Abstract
Objective
To validate malnutrition screening tool of nutrition risk index (NRI) against patient-generated subjective global assessment (PG-SGA) as a gold standard tool in colorectal cancer patients before radiotherapy.
Methods
Nutritional status of 52 volunteer colorectal cancer patients with a mean age of 54.1±16.8 years who referred to radiotherapy center were assessed by PG-SGA (gold standard method) and NRI. Serum albumin levels of patients were determined by colorimetric method. A contingency table was used to determine the sensitivity, specificity, and predictive value of the NRI in screening patients at risk of malnutrition, in comparison with the PG-SGA in patients before radiotherapy.
Results
The findings of PG-SGA and NRI showed that 52% and 45% of patients in our study were moderately or severely malnourished respectively. The NRI had a sensitivity of 66% and a specificity of 60% against PG-SGA. The positive predictive value was 64% and the negative predicative value was 62%. The agreement between NRI and PG-SGA was statistically insignificant (kappa =0.267; P>0.05).
Conclusions
The findings of present study showed that the prevalence of malnutrition was high in patients with colorectal cancer. Moreover, NRI method had low sensitivity and specificity in assessing nutritional status of patients with cancer. It seems that the combination of anthropometric, laboratory parameters and a subjective scoring system may be helpful tools in screening of malnutrition in cancer patients.
Keywords: Colorectal cancer patients, malnutrition, patient-generated subjective global assessment, nutrition risk index (NRI)
Introduction
Supportive care is becoming increasingly important in the management of cancer patients. Nowadays, oncologists aim to positively influence their quality of life and nutritional status in addition to improving survival rates (1). It is well known that diet and nutrition play important roles during the clinical course of cancer treatments (2). The incidence of malnutrition in patients with cancer ranges from 31% to 87% (3) and its risk and severity are affected by tumor type, stage of disease, and the type of anticancer therapy applied (4). Moreover, malnutrition in cancer patients has many consequences. These include increased risk of complications, decreased response and tolerance to treatments, a lower quality of life, reduced survival, and higher health care costs (4-6). Thus, early detection of malnutrition and dietary intervention can be the important factor in preventing symptoms of malnutrition (7).
It is critical that cancer patients undergo nutritional assessments using highly sensitive and specific tools in all stages of anticancer therapy: from baseline at diagnosis, to the beginning and duration of anticancer treatments (4). Various tools, such as patient-generated subjective global assessment (PG-SGA), nutrition risk index (NRI) and malnutrition universal screening tool (MUST), are available to assess the nutritional status of cancer patients (8). Among these different tools, PG-SGA is considered to be the most valid and useful tool. The oncology nutrition dietetic practice groups of the American Dietetic Association and Australian Dietetic Association recommend it as the standard tool for nutrition assessment of patients with cancer (9). Bauer et al. reported that PG-SGA had high sensitivity of 98% and specificity of 82% in determining malnutrition in cancer patients (10). It has also been suggested that PG-SGA provides a suitable gold standard tool against which other nutrition screening tools can be evaluated (11). Although PG-SGA is easy to use, cost-effective, and valid (12), most oncology departments do not use it to assess their patients’ nutritional statuses (11). Instead, they evaluate nutritional status by measuring serum albumin levels or weight changes, elements of the NRI screening tool.
NRI is an objective nutritional screening tool that is based on serum albumin and weight loss (8) and there are few studies validating NRI as a malnutrition screening tool in cancer outpatients. We aim at validating NRI against PG-SGA in colorectal cancer patients before they undergo radiotherapy.
Methods and materials
Subjects
In this study, fifty-two volunteer patients with colorectal cancer (40 males and 12 females) were recruited. Patients had a mean age of 54.1±16.8 years, and were referred to the radiotherapy center of Imam Khomeini hospital. Protocol of present study was approved by Ethics Committee of Tabriz University of Medical Sciences. Inclusion criteria included ambulatory colorectal cancer patients, who were slated to receive standard radiotherapy treatment. Exclusion criteria were: history of previous cancer treatments, patients with diabetes, and liver or endocrine dysfunction.
