Abstract
Background
Several initiatives aim to reduce postoperative infection across a variety of surgical patients as a means to improve overall quality of care and reduce variation across centers. However, the association of infection rates with hospital-level outcomes and resource utilization has not been well described. We evaluated this association across a multicenter cohort undergoing congenital heart surgery.
Methods
The Society of Thoracic Surgeons Congenital Heart Surgery Database was linked to resource utilization data from the Pediatric Health Information Systems Database for hospitals participating in both (2006 to 2010). Hospital-level infection rates (sepsis, wound infection, mediastinitis, endocarditis, pneumonia) adjusted for patient risk factors and case mix were calculated using Bayesian methodology, and association with hospital mortality rates, postoperative length of stay (LOS), and total costs evaluated.
Results
The cohort included 32,856 patients (28 centers); 3.7% had a postoperative infection. Across hospitals, the adjusted infection rate varied from 0.9% to 9.8%. Hospitals with the highest infection rates had longer (LOS) (13.2 vs 11.7 days, p < 0.001) and increased hospital costs ($71,100 vs $65,100, p < 0.001), but similar mortality rates (odds ratio 0.99, 95% confidence interval 0.80 to 1.21, p = 0.9). The proportion of variation in costs and LOS explained by infection was 15% and 6%, respectively.
Conclusions
Infection after congenital heart surgery contributes to prolonged LOS and increased costs on a hospital level. However, given that infection rates alone explained relatively little of the variation in these outcomes across hospitals, further study is needed to identify additional factors that may be targeted in initiatives to reduce variation and improve outcomes across centers.
Previous studies have demonstrated that outcomes and hospital costs after congenital heart surgery are variable across centers [1–3]. However, the mechanisms underlying this variation are unclear. Several recent federal initiatives have aimed to reduce the occurrence of postoperative complications and morbidities, including postoperative infection, in patients undergoing a variety of surgical procedures as a means to improve overall quality of care and reduce resource utilization [4, 5].
In the congenital heart surgery population, it has been shown that patients with postoperative infection have higher morbidity and mortality. In the largest series, analysis of 30,078 operations in the Society of Thoracic Surgeons Congenital Heart Surgery (STS-CHS) Database from 2002 to 2006 showed that those with postoperative infection had higher in-hospital mortality and longer length of stay (LOS) when compared with those who did not have infection [6]. However, although postoperative infection is known to contribute to adverse outcomes on a patient level, the association of hospital-level infection rates with hospital performance, measured by outcomes such as survival or resource utilization, has not been well described. Thus, the extent to which differences in hospital infection rates may contribute to variability in outcomes such as mortality, LOS, and costs across hospitals performing congenital heart surgery is unclear [1–3].
This study utilizes linked clinical data from the STS-CHS Database and resource utilization data from Pediatric Health Information Systems (PHIS) Database to describe hospital infection rates across a large multicenter cohort undergoing congenital heart surgery, and association with hospital-level outcome measures including mortality, LOS, and costs.
Material and Methods
Data Source
De-identified data from the STS-CHS and PHIS databases were linked at the individual patient level using the method of “indirect identifiers” as previously described and verified [7–9]. The STS-CHS Database is the largest existing pediatric heart surgery registry, and collects data on all children undergoing heart surgery at greater than 100 participating North American centers. The PHIS Database is a large administrative database that collects information from the hospital bill from 41 US children’s hospitals. Linking these datasets enables us to capitalize upon the strengths of both datasets, including the detailed information captured in the STS-CHS Database for case ascertainment, risk adjustment, and capture of postoperative complications (including infection), and the resource utilization information collected in the PHIS Database [7]. This study was not considered human subjects research by the Duke Institutional Review Board in accordance with the Common Rule (45 CFR 46.102(f)).
