Abstract
Background
Obesity is more prevalent in asthmatics. Short sleep duration is a novel risk factor for obesity in general populations.
Objective
We tested the association of sleep duration and asthma characteristics with obesity.
Methods
Adults at tertiary clinics were surveyed on asthma symptoms and habitual sleep duration. Medical records were used to assess asthma severity step (1-4), extract height and weight, current medications and diagnosed comorbid conditions. BMI≥30 kg/m2 defined obesity. Habitual sleep was categorized as <6 (very short), 6 to <7h (short), 7-8h (normal), >8 to ≤9h (long) and >9h (very long). Inhaled corticosteroid doses were categorized as low, moderate and high.
Results
Among 611 participants (mean BMI 30±8), 249 (41%) were obese. After adjustment for covariates, obesity was associated with short and very long sleep: as compared to normal sleepers, the odds of being obese were on average 66% higher ([95% Confidence Interval: 1.07-2.57], p=0.02) among short and 124% higher ([1.08-1.65], p=0.03) among very long sleepers, and the association with very short sleep approached significance (1.74 [0.96-3.14], p=0.06). Obesity was also significantly related to highest asthma step (1.87 [1.09-3.21], p=0.02) and psychopathology (1.64 [1.08-2.48], p=0.02), and a trend was seen with high dose inhaled corticosteroids (1.82 [0.93-3.56], p=0.08).
Conclusions
Obesity in asthmatics is associated with shorter and very long sleep duration, worse asthma severity, psychopathology, and high dose inhaled corticosteroids. Although this cross-sectional study cannot prove causality, we speculate that further investigation of sleep may provide new opportunities to reduce the rising prevalence of obesity among asthmatics.
Keywords: asthma, sleep duration, obesity
1. Introduction
Obesity is more prevalent in individuals with asthma than those without asthma.1 While this relationship may be bidirectional, the vast majority of studies have focused on the influence obesity has on incident asthma or asthma control and underlying mechanisms.2, 3
Earlier studies have suggested that antecedent asthma may lead to obesity, however this direction of the relationship has received far less attention. In a large cross-sectional study of women of reproductive age, those who were overweight or obese were more likely to have had a history of asthma; women who had gained ≥20 kg during their adult years (since age 18) compared with those who maintained their weight had a 2.7-fold increased odds of asthma.4 Furthermore, in the Zurich Cohort Study—a prospective cohort study of 591 subjects followed for over 20 years—subjects with vs. those without asthma experienced a higher rate of weight gain.5 This relationship was stronger in women than men.5
What may predispose individuals with asthma to weight gain is unknown. It was initially hypothesized that poor asthma control may lead to a more sedentary lifestyle, which could result in more obesity in this population. However, energy expenditure and activity levels do not appear to be less when comparing asthmatic and non-asthmatic children.6, 7 For years, it has been thought that corticosteroids used to treat asthma might be associated with weight gain, as a side effect.8 More recent data has been less favorable to this association. While use of oral corticosteroids may affect body composition,9 self-reported use of more modern asthma medications was not associated with obesity in one large cross-sectional study of Swedish population.8 Although psychopathology appears to play important roles in the development and persistence of both asthma10 and obesity,11 this has rarely been considered as a shared risk factor in studies of asthma–obesity comorbidity.5 Depression on during childhood was associated with adult obesity and asthma, partially explaining the asthma-obesity comorbidity.5
Sleep duration is emerging as a novel risk factor for obesity in population-based studies.12-15 In a prospective cohort of young adults, there was a negative association between sleep duration and obesity.13 Furthermore, short sleep duration appears independently associated with weight gain, particularly in younger age.16 Asthmatics have shorter and more disrupted sleep than normal individuals, in relation with disease control.17, 18 For example, in a large sample representative of adult Israeli population (age 21 and older), almost 45% of asthmatics reported sleeping less than 6 hours/night.10
We aimed to study the relationship of asthma and related characteristics with obesity, to test potential risk factors for obesity in asthma. Specifically, we assessed the association of obesity with sleep duration, asthma itself, and related characteristics such as medications and comorbidities. We hypothesized that short sleep duration, asthma severity, corticosteroid use and psychopathology will be each related to obesity. A report of preliminary findings of this work was previously published.19
2. Methods
2.1. Subjects
The sample included asthma patients seen in routine follow-up at the Allergy and Pulmonary subspecialty clinics at the University of Michigan (UM)- Ann Arbor between February 2005 (when the question on habitual sleep duration was added to the original survey) to April 2006 and the University of Wisconsin (UW)- Madison between July 2007 and December 2008. Subjects were enrolled as part of an ongoing study on the relationship between OSA and asthma, approved by University of Michigan Medical School Institutional Review Board for Human Subject Research and University of Wisconsin Health Sciences Institutional Review Board. Each participant provided written informed consent. To be eligible, subjects must have been 18-75 years old and have had specialist-diagnosed20 and managed asthma. Urgent asthma visits and current pregnancy were exclusion criteria. As part of their clinic visits, patients underwent history, physical examination, asthma control assessment and spirometry.
