Abstract
In Drosophila, the non-LTR retrotransposons HeT-A, TART and TAHRE build a head-to-tail array of repetitions that constitute the telomere domain by targeted transposition at the end of the chromosome whenever needed. As a consequence, Drosophila telomeres have the peculiarity to harbor the genes in charge of telomere elongation. Understanding telomere expression is important in Drosophila since telomere homeostasis depends in part on the expression of this genomic compartment. We have recently shown that the essential kinase JIL-1 is the first positive regulator of the telomere retrotransposons. JIL-1 mediates chromatin changes at the promoter of the HeT-A retrotransposon that are necessary to obtain wild type levels of expression of these telomere transposons. With the present study, we show how JIL-1 is also needed for the expression of a reporter gene embedded in the telomere domain. Our analysis, using different reporter lines from the telomere and subtelomere domains of different chromosomes, indicates that JIL-1 likely acts protecting the telomere domain from the spreading of repressive chromatin from the adjacent subtelomere domain. Moreover, the analysis of the 4R telomere suggests a slightly different chromatin structure at this telomere. In summary, our results strongly suggest that the action of JIL-1 depends on which telomere domain, which chromosome and which promoter is embedded in the telomere chromatin.
Introduction
Drosophila telomeres are the best-studied telomerase exception to date. The transposition of three special non-LTR retrotransposons; HeT-A, TART and TAHRE (HTT), buffers the receding chromosome ends when needed [1,2]. Therefore, in Drosophila the genes responsible for telomere elongation are embedded in the telomere chromatin and to understand telomere regulation in this organism it is necessary to understand gene expression from this genomic compartment.
Drosophila telomeres are organized in two different domains; the cap domain protecting the very end of the chromosome, and the telomere domain composed of the HTT array. Additionally, immediately adjacent to the HTT array exists the subtelomere domain, composed of the Telomere Associated Sequences (TAS) [3-5]. Although the subtelomere domain is not strictly part of the telomere, its closeness to the HTT array influences some of its functions.
Previous studies, based on the variegation of reporter genes, suggested that TAS sequences nucleate a compacted chromatin restrictive of gene expression at the telomeres, causing a telomere position effect (TPE) [6]. Later, molecular studies detected the presence of chromatin marks typical of repressive and compacted chromatin in TAS, such as H3K27me3 and Polycomb [3,4,6,7].
We aimed to better understand the regulation of gene expression in Drosophila telomeres. With this objective we assayed the influence of the JIL-1 kinase over Drosophila telomere and subtelomere domains. In Drosophila, JIL-1 is an essential gene responsible for the phosphorylation of the Ser10 of Histone H3 when cells are in interphase or when a stress response is needed [8-10]. Besides the telomeres, JIL-1 localizes at the interband region of polytene chromosomes and has been shown to be involved in maintaining chromosome structure and in protecting genomic regions from an excess of heterochromatinization [11-15]. JIL-1 has been found to localize among other genomic locations, at the HTT array and to be the first positive regulator of the expression of the telomeric retrotransposons [3,16]. In this study, we report that JIL-1 protects the telomere domain from the spreading of repressive chromatin from the flanking subtelomere domain and that JIL-1 action depends on which telomere domain, which chromosome and which promoter is embedded in the telomere chromatin.
Results
In order to assess the possible role of JIL-1 regulating gene expression at the HTT array, we chose several lines that contain a mini-white reporter gene at the telomere domain (HTT array), or at the subtelomere domain (TAS sequences) from different chromosomes [6,17,18]. The mini-white gene from the chosen reporter lines is expressed from the same basic promoter, which does not include the eye-specific enhancers (see references above). All flies in this study are in a white minus background (w-, white eyes). The amount of red color that is observed in the eyes of the reporter lines (EY08176, EY00453, EY09966, 39C-27, 39C-5, 39C-72 and 118E-5), depends on where in the chromosome the mini-white gene has been inserted. In order to evaluate the effect of the different mutations assayed in this study over the reporter lines, we have compared the descendants of the crosses with the JIL-1 and the Su(var)2-5 mutant flies (also in a w- background and with white eyes) with the descendants resulting from crossing the flies containing the reporter lines with a w 1118 strain (white eyes). This control cross is necessary in order to be able to evaluate the presence of the reporter gene in heterozygous state. We search for changes in eye color revealing a TPE effect.