Study Population
Hospitals participating in both the STS-CHS and PHIS Databases from 2006 to 2010 were eligible for inclusion (n = 33 hospitals). From these hospitals, children (0 to 18 years) undergoing any cardiovascular operation (with or without cardiopulmonary bypass) classified in the Society of Thoracic Surgeons-European Association for Cardiothoracic Surgery (STAT) risk stratification system were included (n = 44,198 patients) [10]. For the purposes of this analysis, those undergoing heart transplant (n = 676 patients) and those with preoperative sepsis or endocarditis (n = 429 patients) were excluded. Hospitals with greater than 15% missing data on key study variables (n = 5 hospitals) were excluded. This exclusion was applied, in part, in order to exclude hospitals who did not code complications, including infection, consistently. Finally, from the remaining hospitals, patients with missing LOS or postoperative complications data (n = 148 patients) were excluded.
Data Collection: STS-CHS Database
Data collected from the STS-CHS Database included patient demographics, preoperative risk factors, and noncardiac or genetic abnormalities (of note, this included the presence of DiGeorge syndrome/22q11 deletion), history of previous cardiothoracic surgery, primary procedure of the index (first) operation of the admission, use of delayed sternal closure, postoperative complications (including postoperative infection) and LOS, and in-hospital mortality [11]. Postoperative infection included sepsis, wound infection, mediastinitis, endocarditis, or pneumonia, as collected and defined in the STS-CHS Database [11].
Data Collection: PHIS Database
Resource utilization information collected from the PHIS Database included payer type, medication utilization (perioperative corticosteroids), and total hospital charges. Costs were estimated from charges using hospital- and department-specific cost or charge ratios, adjusted for regional differences using the Centers for Medicare and Medicaid Services price and wage index, and indexed to 2006 dollars.
Analysis
Preoperative, operative, and postoperative variables were described using standard summary statistics in the overall cohort, and in those with and without postoperative infection.
On a patient-level, the association of postoperative infection with outcome was first examined. This was done in order to examine the validity of our dataset before proceeding with any of the hospital-level analyses. Based on multiple previous studies, we expected to find that on a patient level, infection was associated with adverse outcomes [6, 12–15]. In the present analysis, the association of postoperative infection with outcome was evaluated using multivariable regression adjusting for age, weight, sex, prematurity, preoperative length of stay, any noncardiac or genetic abnormality, any STS-CHS preoperative risk factor, previous cardiothoracic surgery, payer type, STAT mortality category (category 1 = operations with lowest mortality risk, category 5 = operations with highest mortality risk), perioperative corticosteroids, use of delayed sternal closure, postoperative mechanical circulatory support, and year of surgery [10]. Logistic regression was used for the in-hospital mortality endpoint and negative binomial regression with log link for the continuous endpoints (postoperative LOS and total hospital costs). These models included hospital indicator variables to account for center effects. For the purposes of this analysis, LOS and hospital costs were evaluated in hospital survivors. In order to examine the potential impact of hospitals at the extremes of complication coding on our results, a sensitivity analysis was performed. In this analysis, approximately 20% (n = 6) of the hospitals at the extremes of rates of postoperative infection were excluded, and the analysis repeated to evaluate the sensitivity the results regarding the association of infection and outcome to inclusion or exclusion of these hospitals.
Next, in order to examine infection at the hospital level, Bayesian hierarchic models were used to calculate adjusted infection rates for each hospital. The models were adjusted for patient characteristics and case mix (as described above) in order to account for any differences across hospitals. This methodology also accounts for increased variability in outcomes from centers with smaller sample size and shrinks estimates from smaller centers toward the population average to provide more stable estimates [16]. Hospitals were categorized based on adjusted infection rate (top 25%, middle 50%, and bottom 25%). Outcomes (in-hospital mortality, LOS, and total hospital costs) across hospital infection groups were examined using logistic or negative binomial regression as described above, with the addition of group indicators. Adjusted outcomes for hospital infection groups were calculated by averaging the predicted rates of patients from these hospitals assuming they all had the given group’s estimated effect. Because those who die early may not be at risk of developing infection (and thus hospitals with more early deaths may appear to have lower infection rates), for this portion of the analysis we excluded those who died within 7 days of surgery. Finally, we estimated the amount of between-center variation in outcome explained by hospital infection rates by fitting another set of Bayesian hierarchic models with hospital infection rates in addition to patient risk factors. The estimated variances of the center level intercept of these models were compared with those from models without infection rate. The percentage reduction in estimated variance was used to quantify the amount of between-center variation explained by hospital infection rates. All analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC), R version 2.15 (R Foundation for Statistical Computing, Vienna, Austria), and WinBUGS version 1.4.3 (the BUGS project, Cambridge, UK). A p value less than 0.05 was considered statistically significant.
