of the predictive marker hypothesis.
| model | type | Ang2 ng/g | Ang1 ng/g | VEGF ng/g | EGF ng/g | EGFR µg/g | Emmprin µg/g | Predicted TGI% | Observed TGI% | Prediction within 15% of observed? |
|---|---|---|---|---|---|---|---|---|---|---|
| U251 | GBM | 5 | 293 | 15719 | 5.8 | 26 | 99 | 19 | 36 | N |
| NCI-H441 | NSCLC | 20 | 91 | 2876 | 9.9 | 13 | 67 | 24 | 8 | N |
| D54MG | GBM | 5 | 14 | 607 | 3.8 | 3 | 102 | 26 | 39 | Y |
| NCI-H720 | NSCLC | 6907 | 266 | 305 | 2.9 | 14 | 34 | 29 | 45 | N |
| SKMEL1 | melanoma | 3948 | 483 | 30 | 11.1 | 3 | 21 | 29 | 23 | Y |
| A431 | melanoma | 41 | 56 | 3392 | 2.6 | 361 | 46 | 30 | 12 | N |
| NCI-H209 | SCLC | 1966 | 258 | 695 | 2.4 | 9 | 27 | 31 | 18 | Y |
| SHP-77 | SCLC | 3331 | 164 | 365 | 1.2 | 10 | 31 | 32 | 16 | N |
| MDA-MB435 | Breast/melanoma | 8 | 16 | 552 | 2.8 | 1 | 13 | 43 | 41 | Y |
| U87 | GBM | 6 | 14 | 263 | 0.8 | 3 | 17 | 43 | 37 | Y |
| A549 | NSCLC | 4 | 28 | 124 | 6.3 | 1 | 5 | 48 | 50 | Y |
Marker levels shown in median ng target/g total protein in untreated 500 mm3 tumors (n = 2-10), except for EGFR and Emmprin which are shown at µg/g levels. CVX-060 dosed and TGI% defined as in Table 1.
Discussion
Biomarkers predicting clinical antiangiogenic therapy response have proved elusive, yet are sorely needed due to lack of consistent response of the therapeutic class in any given oncological indication. While clearly not perfect mirrors of the clinical situation animal models of cancer are useful tools for building and validating predictive hypotheses, particularly for anti-angiogenic agents which usually only show (indirect) anti-tumor cell effect in vivo. Building and testing predictive hypotheses however often requires large number of patients/models and so the cost, time, and labor involved in doing this entirely with in vivo models is daunting and without rigor can yield no significant findings. Here we describe an attempt to address this problem and in doing so reveal a multi-protein signature that may be predictive of anti-Ang2 therapy response in animal models.
CVX-060 has been well tolerated and exhibited promising efficacy in a phase 1 trial (NCT00879684) [23,24]. As CVX-060 is the first clinical compound to specifically target Ang2 the potential for identifying biomarkers is entirely unknown. Indeed at this time there are no published reports of biomarkers for any angiopoietin targeting compounds. The results described here suggest that the levels of 3 specific tumor proteins (Ang1, EGF, Emmprin) taken together as a multi-expression signature may be predictive of anti-Ang2 therapy in XG models. As confirmation of the findings the direction of each markers correlation with CVX-060 response is intuitive: higher levels of the CVX-060 target Ang2 correlated with better response, and lower levels of all the other markers correlated with better response. Ang1 and Ang2 are thought to compete for binding to the signaling receptor Tie2, thus lower levels of Ang1 would allow Tie2 signaling to be predominantly driven by Ang2 hence pre-establishing a enhanced sensitivity to Ang2-therapy. EGF/EGFR and Emmprin are known to have roles in angiogenesis and VEGF-therapy response [44-49], and so could be considered as compensatory pathways to circumvent angiopoietin therapy-induced hypoxia.
The lack of statistical significance seen with the individual biomarkers in the training set, or the 3-marker signature in the testing set, may be explained by small sample size. Indeed the findings themselves are intuitive as suggested above, and the combination of training and testing sets allowed for statistical significance with the 3-marker signature. However, as statistical significance is lacking in the blinded analyses the conclusions presented here should be taken with caution and require further evaluation.
