Abstract
Olfactory sensitivity exhibits daily fluctuations. Several studies have suggested that the olfactory system in insects is modulated by both biogenic amines and neuropeptides. However, molecular and neural mechanisms underlying olfactory modulation in the periphery remain unclear since neuronal circuits regulating olfactory sensitivity have not been identified. Here, we investigated the structure and function of these signaling pathways in the peripheral olfactory system of the American cockroach, Periplaneta americana, utilizing in situ hybridization, qRT-PCR, and electrophysiological approaches. We showed that tachykinin was co-localized with the octopamine receptor in antennal neurons located near the antennal nerves. In addition, the tachykinin receptor was found to be expressed in most of the olfactory receptor neurons in antennae. Functionally, the effects of direct injection of tachykinin peptides, dsRNAs of tachykinin, tachykinin receptors, and octopamine receptors provided further support for the view that both octopamine and tachykinin modulate olfactory sensitivity. Taken together, these findings demonstrated that octopamine and tachykinin in antennal neurons are olfactory regulators in the periphery. We propose here the hypothesis that octopamine released from neurons in the brain regulates the release of tachykinin from the octopamine receptor neurons in antennae, which in turn modulates the olfactory sensitivity of olfactory receptor neurons, which house tachykinin receptors.
Introduction
Olfaction is an important sensory modality for social interactions, perception, and efficient orientation to food sources in most animals [1]. Moreover, olfactory neural processing is closely related to the physiological state of the organisms [2-4]. There is substantial evidence that numerous types of neurotransmitters modulate the central and peripheral steps of odor detection in most animals [5-7]. While this relationship has been widely known for several years, the molecular neural mechanism underlying the alteration of the peripheral olfactory sensitivity induced by the neurotransmitters are not yet understood.
Available evidence suggests that modulation of olfactory sensitivity is controlled by both neuropeptides and biogenic amines [6,8,9]. Among them, octopamine (OA) is known as a neurotransmitter, a neuromodulator, and a neurohormone in insects [10]. OA is released into the antennal heart of the cockroach [11], and the injection of OA can either decrease or enhance olfactory responses depending on the stimulus [12,13]. While the molecular mechanisms underlying the role of the OA in olfactory modulation are not known, it seems to have several roles on the regulation. In addition to OA, it is likely that certain neuropeptides are also employed as neuromodulators in insect olfactory systems. One neuropeptide of particular interest is tachykinin (TK) [14]. Several isoforms of TK peptides have been shown to be expressed in the central and peripheral nervous systems [15-17]. A recent study found that flies deficient with tachykinin-related peptides exhibited a decrease of sensitivity for odor perception, implicating their significant role of neural modulation in olfactory systems [18]. In addition, it has been reported that the Drosophila tachykinin receptor (DTKR), expressed in axon terminals of olfactory receptor neurons (ORNs), is modulated by TK released from local interneurons (LNs) of antennal lobes (ALs) and inhibits the olfactory responses of ORNs [19]. However, most research has focused on the central nervous system (CNS), and thus information on how the function of peripheral olfactory systems can be regulated is largely unknown.
In this regard, we have employed in situ hybridization, immunostaining, and quantitative real-time polymerase chain reaction (qRT-PCR) methods to investigate the anatomical organization of neuronal circuits governing the alteration of olfactory sensitivity by both OA and TK signaling in the cockroach antenna. In addition, we report the observations on the physiological effects of tachykinin, octopamine, and octopamine receptors on olfactory sensitivity in the antennae of Periplaneta americana. Our current data demonstrate that the molecular machinery involved in the modulation of olfactory sensitivity exists in the peripheral olfactory organ.
Materials and Methods
Animals and tissue preparation
P. americana were raised under a light cycle of 12:12 hours light and a dark (L/D) cycle at 26±1°C as described [20]. Intact adult male cockroaches were used for qRT-PCR and electrophysiological recordings. Animals with external damage such as missing antennal segments were discarded for tissue preparation. Male cockroaches were anesthetized by chilling on ice before dissection after which antennae were immediately placed on dry ice. Further experimental processing of the tissue for histology is described below.
Gene Cloning of octopamine receptor (PaOA1), tachykinin (PaTK), and tachykinin receptor (PaTKR) and RNA probes
Total RNA was extracted from three male P. americana antennae and brains using the Qiagen RNasy kit by manufacturer’s instruction (Qiagen, Valencia, CA, USA), after which RQ1 RNase-free DNase I (Promega, Madison, WI, USA) was treated by manufacturer’s instruction. Reverse transcription procedures were carried out as described previously [21]. In order to clone PaTKR mRNA, TKR sequences of L. maderae [22] (CAC36957.1), Tribolium castaneum (XP_970102.1), Anopheles gambiae (XP_312088.3), Apis mellifera (XP_395081), and D. melanogaster (AAA28722.1) were used for multiple alignment for conserved domain of the transmembrane protein regions (Figure 1A). Partial cDNA clones for PaOA1 and PaTKR were generated using specific primer sets as shown in Table S1. These genes were inserted into a pGEM-T vector (Promega), after which RNA probes were made using a DIG RNA labeling mix as manufacturer’s instructions (Roche, Indianapolis, IN).
Figure 1. Localization of octopamine receptors, tachykinin, and tachykinin receptors in the antennae of P. americana.