Results
Study Population
The study cohort included 32,856 patients from 28 centers. Patient characteristics overall and in those with and without infection are displayed in Table 1. The unadjusted rate of postoperative infection in the overall study population was 3.7%, and was highest in the following subgroups of patients: any noncardiac or genetic abnormality (5.3%), STAT categories 4–5 (7.5%), neonates (8.4%), and those with delayed sternal closure (11.5%). The types of infection are displayed in Table 2. Sepsis and wound infection were the most common.
Table 1.
Study Population Characteristics
| Variable | Overall (n = 32,856) | Without Infection (n = 31,631) | With Infection (n = 1,225) |
|---|---|---|---|
| Age, days | 207 (52–1,249) | 216 (61–1,295) | 31 (7–192) |
| Weight, kg | 6.6 (3.9–14.2) | 6.8 (4.0–14.5) | 3.8 (3.1–6.1) |
| Gender, male | 18,182 (55.3%) | 17,519 (55.4%) | 663 (54.1%) |
| Any non-CV/genetic abnormality | 9,234 (28.1%) | 8,741 (27.6%) | 493 (40.2%) |
| Any STS preop risk factor | 7,621 (23.2%) | 7,049 (22.3%) | 572 (46.7%) |
| Previous CT surgery | 9,888 (30.1%) | 9,570 (30.3%) | 318 (26.0%) |
| Preop LOS, days | 0 (0–3) | 0 (0–3) | 3 (0–8) |
| Payer | |||
| Government | 13,780 (41.9%) | 13,190 (41.7%) | 590 (48.2%) |
| Private | 12,436 (37.9%) | 12,032 (38.0%) | 404 (32.9%) |
| Other | 6,013 (18.2%) | 5,806 (18.3%) | 207 (16.9%) |
| STAT category | |||
| 1–3 | 23,897 (72.7%) | 23,342 (73.8%) | 555 (45.3%) |
| 4–5 | 8,959 (27.3%) | 8,289 (26.2%) | 670 (54.7%) |
| CPB (vs non-CPB) operation | 26,801 (81.6%) | 25,801 (81.6%) | 1,000 (81.6%) |
| Perioperative corticosteroids | 17,543 (53.4%) | 16,764 (53.0%) | 779 (63.6%) |
| Delayed sternal closure | 2,949 (9.0%) | 2,611 (8.3%) | 338 (27.6%) |
| Postop mechanical circulatory support | 594 (1.8%) | 488 (1.5%) | 106 (8.7%) |
Data are presented as median (interquartile range) or frequency (percent).
CPB = cardiopulmonary bypass; CT = cardiothoracic; CV = cardiovascular; LOS = length of stay; STS = Society of Thoracic Surgeons; STAT = Society of Thoracic Surgeons-European Association of Cardiothoracic Surgery.
Table 2.
Type of Postoperative Infection
| Infection | Overall Cohort (n = 32,856) | Those With Infection (n = 1,225)a |
|---|---|---|
| Sepsis | 628 (1.9%) | 628 (51.3%) |
| Wound infection | 430 (1.3%) | 430 (35.1%) |
| Pneumonia | 142 (0.4%) | 142 (11.6%) |
| Mediastinitis | 108 (0.3%) | 108 (8.8%) |
| Endocarditis | 33 (0.1%) | 33 (2.7%) |
Values sum to greater than 100% due to some patients having greater than 1 type of infection.
Association of Infection With Patient-Level Outcomes
Unadjusted outcomes in the overall study cohort included the following: an in-hospital mortality rate of 3.1%; median postoperative LOS of 6 days, interquartile range 4 to 11 days (mean LOS 11.8 ± 19.3 days); and median total hospital costs of $37,819, interquartile range $25,384 to $68,337 (mean hospital costs $65,257 ± $93,524). On a patient level, infection was associated with significantly increased in-hospital mortality, prolonged postoperative LOS, and increased total hospital costs [Table 3]. Similar results were observed when hospitals at the extremes of infection rates were excluded (as described in detail in Materials and Methods).