It is unclear if the predictive power of this multi-expression signature will hold true for other anti-angiopoietin compounds that are not as specific for Ang2 as CVX-060 [22]. In particular these results indicate that lower Ang1 levels would correlate with better CVX-060 affect, however compounds that target both Ang1 and Ang2 might theoretically work better with high levels of both targets.
All assays used here to detect somatic mutations, SNPs, and proteins were designed to be human specific in order to focus on variable provided by the implanted tumor, rather than the mouse host, and so can be described as being tumor derived rather than endothelial, stromal, or serum components. While this approach would not detect any of the myriad of supporting cell components involved in tuning angiogenesis/hypoxia detection we felt that a focused approach was needed in order to tease out any significant correlations within the complex choreography of angiogenesis. By examining only tumor expressed components in effect this study is designed to capture importance of target and Tie2-competitor ligands (Angiopoietins) and the tumors response to the downstream hypoxia induced by vascular collapse. In this study there is no evaluation of the intermediate step in CVX-060 therapy, the endothelial cell response to Ang2 deprivation, and indeed some evidence indicates that variation in the endothelial proteome can impact Ang2 function [10].
It should be noted that the work done here employed a large number of tumor types (multiple models of RCC, Ovarian, NSCLC, SCLC, GBM, and melanoma) and thus the multi-expression signature can be defined as a pan-tumor predictive model. Despite the fact that the training and testing cohorts employed different tumors types the correlation between predicted and observed TGI values was similar in the both sets. Typically one would desire to work with more homogeneous sets in biomarker work, in particular by working entirely within one tumor type, however due to requiring a minimal sample size for statistics this may not often be possible using XGs. By building our training set in RCC and Ovarian models and then switching to other tumor types for the validation set the bar for predictive performance was potentially higher. This suggests that the multi-expression signature we define here could be pan-tumor in nature and/or that enrichment in a specific tumor type could improve the predictive power of this model.
Beyond the results of this work an examination of the study design employed could be useful for guiding future biomarker discovery. To search for biomarkers we first ran a differential gene expression screen for acutely modified genes following CVX-060 treatment in a responsive XG model. Additionally we compiled a list of all biomarkers reported to play a role in VEGF-inhibitor response, since the key predictive markers may be difficult to find from unbiased, whole-genome approaches on a small set of samples [50]. This large set of biomarkers was used to run a number of different correlation analyses with CVX-060 response in a focused XG panel. Finally, a second XG panel was employed to prospectively evaluate the predictive power of the findings.
Whether the predictive markers identified here for CVX-060 will translate to clinic is unknown. Despite intensive preclinical and clinical analyses over the past decade with VEGF/VEGFR-targeting agents very few markers have correlated with efficacy, and contradicting findings have also been reported [2-4]. This may be a phenomenon of VEGF-axis therapies or anti-angiogenic therapies in general. Angiogenesis is a complex and dynamic process, with numerous growth factors and receptors involved, suggesting that efficacy of non-VEGF targeting agents (particularly those with different roles) might be governed by a unique set of markers. VEGFs role in angiogenesis is believed to be primarily in invasive capillary sprouting, while the angiopoietin family role is temporally and functionally different (vessel maturation/remodeling) [5-8]. In sum, identifying a simple predictive assay for anti-angiogenics may be a difficult task however there is hope for greater translational impact with angiopoietin biomarker work than has been seen with prior VEGF results.
Supporting Information
Least Angle Regression (LAR) code used for identifying predictive biomarkers. Full analysis settings and results are shown.
(PDF)
Tumor inhibition curves in training set XG models. Efficacy data used for table 1 TGI calculations is shown. Statistical difference between vehicle (PBS) and CVX-060 groups (*,**,*** = P <0.05, 0.01, or 0.001, respectively) determined by paired t-test.
(TIFF)
Data used for statistical analysis. Tab 1: Tumor growth inhibition (TGI) achieved with CVX-060 treatment (10 mg/kg IP, QW until study termination) for each cell line xenograft (XG) or patient derived xenograft (PDX) model. Data represents median of 10 animals per XG. Training Set represents the 15 XG/PDX models used for building the biomarker hypothesis, Testing Set represents the 11 XG models used for prospective analysis of the hypothesis.
