Abstract
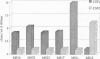
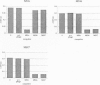
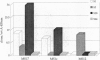
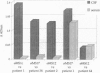
The study of the origin and pathogenetic relevance of the oligoclonal antibodies present in the cerebrospinal fluid (CSF) of multiple sclerosis (MS) patients has been hampered by a lack of specific ligands. We recently reported a general strategy, based on phage-displayed random peptide libraries, to identify ligands for disease-specific antibodies even in the absence of any information on the nature of the pathologic antigen. With this procedure, we identified several peptides specifically recognized by antibodies present in the CSF of MS patients. Using these peptides as reagents, we demonstrated that they mimic different natural epitopes and react with antibodies enriched in the CSF of MS patients. Antibodies recognizing the selected peptides are commonly found with equal frequency in the sera of MS patients and of normal individuals. In contrast, the repertoire of CSF antibodies appears to be individual-specific and is probably the result of a nonspecific immunodysregulation rather than a stereotyped response to a single antigen/agent.
Full text
PDF
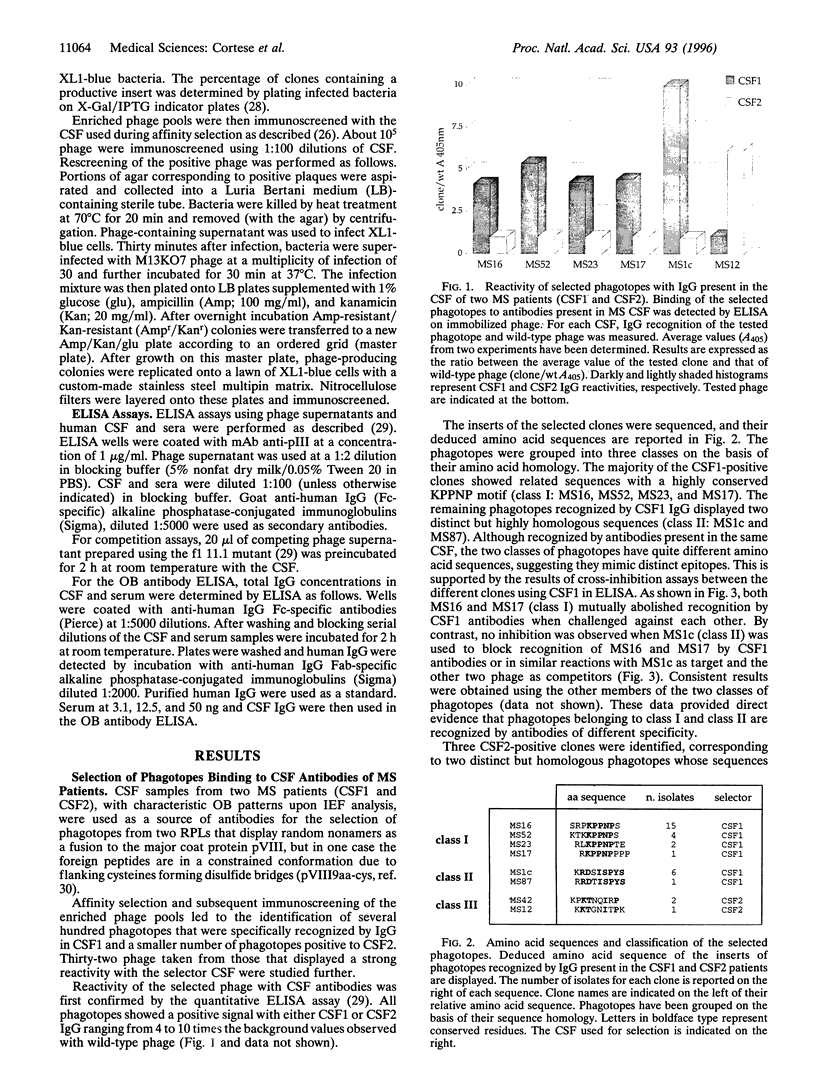
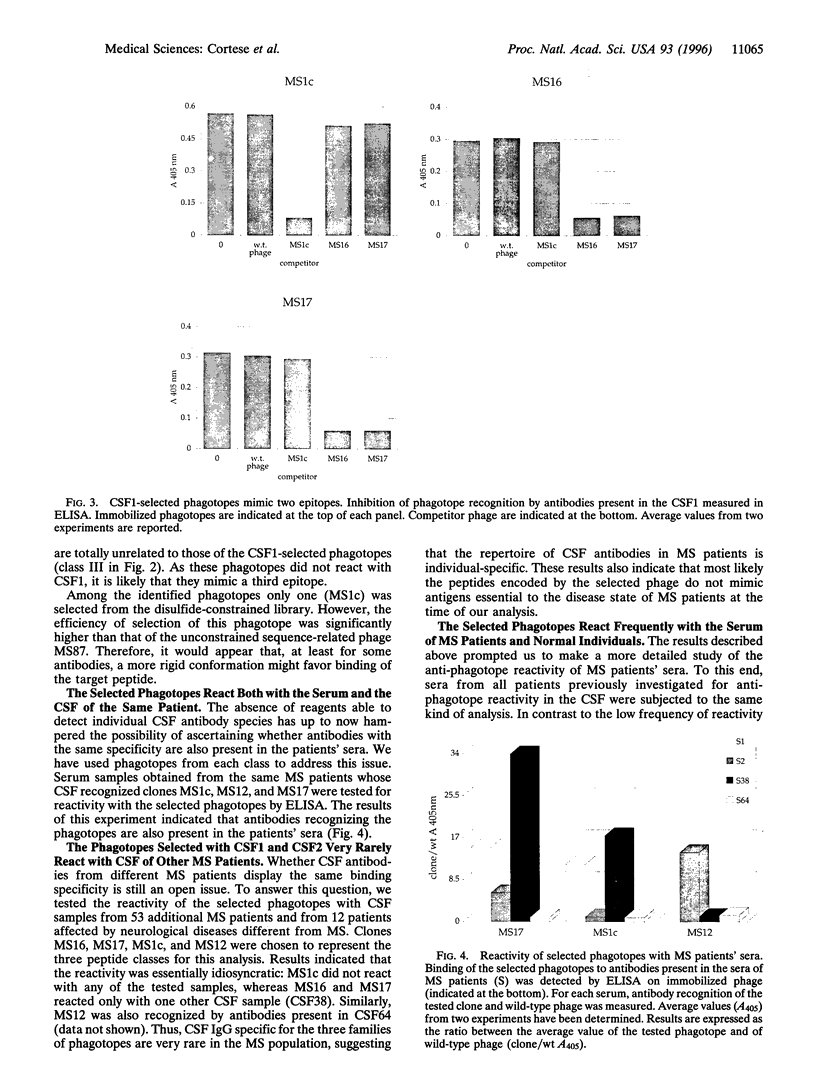
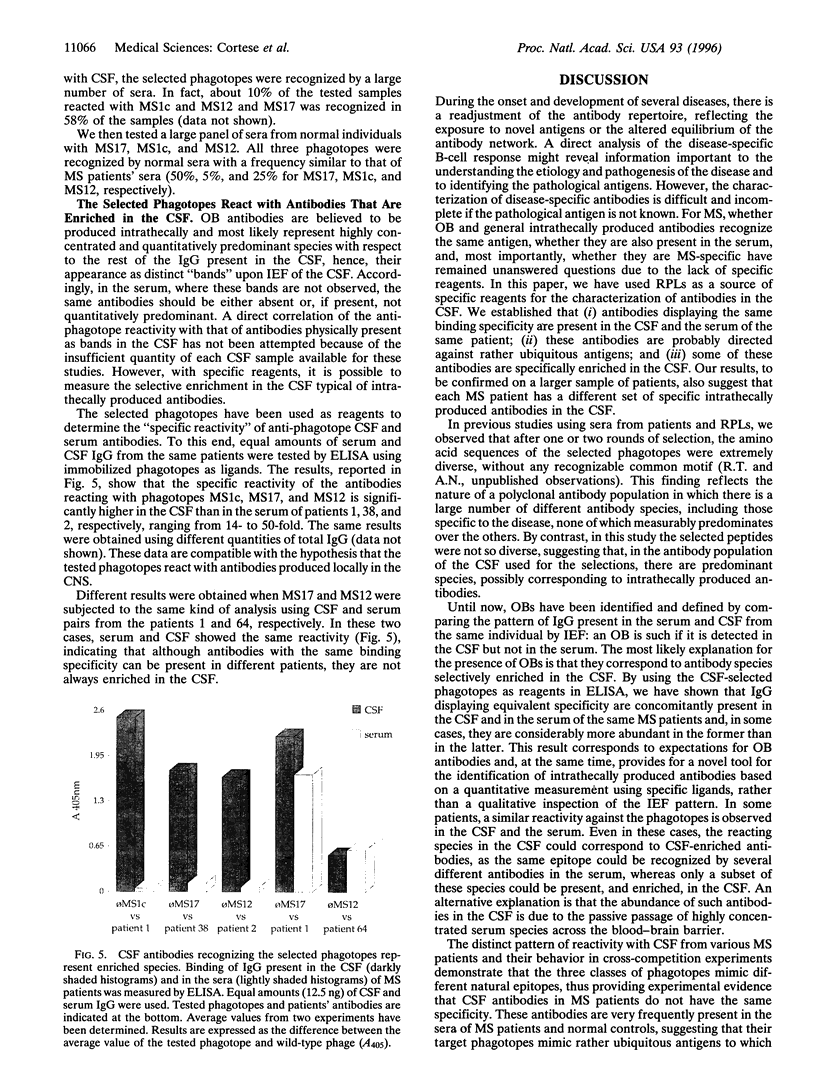

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cortese R., Felici F., Galfre G., Luzzago A., Monaci P., Nicosia A. Epitope discovery using peptide libraries displayed on phage. Trends Biotechnol. 1994 Jul;12(7):262–267. doi: 10.1016/0167-7799(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Cortese R., Monaci P., Nicosia A., Luzzago A., Felici F., Galfré G., Pessi A., Tramontano A., Sollazzo M. Identification of biologically active peptides using random libraries displayed on phage. Curr Opin Biotechnol. 