Table 3.
Association of Infection With Patient-Level Outcomes
| Outcome | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) | Adjusted Estimate Without Infection | Adjusted Estimate With Infection | p Value |
|---|---|---|---|---|---|
| In-hospital mortality | 6.1 (5.1–7.3) | 2.8 (2.2–3.6) | 2.8% | 6.0% | <0.0001 |
|
| |||||
| Unadjusted Ratio (95% CI) | Adjusted Ratio (85% CI) | Adjusted Estimate Without Infection | Adjusted Estimate With Infection | p Value | |
|
| |||||
| Length of stay | 3.6 (3.4–3.9) | 2.3 (2.1–2.4) | 11.2 days | 25.5 days | <0.0001 |
| Hospital costs | 2.9 (2.7–3.1) | 1.8 (1.7–2.0) | $63,300 | $115,800 | <0.0001 |
For dichotomous variables (mortality), unadjusted and adjusted odds ratios and 95% confidence intervals are presented. For continuous variables, the ratio of length of stay or hospital costs in those with and without infection, and 95% confidence interval, is presented. The p value presented refers to the adjusted data.
CI = confidence interval; LOS = length of stay.
Association of Infection With Hospital-Level Outcome Measures
Across hospitals, the unadjusted infection rate ranged from 0.9% to 9.5%. The infection rate adjusted for patient characteristics and case mix varied from 0.9% to 9.8% (Fig 1). When hospitals were ranked according to adjusted infection rates (Fig 2A–C), those with the highest rates had significantly longer LOS and higher hospital costs, but similar mortality rates, as displayed in Table 4. The proportion of variation in total hospital costs and LOS explained by differences in postoperative infection was 15% and 6%, respectively.
Fig 1.
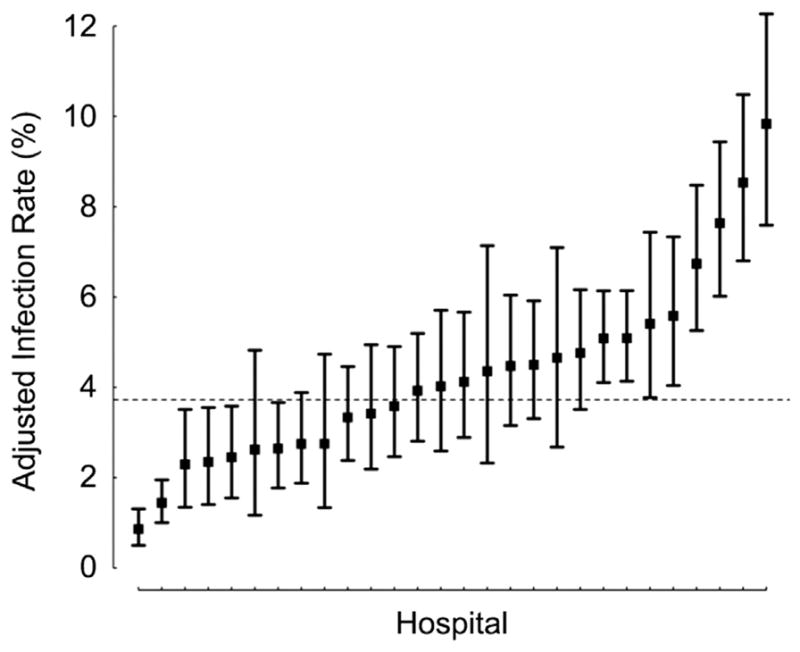
Adjusted infection rate across hospitals. (Boxes = adjusted estimates for each hospital; lines = 95% Bayesian probability intervals; dashed line = infection rate in overall cohort.)
Fig 2.