JIL-1 is necessary for gene expression at the telomere domain (HTT)
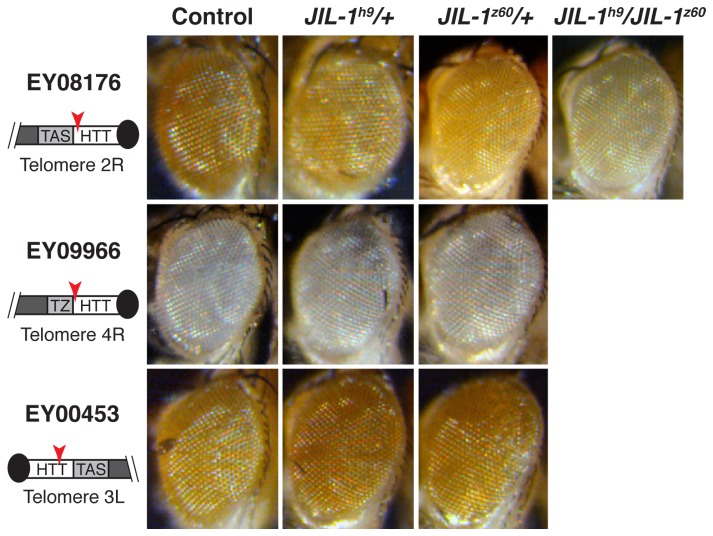
By crossing flies containing mutant alleles of JIL-1 with lines with a mini-white gene inserted at the HTT array from different chromosomes, we screened for TPE effects in the eyes of the first generation (Figure 1). In simple heterozygous combinations of JIL-1 alleles (Figure 1, 2nd and 3rd columns), we observed subtle enhancer position effect variegation in the telomere line EY08176 (1st row) and no effect with the telomere line EY00453 (3rd row). We did not observe any effect in the EY09966 line corresponding to the 4R telomere, likely because the reporter gene is already heavily repressed in this line (2nd row). Nevertheless, when we combined both JIL-1 alleles in a trans-heterozygous mutant (JIL-1z60/JIL-1 h9) with the line EY08176, a clear enhancer effect of TPE was revealed (1st row, 4th column).
Figure 1. JIL-1 affects the expression of genes embedded in the HTT array.
For all crosses, eyes from F1 males are shown. 1st column: control: F1 male descendants from crossing w 1118 females with male flies with the indicated reporter line, 2nd and 3rd columns: results from the crosses of the JIL-1 heterozygous, JIL-1 z60/+ or JIL-1 h9 /+, with the indicated reporter line. 4th column: Result from crossing the JIL-1z60/JIL-1 h9 transheterozygous allele with the indicated mini-white insertion. The schematic drawings on the left indicate the position of the insertion (red arrow head) of the mini-white reporter gene in each of the reporter lines. Black circle represents telomere cap, white rectangle the telomeric retrotransposon array (HTT), light grey rectangle the subtelomeric domain TAS, telomere associated sequences, or TZ, transition zone, and dark grey rectangle represents the chromosome arm. EY08176 for the 2R telomere (1st row), EY09966 for the 4R telomere (2nd row), and EY00453 for the 3L telomere (3rd row). Note that descendants from the JIL-1 heterozygous, JIL-1 z60/+ or JIL-1 h9 /+, show a subtle enhancer effect in the telomere line EY08176 (1st row) and no effect with the telomere line EY00453 (3rd row). We did not observe any effect in the EY09966 line corresponding to the 4R telomere, likely because the reporter gene is already heavily repressed in this line (2nd row). When the JIL-1z60/JIL-1 h9 transheterozygous allele is assayed with the EY08176 reporter line, an enhancer effect of TPE is observed (4th column).
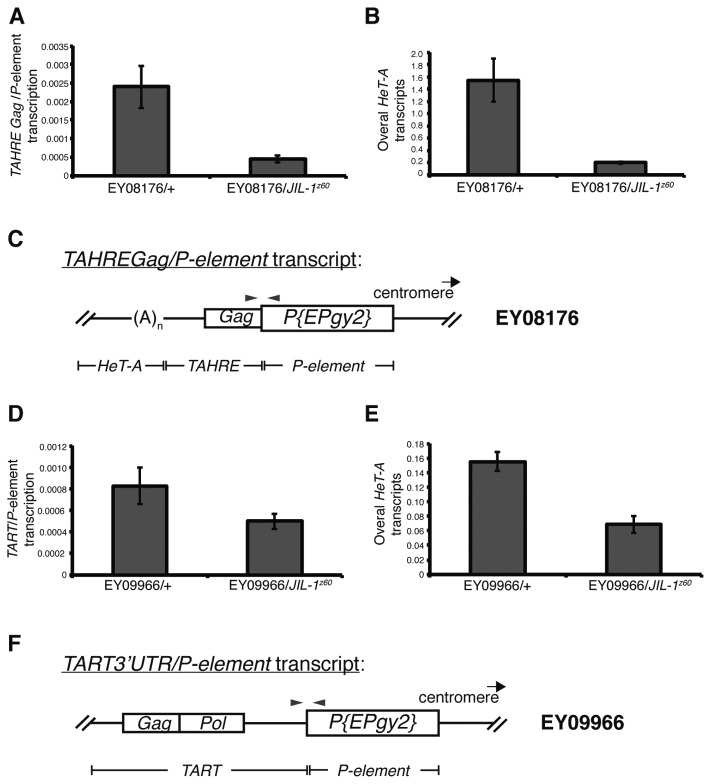
Following, we investigated if, in comparison with the expression in the wild type situation of the reporter line, a decrease in the expression of the HTT retroelements upstream to the mini-white insertion was observed in JIL-1 mutants. Quantitative real-time PCR was used to detect expression of the transcript containing both, the telomeric element and the mini-white reporter (Figure 2A and D). When compared with the expression detected in the reporter in a wild type background, JIL-1 heterozygous mutants show lower telomere transcription from the HTT element adjacent to the insertion in the 2nd and in the 4th chromosomes (EY08176, Figure 2A and D) (EY09966, Figure 2B and F). This reduction is in agreement with a reduction of the overall expression of HeT-A in the same reporter lines (Figure 2B, 2D and [16]) suggesting a global role of JIL-1 over the HTT array.