2.2 Survey Content and Medical Records Review
Surveys were self-administered and included questionnaires and items regarding demographics (age, gender, height, weight, race, and smoking status). In February 2005, a question on the average sleep duration over the 24 hour period was added to the initial survey. A similar question has been previously validated against sleep diaries21 and used extensively in other studies of the relationship between sleep duration and health.21-27 The questionnaire also assessed the frequency of daytime and nighttime asthma symptoms categorized following the National Asthma Education and Prevention Program (NAEPP) classification of asthma severity based on clinical features.28
Medical records were subsequently reviewed by study physicians, unblinded to the study hypothesis, but who were experienced physicians, very familiar with the record systems, and following study-specific data collection forms and protocol guidelines. The purpose of the reviews was to exclude subjects with any additional lung disease (chronic obstructive pulmonary disease, allergic bronchopulmonary aspergillosis and interstitial lung diseases), and obtain spirometric information (associated with the respective screening visits) necessary to assess asthma severity step. Medical records were also reviewed (clinic visit notes and/or spirometry reports) to verify self-reported height and weight, and if mismatch was noted, the clinically-recorded values were used. Last, noted from charts were established diagnoses of comorbidities (rhinitis, chronic sinusitis, nasal polyposis, and psychiatric disease specifically depression, anxiety, panic or bipolar disorders) possibly linked etiologically to obesity, and current asthma medications. These included inhaled and oral corticosteroids, long acting β-agonists, theophylline and leukotriene modifiers. Due to limited availability, peak flow variability was not used in asthma step assessment.
In order to separately evaluate associations of both, clinical features of asthma and asthma medications respectively with obesity, asthma step was evaluated based on symptoms and FEV1% predicted, using their categories from NAEPP guidelines28: (i) step 1, patients with daytime symptoms ≤2 days/week, nighttime symptoms ≤2 nights/month and FEV1≥80%; (ii) step 2, patients with daytime symptoms 3-6 days/week, nighttime symptoms more than 2 nights/month (3-4 nights/month) and FEV1≥80%; (iii) step 3, patients with daily asthma symptoms, nocturnal symptoms ≥ 5/month (or >once /week) or FEV1 between >60%-<80%; (iv) step 4, patients with continuous daytime symptoms, frequent nighttime symptoms or FEV1≤60%. The asthma step was determined by the most severe qualifying feature among those listed.
2.3 Data analysis
A body mass index (BMI) ≥30kg/m2 defined obesity (Center for Disease Control and Prevention). Habitual sleep duration was categorized as <5h, 5 to <6, 6 to <7h, 7-8h (normal) , >8 to 9h and >9h, as previously reported.21 Because only 22 subjects reported sleeping <5h, the two shortest sleep categories were collapsed together, thus, the following five categories were used for analysis: <6 (very short), 6 to <7h (short), 7-8h (normal), >8 to ≤9h (long) and >9h (very long) sleepers. For each sleep duration category other than normal (7-8h), dummy variables were created and referred to this category in all subsequent analyses. Asthma step ≥2 defined persistent asthma. Dummy variables referenced to asthma step 1 were created for each of the other asthma steps, to use in the regression models. The doses of inhaled corticosteroids (ICS) were classified as: low, medium and high, per NAEPP guidelines for adults.29 For each ICS dose category, a dummy variable was created and compared with the “no ICS use” as the referent. Age ≥ 30 was stratified in decades, and the 18-29 years old group was used as the referent. African-Americans were compared to all other races combined.