1995 Feb;6(1):73–80. doi: 10.1016/0958-1669(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Delpech B., Lichtblau E. Etude quantitative des immunoglobulines G et de l'albumine du liquide cephalo rachidien. Clin Chim Acta. 1972 Mar;37:15–23. doi: 10.1016/0009-8981(72)90410-x. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Micheli G., Felici F., Folgori A., Luzzago A., Monaci P., Nicosia A., Delmastro P. Monoclonal antibodies that recognise filamentous phage: tools for phage display technology. Gene. 1994 Oct 11;148(1):7–13. doi: 10.1016/0378-1119(94)90227-5. [DOI] [PubMed] [Google Scholar]
- Dybwad A., Førre O., Natvig J. B., Sioud M. Structural characterization of peptides that bind synovial fluid antibodies from RA patients: a novel strategy for identification of disease-related epitopes using a random peptide library. Clin Immunol Immunopathol. 1995 Apr;75(1):45–50. doi: 10.1006/clin.1995.1051. [DOI] [PubMed] [Google Scholar]
- Ebers G. C. Oligoclonal banding in MS. Ann N Y Acad Sci. 1984;436:206–212. doi: 10.1111/j.1749-6632.1984.tb14791.x. [DOI] [PubMed] [Google Scholar]
- FRICK E., SCHEID-SEYDEL L. Untersuchungen mit J 131-markiertem gamma-Globulin zur Frage der Abstammung der Liquoreiweisskörper. Klin Wochenschr. 1958 Sep 15;36(18):857–863. doi: 10.1007/BF01485232. [DOI] [PubMed] [Google Scholar]
- Felici F., Castagnoli L., Musacchio A., Jappelli R., Cesareni G. Selection of antibody ligands from a large library of oligopeptides expressed on a multivalent exposition vector. J Mol Biol. 1991 Nov 20;222(2):301–310. doi: 10.1016/0022-2836(91)90213-p. [DOI] [PubMed] [Google Scholar]
- Folgori A., Tafi R., Meola A., Felici F., Galfré G., Cortese R., Monaci P., Nicosia A. A general strategy to identify mimotopes of pathological antigens using only random peptide libraries and human sera. EMBO J. 1994 May 1;13(9):2236–2243. doi: 10.1002/j.1460-2075.1994.tb06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryden A., Link H., Norrby E. Cerebrospinal fluid and serum immunoglobulins and antibody titers in mumps meningitis and aseptic meningitis of other etiology. Infect Immun. 