Tab 1: Median concentration of each biomarker in each XG model, as determined by ELISA of tumor lysate (500 mm3 size tumors). Values shown are normalized to total protein concentration (BCA) and are shown in ug/g, ng/g, or pg/g.
Tab 2: Number of individual tumors analyzed by ELISA for each XG and biomarker.
(XLSX)
Lack of correlation between TGI and common somatic mutations. Common somatic mutations observed in cancer were queried in the XG models via comparison with COSMIC public database or via Sequenom OncoCarta v1.0 analysis of XG tumor lysate (500 mm3 tumors) and plotted against tumor growth inhibition (TGI). Blank box = no data available, wt = wild type.
(TIF)
Lack of correlation between TGI and anti-angiogenic related SNPs. Haplotypes for 8 single nucleotide polymorphisms (SNPs) potentially related to anti-angiogenic therapeutic response were detected in tumor lysate (500 mm3 tumors) from the XGs by qPCR and plotted against tumor growth inhibition (TGI). The SNPs evaluated here have been previously reported as correlating with bevacizumab37 and/or pazopanib response in RCC38. Blank box = no data available.
(TIF)
Acknowledgments
We thank Gary Woodnutt for expert discussion, and Zhou Zhu for computational assistance.
Funding Statement
This work was supported by Pfizer, Inc. All authors were employees of Pfizer at the time of this work and played roles in study design, data collection and analysis, decision to publish, and preparation of the manuscript.
References
- 1. Shojaei F (2012) Anti-angiogenesis therapy in cancer: Current challenges and future perspectives. Cancer Lett 320: 130-137. doi: 10.1016/j.canlet.2012.03.008. PubMed: 22425960. [DOI] [PubMed] [Google Scholar]
- 2. Gerger A, LaBonte M, Lenz HJ (2011) Molecular predictors of response to antiangiogenesis therapies. Cancer J 17: 134-141. doi: 10.1097/PPO.0b013e318212db3c. PubMed: 21427557. [DOI] [PubMed] [Google Scholar]
- 3. Jubb AM, Harris AL (2010) Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol 11: 1172-1183. doi: 10.1016/S1470-2045(10)70232-1. PubMed: 21126687. [DOI] [PubMed] [Google Scholar]
- 4. Jahangiri A, Aghi MK (2012) Biomarkers predicting tumor response and evasion to anti-angiogenic therapy. Biochim Biophys Acta 1825: 86-100. PubMed: 22067555. [DOI] [PubMed] [Google Scholar]
- 5. Folkman J (2003) Fundamental concepts of the angiogenic process. Curr Mol Med 3: 643-651. doi: 10.2174/1566524033479465. PubMed: 14601638. [DOI] [PubMed] [Google Scholar]
- 6. Marti HH (2005) Angiogenesis—a self adapting principle in hypoxia. EXS 94: 163-180. PubMed: 15617478. [DOI] [PubMed] [Google Scholar]
- 7. Thurston G (2003) Role of Angiopoiteins and Tie receptor tyrosine kinases in angiogenesis and lympangiogenesis. Cell Tissue Res 314: 61-68. doi: 10.1007/s00441-003-0749-6. PubMed: 12915980. [DOI] [PubMed] [Google Scholar]
- 8. Ferrara N (2000) Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res 55: 15-35. PubMed: 11036931. [PubMed] [Google Scholar]
- 9. Huang H, Bhat A, Woodnutt G, Lappe R (2010) Targeting the ANGPT2-TIE2 pathway in malignancy. Nat Rev Cancer 10: 575-585. doi: 10.1038/nrc2894. PubMed: 20651738. [DOI] [PubMed] [Google Scholar]
- 10. Felcht M, Luck R, Schering A, Seidel P, Srivastava K et al. (2012) Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest 122: 1991-2005. doi: 10.1172/JCI58832. PubMed: 22585576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koh YJ, Kim HZ, Hwang SI, Lee JE, Oh N et al. (2010) Double antiangiogenic protein, DAAP, targeting VEGF-A and angiopoietins in tumor angiogenesis, metastasis, and vascular leakage. Cancer Cell 18: 171-184. doi: 10.1016/j.ccr.2010.07.001. PubMed: 20708158. [DOI] [PubMed] [Google Scholar]