Statistical analyses were conducted using SAS statistical software (Version 9.2, SAS Institute Inc. Cary, NC). Continuous variables were summarized as means ± standard deviation (s.d.) and categorical variables as numbers (%). Two sample t-tests were used to test for site differences in BMI. Linear relationships were assessed with Cochrane-Armitage tests for trends. Chi-square tests and logistic regression were used to assess for univariate relationships. Multivariate models with obesity as the dependent variables were constructed with sequential addition to asthma severity step of demographics, asthma medications and comorbidities (that demonstrated statistically significant associations or trends in the univariate analyses), and sleep duration; study site was included in all these models. Only two-sided hypotheses were tested and the significance level was set at p<0.05. Trends were noted when p-values ranged 0.05-0.10.
3. Results
Of the 709 subjects approached, 658 (93%) consented to the study of whom 47 were excluded because of comorbid lung disease. Among the remaining n=611 subjects, 142 were recruited at the UM and 469 at the UW.
Table 1 presents demographic, physiologic and clinical characteristics of the study population. The mean BMI was 30±8 kg/m2 and 249 (41%) subjects were obese; about half (51%) had persistent asthma (step ≥2). Subjects at UM had on average higher BMI (32.4±9.3 vs. 28.9±6.8, p<0.0001) and were more often obese (60% vs. 35%, p<0.0001). Mean habitual sleep duration was 7.16±1.57h overall; within its categories, 4.63±0.94h among very short, 6.16±0.24h in short, 7.44±0.46h in normal, 8.85±0.23h in long and 10.79±1.50h in very long sleepers. Very short habitual sleep was associated with higher asthma steps (odds ratio [95% confidence interval] =2.77 [1.71-4.49], p<0.0001)) and more often persistent (step≥2) asthma (2.69 [1.53-4.71], p=0.0006); the relationship of short sleep with persistent asthma approached significance (1.46 [0.98-2.17], p=0.06), but no associations between other sleep duration categories with asthma severity were noted (all p-values >0.10).
Table 1.
Demographic, physiologic and clinical characteristics s of n=611 asthma subjects.
| Characteristic | Number (%), Mean ± SD |
|---|---|
| Gender (females) | 395 (65%) |
| Age (years) | 47±14 |
| BMI (kg/m2) | 30±8 (range: 17-62) |
| Obese (BMI≥30) | 249 (41%) |
| Race: | |
| African-Americans | 31 (5%) |
| Caucasians | 562 (92%) |
| Others* | 18 (3%) |
| Current smokers | 28 (5%) |
| FEV1% predicted | 92±19 |
| FEV1/FVC | 77±9 |
| FEF25-75% predicted | 68±31 |
| Asthma Step: | |
| 1 | 297 (49%) |
| 2 | 69 (11%) |
| 3 | 134 (22%) |
| 4 | 111 (18%) |
| Habitual sleep duration (h): | 7.16±1.57 |
| very short (<6h) | 68 (11%) |
| short (≥6 and <7h) | 144 (24%) |
| normal (7-8h) | 308 (50%) |
| long (>8 and ≤9h) | 51 (8%) |
| very long (>9h) | 40 (7%) |
| History of rhinitis | 559 (91%) |
| History of chronic sinusitis | 193 (32%) |
| History of nasal polyps | 93 (15%) |
| History of psychiatric disease | 158 (26%) |
| History of GERD | 281 (46%) |
| Using inhaled corticosteroids (ICS) | 471 (77%) |
| ICS dose: | |
| low | 142 (23%) |
| medium | 176 (29%) |
| high | 153 (25%) |
| Using oral corticosteroids | 49 (8%) |
| Using inhaled long acting β2-agonist (LABA) | 371 (61%) |
| Using leukotriene modifiers (LTM) | 156 (26%) |
| Using theophylline | 11 (2%) |
included Asians, Hawaiian/Pacific Islanders and American Indians/Alaskans.