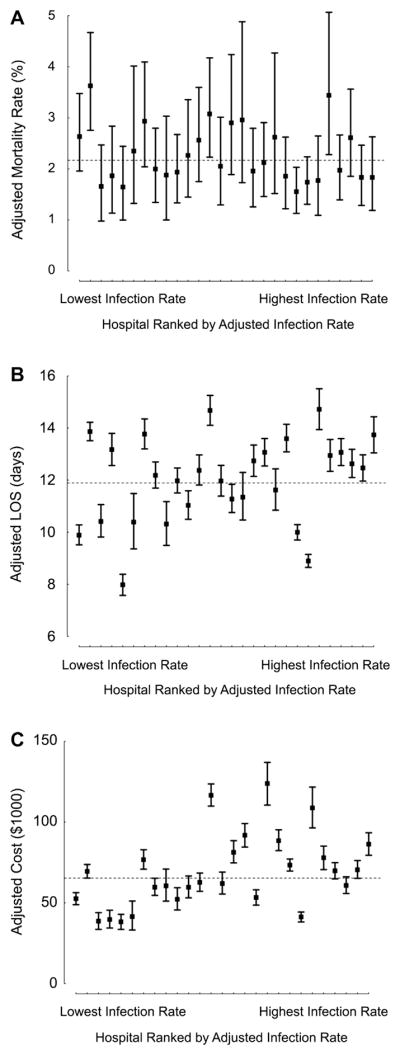
Adjusted outcomes across hospitals ranked by infection rate. (A) Mortality; (B) postoperative LOS; (C) total hospital costs. See Table 2 for corresponding numeric data regarding adjusted outcomes across hospital infection groups. Of note, in the hospital-level analyses, patients who died within 7 days of surgery were excluded as described in Materials and Methods. (Boxes = adjusted estimates for each hospital; lines = 95% Bayesian probability intervals; dashed line = mean value in overall cohort; LOS = length of stay.)
Table 4.
Association of Infection With Hospital-Level Outcome Measures
| Hospital Infection Group | Outcome | p Value | Adjusted Estimate |
|---|---|---|---|
| Mortality Odds ratio (95% CI) |
|||
| High Middle/Low |
0.99 (0.80–1.21) Reference |
0.89 | 2.2% 2.2% |
| LOS Ratio (95% CI) |
|||
| High Middle/Low |
1.12 (1.10–1.15) Reference |
<0.001 | 13.2 days 11.7 days |
| Cost Ratio (95% CI) |
|||
| High Middle/Low |
1.09 (1.07–1.11) Reference |
<0.001 | $71,100 $65,100 |
For dichotomous variables (mortality), adjusted odds ratios and 95% confidence intervals are presented. For continuous variables, the ratio of LOS or hospital costs in those with and without infection, and 95% confidence interval, is presented.
CI = confidence interval; LOS = length of stay.
Comment
In this multi-institutional analysis, we found that postoperative infection rates after congenital heart surgery varied widely across hospitals. Hospitals with higher infection rates had significantly longer LOS and increased costs. However, the magnitude of these differences was relatively small, with infection rates alone explaining only 15% of the total variation in costs and 6% of the total variation in LOS across hospitals.
This study supports the results of previous analyses which have shown that postoperative infection after congenital heart surgery is associated with increased morbidity and mortality on a patient level [6, 12–15]. However, prior studies did not examine the relationship between infection and hospital-level outcome measures. Our group and others have shown that many of these measures, including mortality, LOS, and hospital costs vary across institutions performing congenital heart surgery [1–3]. It was not known whether differences in infection rates contribute to the variation in these measures across centers [4, 5].
In the present analysis we found that differences in hospital infection rates alone were not associated with differences in hospital mortality rates. Given the wide variety of operative and perioperative factors that may influence survival, this finding is not surprising. Recent studies in the adult literature have demonstrated similar findings, in that metrics reflecting certain intermediate aspects of perioperative care and quality that may have some association with outcome on a patient level do not necessarily correlate well with differences in survival or other outcomes when measured on a hospital level [17, 18]. It has been hypothesized that this may be due to the relatively rarity of these intermediate events or their weak association with the outcome of interest [17, 18]. Our results are also consistent with previous analyses in the congenital heart surgery population, as well as in those undergoing other surgical procedures, which have shown that postoperative complication rates in general do not appear to explain differences in performance across hospitals (as measured by mortality rate) [19, 20]. Rather, it is the rate of death in patients who develop a complication (the “failure to rescue rate”) that appears to be more important [19, 20]. There are ongoing studies in the congenital heart surgery population further investigating this concept.