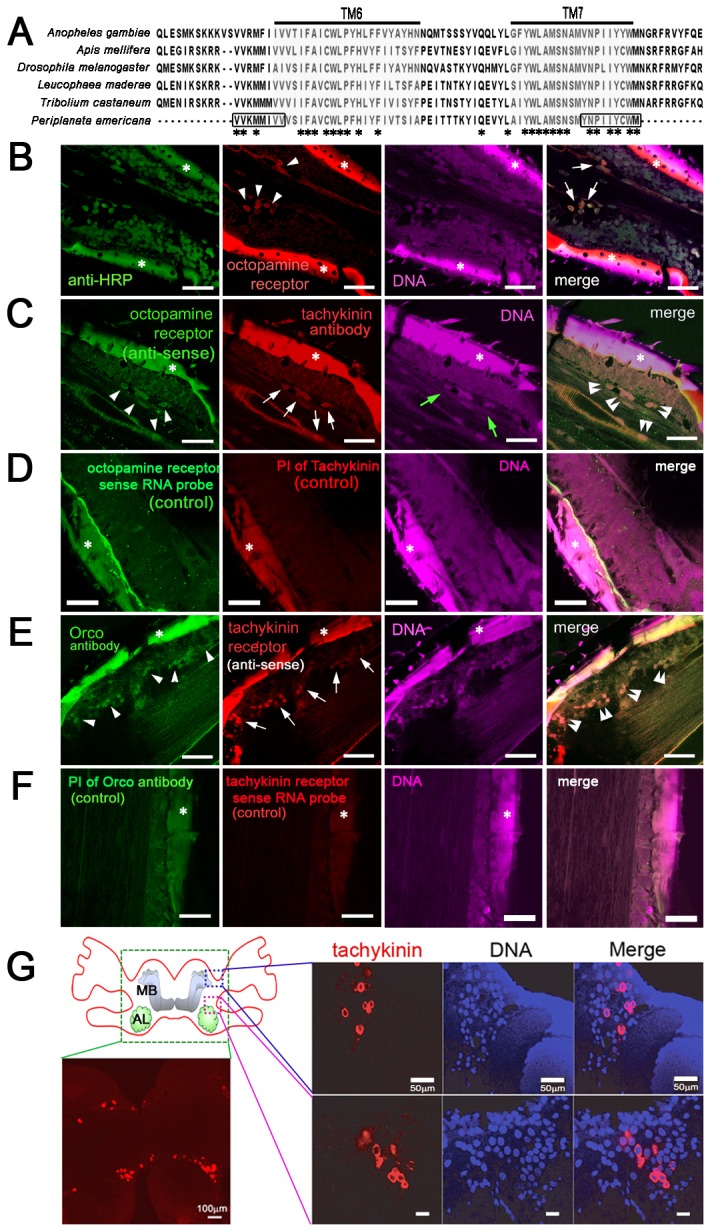
(A) Partial amino acid sequence of tachykinin receptors of Periplaneta americana (PaTKR) and its alignment with other insect tachykinin receptors present in mosquito (Aedes aegypti), honey bee (Apis mellifera), fruit fly (Drosophila melanogaster), red flour beetle (Tribolium castaneum), and Madeira cockroach (Leucophaea maderae). Transmembrane regions 6 and 7 are shaded in gray. Amino acid residues selected for gene cloning were indicated by boxes. (B) In situ hybridization with antisense RNA probes of octopamine receptor (red), where octopamine receptors (arrowheads) were co-localized with neuronal marker anti-HRP antibody (arrows). (C) Expression of tachykinin (arrows) in octopamine receptor neurons (green, arrowheads) were co-localized (double arrowheads). Green arrows indicate antennal nerves. (D) Control of in situ hybridization using sense RNA probes of octopamine receptor and pre-immune serum of tachykinin. (E) Tachykinin receptor (arrows) of P. americana was co-localized (double arrowheads) with odorant receptor co-receptor (Orco, arrowheads) in olfactory sensory neurons of the antennae. (F) Control of in situ hybridization using sense RNA probes of tachykinin receptor and pre-immune serum of Orco. Scale bars demonstrate 20 μm. (G) Immunostaining with tachykinin antibody in brain tissue of cockroach. A schematic diagram of the cockroach brain showed mushroom body (MB) and antennal lobes (AL) where tachykinin (red) was mainly localized. Images demonstrate tachykinin staining in the brain area indicated with a dotted box. Cell bodies were stained with DAPI (blue). Scale bars demonstrate 20 μm unless indicated. Asterisks demonstrate the autofluorescence from antennal cuticle.
Quantitative real-time PCR (qRT-PCR)
Adult male cockroaches were isolated in a plastic cage and were provided with food and water ad lib. cDNA synthesis was conducted as described above. qRT-PCR was carried out with the StepOnePlus (Applied Biosystems, Foster City, CA, USA) using SYBR green qPCR Master Mix (Fermentas, Ontario, Canada). Primer information for qPT-PCR was described in Table S1. Quantitative analysis was employed by StepOne plus Software V. 2.0 (Applied Biosystems). Results were normalized to a validated control gene, actin, using the ΔΔCt method [23]. pQE30 vector sequence was used as a control. Gene-specific primers used for qRT-PCR were as follows: PaOA1 (AY333178.1): Forward primer (FP): 5'-CTCTTCTGGCTGGGCTATTG-3', Reverse Primer (RP): 5'-TCCTTGCTAAAGAGGGCG TA-3'; PaTK: FP: 5'-GCAAGAAGGCACCATCAGC-3', RP: 5'-ATGCCCATAAACCCGG AAC-3', PaAct (AY116670.1): FP: 5'-GCTATCCAGGCTGTGCTTTC-3', RP: 5'-ACCGGAA TCCAGCACAATAC-3'
In situ Hybridization, immunostaining, and imaging
Antennae and heads of male Periplaneta americana were fixed with 4% paraformaldehyde (PFA) solution overnight, after which antennae were cut at 3-5mm long and brains were dissected out. Tissues were then washed with PBS buffer (pH 7.4), followed by dehydration and rehydration using a series of ethanol from 25%~100%. Tissues were then hybridized with a hybridization solution containing Dig-labeled RNA probes for 20 hrs at 58°C. After several washes with PBS buffer containing 0.2% Tween 20, tissues were incubated with peroxidase (POD)-conjugated anti-DIG antibodies (Roche, Indianapolis, IN) in a blocking reagent (Roche) overnight at 4°C, followed by signal visualization process using a Tyramid signal amplification (TSA) kit by manufacturer’s instruction (PerkinElmer, Waltham, MA, USA). After in situ hybridization, immunostaining using several different antibodies were performed as described previously [21]. Tissue preparations after in situ hybridization were subsequently incubated with rabbit anti-tachykinin polyclonal antibodies (1:2000 in PBS with 0.1% Tween 20, PBSTw) against Locusta migratoria tachykinin [24] (gifted from Dr. Yoon-Seong Park at Kansas State University) or anti-DmOrco polyclonal antibody (gifted from Dr. Leslie Vosshall at Rockefeller University) to immuno-localize Orco of P. americana at 4°C overnight. After 3-4 times washing with PBSTw, anti-rabbit secondary antibodies conjugated with cy3 fluorophore were incubated overnight at 4°C. Cell and neuronal staining was conducted with TOTO-3 (Invitrogen) and anti-horseradish peroxidase (HRP) antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), respectively.
Stained tissue preparations were dehydrated with a series of ethanol and 100% acetone to embed into Spurr’s epoxy resin [21]. Resin-embedded tissues were incubated at 60°C oven overnight and sectioned at 20μm thickness by using an automatic sliding microtome (HM 355S; Microm, Thermo Scientific). Sectioned tissues were mounted with Serva fluoromount (Crescent Chemical Co). Images were captured with a LSM 700 confocal microscope (Zeiss, Thornwood, NY) and were processed with ZEN 2009 software. Gene-specific primers for preparing RNA probes were as follows: PaOA1 (AY333178.1): FP: 5’-CAACAGCTCCAAGAAGTCCAG-3’, RP: 5’-GCTGTCCTCTCCTACCGAGTT-3’; PaTK (AY766012.1): FP: 5’-CCCCATCACACAACAAGAGTT-3’, RP: 5’-CCCTCCATCTC TGAGTCCTTT-3’; PaTKR- FP: 5’-GGGTAGTGAAGATGATGATTGTGGTGG-3’, RP: 5’-TCATCCAACAGTAGATGATGGGATTGTAC-3’
Injection of octopamine, tachykinins, dsRNAs, and octopamine agonist and antagonist
Fifteen tachykinin peptides identified in the cockroach, P. americana (PaTKs) [15] were synthesized with 95% purity (Anygen, Gwang-Ju, Korea) (Table S2). dsRNAs for PaOA1, PaTK, PaTKR mRNA as well as pQE30 as a control gene were generated using MEGAscript RNAi kit (Ambion) with specific primer sets (Table S1). For the injection of tachykinins and dsRNAs, 3 μl of each active tachykinin with 60 nmol and 0.5 μl of synthesized dsRNA (5 μg/μl) were injected into the antennal base of intact adult male cockroaches caught at CT11 using a Hamilton micro syringe, needle G30 (Becton Dickinson). For injection experiments of octopamine and TK-dsRNA and octopamine alone, 5 μl of 10mM octopamine were used. Injected cockroaches were kept individually. Control cockroaches were injected with saline (PBS. pH=7.4). 2 μl of octopamine agonist (clonidine) and antagonist (yohimbine) (Sigma-Aldrich) were injected at the concentration of 1M into the same areas of cockroach antennae described above.