- 12. Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO (2001) Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem 276: 26516-26525. doi: 10.1074/jbc.M100282200. PubMed: 11346644. [DOI] [PubMed] [Google Scholar]
- 13. Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL et al. (1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87: 1161-1169. doi: 10.1016/S0092-8674(00)81812-7. PubMed: 8980223. [DOI] [PubMed] [Google Scholar]
- 14. Gaengel K, Genové G, Armulik A, Betsholtz C (2009) Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 29: 630-638. doi: 10.1161/ATVBAHA.107.161521. PubMed: 19164813. [DOI] [PubMed] [Google Scholar]
- 15. Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L et al. (2000) Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 6: 460-463. doi: 10.1038/74725. PubMed: 10742156. [DOI] [PubMed] [Google Scholar]
- 16. Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A et al. (2008) Angiopoietins assemble distinct Tie2 signaling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol 10: 527-537. doi: 10.1038/ncb1715. PubMed: 18425119. [DOI] [PubMed] [Google Scholar]
- 17. Fukuhara S, Sako K, Minami T, Noda K, Kim HZ et al. (2008) Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol 10: 513-526. doi: 10.1038/ncb1714. PubMed: 18425120. [DOI] [PubMed] [Google Scholar]
- 18. Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ et al. (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277: 55-60. doi: 10.1126/science.277.5322.55. PubMed: 9204896. [DOI] [PubMed] [Google Scholar]
- 19. Yuan HT, Khankin EV, Karumanchi SA, Parikh SM (2009) Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in endothelium. Mol Cell Biol 29: 2011-2022. doi: 10.1128/MCB.01472-08. PubMed: 19223473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim HZ, Jung K, Kim HM, Cheng Y, Koh GY (2009) A designed angiopoietin-2 variant, pentameric COMP-Ang2, strongly activates Tie2 receptor and stimulates angiogenesis. Biochim Biophys Acta 1793: 772-780. doi: 10.1016/j.bbamcr.2009.01.018. PubMed: 19339208. [DOI] [PubMed] [Google Scholar]
- 21. Kim I, Kim JH, Moon SO, Kwak HJ, Kim NG et al. (2000) Angiopoietin-2 at high concentrations can enhance endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Oncogene 19: 4549-4552. doi: 10.1038/sj.onc.1203800. PubMed: 11002428. [DOI] [PubMed] [Google Scholar]
- 22. Huang H, Lai JY, Do J, Liu D, Li L et al. (2011) Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration, and tumor growth. Clin Cancer Res 17: 1001-1011. doi: 10.1158/1078-0432.CCR-10-2317. PubMed: 21233403. [DOI] [PubMed] [Google Scholar]
- 23. Rosen LS, Mendelson DS, Cohen RB, Gordon MS, Goldman JW et al. (2010) First-in-human dose-escalation safety and PK trial of a novel intravenous humanized monoclonal CovX body inhibiting angiopoietin 2. J Clin Oncol 28 (suppl: 15s; abstr: [Google Scholar]
- 24. Rosen LS, Mendelson DS, Gordon MS, Goldman JW, Allen JW et al. (2012) Phase Ib safety trial of CVX-060, an intravenous humanized monoclonal CovX body inhibiting angiopoietin (Ang-2), with sunitinib. J Clin Oncol 30 (suppl. p. 2; abstr: 3032) [Google Scholar]
- 25. Simon R, Lam A, Li MC, Ngan M, Menenzes S et al. (2007) Analysis of gene expression data using BRB-Array Tools. Cancer INFORM 3: 11-17. PubMed: 19455231. [PMC free article] [PubMed] [Google Scholar]
- 26. Efron B, Hastie T, Johnstone I, Tibshirani R (2004) Least angle regression (with discussions). Ann Statist 32: 409–499. [Google Scholar]
- 27. Maxwell PH, Pugh CW, Ratcliffe PJ (2001) The pVHL-hIF-1 system. A key mediator of oxygen homeostasis. Adv Exp Med Biol 502: 365-376. doi: 10.1007/978-1-4757-3401-0_24. PubMed: 11950150. [DOI] [PubMed] [Google Scholar]
- 28. Choueiri TK, Vaziri SA, Jaeger E, Elson P, Wood L et al. (2008) von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol 180: 860-865. doi: 10.1016/j.juro.2008.05.015. PubMed: 18635227. [DOI] [PubMed] [Google Scholar]