Figure 2. Quantification of the decrease in telomere expression in JIL-1 mutants.
JIL-1 mutations lead to a decrease in the transcription of the telomeric retrotransposon immediately downstream of the P-element insertion (A and D), as well as in the overall HeT-A transcripts (B and E). Primers used to quantify overall HeT-A transcripts anneal at the 3’UTR region of the element (see M&M for more details). (C and F) correspond to a schematic representation of the telomeric retrotransposon/P-element transcript analyzed by quantitative real-time PCR in A and D, respectively. Primers shown with grey arrowheads indicate the fragment amplified and quantified.
In addition, we assayed the possible effect of flies containing mutant alleles of the Su(var)2-5 gene (HP1a), over the reporter lines inserted at the HTT array (Figure 3). Interestingly, only a subtle change in mini-white expression was detected when we assayed the Su(var)2-5 05 mutation over the lines EY08176, EY09966 and EY00453 (Figure 3 and [18]).
Figure 3. Effect of HP1 over genes embedded at the HTT array.
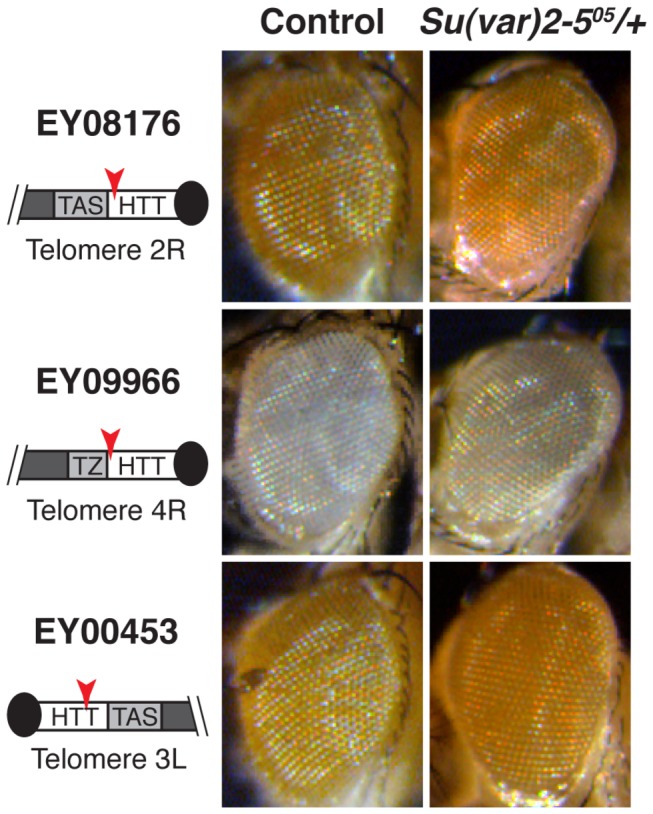
Su(var)2-5 05, a HP1a mutant allele, was assayed with the same reporter lines from Figure 1. Although it has been demonstrated that Su(var)2-5 05 shows a strong de-repression of HeT-A transcription (see main text), no substantial change in the expression of the mini-white reporter is detected in the telomeric lines (1st, 2nd and 3rd rows). As in Figure 1, schematic drawings on the left. Black circle represents telomere cap, white rectangle the telomeric retrotransposon array (HTT), light grey rectangle the subtelomeric domain TAS, telomere associated sequences, or TZ, transition zone, and dark grey rectangle represents the chromosome arm.
JIL-1 acts as a suppressor of TPE over the TAS domain
JIL-1 z60/+ and JIL-1 h9/+ heterozygous alleles did not produce an appreciable effect over the mini-white inserted at the TAS domain of chromosomes 2R (39C-27) and 2L (39C-5) (Figure 4A, 2nd and 3rd columns). Nevertheless, when the dose of JIL-1 was lowered in a trans-heterozygous combination, a significant increase in the mini-white expression was observed when compared with the control cross (Figure 4A 1st column), revealing a suppressor effect of JIL-1 over the TAS domain of these two telomeres (Figure 4A, 4th column). As expected, we did not observe any change in mini-white expression when the TAS lines (39C-27 and 39C-5) were combined with the HP1a allele Su(var)2-5 05 (Figure 4A, 5th column) [7].