SD= standard deviation; BMI= body mass index; FEV1%= forced expiratory volume in the first second of the FVC maneuver; FVC%= forced vital capacity; FEF 25-75%= forced expiratory flow between 25% and 75% of vital capacity (all these physiologic variables are expressed as percentages of predicted values); GERD= gastroesophageal reflux disease.
The proportion of obesity was higher among short sleep duration categories and among very long sleepers, and lowest among normal and long sleepers (Figure 1, chi-square statistic=22.83, p=0.0001). The proportion of subjects with obesity increased with asthma severity step (Figure 2, Cochrane-Armitage Z-statistic=-6.01, p<0.0001 for the linear trend) and with ICS dose (Figure 3, Cochrane- Armitage Z-statistic=-4.42, p<0.0001).
Figure 1. Prevalence of obesity in each habitual sleep duration category.
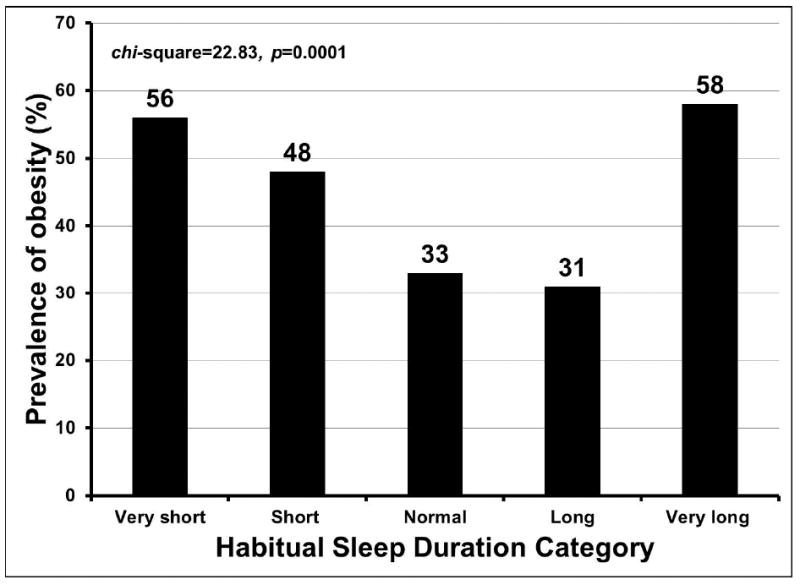
The relationship of sleep duration with obesity: the proportion of obesity was higher among shorter sleep duration categories and very long sleepers, and lowest among normal and long sleepers (chi-square statistic=22.83, p=0.0001).
Figure 2. Prevalence of obesity in each asthma severity step.
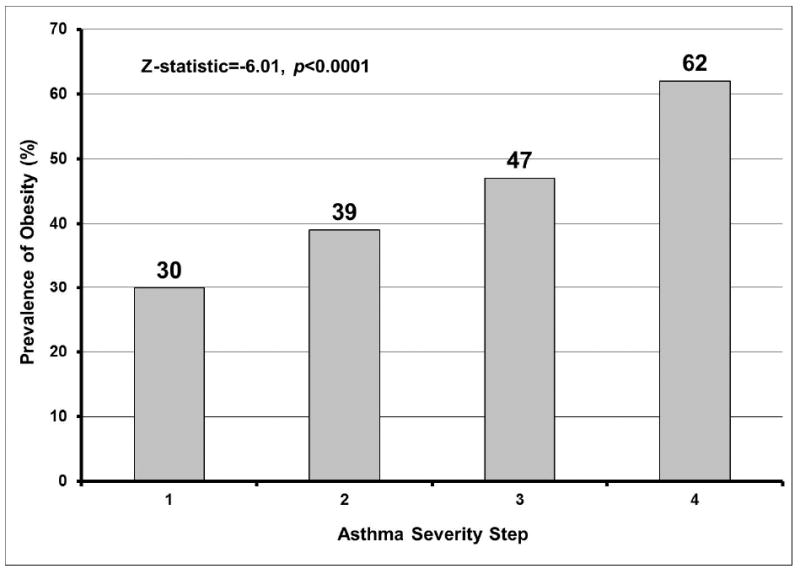
Obesity prevalence increased linearly with asthma severity step (Cochrane-Armitage test for linear trend Z-statistic=-6.01, p<0.0001).