Electroantennogram (EAG)
A male cockroach was restrained with one antenna fixed in an EAG setup, as previously described [25]. The electrical signals were amplified (IDAC4, Syntech, The Netherlands) and processed with EAGPro software (Syntech). Prior to injection of tachykinin and dsRNA, three to five pre-recordings with 5 min intervals for olfactory responses to ethyl acetate (1 sec stimulation), diluted to 10-2 (vol/vol) with mineral oil to a final concentration of 10 mM (Sigma-Aldrich), were measured. Five min after injection of tachykinin and dsRNA, 13 to 20 consecutive post-recordings were made to ethyl acetate delivered every 5 min. EAG amplitudes were normalized by dividing a mean amplitude value of pre-recording (before injection) from each olfactory amplitude during post-recordings (after injection). EAG recordings were conducted in the middle of the animal’s subjective day. This is near the peak sensitivity of the circadian rhythm in olfactory response in cockroaches [20,25].
Single sensillum recording (SSR)
Olfactory responses using SSR were measured from single-walled type C sensilla by inserting the tip of glass electrode into the cuticle at the base of the sensillum. Odorant stimulation was the same as in the EAG recording setup described above. Olfactory responses were measured by the number of spikes (action potentials) elicited by ethyl acetate stimulation by deducting spike numbers during 1 sec post-stimulation from 1-sec pre-stimulation. Three to five recordings prior to injection and 13 to 21 consecutive post-injection recordings were analyzed (N=12). Antennal preparation was viewed at 1,000 magnification by a BX-51 microscope (Olympus). Electrical signals elicited by odorant stimulation were imported to a 4-channel IDAC-USB and analyzed with AutoSpike software (Syntech, The Netherlands). Spike sorting and counting by the AutoSpike program were clearly distinguishable according to spike amplitude compared to background noises. The number of spikes, as an indicator of olfactory responses, was calculated as follows: the number of spikes that occurred during the first 1 s after the onset of odor stimulation minus the number of action potentials during the 1 s immediately prior to the onset of the stimulus. .
Statistical analysis
Gene expression data were analyzed by Student’s t-test (SPSS, Version 20, IBM, NY, USA). Comparisons of EAG and SSR responses between pre-injection and post-injection were analyzed using a one-way ANOVA test followed by Bonferroni correction with multiple comparisons (SPSS, Version 20, IBM, NY, USA). Time frames were divided as a group before and after injection during EAG and SSR. Statistical analysis was conducted among these groups.
Results
Cloning of octopamine receptor, tachykinin, and tachykinin receptor of P. americana
The P. americana genome has been reported to contain genes encoding octopamine receptor (PaOA1) and TK (PaTK) neuropeptides [15,26]. We subcloned these genes using gene-specific primer sets as shown in Table S1. In contrast, the tachykinin receptor gene of P. americana (PaTKR) was not available in the GenBank. For this reason, the conserved regions of TKR protein sequences from several different species of insects were aligned, especially in transmembrane regions 6 and 7 (TM6 and TM7) (Figure 1A). Specific primer sets were able to clone partial PaTKR gene sequence (192bp) as a novel gene (Table S1). The cloning of the full coding sequence of PaTKR gene remains to be accomplished.
Localization of PaOA1, PaTK, and PaTKR mRNA in antennae
PaOA1, PaTK and PaTKR in cockroach antennae were localized by using double-labeling studies with in situ hybridization and immunostaining (Figure 1). First, PaOA1 mRNA were localized in the antennal neurons (Figure 1B) compared to control (Figure 1D). Neuronal clusters consisting of 5~6 neurons that expressed PaOA1 mRNA were found in each antennal segment near the area of antennal nerves (arrowheads in Figure 1B and C) where PaOA1 and PaTK (arrows in Figure 1C) were co-localized in the same neurons of the antennae (double arrowheads in Figure 1C). Immunohistochemistry using tachykinin antibody in the brain of the cockroach showed that tachykinin-positive neurons are located in the vicinity of antennal lobe (AL) and mushroom body (MB) calyces (Figure 1G). Double-label studies using an anti-sense RNA probe of the PaTKR and an antiserum to the D. melanogaster odorant receptor co-receptor (DmOrco) overlapped in ORNs of the antennae (Figure 1E) when compared to control (Figure 1F). DmOrco gene is well-conserved among different insect species and forms heterodimers with ligand-specific conventional odorant receptors (ORs) in the ORNs, as reported previously [27]. Intriguingly, our results demonstrated that most ORNs (arrowheads in Figure 1E) also expressed the TKRs (arrows and double arrowheads in Figure 1E).
Effects of tachykinin (TK) on olfactory sensitivity in antennae
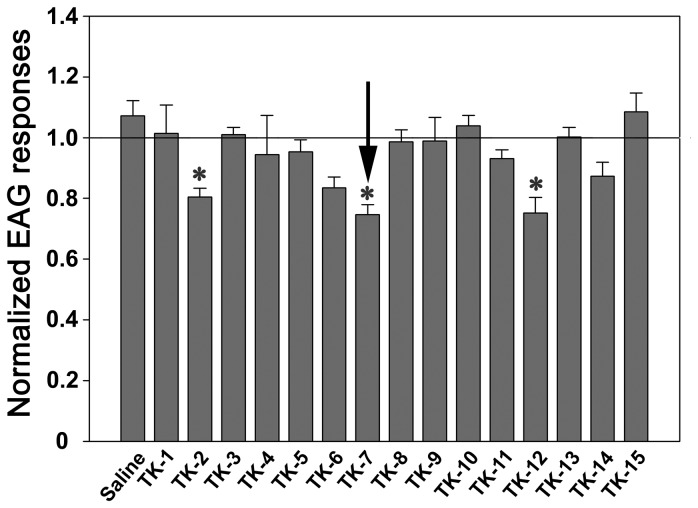
In order to better understand the functional roles of TK on olfactory responses in antennae, 15 synthesized active TK peptides were used to identify the effects on olfactory responses in the antennae (see Figure 2 legend). Among them, TK-2, TK-7 and TK-12 peptides decreased the olfactory responses after TK application (Figure 2, p < 0.05), while no detectable differences from control saline and other TKs were found (Figure 2 and Figure 3A, p > 0.6). TK-7 injection elicited the strongest decrease in EAG responses (Figure 2 and Figure 3B and G, p < 0.05). Notably, a significant decrease in EAG responses was observed at 25 min and the greatest reduction in EAG amplitude was shown 45 min after TK-7 injections (Phase II in Figure 3B). At this point there was approximately a 30% reduction in amplitude after TK-7 application (Figure 3B). Furthermore, recording from single trichoid sensilla after TK-7 injection also showed a decrease in the number of action potentials compared to saline injection as control (Figure 4A, B, and G).
Figure 2. Normalized EAG responses of cockroach antennae to ethyl acetate (10-2 dilution) after injection of tachykinin (TK) peptides.