1978 Sep;21(3):852–861. doi: 10.1128/iai.21.3.852-861.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey L. A., Trotter J. L. The use and abuse of the cerebrospinal fluid IgG profile in the adult: a practical evaluation. Ann Neurol. 1980 Oct;8(4):426–434. doi: 10.1002/ana.410080415. [DOI] [PubMed] [Google Scholar]
- Kabat E. A., Moore D. H., Landow H. AN ELECTROPHORETIC STUDY OF THE PROTEIN COMPONENTS IN CEREBROSPINAL FLUID AND THEIR RELATIONSHIP TO THE SERUM PROTEINS. J Clin Invest. 1942 Sep;21(5):571–577. doi: 10.1172/JCI101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterre E. C., Callewaert A., Heremans J. F., Sfaello Z. Electrophoretic morphology of gamma globulins in cerebrospinal fluid of multiple sclerosis and other diseases of the nervous system. Neurology. 1970 Oct;20(10):982–990. doi: 10.1212/wnl.20.10.982. [DOI] [PubMed] [Google Scholar]
- Link H., Kostulas V. Utility of isoelectric focusing of cerebrospinal fluid and serum on agarose evaluated for neurological patients. Clin Chem. 1983 May;29(5):810–815. [PubMed] [Google Scholar]
- Link H., Müller R. Immunoglobulins in multiple sclerosis and infections of the nervous system. Arch Neurol. 1971 Oct;25(4):326–344. doi: 10.1001/archneur.1971.00490040052007. [DOI] [PubMed] [Google Scholar]
- Link H., Zettervall O. Multiple sclerosis: disturbed kappa: lambda chain ratio of immunoglobulin G in cerebrospinal fluid. Clin Exp Immunol. 1970 Mar;6(3):435–438. [PMC free article] [PubMed] [Google Scholar]
- Luzzago A., Felici F., Tramontano A., Pessi A., Cortese R. Mimicking of discontinuous epitopes by phage-displayed peptides, I. Epitope mapping of human H ferritin using a phage library of constrained peptides. Gene. 1993 Jun 15;128(1):51–57. doi: 10.1016/0378-1119(93)90152-s. [DOI] [PubMed] [Google Scholar]
- Martin R., McFarland H. F., McFarlin D. E. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- McFarland H. F. The multiple sclerosis lesion. Ann Neurol. 1995 Apr;37(4):419–421. doi: 10.1002/ana.410370402. [DOI] [PubMed] [Google Scholar]