Data collection
Before radiotherapy, a nutritionist assessed the nutritional status of all patients. Height was measured using a mounted tape, with the subject’s arm hanging freely by their sides, and recorded to the nearest 0.5 cm. After ensuring that subjects were barefoot and wore light clothing, their weight was recorded to the nearest 0.1 kg with a Seca scale. Nutritional status of patients was assessed by PG-SGA and NRI. Both tools address:
Weight changes, symptoms (anorexia, nausea, constipation, mouth sores, vomiting, diarrhea, dry mouth, hypogeusia, and dysphagia), alterations in food intake, and functional capacity;
Components of metabolic stress (sepsis, neutropenic or tumor fever, corticosteroids) and physical examination, subcutaneous fat, ankle/sacral edema, or ascites. By this tool, nutritional status is categorized as normal (PG-SGA A), moderate (PG-SGA B) or severe malnutrition (PG-SGA C).
NRI was calculated on the basis of this equation: 1.519 (serum albumin; g/dL) + 41.7 (current weight/usual weight). NRI >100 indicates that the patient is not malnourished, 97.5-100 indicates mild malnourishment, 83.5-97.5 indicates moderate malnourishment, and NRI <83.5 indicates severe malnourishment (13).
Blood samples were collected after an overnight fasting of 12 h. Serum albumin was measured by the colorimetric method (14).
Statistical analysis
A contingency table was used to determine the sensitivity, specificity, and predictive value of NRI in screening patients at risk of malnutrition, in comparison with the PG-SGA. The positive likelihood ratio (sensitivity/1-specificity) and negative likelihood ratio (1-sensitivity/specificity) were calculated. Agreement between PG-SGA and NRI was analyzed by Kappa tests. P<0.05 was considered statistically significant.
Results
Prevalence of malnutrition
Patients’ characteristics are shown in Table 1. Nutritional status of patients on the basis of PG-SGA and NRI are presented in Figure 1. According to PG-SGA, 48% of patients were well nourished, and 33% and 19% of patients were moderately and severely malnourished, respectively. The results of NRI showed that 35% of patients were well nourished, and 35% and 10% of patients were moderately malnourished or at risk of severe malnutrition, respectively.
Table 1. Baseline characteristics of patients with colorectal cancer (N=52).
| Baseline characteristics | N |
|---|---|
| Age (year) | 54.1±16.8 |
| Gender | |
| Female | 12 |
| Male | 40 |
| Weight (kg) | 63.95±13.4 |
| Height (cm) | 164.09±11.44 |
| BMI (kg/m2) | 23.85±4.9 |
| Stage of tumor | |
| II | 20 |
| III | 25 |
| IV | 7 |
Figure 1.
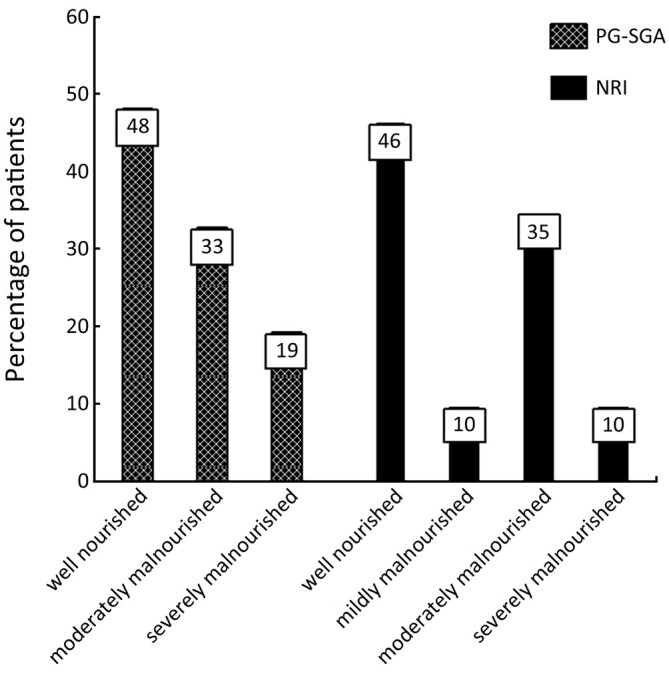
Nutritional status of patients according to PG-SGA and NRI.