Figure 3. Prevalence of obesity within each inhaled corticosteroid (ICS) dose group.
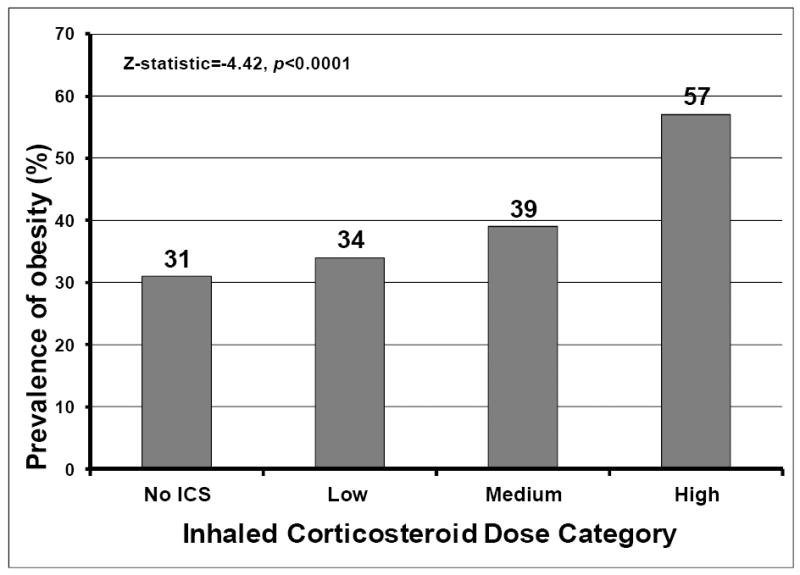
Obesity prevalence increased linearly with higher ICS dose group (Cochrane-Armitage test for linear trend Z-statistic=-4.42, p<0.0001).
Table 2 presents the univariate associations of obesity with sleep duration, asthma severity step, demographics, corticosteroid and other asthma medications use, and comorbidities. Obesity was significantly associated with shorter and very long sleep duration categories, higher asthma steps (3 and 4), age and African-American race, ICS use particularly in high doses, oral corticosteroid and LABA use, rhinitis and psychiatric disease. Trends were noted with LTM use. No significant relationships were seen with long sleep duration and other medications. When these variables were sequentially included, along with the study site, in multivariate regression models (Table 3), although the odds ratios estimates were slightly attenuated, the association of obesity with both short sleep and very long sleep duration were maintained, and the association with very short sleep duration approached significance. In the final model, independent of all these covariates, the odds of being obese were on average 74% higher ([95% CI: 0.96-3.14], p=0.06) among very short sleepers as compared to normal sleepers, 66% higher ([1.07-2.57], p=0.02) among short sleepers and 124% higher ([1.08-1.65], p=0.03) among very long sleepers. Significant associations with obesity were also maintained for highest asthma step, psychiatric disease, age and African-American race. As compared to asthma severity step 1, for individuals in step 4 the likelihood of being obese was on average 87% higher ( [1.09-3.21], p=0.02). While reduced to a trend in Model III, the relationship with high ICS dose somewhat strengthened with progressive adjustment for all covariates in the final model (1.82 [0.93-3.56], p=0.08). No statistically significant relationships or trends were seen for long sleep duration, the other asthma steps, lower ICS doses and other asthma medications, and rhinitis.
Table 2.