- Olsson J. E., Link H. Immunoglobulin abnormalities in multiple sclerosis. Relation to clinical parameters: exacerbations and remissions. Arch Neurol. 1973 Jun;28(6):392–399. doi: 10.1001/archneur.1973.00490240052009. [DOI] [PubMed] [Google Scholar]
- Poser C. M., Paty D. W., Scheinberg L., McDonald W. I., Davis F. A., Ebers G. C., Johnson K. P., Sibley W. A., Silberberg D. H., Tourtellotte W. W. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983 Mar;13(3):227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Reingold S. C. Advances in the understanding and treatment of multiple sclerosis. Boston, Massachusetts, USA, 28-29 October 1992. J Neuroimmunol. 1993 May;44(2):221–224. doi: 10.1016/0165-5728(93)90047-3. [DOI] [PubMed] [Google Scholar]
- Ryberg B., Jacque C. Are anti-brain antibodies in multiple sclerosis directed to myelin basic protein? Studies employing the Shiverer mouse mutant. Acta Neurol Scand. 1986 Mar;73(3):247–252. doi: 10.1111/j.1600-0404.1986.tb03270.x. [DOI] [PubMed] [Google Scholar]
- Sköldenberg B., Carlström A., Forsgren M., Norrby E. Transient appearance of oligoclonal immunoglobulins and measles virus antibodies in the cerebrospinal fluid in a case of acute measles encephalitis. Clin Exp Immunol. 1976 Mar;23(3):451–455. [PMC free article] [PubMed] [Google Scholar]
- Smith G. P. Surface presentation of protein epitopes using bacteriophage expression systems. Curr Opin Biotechnol. 1991 Oct;2(5):668–673. doi: 10.1016/0958-1669(91)90032-z. [DOI] [PubMed] [Google Scholar]
- Tibbling G., Link H., Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest. 1977 Sep;37(5):385–390. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- Weil M. L., Itabashi H., Cremer N. E., Oshiro L., Lennette E. H., Carnay L. Chronic progressive panencephalitis due to rubella virus simulating subacute sclerosing panencephalitis. N Engl J Med. 1975 May 8;292(19):994–998. doi: 10.1056/NEJM197505082921903. [DOI] [PubMed] [Google Scholar]