EAG amplitudes were decreased after injection of TK-2, -7, and -12, compared to other TK peptides and control saline injection (N=4-8, ANOVA with Bonferroni correction, *=p<0.05). TK-7 (arrow) showed strongest effects on decrease of olfactory responses, which was in turn used for further experiments. Effects of other TKs on EAG responses were not significantly different from saline control injection. Information on synthesized TK peptides was as follows: PaTK1: APSGFLGVR-NH2, PaTK2: APEESPKRAPSGFLGVR-NH2, PaTK3: NGERAPASKKAPSGFLGTR-NH2, PaTK4: APSGFLGTR-NH2, PaTK5: APGSGFMGMR-NH2, PaTK6: APAMGFQGVR-NH2, PaTK7: APASGFFGMR-NH2, PaTK8: VPASGFFGMR-NH2, PaTK9: GPSMGFHGMR-NH2, PaTK10: APSLGFQGMR-NH2, PaTK11: APNMGFMGMR-NH2, PaTK12: MGFMGMR-NH2, PaTK13: GPSVGFFAMR-NH2, PaTK14: APSAGFMGMR-NH2, PaTK15: APSAGFHGMR-NH2.
Figure 3. Effects of “before and after injection” of tachykinin, octopamine receptors, tachykinin receptors on olfactory responses (EAG) in antennae and quantitative real-time PCR after dsRNA injection of tachykinin and octopamine receptor.
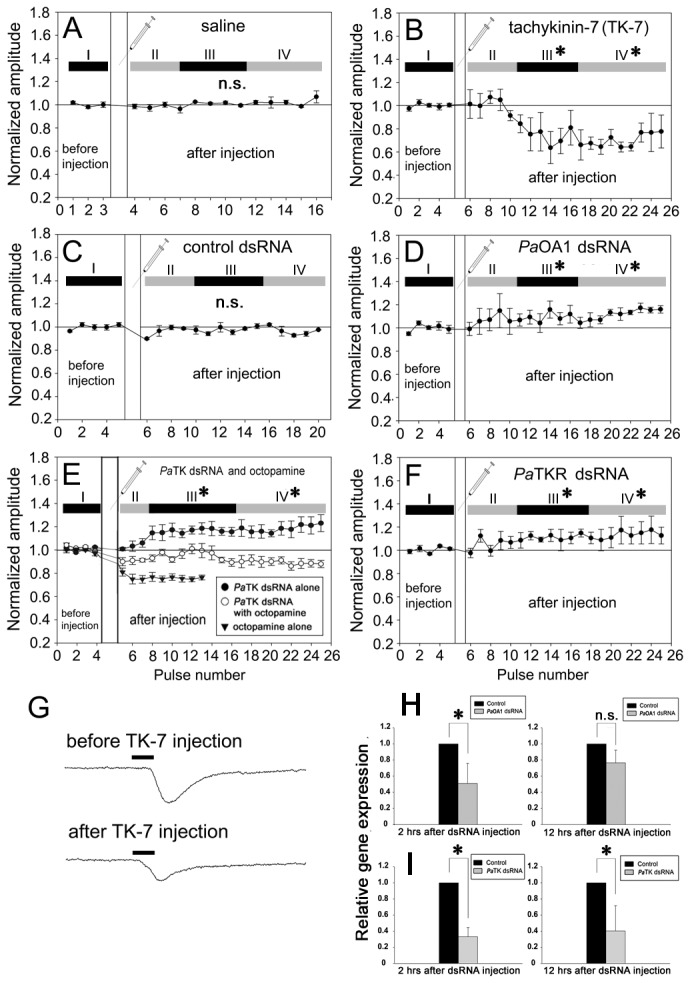
(A) There was no significant difference before and after a control injection of saline (N=3, p>0.6). (B) Tachykinin-7 (TK-7) injection produced a significant decrease in olfactory responses phase II and phase III after injection compared to before injection (phase I) (N=5, *=p<0.001). (C) Injection of control dsRNA from an expression vector (pQE30) did not significantly alter EAG responses (N=5, p>0.7). (D) Injection of octopamine receptor (PaOA1) dsRNA elicited significant increase in EAG amplitude in Phase III and IV, which continued to over 2 hours after injection (N=5, p<0.001). (E) Three EAG traces showed interaction between octopamine and tachykinin on the modulation of olfactory sensitivity in cockroach antennae. Injection of octopamine itself (inverted triangle) decreased EAG response after injection in Phase III and IV, while TK-dsRNA (filled circle) induced significant increase of EAG amplitude (N=5, p<0.01). Notably, the enhanced effect of TK-dsRNA on EAG responses was eliminated when co-injected with octopamine (empty circle). (F) Injection of tachykinin receptor (PaTKR) dsRNA had a significant positive effect on EAG amplitude (N=3, p<0.001). (G) Representative EAG traces before and after TK-7 injection, showing that TK-7 decreased EAG responses. (H) Relative RNA expression levels of octopamine receptor (PaOA1) in antennae after injection of octopamine receptor dsRNA were strongly reduced at 2 hours after injection but not significantly different 12 hours after injection (Student’s t-test: *, P≤0.05). (I) Relative RNA expression levels of tachykinin gene (PaTK) in antennae showed significant reduction at 2 and 12 hours after injection. Values depict mean ± SE. One-way ANOVA test followed by Bonferroni correction for multiple comparisons was employed to test the difference in EAG responses, while Student t-test was used to test the difference of gene expression levels. Pulse number indicates the number of ethyl acetate stimulation every five minutes. Phase I depicts EAG responses during “before injection” and phases II, III, and IV indicate EAG responses during “after injection”.
Figure 4. Olfactory responses to ethyl acetate by TK-7 and other dsRNA treatment using single sensillum recording.
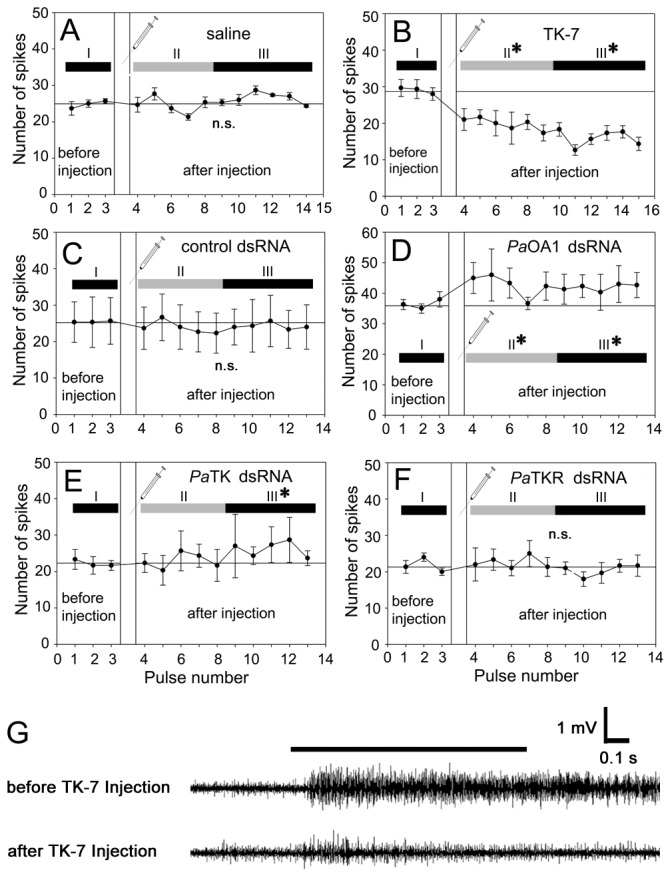
(A) Saline injection caused no significant changes after injection (N=5, p>0.5). (B) Application of TK-7 induced a substantial decrease in the spike activities of olfactory receptor neurons (N=4, p<0.001). (C) Control non-specific dsRNA did not alter olfactory responses (N=4, p>0.2). (D) Octopamine receptor (PaOA1) dsRNA treatment resulted in significant increase in spike numbers (N=5, p<0.01). (E) Tachykinin (PaTK) dsRNA injection exhibited no significant changes in olfactory responses at phase II (4~8th pulses) (N=4, p>0.9) but significant increase in phase III (after 9th pulse) (N=4, p<0.02). (F) Tachykinin receptor (PaTKR) dsRNA injection did not show significant changes (N=5, p>0.7). (G) Representative olfactory response traces by SSR upon TK-7 injection. Significant decrease of spike trains was shown, indicative of decrease of olfactory sensitivity. One-way ANOVA test followed by Bonferroni correction for multiple comparisons was employed for statistical analysis.