Figure 4. JIL-1 act as suppressor of TPE over the TAS domain.
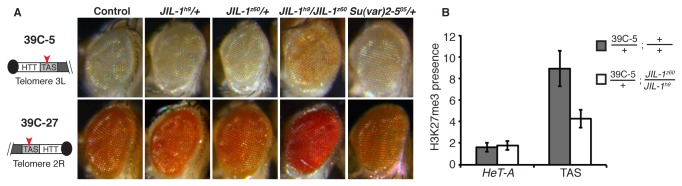
(A) TPE assays in the TAS domain were performed with the reporter lines 39C-5 (chromosome 2L, TAS-L) and 39C-27 (chromosome 2R, TAS-R). No effect on the mini-white gene expression was observed when the reporter lines were crossed with the JIL-1 heterozygous mutants JIL-1 z60/+ and JIL-1 h9 /+ (2nd and 3rd column). However, when the same lines were crossed with the trans-heterozygous mutant allele (JIL-1z60/JIL-1 h9), a clear increase in the eye color was observed revealing a suppressor effect of JIL-1 over the TAS domain (4th column). 5th column: control cross of the TAS reporter lines with the Su(var)2-5 05, a HP1a mutant allele, does not show any differences in the expression of the mini-white gene inserted in TAS as expected by the literature (see main text). (B) Chromatin immunoprecipitation (ChIP) experiments reveal that the JIL-1 trans-heterozygous mutant allele (JIL-1z60/JIL-1 h9) lead to a decrease in the presence of the H3K27me3 mark at the TAS domain. Three independent ChIP samples were analyzed and the amount of immunoprecipitated DNA was calculated by three independent quantitative real-time PCRs. Specific primers were used to quantify the amount of TAS or HeT-A DNA immunoprecipitated by the H3K27me3 antibody (see M&M).
In order to understand which changes in the TAS chromatin could explain the increase in mini-white transcription in the JIL-1z60/JIL-1 h9 allelic combination, we performed Chromatin Immunoprecipitation (ChIP) experiments. The chromatin environment at the TAS domain has been shown to be enriched in H3K27me3, which serves for targeting the Polycomb complex to the subtelomere domain [3,7]. We therefore developed the ChIP assay with an antibody against H3K27me3 (Figure 4B). The ChIP data showed a significant decrease in the presence of the H3K27me3 mark inside the TAS domain in JIL-1 mutant alleles, suggesting a possible spreading of this mark into flanking domains. We have not been able to detect an increase in H3K27me3 at the HTT array indicative of the consequential spreading of this mark from the TAS towards the HTT array, (Figure 4B and 5). Because the HTT array flanking the TAS domain at the 2R (39C-27) and 2L (39C-5) telomeres cannot be distinguished from the HTT arrays of other chromosomes, it is not surprising that the ChIP assay was not sensitive enough to reveal a slight increase in H3K27me3 in the vicinity of the HTT array.
Figure 5. Possible scenarios for the TAS and the HTT chromatin in a JIL-1 mutant background.
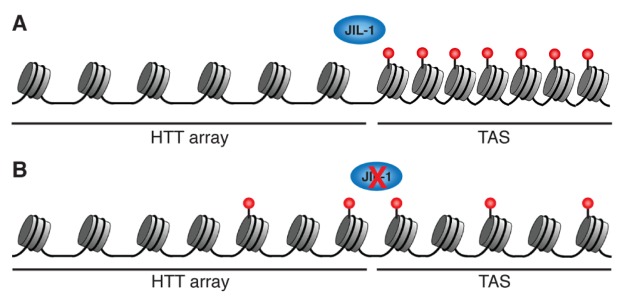
(A) Wild type: the presence of the H3K27me3 mark nucleates a highly repressed chromatin (note the tight packaging of the nucleosomes) in the TAS domain, regulated by the Polycomb group of proteins (Table S1). (B) JIL-1 mutant background: The loss of the JIL-1 boundary, causes a spread of the H3K27me3 mark into the HTT array, resulting in a local decrease of the H3K27me3 mark in the TAS chromatin and as a consequence making this compartment more permissive to gene expression.
The TPE assays with the JIL-1 mutant alleles have shown the same result for both TAS domains, TAS-L (2L, 3L telomeres, line 39C-5) and TAS-R (2R, 3R and XL telomeres, line 39C-27) [19], demonstrated by the increase in eye color of the resulting descendants (Figure 4A). These results suggest that the protective boundary built by JIL-1 also exists in the other Drosophila telomeres.
As an additional control, we analyzed if the TAS reporter lines 39C-27 and 39C-5 behave as had been previously described for TAS sequences [6,7]. Therefore, we performed control crosses with mutant alleles of genes whose effect over the expression in the subtelomeric domain had been already reported (Table S1) and obtained results consistent with previous studies [6,7].