Univariate associations of obesity with sleep duration category, asthma severity y step, asthma medications, comorbidities and demographics.
| OBESITY
|
|||
|---|---|---|---|
| OR (95% CI) | P-value | ||
| Habitual sleep duration | |||
| category: | |||
| very short (<6h)* | 2.52 (1.48-4.30) | 0.0007 | |
| short (≥6 and <7h)* | 1.83(1.22-2.74) | 0.003 | |
| long (>8 and ≤9h )* | 0.91 (0.48-1.72) | 0.77 | |
| very long (>9h)* | 2.69 (1.38-5.26) | 0.004 | |
| Asthma steps: | |||
| step 2# | 1.48 (0.86-2.55) | 0.16 | |
| step 3# | 2.04 (1.34-3.11) | 0.0009 | |
| step 4# | 3.78 (2.39-5.96) | <0.0001 | |
| Using ICS | 1.61 (1.08-2.40) | 0.02 | |
| ICS dose: | |||
| low† | 1.10 (0.67-1.81) | 0.70 | |
| medium† | 1.38 (0.86-2.19) | 0.18 | |
| high† | 2.81 (1.75-4.53) | <0.0001 | |
| Using oral corticosteroids | 2.05 (1.14-3.71) | 0.02 | |
| Using LABA | 1.77 (1.26-2.49) | 0.0009 | |
| Using LTM | 1.39 (0.97-2.01) | 0.08 | |
| Using theophylline | 1.22 (0.37-4.09) | 0.75 | |
| Rhinitis | 0.43 (0.24-0.77) | 0.005 | |
| Chronic sinusitis | 1.26 (0.89-1.18) | 0.19 | |
| Polyps | 1.37 (0.88-2.14) | 0.16 | |
| Psychiatric Disease | 1.55 (1.08-2.24) | 0.02 | |
| Age (in decades)‡ | 1.19 (1.07-1.33) | 0.001 | |
| Gender (female vs. male) | 1.20 (0.85-1.68) | 0.30 | |
| African American§ | 6.62 (2.68-16.39) | <0.0001 | |
relative to normal sleeper (7-8h);
relative to asthma step 1;
relative to no ICS use;
age 18-29 was the reference category;
African American vs. all other races combined.
Abbreviations: OR: Odds Ratios; CI: 95% Confidence Intervals; ICS: inhaled corticosteroid; LABA: long acting β2-agonist; LTM: leukotriene modifiers.
Table 3.
Multivariate logistic regression models of obesity on sleep duration category with adjustment for asthma severity steps, asthma medications, comorbidities and demographics, and inclusion of study site in all models.
| MODEL I* | MODEL II* (ADJUSTED FOR ASTHMA STEPS) | MODEL III* (ADJUSTED FOR ASTHMA STEPS AND MEDICATIONS) | MODEL IV* (ADJUSTED FOR ASTHMA STEPS, MEDICATIONS AND COMORBIDITIES) | MODEL V* (ADJUSTED FOR ASTHMA STEPS, MEDICATIONS, COMORBIDITIES AND DEMOGRAPHICS) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
|
|
||||||||||
| Habitual sleep duration | ||||||||||
| category: | ||||||||||
| very short# | 2.14 (1.24-3.71) | 0.007 | 1.83 (1.04-3.28) | 0.04 | 1.87 (1.05-3.30) | 0.03 | 1.82 (1.02-3.24) | 0.04 | 1.74 (0.96-3.14) | 0.06 |
| short# | 1.78 (1.18-2.68) | 0.006 | 1.76 (1.16-2.68) | 0.009 | 1.76 (1.15-2.70) | 0.009 | 1.77 (1.15-2.71) | 0.009 | 1.66 (1.07-2.57) | 0.02 |
| long# | 0.90 (0.47-1.72) | 0.75 | 0.95 (0.49-1.84) | 0.88 | 0.97 (0.49-1.90) | 0.93 | 0.90 (0.46-1.77) | 0.76 | 0.82 (0.41-1.65) | 0.58 |
| very long# | 2.51 (1.27-4.98) | 0.008 | 2.56 (1.28-5.11) | 0.008 | 2.63 (1.31-5.28) | 0.007 | 2.34 (1.15-4.77) | 0.02 | 2.24 (1.08-4.63) | 0.03 |
| Asthma steps | ||||||||||
| step 2† | - | - | 1.47 (0.84-2.57) | 0.17 | 1.35 (0.77-2.38) | 0.29 | 1.28 (0.72-2.26) | 0.40 | 1.39 (0.78-2.47) | 0.26 |
| step 3† | - | - | 1.66 (1.07-2.57) | 0.02 | 1.42 (0.87-2.25) | 0.14 | 1.41 (0.89-2.24) | 0.14 | 1.31 (0.82-2.10) | 0.26 |