- 29. Newbold RF, Mokbel K (2010) Evidence for a tumour suppressor function of SETD2 in human breast cancer: a new hypothesis. Anticancer Res 30: 3309-3311. PubMed: 20944102. [PubMed] [Google Scholar]
- 30. Duns G, van den Berg E, van Duivenbode I, Osinga J, Hollema H et al. (2010) Histone methyltransferase gene SETD2 is a novel tumor suppressor gene in clear cell renal cell carcinoma. Cancer Res 70: 4287-4291. doi: 10.1158/0008-5472.CAN-10-0120. PubMed: 20501857. [DOI] [PubMed] [Google Scholar]
- 31. Hu M, Sun XJ, Zhang YL, Kuang Y, Hu CQ et al. (2010) Histone H3 lysine 36 methyltransferase Hypb/Setd2 is required for embryonic vascular remodeling. Proc Natl Acad Sci U_S_A 107: 2956-2961. doi: 10.1073/pnas.0915033107. PubMed: 20133625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walter JW, North PE, Waner M, Mizeracki A, Blei F et al. (2002) Somatic mutation of vascular endothelial growth factors in juvenile hemangioma. Genes Chromosomes Cancer 33: 295-303. doi: 10.1002/gcc.10028. PubMed: 11807987. [DOI] [PubMed] [Google Scholar]
- 33. Jinnin M, Medici D, Park L, Limaye N, Liu Y et al. (2008) Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med 14: 1236-1246. doi: 10.1038/nm.1877. PubMed: 18931684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giatromanolaki A, Bai M, Margaritis D, Bourantas KL, Koukourakis MI et al. (2010) Hypoxia and activated VEGF/receptor pathway in multiple myeloma. Anticancer Res 30: 2831-2836. PubMed: 20683019. [PubMed] [Google Scholar]
- 35. Shigematsu H, Gazdar AF (2006) Somatic mutations of epidermal growth factor signaling pathway in lung cancers. Int J Cancer 118: 257-262. doi: 10.1002/ijc.21496. PubMed: 16231326. [DOI] [PubMed] [Google Scholar]
- 36. Lièvre A, Blons H, Laurent-Puig P (2010) Oncogenic mutations as predictive factors in colorectal cancer. Oncogene 29: 3033-3043. doi: 10.1038/onc.2010.89. PubMed: 20383189. [DOI] [PubMed] [Google Scholar]
- 37. Schneider BP, Wang M, Radovich M, Sledge GW, Badve S et al. (2008) Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol 26: 4672-4678. doi: 10.1200/JCO.2008.16.1612. PubMed: 18824714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu CF, Bing NX, Ball HA, Rajagopalan D, Sternberg CN et al. (2011) Pazopanib efficacy in renal cell carcinoma: evidence for predictive genetic markers in angiogenesis-related and exposure-related genes. J Clin Oncol 29: 2557-2564. doi: 10.1200/JCO.2010.32.9110. PubMed: 21576632. [DOI] [PubMed] [Google Scholar]
- 39. Bergers G, Hanahan D (2008) Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 8: 592-603. doi: 10.1038/nrc2442. PubMed: 18650835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Monnier J, Samson M (2010) Prokineticins in angiogenesis and cancer. Cancer Lett 296: 144-149. doi: 10.1016/j.canlet.2010.06.011. PubMed: 20633984. [DOI] [PubMed] [Google Scholar]
- 41. di Tomaso E, Snuderl M, Kamoun WS, Duda DG, Auluck PK et al. (2011) Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res 71: 19-28. doi: 10.1158/0008-5472.CAN-10-2602. PubMed: 21199795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hanrahan EO, Lin HY, Kim ES, Yan S, Du DZ et al. (2010) Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol 28: 193-201. doi: 10.1200/JCO.2009.22.4279. PubMed: 19949019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Newman JR, Helman EE, Safavy S, Zhang W, Rosenthal EL (2009) EMMPRIN expression is required for response to bevacizumab therapy in HNSCC xenografts. Cancer Lett 274: 313-318. doi: 10.1016/j.canlet.2008.09.033. PubMed: 18990485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bozec A, Fischel JL, Milano G (2006) Epidermal growth factor receptor/angiogenesis dual targeting: preclinical experience. Curr Opin Oncol 18: 330-334. doi: 10.1097/01.cco.0000228737.78003.06. PubMed: 16721126. [DOI] [PubMed] [Google Scholar]