The subtelomere domain of the 4th chromosome and the TAS domains have a different chromatin
The 4R telomeres of the reporter lines here analyzed (39C-72 and 118E-5) do not have a TAS domain flanking the HTT array. Instead, a small transition zone (≈ 5Kb) composed of decayed transposable elements together with pieces of the HTT elements lies at the subtelomere region of the 4R arm (Flybase GBrowse and [6,20]). Our experiments have revealed that the JIL-1 kinase and HP1a easily affect the 4th chromosome subtelomeric lines 39C-72 and 118E-5 (Figure 6). The effects that these two proteins exert at the 4R subtelomere are similar to the ones that they produce at the HTT array of the other chromosomes (Figure 1 4th column and Figure 2A, B, D, E and Figure 3). JIL-1 behaves as an enhancer of TPE while HP1a shows a strong suppressor effect in all cases where the reporter is inserted at the telomere domain. Additionally, we performed control crosses with insertions inside the arms of the 4th chromosome, the lines 39C-12 and 39C-42 [21]. No change in the eye color of the JIL-1 mutant descendants was observed, while in Su(var)2-5 05 mutants the expected increase in red eye color with respect to the control cross was observed (Figure S1) [21]. Therefore, our results suggest that the subtelomere of the 4th chromosome depends on JIL-1 for gene expression and on HP1a for gene silencing (Figure 6).
Figure 6. JIL-1 and HP1 control expression from the subtelomeric region of the fourth chromosome.
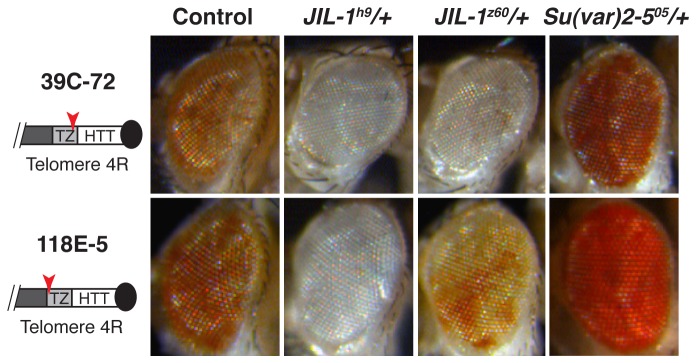
Reporter lines from the subtelomere domain (transition zone) of chromosome 4R, 39C-72 and 118E-5, were crossed with the heterozygous mutant alleles JIL-1 z60/+, JIL-1 h9 /+ and Su(var)2-5 05. The subtelomere domain of the 4th chromosome shows a clear descent in eye color already with the simple JIL-1 heterozygous alleles, (2nd and 3rd columns) and a strong suppressor effect of TPE in the Su(var)2-5 05 allele (4th column).
Discussion
JIL-1 is a positive regulator of gene expression at the telomere domain (HTT)
Both the experiments of telomere position effect on the mini-white insertions (Figure 1) and the real-time PCR quantifications of the expression of the different telomeric elements (Figure 2), indicate that the lower amount of JIL-1 present in the mutant alleles JIL-1 z60 and JIL-1 h9 here assayed, results in a decreased expression of the HTT array. These experiments, in accordance with our recent published data [16], confirm that JIL-1 is necessary to obtain wild type levels of gene expression from the HTT array.
Additionally, these experiments have also revealed that the effect of JIL-1 is stronger over the HeT-A promoter than over the mini-white promoter existent in the reporter lines used in this study. Similarly, when we assayed the effect of mutations of the Su(var)2-5 gene, known to greatly de-repress the expression of the HeT-A retrotransposon [16,22-24], we only obtained a faint de-repression of the mini-white gene of the lines EY08176, EY09966 and EY00453 (Figure 3). In agreement with our observations, Frydrychova and colleagues found similar results using two additional Su(var)2-5 mutant alleles, Su(var)2-5 02 and Su(var)2-5 04, over the same reporter lines [23].
In summary, JIL-1 and HP1a control gene expression from the telomere domain in Drosophila, being the HeT-A promoter especially sensitive to their effect.
JIL-1 protects the HTT array of the chromatin spreading from the TAS domain
Although JIL-1 has not been found to localize at the subtelomere domain, TAS, the observation that gene expression varies in this domain in a JIL-1 mutant background, suggests a role of this protein related with a putative boundary between the telomere and subtelomere domains. We have observed a pronounced increase in mini-white expression at the TAS domains from the 2L and 2R telomeres when placed in a JIL-1 trans-heterozygous (JIL-1z60/JIL-1 h9) mutant background (Figure 4A, 4th column). Accordingly, the ChIP data reveals a significant decrease of H3K27me3 upstream of the mini-white insertion when a JIL-1 trans-heterozygous background is present (Figure 4B). Integrating these results, we propose a model in which the JIL-1 kinase acts as a boundary protecting the promoters of the HTT retrotransposons from the highly compacted chromatin of the adjacent TAS domain (see model in Figure 5). This model is in agreement with an increase of repressive chromatin at the HTT array in JIL-1 mutations (Figure 1 4th column and [16]). Moreover, our model also reflects the previously reported role of JIL-1 in the protection of the excessive spreading of heterochromatin to adjacent domains [13,14], and suggests that JIL-1 could exert a barrier function at the HTT-TAS boundary, based on the results obtained from this study.