In contrast to our findings regarding mortality, we found that hospitals with higher infection rates did have significantly longer LOS and higher hospital costs. Previous studies have suggested that LOS is one of the primary drivers of hospital costs; thus it follows that factors which influence LOS will also have an impact on costs [3]. While our data support that reducing postoperative infection may be 1 potential mechanism to reduce resource utilization, it is also important to note that we found that the proportion of total variation in hospital costs and LOS explained by differences in postoperative infection was relatively small (15% and 6%, respectively). Therefore, there are likely other factors that may play a more important role and require further study.
Finally, this study highlights the advantages of linking administrative and clinical datasets together. While administrative datasets contain valuable resource utilization information, it has been shown that their accuracy regarding case ascertainment, risk adjustment, and outcomes assessment can be limited in comparison with clinical registry data [21, 22]. Combining these datasets together optimizes the strengths and minimizes the weaknesses of each, and allows analyses not possible with either dataset alone. Specifically, in this study we were able to utilize the information regarding postoperative infection collected in the STS-CHS Database, and relate this to resource utilization data collected in the PHIS Database.
Limitations
The limitations of this study are primarily related to the nature of the datasets utilized in this analysis. While there are standard definitions for each of the postoperative complications collected in the STS Database, it may be possible that there is variation in how these are coded across hospitals. However, our findings of a significant association of infection with morbidity and mortality on a patient level similar to that demonstrated by previous studies, and consistent findings when centers at the extremes of the distribution of coding of infection were excluded, support the validity of the data. In this study, we were limited to evaluating infections captured in the STS Database. For example, the database currently does not collect information on urinary tract infections. We were also unable to evaluate infection occurring after hospital discharge, as the datasets utilized primarily collect in-hospital data. Future evaluation of readmission data from these datasets could allow analysis of later infection requiring rehospitalization. Our study was limited to the hospitals submitting data to both the STS and PHIS databases, and it is possible the extent of variation in infection or other variables may be greater on a national level. Finally, a complete understanding of mechanisms underlying variation in outcome and resource utilization across centers will require future analyses of factors in addition to postoperative infection.
Conclusions
This study suggests that initiatives aimed at reducing infection after congenital heart surgery may play a role in reducing variation in LOS and costs across centers. However, given that infection rates alone explained relatively little of the total variation in these outcomes across hospitals, further study is needed to identify other factors in addition to postoperative infection which may be targeted in quality improvement initiatives.
Acknowledgments
The following authors have received support: National Heart, Lung, and Blood Institute (1K08HL103631, principal investigator (PI): Pasquali; 1RC1HL099941, co-PIs: J Jacobs, Li); Dr Shah, National Institute of Allergy and Infectious Diseases (K01AI73729) and Robert Wood Johnson Foundation Physician Faculty Scholar program.
Footnotes
Presented at the Forty-ninth Annual Meeting of The Society of Thoracic Surgeons, Los Angeles, CA, Jan 26–30, 2013.
References
- 1.Jacobs JP, O’Brien SM, Pasquali SK, et al. Variation in outcomes for benchmark operations: an analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg. 2011;92:2184–92. doi: 10.1016/j.athoracsur.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs JP, O’Brien SM, Pasquali SK, et al. Variation in outcomes for risk-stratified pediatric cardiac surgical operations: an analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg. 2012;94:564–71. doi: 10.1016/j.athoracsur.2012.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasquali SK, Sun JL, d’Almada P, et al. Center variation in hospital costs for patients undergoing congenital heart surgery. Circ Cardiovasc Qual Outcomes. 2011;1:306–12. doi: 10.1161/CIRCOUTCOMES.110.958959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. [Accessed December 6, 2012];CDC recommendations to prevent healthcare-associated infections. Available at: http://www.cdc.gov/HAI/prevent/top-cdc-recs-prevent-hai.html.