- 45. Wu W, Onn A, Isobe T, Itasaka S, Langley RR et al. (2007) Targeted therapy of orthotopic human lung cancer by combined vascular endothelial growth factor and epidermal growth factor receptor signaling blockade. Mol Cancer Ther 6: 471-483. doi: 10.1158/1535-7163.MCT-06-0416. PubMed: 17308046. [DOI] [PubMed] [Google Scholar]
- 46. Herbst RS, Johnson DH, Mininberg E, Carbone DP, Henderson T et al. (2005) Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the Her-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol 23: 2544-2555. doi: 10.1200/JCO.2005.02.477. PubMed: 15753462. [DOI] [PubMed] [Google Scholar]
- 47. De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR et al. (2008) The role of the EGFR signaling in tumor microenvironment. J Cell Physiol 214: 559-567. doi: 10.1002/jcp.21260. PubMed: 17894407. [DOI] [PubMed] [Google Scholar]
- 48. Bougatef F, Quemener C, Kellouche S, Naïmi B, Podgorniak MP et al. (2009) EMMPRIN promotes angiogenesis through hypoxia-inducible factor-2alpha-mediated regulation of soluble VEGF isoforms and their receptor VEGFR-2. Blood 114: 5547-5556. doi: 10.1182/blood-2009-04-217380. PubMed: 19837976. [DOI] [PubMed] [Google Scholar]
- 49. Voigt H, Vetter-Kauczok CS, Schrama D, Hofmann UB, Becker JC et al. (2009) CD147 impacts angiogenesis and metastasis formation. Cancer Invest 27: 329-333. doi: 10.1080/07357900802392675. PubMed: 19160100. [DOI] [PubMed] [Google Scholar]
- 50. Pusztai L, Anderson K, Hess KR (2007) Pharmacogenomic predictor discovery in phase II clinical trials for breast cancer. Clin Cancer Res 13: 6080-6086. doi: 10.1158/1078-0432.CCR-07-0809. PubMed: 17947471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Least Angle Regression (LAR) code used for identifying predictive biomarkers. Full analysis settings and results are shown.
(PDF)
Tumor inhibition curves in training set XG models. Efficacy data used for table 1 TGI calculations is shown. Statistical difference between vehicle (PBS) and CVX-060 groups (*,**,*** = P <0.05, 0.01, or 0.001, respectively) determined by paired t-test.
(TIFF)
Data used for statistical analysis. Tab 1: Tumor growth inhibition (TGI) achieved with CVX-060 treatment (10 mg/kg IP, QW until study termination) for each cell line xenograft (XG) or patient derived xenograft (PDX) model. Data represents median of 10 animals per XG. Training Set represents the 15 XG/PDX models used for building the biomarker hypothesis, Testing Set represents the 11 XG models used for prospective analysis of the hypothesis.
Tab 1: Median concentration of each biomarker in each XG model, as determined by ELISA of tumor lysate (500 mm3 size tumors). Values shown are normalized to total protein concentration (BCA) and are shown in ug/g, ng/g, or pg/g.
Tab 2: Number of individual tumors analyzed by ELISA for each XG and biomarker.
(XLSX)
Lack of correlation between TGI and common somatic mutations. Common somatic mutations observed in cancer were queried in the XG models via comparison with COSMIC public database or via Sequenom OncoCarta v1.0 analysis of XG tumor lysate (500 mm3 tumors) and plotted against tumor growth inhibition (TGI). Blank box = no data available, wt = wild type.
(TIF)
Lack of correlation between TGI and anti-angiogenic related SNPs. Haplotypes for 8 single nucleotide polymorphisms (SNPs) potentially related to anti-angiogenic therapeutic response were detected in tumor lysate (500 mm3 tumors) from the XGs by qPCR and plotted against tumor growth inhibition (TGI). The SNPs evaluated here have been previously reported as correlating with bevacizumab37 and/or pazopanib response in RCC38. Blank box = no data available.
(TIF)