Validation of NRI against PG-SGA
Comparison of NRI in screening of malnutrition against PG-SGA is indicated in Table 2. Twenty-nine percent of well nourished (true negative) patients and 34.6% of malnourished patients (true positive) were correctly classified by the NRI. Nineteen percent and seventeen percent of patients were misclassified as being malnourished (false positive) and well nourished (false negative), respectively.
Table 2. Comparison of NRI in screening of malnutrition against PG-SGA in cancer patients.
| Malnourished (PG-SGA B and C) | Well nourished (PG-SGA A) | Total | |
|---|---|---|---|
| Malnourished (NRI: <83.5-100) | 18 (TP) | 10 (FP) | 28 |
| Not at risk of malnutrition (NRI >100) | 9 (FN) | 15 (TN) | 24 |
| Total | 27 | 25 | 52 |
TP, true positive; FP, false positive; TN, true Negative; FN, false negative.
In comparison with PG-SGA, NRI had a sensitivity of 66% and a specificity of 60%. The positive predictive value was 64% and the negative predicative value was 62%. The positive likelihood ratio and negative likelihood ratio were 1.65 and 0.56, respectively. The agreement between NRI and PG-SGA was statistically insignificant (kappa =0.267; P>0.05).
Discussion
The findings of PG-SGA and NRI showed that 52% and 45% of patients in our study were moderately or severely malnourished, respectively. These results were in agreement with the findings of previous studies, which had reported higher prevalence of malnutrition in colorectal cancer patients (15,16). Malnutrition is a common problem in cancer patients and it is associated with increased risk of complications and decreased response and tolerance to anticancer treatments (4,5). Early detection of malnutrition would ideally allow early interventions which may prevent later complications (7), but different oncology departments do not make nutritional assessments with valid and standard tools (11). McWhirter et al. noted that up to 52% of malnourished cancer patients were not detected based on their nutritional documentation (17). The result of our present study indicated that NRI had low sensitivity (66%) and specificity (60%) in comparison with PG-SGA. We examined the validation of NRI against PG-SGA in cancer patients before radiotherapy. The findings of only one study which carried out by Ryu et al. indicated that NRI had a sensitivity of 72.7% and a specificity of 40% against SGA in patients with gastric cancer (18). Bauer et al. has already reported that PG-SGA, in comparison with SGA, has higher sensitivity and specificity (98% and 82%, respectively) in cancer patients (10). Thus, NRI may have low sensitivity and specificity in comparison with SGA. The results of present study showed that NRI had low sensitivity and specificity against PG-SGA, and these results were in line with the findings of former mentioned study (18).
In hospitalized patients, results of Galvan et al.’s study indicated that NRI had a sensitivity of 33% and a specificity of 92% (19) in determining malnutrition. Also Doley et al. reported that NRI had low sensitivity (71%) and high specificity (90%) in inpatients (20). In spite of low sensitivity of NRI in hospitalized patients, some researchers have suggested that it is a useful tool in identifying patients at risk for postoperative complication (21,22).
Taking into account, then, that NRI is based on the serum albumin concentration, low sensitivity and specificity of NRI against PG-SGA may be due to non-nutritional factors such as fluid overload, inflammation, renal and liver diseases that influence albumin synthesis (23). Since low serum albumin does not always indicate malnutrition, it can be concluded that serum albumin may not be as sensitive as anthropometric measurements in the assessment of nutritional status in cancer patients.
In conclusion, the findings of present study showed that the prevalence of malnutrition was high in patients with colorectal cancer before radiotherapy. Moreover, our results indicated that NRI had low sensitivity and specificity in assessing nutritional status of patients with cancer. Since each method has its own advantages and disadvantages, it seems that a combination of anthropometric, laboratory parameters and a subjective scoring system may be helpful tools in the screening of malnutrition in cancer patients.
Acknowledgements
This article was written based on a dataset of PhD thesis (Elnaz Faramarzi) registered in Tabriz university of Medical sciences. The authors are grateful for the financial support of Nutrition Research Center, Tabriz University of Medical Sciences.
Disclosure: The authors declare no conflict of interest.