Alteration of olfactory sensitivity by dsRNA
dsRNA of each gene demonstrated that the transcript levels of both PaOA1 and PaTK mRNAs were prominently reduced for 2 hours after injection (Figure 3H and I, p < 0.05). The RNAi effect was noticeable during a 12-hour period after dsRNA injection. dsRNA injections of PaOA1, PaTK, and PaTKR significantly increased EAG amplitudes about 50 minutes after injection (Phase III and IV), compared to “before injection” (Figure 3D, E, and F, p < 0.01, ANOVA with Bonferroni correction test). Olfactory responses in single sensillum levels were also affected by dsRNA injection (Figure 4). The number of action potentials was slightly increased after injection of PaOA1 dsRNA (Figure 4D, p < 0.01). In contrast, although we did not observe a strong increase in the number of spikes overall before and after injection of PaTK dsRNA (Figure 4E, p > 0.2, ANOVA, Bonferroni correction test), we found significant differences in phase III (9~13th pulse numbers), compared to spike numbers in “before injection” (Figure 4E, p <0.02). Next, we tried to test whether antennal olfactory sensitivity might be affected by both tachykinin and octopamine. Obviously, the injection of octopamine alone showed the decrease of olfactory responses (inverted triangles in Figure 3E, N=6, p<0.01). With co-injection of PaTK dsRNA and octopamine, we observed no significant increase or decrease in olfactory responses, compared to “octopamine alone” and “PaTK dsRNA alone” (empty circles in Figure 3E). However, EAG responses after co-injection were significantly different, compared to “before injection” (N=4, p<0.01), indicating that octopamine concentration used in this experiment, 5 μl of 10mM octopamine, were rather high to compensate the effect of PaTK dsRNA.
Spike numbers upon the dsRNA injection of PaTKR did not show significant differences throughout pulse numbers (Figure 4F, p > 0.7). Spike frequency in response to ethyl acetate stimulation was significantly decreased by clonidine treatment (an octopamine agonist) (Figure 5A, p < 0.001), whereas yohimbine (an octopamine receptor antagonist) elevated the olfactory responses after 6th to 9th odor pulses (phase II-b in Figure 5B, p < 0.001). Other pulses showed no significantly differences. * indicates significant difference of the group compared to before injection.
Figure 5. Effect of octopaminergic agonist and antagonist on olfactory responses using single sensillum recordings.
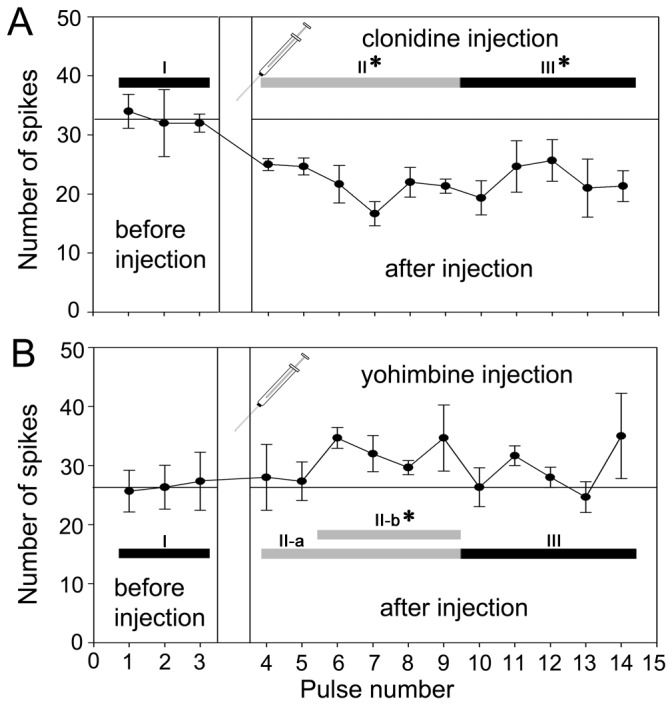
(A) Injection of clonidine, an octopamine receptor agonist, into the antennae resulted in a drastic decrease in the number of spikes right after injection until the end of the experiment (Phase II and III), compared with “before injection” (Phase I) (N=5, p<0.001). (B) Injection of the octopamine receptor antagonist, yohimbine, induced a slight increase in the middle of time phase (II-b, 6th~9th pulses), compared with “before injection” (N=5, p<0.001). One-way ANOVA test followed by Bonferroni correction for multiple comparisons was employed for statistical analysis.
Discussion
A variety of studies have examined the effects of octopamine on olfactory responses in insect antennae. Consistent with our findings here, EAG amplitude has been shown to be decreased by treatment with exogenous octopamine in P. americana [28,29]. In contrast, while we found a decrease in action potentials in response to ethyl acetate stimulation, Zhukovskaya and Kapitsky (2006) reported an increase in nerve impulse responses recorded in single sensillar responses to sex pheromone following octopamine injection. Similarly, octopamine was reported to increase the response of pheromone sensitive ORNs in the hawkmoth, Manduca sexta [30], the silkmoth, Antheraea polyphemus [31], and the cabbage moth, Mamestra brassicae [32]. These findings raise the possibility that octopamine may differentially impact the response of sensilla tuned to respond to sex pheromone and those that respond to at least some other odors (e.g., ethyl acetate) and dominate the EAG response. This view is consistent with recent observations in P. americana that exogenous octopamine does have differential effects on both the behavioral [12] and olfactory receptor neuron responses [28].
In the present study we also find evidence that tachykinin may also play a major role in olfactory modulation. We showed that tachykinin (TK) neuropeptides co-localized with octopamine receptors in antennal neurons in P. americana, and may act via tachykinin receptors (TKR) present in ORNs and may act to regulate olfactory sensitivity. These neurotransmitters have previously been found to act as neuromodulators in the visual system by altering the amplitude of responses to light [33]. Substance P, which is a member of TK peptide family in mammals, and octopamine (OA) efferent systems act in concert to increase electroretinogram (ERG) response and regulate the circadian rhythm in sensitivity in visual system of the horseshoe crab, Limulus polyphemus [34]. However, the chemical and electrophysiological studies of interactions between OA and substance P are largely unknown. Evidence for OA modulation in the moth Bombyx mori demonstrated that OARs were located somewhere near the base of sensilla hairs [35], implying that OA plays an important role in fine tuning of the olfactory responses [28]. Interestingly, cockroach antennae have shown that OA is located in the antennal heart of a neurohemal area, which is secreted from octopaminergic dorsal unpaired median (DUM) neurons in P. americana [36].