Not all the subtelomere domains have the same chromatin characteristics
We have not observed a different behavior in the control of gene expression when studying the HTT array of the 4th and the 2nd chromosomes. Interestingly, we did find a significant difference when looking at the subtelomere of the 4R telomere (Figure 6). In the reporter lines 39C-72 and 118E-5 from the subtelomere domain of the 4th chromosome, we have found that JIL-1 is important to allow gene expression and HP1a is necessary to repress it. This finding indicates that the chromatin of the subtelomere domain in the 4th chromosome is not equivalent to the TAS chromatin in the other chromosomes.
In contrast, the results here described indicate that the chromatin at the 4R subtelomere of the reporter lines used in this study is less compacted and more permissive to gene expression than the TAS domains from the other chromosomes, and even than the HTT array. This scenario suggests that in the 4R telomere of these lines the compaction of the chromatin is in the opposite orientation than in the rest of the telomeres. It would be interesting to study if this reversed chromatin organization with respect to the other telomeres has a particular role in the general telomere function in Drosophila.
Conclusions
We report how the presence of the JIL-1 kinase is needed at the telomere in Drosophila in order to allow gene expression of the promoters embedded in this domain. The lack of JIL-1 causes an increase of silencing at the HTT array and a release of silencing at the subtelomeric domain likely by the spreading of heterochromatin towards adjacent domains. Finally, we discovered that the telomere and subtelomere domain of the 4R arm of some lines might have a different chromatin organization with respect to the other Drosophila telomeres.
Materials and Methods
Fly stocks and crosses
Fly stocks were maintained and crosses performed at 25°C on standard Drosophila corn meal medium. JIL-1z60/TM6 and JIL-1h9/TM6 stocks were provided by Kristin M. Johansen. Both JIL-1 mutations are hypomorph. The JIL-1 h9 allele contains a molecular lesion that results in a deletion inside the C-terminal of the protein and the JIL-1 z60 contains a mutation that eliminates the promoter and 5’UTR of the JIL-1 gene [9]. Su(var)2-5 05 contains a frame shift that results in a peptide containing only the first 10 amino acids of the original HP1 protein [22]. w 1118, Su(var)2-505/CyO, Su(Z)21.a1/CyO and Su(Z)21.b7/CyO were obtained from Bloomington Stock Center. Su(var)3-917/TM3 and Bmx2trx/TM6 were provided by Fernando Azorin laboratory. Lori Walrath provided the reporter lines 39C-5, 39C-27, 118E-5, 39C-72, 39C-42, and 39C-12, and James Mason the lines EY08176, EY00453 and EY09966.
To assay for TPE modifications, males from homozygous reporter gene stocks, with a P-element inserted at a telomere or at the subtelomere domains, were crossed with virgin females from mutant stocks of the proteins of interest. The eye phenotype of the resulting F1 progeny with the mutation was compared with their siblings lacking the mutation. For each cross 20 flies were analyzed (males and females) and collected 2 days after eclosion and briefly frozen. Each cross was repeated three times. Images were obtained from mutant and control F1 males using Zeiss Discovery V.8 microscope with AxioCam MRE5 camera and Axiovision 4.7 software.
RNA Extraction and cDNA synthesis
Total RNA was isolated from ten whole third instar larvae and extracted using RNeasy Mini Kit (Qiagen) according to manufacturer’s protocol. RNase Free DNase Set (Qiagen) was used to remove genomic DNA contaminations as follows: one on column during the extraction accordingly to manufacturer’s protocol, and two in solution for 2 hours at 37°C. RNA was cleaned by precipitation and its quality was assessed using NanoDrop spectrophotometry.
One microgram of RNA was reverse transcribed into cDNA using Transcriptor First Strand cDNA Synthesis Kit (Roche) with Oligo d(T) primers, and the expression of the different transcripts analyzed by quantitative real-time PCR. For each fly strain, two independent RNA extractions were prepared and analyzed three independent times. Primers used for real time PCR: HeT-A_F (CCCCGCCAGAAGGACGGA) and HeT-A_R (TGTTGCAAGTGGCGCGCA) included in the 3’UTR, EY0176_F (GTGGATGTCTCTTGCCGACG) and EY8176_R (GCTCTTACTCACACTGACGCTTCTC), EY09966_F (CTATAACATCGAGTACCAGCCG) and EY09966_R (GCTCTTACTCACACTGACGCTTCTC), Actin_F (GCGCCCTTACTCTTTCACCA) and Actin_R (ATGTCACGGACGATTTCACG).