References
- 1.Ottery FD. Supportive nutrition to prevent cachexia and improve quality of life. Semin Oncol 1995;22:98-111 [PubMed] [Google Scholar]
- 2.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62:243-74 [DOI] [PubMed] [Google Scholar]
- 3.Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Coperative Oncology Group. Am J Med 1980;69:491-7 [DOI] [PubMed] [Google Scholar]
- 4.Nitenberg G, Raynard B. Nutritional support of the cancer patient: issues and dilemmas. Crit Rev oncol Hemato 2000;34:137-68. [DOI] [PubMed]
- 5.Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996;12:S15-9 [DOI] [PubMed] [Google Scholar]
- 6.Langer CJ, Hoffman JP, Ottery FD. Clinical significance of weight loss in cancer patients: Rationale for the use of anabolic agents in treatment of cancer-related cachexia. Nutrition 2001;17:S1-20 [DOI] [PubMed] [Google Scholar]
- 7.Santarpia L, Contaldo F, Pasanisi F.Nutritional screening and early treatment of malnutrition in cancer patients. J Cachexia Sarcopenia Muscle 2011;2:27-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patricia Fuhrman M. Nutrition support for oncology patients. In: Marrian M, Roberts S. eds. Clinical nutrition for oncology patients. United States of America: Jones & Bartlett, 2010:32. [Google Scholar]
- 9.Bauer JD, Ash S, Davidson WL, et al. Evidence-based practice guidelines for the nutritional management of cancer cachexia. Nutr Diet 2006;63:S3-32 [Google Scholar]
- 10.Bauer J, Capra S, Ferguson M.Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in cancer patients. Eur J Clin Nutr 2002;56:779-85 [DOI] [PubMed] [Google Scholar]
- 11.Lewis S. Nutrition screening. In: Shaw C. eds. Nutrition and cancer. Singapore: Wiley Blackwell, 2011:85,88. [Google Scholar]
- 12.Patricia Fuhrman M. Nutrition support for oncology patients. In: Marrian M, Roberts S. eds. Clinical nutrition for oncology patients. United States of America: Jones &Bartlett, 2010:38. [Google Scholar]
- 13.Width M, Reinhard T. The clinical dietitian’s essential pocket guide. Philadelphia: Lippincott Williams &Wilkins, 2009. [Google Scholar]
- 14.Johnson AM, Rohlfs EM, Silverman LM. Proteins. In: Burtis CA, Ashwood ER, Bruns DE. eds. Tietz Text Book of Clinical Chemistry and molecular diagnostic. 4th ed, Philadelphia: Saunders, 2006:546. [Google Scholar]
- 15.Gupta D, Lis CG, Granick J, et al. Malnutrition was associated with poor quality of life in colorectal cancer: a retrospective analysis. J Clin Epidemiol 2006;59:704-9 [DOI] [PubMed] [Google Scholar]
- 16.Gupta D, Lis CG, Dahlk SL, et al. The relationship between bioelectrical impedance phase angle and subjective global assessment in advanced colorectal cancer. Nutr J 2008;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ 1994;308:945-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryu SW, Kim IN. Comparison of different nutritional assessments in detecting malnutrition among gastric cancer patients. World J Gastroenterol 2010;16:3310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galvan O, Joannidis M, Widschwendter A, et al. Comparison of different scoring methods for assessing the nutritional status of hospitalised patients. Wien Klin Wochenschr 2004;116:596-602 [DOI] [PubMed] [Google Scholar]
- 20.Doyle MP, Barnes E, Moloney M. The evaluation of an under nutrition risk score to be used by nursing staff in a teaching hospital to identify surgical patients at risk of malnutrition onadmission: a pilot study. J Hum Nutr Diet 2000;13:433-41 [Google Scholar]
- 21.The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group : perioperative total parenteral nutrition in surgical patients. N Engl J Med 1991;325:525-32 [DOI] [PubMed] [Google Scholar]
- 22.Sungurtekin H, Sungurtekin U, Balci C, et al. The influence of nutritional status on complications after major intraabdominal surgery. J Am Coll Nutr 2004;23:227-32 [DOI] [PubMed] [Google Scholar]
- 23.Fanali G, di Masi A, Trezza V, et al. Human serum albumin: From bench to bedside. Mol Aspects Med 2012;33:209-90 [DOI] [PubMed] [Google Scholar]