In this regard, our study is the first to demonstrate the fact that OA- and TK-related signaling pathways affect the olfactory regulation of peripheral organs. Co-localization of TK and OARs has suggested the possibility that OA regulates the production or release of TK, which induces the reduction of olfactory responses in ORNs. A previous study has demonstrated that TK-deficient flies exhibit defects in olfactory perception and behaviors responding to specific odorants [18]. Moreover, Drosophila tachykinins (DTKs) present in LNs of the ALs, a primary olfactory processing center in insect brains [37], exert a presynaptic inhibitory action to ORNs [19]. This neuronal circuit may regulate olfactory sensitivity to ORNs from AL neurons, modulating innate food searching behaviors under internal and external cues [38]. Given the importance of peripheral olfactory modulation by neuropeptides, food-searching behaviors are also controlled by regulating the insulin signaling in ORNs [6]. It has also been reported that tachykinin receptors and short neuropeptide F receptors (sNPFR) are affected by starvation via insulin signaling in Drosophila ORNs [6,39]. These studies also imply that low olfactory responses at a day time enhance the food searching behavior in fruit flies [40]. Likewise, lower olfactory sensitivity has been demonstrated at dusk compared to day time and dawn in a cockroach at which cockroaches habitually show strong food preference [25], thus they may share similar molecular and neural mechanisms modulating olfactory sensitivity.
Similar peptidergic pathways for the modulation of olfactory sensitivity also exist in vertebrate systems. In Mexican salamander (Ambystoma mexicanum), centrifugal peptidergic neurons modulate olfactory responses associated with hunger [41]. Moreover, the tetrapeptide Phe-Met-Arg-Phe-NH2 (FMRF-amide) has shown to modulate the neural activities of ORNs in the olfactory epithelium of the mouse and the salamander [42,43]. Recently, several studies has shed light on the olfactory modulation in ORNs in peripheral sites, where acetylcholine is released by micovillar cells of the olfactory epithelium [44] and dopamine is released into the olfactory mucus triggered by exposure to irritants [45]. Another study has found the hormone, leptin and its receptors in olfactory mucosa [7]. Neuropeptide Y (NPY), associated with the regulation of satiety signal of hypothalamus in vertebrates, has also been shown to modulate olfactory responses to a food-related odorant in rat olfactory mucosa by using electro-olfactogram (EOG), where NPY application increase EOG amplitudes in starved rats but not in fed ones [46]. Unlike peripheral modulation, there are ample reports on olfactory sensitivity changes by central mechanisms focused on CNS [19,47-49].
Our results have demonstrated that levels of TK regulate the olfactory responses in ORNs of the peripheral olfactory organs. The results suggest that the reduction of olfactory responses by TK reflects either a decrease of the number of responding neurons or efficiency of olfactory signal transduction in ORNs. However, the mechanism by which TK alters olfactory sensitivity in ORNs remains unclear. Further experiments are necessary to demonstrate which olfactory signal transduction components are involved in TK-related signaling in ORNs. Our study has also demonstrated that TK-mediated neural circuits exist in antennal olfactory systems. Although neural circuits associated with sensory facilitation by peptidergic presynaptic modulation has been reported in the ALs of the brain [6,49], our results indicate that TK may have an important role on the fine tuning of peripheral olfactory responses in ORNs, which in turn may subsequently facilitate olfactory perception and recognition. One potential hypothesis is that the release of octopamine into the hemolymph of the antennal heart ultimately activates receptors in antennal neurons which in response release tachykinin. Tachykinin, in turn, could activate its receptors in the ORN to modulate olfactory responses (Figure 6).
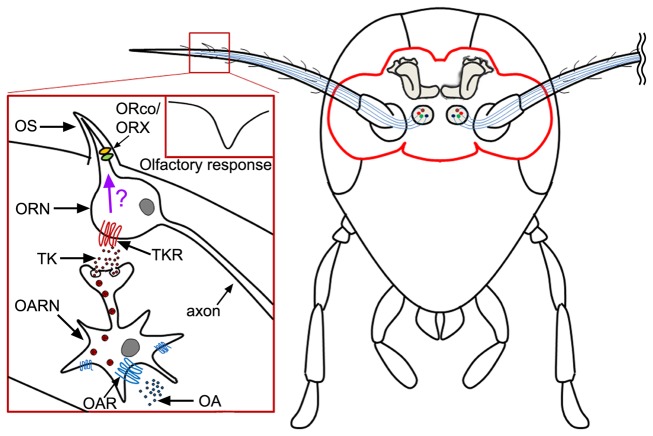
Figure 6. Schematic diagram of olfactory neuromodulation models in the peripheral olfactory organ in the cockroach.
Tachykinin is produced from octopamine receptor neurons in antennae. The tachykinin displays the neuro-inhibitory function on olfactory sensitivity changes in the antennal ORNs by unknown mechanisms (arrow with a question mark). OA: Octopamine, OARN: Octopamine receptor neuron, ORN: Odorant receptor neuron, OS: Olfactory sensillum, TK: Tachykinin, TKR: Tachykinin receptor.
A recent study has also shown that the cyclic nucleotides cAMP and cGMP modulate the olfactory sensitivities in ORNs by octopamine and adaptation to pheromone concentration, respectively [50]. Thus, it will be interesting to observe whether there are correlations between TKR components and these cyclic nucleotides in ORNs. Taken together, our study indicates that neuronal networks are present in the peripheral olfactory organ in the cockroach, which is regulated by neuropeptides and biogenic amine systems.
Supporting Information
Gene-specific primers used for qRT-PCR and preparation for RNA probes.
(DOCX)
Active forms of tachykinin peptides in Periplaneta americana.
(DOCX)
Acknowledgments
We appreciate Dr. Leslie Vosshall at Rockefeller University and Dr. Yoon-Seong Park at Kansas State University for kind gifts for antibodies.