Chromatin Immunoprecipitation experiments (ChIPs)
Chromatin immunoprecipitation experiments were preformed has described in [16]. Chromatin was immunoprecipitated with anti-H3K27me3 (ab6002, Abcam). Three independent ChIP samples were analyzed and the amount of immunoprecipitated DNA was calculated by three independent quantitative real-time PCR using iQTM SYBR® Green Supermix (BioRad). Primers used for quantitative real-time PCR: HeT-A_5’UTR_F (TCGCGCTTCCTCCGGCAT) and HeT-A_5’UTR_R (GCGGTTATTACATTACGGCGG), TAS_X_F (TTGTAATTTGGTGCGGCAGC) and TAS_X_R (CAGCGTGACTGTTCGCATTC), RpL32_F (CAAGAAGTTCCTGGTGCACAA) and RpL32-R (AAACGCGGTTCTGCATGAG).
Quantitative real-time PCR
Quantitative real-time PCR was performed to determine HeT-A expression and in ChIP experiments. The iQ5 Multicolor Real-Time PCR Detection System was used and the iQTM SYBR® Green Supermix (BioRad) was used to prepare the reactions. Relative levels of HeT-A expression were determined using the threshold cycle and normalized to actin levels (or RpL32 for ChIPs). Three independent experiments of two samples each strain were performed.
Supporting Information
JIL-1 control crosses. JIL-1 mutations have no effect on the expression of a reporter gene inserted in the arms of the 4th chromosome (39C-12 and 39C-42). The Su(var)2-505 allele has the expected suppressor of variegation effect in these same lanes [21].
(TIF)
Subtelomeric domain control crosses. Previously reported mutant alleles from su(var)3-9, polycomb and trithorax were crossed with lines containing the reporter gene inserted in the subtelomeric domains of the 4th (39C-72 and 118E-5) and 2nd chromosomes respectively (39C-5 and 39C-27).
(DOC)
Acknowledgments
Ma LLuisa Espinàs, Mary-Lou Pardue and the Casacuberta Laboratory for critical reading of the manuscript, Lori Wallrath, Fernando Azorín, Kristin Johansen and James Mason for kindly passing reagents.
Funding Statement
This work was supported by a grant from the Spanish Ministry of Science and Innovation BFU2009-08318/BMC to EC and a PhD Fellowship Fundação para a Ciência e Tecnologia, Portugal SFRH / BD / 36291 / 2007 to RSS. These funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Capkova Frydrychova R, Biessmann H, Mason JM (2008) Regulation of telomere length in Drosophila. Cytogenet Genome Res 122: 356-364. doi: 10.1159/000167823. PubMed: 19188706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pardue ML, DeBaryshe PG (2003) Retrotransposons provide an evolutionarily robust non-telomerase mechanism to maintain telomeres. Annu Rev Genet 37: 485-511. doi: 10.1146/annurev.genet.38.072902.093115. PubMed: 14616071. [DOI] [PubMed] [Google Scholar]
- 3. Andreyeva EN, Belyaeva ES, Semeshin VF, Pokholkova GV, Zhimulev IF (2005) Three distinct chromatin domains in telomere ends of polytene chromosomes in Drosophila melanogaster Tel mutants. J Cell Sci 118: 5465-5477. doi: 10.1242/jcs.02654. PubMed: 16278293. [DOI] [PubMed] [Google Scholar]
- 4. Mason JM, Frydrychova RC, Biessmann H (2008) Drosophila telomeres: an exception providing new insights. Bioessays 30: 25-37. doi: 10.1002/bies.20688. PubMed: 18081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silva-Sousa R, López-Panadès E, Casacuberta E (2012) Drosophila telomeres: An example of co-evolution with transposable elements, in Repetitive DNA. Karger AG. Genome Dynamics 7: 46-67. doi: 10.1159/000337127. PubMed: 22759813. [DOI] [PubMed] [Google Scholar]
- 6. Cryderman DE, Morris EJ, Biessmann H, Elgin SC, Wallrath LL (1999) Silencing at Drosophila telomeres: nuclear organization and chromatin structure play critical roles. EMBO J 18: 3724-3735. doi: 10.1093/emboj/18.13.3724. PubMed: 10393187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boivin A, Gally C, Netter S, Anxolabéhère D, Ronsseray S (2003) Telomeric associated sequences of Drosophila recruit polycomb-group proteins in vivo and can induce pairing-sensitive repression. Genetics 164: 195-208. PubMed: 12750332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin Y, Wang Y, Walker DL, Dong H, Conley C et al. (1999) JIL-1: a novel chromosomal tandem kinase implicated in transcriptional regulation in Drosophila. Mol Cell 4: 129-135. doi: 10.1016/S1097-2765(00)80195-1. PubMed: 10445035. [DOI] [PubMed] [Google Scholar]