- 5. [Accessed December 6, 2012];Partnership for patients: Better care, lower costs. Available at: http://www.healthcare.gov/compare/partnership-for-patients/
- 6.Barker GM, O’Brien SN, Welke KF, et al. Major infection after pediatric cardiac surgery: a risk estimation model. Ann Thorac Surg. 2010;89:843–50. doi: 10.1016/j.athoracsur.2009.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasquali SK, Jacobs JP, Shook GJ, et al. Linking clinical registry data with administrative data using indirect identifiers: implementation and validation in the congenital heart surgery population. Am Heart J. 2010;160:1099–104. doi: 10.1016/j.ahj.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquali SK, Li JS, He X, et al. Comparative analysis of antifibrinolytic medications in pediatric heart surgery. J Thorac Cardiovasc Surg. 2012;143:550–7. doi: 10.1016/j.jtcvs.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasquali SK, Li JS, He X, et al. Perioperative methylprednisolone and outcome in neonates undergoing heart surgery. Pediatrics. 2012;129:e385–91. doi: 10.1542/peds.2011-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien SM, Clarke DR, Jacobs JP, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–53. doi: 10.1016/j.jtcvs.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 11. [Accessed December 6, 2012];STS Congenital Database Full Specifications. Available at http://www.sts.org/sites/default/files/documents/pdf/Congenital_DataSpecs_250.pdf.
- 12.Shah SS, Kagen J, Lautenbach E, et al. Bloodstream infections after median sternotomy at a children’s hospital. J Thorac Cardiovasc Surg. 2007;133:435–40. doi: 10.1016/j.jtcvs.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Long CB, Shah SS, Lautenbach E, et al. Postoperative mediastinitis in children: epidemiology, microbiology and risk factors for gram-negative pathogens. Pediatr Infect Dis J. 2005;24:315–9. doi: 10.1097/01.inf.0000157205.31624.ed. [DOI] [PubMed] [Google Scholar]
- 14.Holzmann-Pazgal G, Hopkins-Broyles D, Recktenwald A, et al. Case-control study of pediatric cardiothoracic surgical site infections. Infect Control Hosp Epidemiol. 2008;29:76–9. doi: 10.1086/524323. [DOI] [PubMed] [Google Scholar]
- 15.Levy I, Ovadia B, Erez E, et al. Nosocomial infections after cardiac surgery in infants and children: incidence and risk factors. J Hosp Infect. 2003;53:111–6. doi: 10.1053/jhin.2002.1359. [DOI] [PubMed] [Google Scholar]
- 16.Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: The importance of reliability adjustment. Health Serv Res. 2010;45(6 Pt 1):1614–29. doi: 10.1111/j.1475-6773.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong SL, Ji J, Hollenbeck BK, Morris AM, Baser O, Birkmeyer JD. Hospital lymph node examination rates and survival after resection for colon cancer. JAMA. 2007;298:2149–54. doi: 10.1001/jama.298.18.2149. [DOI] [PubMed] [Google Scholar]
- 18.Nicholas LH, Osborne NH, Birkmeyer JD, Dimick JB. Hospital process compliance and surgical outcomes in medicare beneficiaries. Arch Surg. 2010;145:999–1004. doi: 10.1001/archsurg.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasquali SK, He X, Jacobs JP, Jacobs ML, O’Brien SM, Gaynor JW. Evaluation of failure to rescue as a quality metric in pediatric heart surgery. Ann Thorac Surg. 2012;94:573–9. doi: 10.1016/j.athoracsur.2012.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. New Engl J Med. 2009;361:1368, 75. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 21.Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256:973–81. doi: 10.1097/SLA.0b013e31826b4c4f. [DOI] [PubMed] [Google Scholar]
- 22.Pasquali SK, Peterson ED, Jacobs JP, et al. Differential case ascertainment in clinical registry vs. administrative data and impact on outcomes assessment in pediatric heart surgery. Ann Thorac Surg. 2012;95:197–203. doi: 10.1016/j.athoracsur.2012.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]