Funding Statement
This work was supported by grants from the World Class University program (R31-10056) and Basic Science Research Program (No. 2012-008656) through the Korea Science and Engineering Foundation funded by the National Research Foundation of Korea to HWK and by National Science Foundation Grant #IOS-1145605 to TLP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kaupp UB (2010) Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci 11: 188–200. PubMed: 20145624. [DOI] [PubMed] [Google Scholar]
- 2. Prud’homme MJ, Lacroix MC, Badonnel K, Gougis S, Baly C et al. (2009) Nutritional status modulates behavioural and olfactory bulb Fos responses to isoamyl acetate or food odour in rats: roles of orexins and leptin. Neuroscience 162: 1287–1298. doi: 10.1016/j.neuroscience.2009.05.043. PubMed: 19477242. [DOI] [PubMed] [Google Scholar]
- 3. Granados-Fuentes D, Tseng A, Herzog ED (2006) A circadian clock in the olfactory bulb controls olfactory responsivity. J Neurosci Off J Soc Neurosci 26: 12219–12225. doi: 10.1523/JNEUROSCI.3445-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merlin C, Lucas P, Rochat D, François M-C, Maïbèche-Coisne M et al. (2007) An Antennal Circadian Clock and Circadian Rhythms in Peripheral Pheromone Reception in the Moth Spodoptera littoralis. J Biol Rhythms 22: 502–514. doi: 10.1177/0748730407307737. PubMed: 18057325. [DOI] [PubMed] [Google Scholar]
- 5. Caillol M, Aı ¨oun J, Baly C, Persuy M-A, Salesse R (2003) Localization of orexins and their receptors in the rat olfactory system: possible modulation of olfactory perception by a neuropeptide synthetized centrally or locally. Brain Res 960: 48–61. doi: 10.1016/S0006-8993(02)03755-1. PubMed: 12505657. [DOI] [PubMed] [Google Scholar]
- 6. Root CM, Ko KI, Jafari A, Wang JW (2011) Presynaptic Facilitation by Neuropeptide Signaling Mediates Odor-Driven Food Search. Cell 145: 133–144. doi: 10.1016/j.cell.2011.02.008. PubMed: 21458672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baly C, Aioun J, Badonnel K, Lacroix M-C, Durieux D et al. (2007) Leptin and its receptors are present in the rat olfactory mucosa and modulated by the nutritional status. Brain Res 1129: 130–141. doi: 10.1016/j.brainres.2006.10.030. PubMed: 17169337. [DOI] [PubMed] [Google Scholar]
- 8. Leinwand SG, Chalasani SH (2011) Olfactory networks: from sensation to perception. Curr Opin Genet Dev 21: 806–811. doi: 10.1016/j.gde.2011.07.006. PubMed: 21889328. [DOI] [PubMed] [Google Scholar]
- 9. Carlsson MA, Diesner M, Schachtner J, Nässel DR (2010) Multiple neuropeptides in the Drosophila antennal lobe suggest complex modulatory circuits. J Comp Neurol 518: 3359–3380. doi: 10.1002/cne.22405. PubMed: 20575072. [DOI] [PubMed] [Google Scholar]
- 10. Roeder T (1999) Octopamine in invertebrates. Prog Neurobiol 59: 533–561. doi: 10.1016/S0301-0082(99)00016-7. PubMed: 10515667. [DOI] [PubMed] [Google Scholar]
- 11. Möbius P, Penzlin HP M, H P (1992) Stress-induced release of octopamine in the American cockroach Periplaneta americana L. Acta Biol Hung 44: 45–50 PubMed; : 8493851 [Google Scholar]
- 12. Zhukovskaya MI (2008) Selective regulation of sensitivity to odours of different behavioural significance in the American cockroach, Periplaneta americana. Physiol Entomol 33: 162–166. doi: 10.1111/j.1365-3032.2008.00615.x. [DOI] [Google Scholar]
- 13. Meer RKV, Preston CA, Hefetz A (2008) Queen regulates biogenic amine level and nestmate recognition in workers of the fire ant, Solenopsis invicta. Naturwissenschaften 95: 1155–1158. doi: 10.1007/s00114-008-0432-6. PubMed: 18704354. [DOI] [PubMed] [Google Scholar]
- 14. Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM et al. (2004) Tachykinins and tachykinin receptors: a growing family. Life Sci 74: 1445–1463. doi: 10.1016/j.lfs.2003.09.039. PubMed: 14729395. [DOI] [PubMed] [Google Scholar]
- 15. Predel R, Neupert S, Roth S, Derst C, Nässel DR (2005) Tachykinin-related peptide precursors in two cockroach species. FEBS J 272: 3365–3375. doi: 10.1111/j.1742-4658.2005.04752.x. PubMed: 15978042. [DOI] [PubMed] [Google Scholar]
- 16. Takeuchi H, Yasuda A, Yasuda-Kamatani Y, Sawata M, Matsuo Y et al. (2004) Prepro-tachykinin gene expression in the brain of the honeybee Apis mellifera. Cell Tissue Res 316: 281–293. doi: 10.1007/s00441-004-0865-y. PubMed: 14999560. [DOI] [PubMed] [Google Scholar]
- 17. Meola SM, Clottens FL, Holman GM, Nachman RJ, Nichols R et al. (1998) Isolation and Immunocytochemical Characterization of Three Tachykinin-Related Peptides from the Mosquito, Culex salinarius. Neurochem Res 23: 189–202. doi: 10.1023/A:1022432909360. PubMed: 9475514. [DOI] [PubMed] [Google Scholar]
- 18. Winther ÅME, Acebes A, Ferrús A (2006) Tachykinin-related peptides modulate odor perception and locomotor activity in Drosophila. Mol Cell Neurosci 31: 399–406. doi: 10.1016/j.mcn.2005.10.010. PubMed: 16289899. [DOI] [PubMed] [Google Scholar]
- 19. Ignell R, Root CM, Birse RT, Wang JW, Nässel DR et al. (2009) Presynaptic peptidergic modulation of olfactory receptor neurons in Drosophila. Proc Natl Acad Sci USA 106: 13070–13075. doi: 10.1073/pnas.0813004106. PubMed: 19625621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saifullah ASM, Page TL (2009) Circadian regulation of olfactory receptor neurons in the cockroach antenna. J Biol Rhythms 24: 144–152. doi: 10.1177/0748730408331166. PubMed: 19346451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwon H-W, Lu T, Rützler M, Zwiebel LJ (2006) Olfactory responses in a gustatory organ of the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci U S A 103: 13526–13531. doi: 10.1073/pnas.0601107103. PubMed: 16938890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johard HA, Muren JE, Nichols R, Larhammar DS, Nässel DR (2001) A putative tachykinin receptor in the cockroach brain: molecular cloning and analysis of expression by means of antisera to portions of the receptor protein. Brain Res 919: 94–105. doi: 10.1016/S0006-8993(01)03004-9. PubMed: 11689166. [DOI] [PubMed] [Google Scholar]
- 23. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29: e45–e45. doi: 10.1093/nar/29.9.e45. PubMed: 11328886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schoofs L, Broeck JV, De Loof A (1993) The myotropic peptides of Locusta migratoria: Structures, distribution, functions and receptors. Insect Biochem Mol Biol 23: 859–881. doi: 10.1016/0965-1748(93)90104-Z. PubMed: 8220386. [DOI] [PubMed] [Google Scholar]
- 25. Page TL, Koelling E (2003) Circadian rhythm in olfactory response in the antennae controlled by the optic lobe in the cockroach. J Insect Physiol 49: 697–707. doi: 10.1016/S0022-1910(03)00071-4. PubMed: 12837322. [DOI] [PubMed] [Google Scholar]