- 9. Zhang W, Jin Y, Ji Y, Girton J, Johansen J et al. (2003) Genetic and phenotypic analysis of alleles of the Drosophila chromosomal JIL-1 kinase reveals a functional requirement at multiple developmental stages. Genetics 165: 1341-1354. PubMed: 14668387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Regnard C, Straub T, Mitterweger A, Dahlsveen IK, Fabian V et al. (2011) Global analysis of the relationship between JIL-1 kinase and transcription. PLOS Genet 7: e1001327 PubMed: 21423663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ebert A, Schotta G, Lein S, Kubicek S, Krauss V et al. (2004) Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev 18: 2973-2983. doi: 10.1101/gad.323004. PubMed: 15574598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng H, Zhang W, Bao X, Martin JN, Girton J et al. (2005) The JIL-1 kinase regulates the structure of Drosophila polytene chromosomes. Chromosoma 114: 173-182. doi: 10.1007/s00412-005-0006-8. PubMed: 15986206. [DOI] [PubMed] [Google Scholar]
- 13. Zhang W, Deng H, Bao X, Lerach S, Girton J et al. (2006) The JIL-1 histone H3S10 kinase regulates dimethyl H3K9 modifications and heterochromatic spreading in Drosophila. Development 133: 229-235. PubMed: 16339185. [DOI] [PubMed] [Google Scholar]
- 14. Wang C, Girton J, Johansen J, Johansen KM (2011) A Balance Between Euchromatic (JIL-1) and Heterochromatic [SU (VAR) 2-5 and SU (VAR) 3-9] Factors Regulates Position-Effect Variegation in Drosophila. Genetics 188: 745–748. doi: 10.1534/genetics.111.129353. PubMed: 21515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang C, Cai W, Li Y, Deng H, Bao X et al. (2011) The epigenetic H3S10 phosphorylation mark is required for counteracting heterochromatic spreading and gene silencing in Drosophila melanogaster. J Cell Sci 124: 4309-4317. doi: 10.1242/jcs.092585. PubMed: 22247192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silva-Sousa R, López-Panadès E, Piñeyro D, Casacuberta E (2012) The chromosomal proteins JIL-1 and Z4/Putzig regulate the telomeric chromatin in Drosophila melanogaster . PLOS Genet, 8; e1003153 PubMed: 23271984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wallrath LL, Elgin SC (1995) Position effect variegation in Drosophila is associated with an altered chromatin structure. Genes Dev 9: 1263-1277. doi: 10.1101/gad.9.10.1263. PubMed: 7758950. [DOI] [PubMed] [Google Scholar]
- 18. Biessmann H, Prasad S, Semeshin VF, Andreyeva EN, Nguyen Q et al. (2005) Two distinct domains in Drosophila melanogaster telomeres. Genetics 171: 1767-1777. doi: 10.1534/genetics.105.048827. PubMed: 16143601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Antão JM, Mason JM, Déjardin J, Kingston RE (2012) Protein landscape at Drosophila melanogaster telomere-associated sequence repeats. Mol Cell Biol 32: 2170-2182. doi: 10.1128/MCB.00010-12. PubMed: 22493064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. George JA, DeBaryshe PG, Traverse KL, Celniker SE, Pardue ML (2006) Genomic organization of the Drosophila telomere retrotransposable elements. Genome Res 16: 1231-1240. doi: 10.1101/gr.5348806. PubMed: 16963706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riddle NC, Leung W, Haynes KA, Granok H, Wuller J et al. (2008) An investigation of heterochromatin domains on the fourth chromosomes of Drosophila melanogaster . Genetics 178: 1177-1191. doi: 10.1534/genetics.107.081828. PubMed: 18245350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savitsky M, Kravchuk O, Melnikova L, Georgiev P (2002) Heterochromatin protein 1 is involved in control of telomere elongation in Drosophila melanogaster. Mol Cell Biol 22: 3204-3218. doi: 10.1128/MCB.22.9.3204-3218.2002. PubMed: 11940677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perrini B, Piacentini L, Fanti L, Altieri F, Chichiarelli S et al. (2004) HP1 controls telomere capping, telomere elongation, and telomere silencing by two different mechanisms in Drosophila. Mol Cell 15: 467-476. doi: 10.1016/j.molcel.2004.06.036. PubMed: 15304225. [DOI] [PubMed] [Google Scholar]
- 24. Frydrychova RC, Mason JM, Archer TK (2008) HP1 is distributed within distinct chromatin domains at Drosophila telomeres. Genetics 180: 121-131. doi: 10.1534/genetics.108.090647. PubMed: 18723888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
JIL-1 control crosses. JIL-1 mutations have no effect on the expression of a reporter gene inserted in the arms of the 4th chromosome (39C-12 and 39C-42). The Su(var)2-505 allele has the expected suppressor of variegation effect in these same lanes [21].
(TIF)
Subtelomeric domain control crosses. Previously reported mutant alleles from su(var)3-9, polycomb and trithorax were crossed with lines containing the reporter gene inserted in the subtelomeric domains of the 4th (39C-72 and 118E-5) and 2nd chromosomes respectively (39C-5 and 39C-27).
(DOC)