- 26. Bischof LJ, Enan EE (2004) Cloning, expression and functional analysis of an octopamine receptor from Periplaneta americana. Insect Biochem Mol Biol 34: 511–521. doi: 10.1016/j.ibmb.2004.02.003. PubMed: 15147753. [DOI] [PubMed] [Google Scholar]
- 27. Jones WD, Nguyen T-AT, Kloss B, Lee KJ, Vosshall LB (2005) Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr Biol CB 15: R119–R121. doi: 10.1016/j.cub.2005.02.007. PubMed: 15723778. [DOI] [PubMed] [Google Scholar]
- 28. Zhukovskaya MI (2012) Modulation by Octopamine of Olfactory Responses to Nonpheromone Odorants in the Cockroach, Periplaneta americana L. Chem Sens. 37: 421-429. doi: 10.1093/chemse/bjr121. PubMed: 22281532. [DOI] [PubMed] [Google Scholar]
- 29. Zhukovskaya MI, Kapitsky SV (2006) Activity modulation in cockroach sensillum: The role of octopamine. J Insect Physiol 52: 76–86. doi: 10.1016/j.jinsphys.2005.09.005. PubMed: 16256134. [DOI] [PubMed] [Google Scholar]
- 30. Flecke C, Stengl M (2009) Octopamine and tyramine modulate pheromone-sensitive olfactory sensilla of the hawkmoth Manduca sexta in a time-dependent manner. J Comp Physiol Neuroethol Sens Neural Behav Physiol 195: 529–545. doi: 10.1007/s00359-009-0429-4. [DOI] [PubMed] [Google Scholar]
- 31. Pophof B (2000) Octopamine modulates the sensitivity of silkmoth pheromone receptor neurons. J Comp Physiol 186: 307–313. doi: 10.1007/s003590050431. PubMed: 10757246. [DOI] [PubMed] [Google Scholar]
- 32. Grosmaitre X, Marion-Poll F, Renou M (2001) Biogenic amines modulate olfactory receptor neurons firing activity in Mamestra brassicae. Chem Sens 26: 653–661. doi: 10.1093/chemse/26.6.653. PubMed: 11473931. [DOI] [PubMed] [Google Scholar]
- 33. Lim-Kessler CCm, Bolbecker AR, Li J, Wasserman GS (2008) Visual efference in Limulus: In vitro temperature-dependent neuromodulation of photoreceptor potential timing by octopamine and substance P. Vis Neurosci 25: 83–94. PubMed: 18282313. [DOI] [PubMed] [Google Scholar]
- 34. Bolbecker AR, Lim-Kessler CCM, Li J, Swan A, Lewis A et al. (2009) Visual Efference Neuromodulates Retinal Timing: In Vivo Roles of Octopamine, Substance P, Circadian Phase, and Efferent Activation in Limulus. J Neurophysiol 102: 1132–1138. doi: 10.1152/jn.91167.2008. PubMed: 19535477. [DOI] [PubMed] [Google Scholar]
- 35. Von Nickisch-Rosenegk E, Krieger J, Kubick S, Laage R, Strobel J et al. (1996) Cloning of biogenic amine receptors from moths (Bombyx mori and Heliothis virescens). Insect Biochem Mol Biol 26: 817–827. doi: 10.1016/S0965-1748(96)00031-8. PubMed: 9014328. [DOI] [PubMed] [Google Scholar]
- 36. Lucero MT (2013) Peripheral modulation of smell: Fact or fiction? Semin Cell Dev Biol 24: 58–70. doi: 10.1016/j.semcdb.2012.09.001. PubMed: 22986099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seki Y, Rybak J, Wicher D, Sachse S, Hansson BS (2010) Physiological and Morphological Characterization of Local Interneurons in the Drosophila Antennal Lobe. J Neurophysiol 104: 1007–1019. doi: 10.1152/jn.00249.2010. PubMed: 20505124. [DOI] [PubMed] [Google Scholar]
- 38. Hewes RS, Taghert PH (2001) Neuropeptides and Neuropeptide Receptors in the Drosophila melanogaster Genome. Genome Res 11: 1126–1142. doi: 10.1101/gr.169901. PubMed: 11381038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Birse RT, Söderberg JAE, Luo J, Winther ÅME, Nässel DR (2011) Regulation of insulin-producing cells in the adult Drosophila brain via the tachykinin peptide receptor DTKR. J Exp Biol 214: 4201–4208. doi: 10.1242/jeb.062091. PubMed: 22116763. [DOI] [PubMed] [Google Scholar]
- 40. Krishnan B, Dryer SE, Hardin PE (1999) Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature 400: 375–378. doi: 10.1038/22566. PubMed: 10432117. [DOI] [PubMed] [Google Scholar]
- 41. Mousley A, Polese G, Marks NJ, Eisthen HL (2006) Terminal Nerve-Derived Neuropeptide Y Modulates Physiological Responses in the Olfactory Epithelium of Hungry Axolotls (Ambystoma mexicanum). J Neurosci 26: 7707–7717. doi: 10.1523/JNEUROSCI.1977-06.2006. PubMed: 16855098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ni M-M, Luo Y, Liu J, Liao D-Q, Tang Y-D (2008) FMRFamide MODULATES OUTWARD POTASSIUM CURRENTS IN MOUSE OLFACTORY SENSORY NEURONS. Clin Exp Pharmacol Physiol 35: 563–567. doi: 10.1111/j.1440-1681.2007.04840.x. PubMed: 18067588. [DOI] [PubMed] [Google Scholar]
- 43. Park D, Zawacki SR, Eisthen HL (2003) Olfactory Signal Modulation by Molluscan Cardioexcitatory Tetrapeptide (FMRFamide) in Axolotls (Ambystoma mexicanum). Chem Sens 28: 339–348. doi: 10.1093/chemse/28.4.339. PubMed: 12771020. [DOI] [PubMed] [Google Scholar]
- 44. Ogura T, Szebenyi SA, Krosnowski K, Sathyanesan A, Jackson J et al. (2011) Cholinergic microvillous cells in the mouse main olfactory epithelium and effect of acetylcholine on olfactory sensory neurons and supporting cells. J Neurophysiol 106: 1274–1287. doi: 10.1152/jn.00186.2011. PubMed: 21676931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lucero MT, Squires A (1998) Catecholamine concentrations in rat nasal mucus are modulated by trigeminal stimulation of the nasal cavity. Brain Res 807: 234–236. doi: 10.1016/S0006-8993(98)00825-7. PubMed: 9757051. [DOI] [PubMed] [Google Scholar]
- 46. Negroni J, Meunier N, Monnerie R, Salesse R, Baly C et al. (2012) Neuropeptide Y enhances olfactory mucosa responses to odorant in hungry rats. PLOS ONE 7: e45266. doi: 10.1371/journal.pone.0045266. PubMed: 23024812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF et al. (2010) Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci 13: 615–621. doi: 10.1038/nn.2526. PubMed: 20364145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kawai T, Abe H, Akazome Y, Oka Y (2010) Neuromodulatory Effect of GnRH on the Synaptic Transmission of the Olfactory Bulbar Neural Circuit in Goldfish, Carassius auratus. J Neurophysiol 104: 3540–3550. doi: 10.1152/jn.00639.2010. PubMed: 20962071. [DOI] [PubMed] [Google Scholar]
- 49. Wang JW (2012) Presynaptic modulation of early olfactory processing in Drosophila. Dev Neurobiol 72: 87–99. doi: 10.1002/dneu.20936. PubMed: 21688402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schendzielorz T, Peters W, Boekhoff I, Stengl M (2012) Time of Day Changes in Cyclic Nucleotides Are Modified via Octopamine and Pheromone in Antennae of the Madeira Cockroach. J Biol Rhythms 27: 388–397. doi: 10.1177/0748730412456265. PubMed: 23010661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene-specific primers used for qRT-PCR and preparation for RNA probes.
(DOCX)
Active forms of tachykinin peptides in Periplaneta americana.
(DOCX)