| step 4† | - | - | 2.99 (1.85-4.84) | <0.0001 | 2.36 (1.39-3.98) | 0.001 | 2.20 (1.30-3.75) | 0.004 | 1.87 (1.09-3.21) | 0.02 |
| ICS dose: | ||||||||||
| low‡ | - | - | - | - | 1.13 (0.61-2.07) | 0.70 | 1.12 (0.61-2.06) | 0.73 | 1.10 (0.59-2.06) | 0.77 |
| medium‡ | - | - | - | - | 0.94 (0.51-1.75) | 0.85 | 0.93 (0.50-1.74) | 0.83 | 0.96 (0.51-1.82) | 0.91 |
| high‡ | - | - | - | - | 1.72 (0.90-3.31) | 0.10 | 1.74 (0.90-3.35) | 0.10 | 1.82 (0.93-3.56) | 0.08 |
| Using oral corticosteroids | - | - | - | - | 1.16 (0.60-2.25) | 0.67 | 1.14 (0.60-2.23) | 0.71 | 1.08 (0.54-2.14) | 0.83 |
| Using LABA | - | - | - | - | 1.29 (0.80-2.09) | 0.30 | 1.33 (0.82-2.17) | 0.25 | 1.32 (0.81-2.16) | 0.27 |
| Using LTM | - | - | - | - | 0.96 (0.63-1.46) | 0.83 | 0.94 (0.62-1.44) | 0.79 | 0.95 (0.62-1.46) | 0.81 |
| Rhinitis | - | - | - | - | - | - | 0.82 (0.43-1.59) | 0.56 | 0.91 (0.46-1.79) | 0.77 |
| Psychiatric disease | - | - | - | - | - | - | 1.45 (0.96-2.17) | 0.08 | 1.64 (1.08-2.48) | 0.02 |
| Age (in decades)§ | - | - | - | - | - | - | - | - | 1.20 (1.07-1.36) | 0.003 |
| African-American race¥ | - | - | - | - | - | - | - | - | 5.32 (1.94-14.59) | 0.001 |
all models are adjusted for study site;
relative to normal sleeper (7-8h);
relative to asthma step 1;
relative to no ICS use.
age 18-29 was the reference category;
African American vs. all other races combined.
Abbreviations: OR: Odds Ratios; CI: 95% Confidence Intervals; ICS: inhaled corticosteroid; LABA: long acting β2-agonist; LTM: leukotriene modifiers.
4. Discussion
In this study of a large sample of asthma patients seen in tertiary care settings, obesity was associated with shorter and very long sleep duration, highest asthma severity step, and psychopathology, each independent of the other. A trend was seen with high doses of ICS. Although this cross-sectional analysis cannot prove causality, our data suggest for the first time in asthma patients that modifiable factors such as sleep duration may contribute to obesity, and offer new opportunities to intervene to abate this epidemic in asthma patients.
The high participation rate in our survey (93%) makes these results generalizable to other populations of asthmatics in tertiary settings. In that context, our results indicate higher prevalence of obesity in asthma compared with that in the general population in two states ranking among the top in the prevalence of obesity in the US.30 Our sample also demonstrates similar obesity dynamics as in the general population in regards to known risk factors such age and race.19 These made our sample particularly well-suited to explore associations between characteristics more unique to this population and obesity.
We found a significant association of short and very long sleep duration with obesity (Figure 1, Table 2 and 3) in these patients inherently predisposed to sleep disturbance; the association with very short sleep duration only approached significance likely as a result of much smaller number of subjects in this group than in the short or normal sleep category. Short sleep duration has emerged as an independent risk factor for weight gain,12, 16 as well as for adverse outcomes in various disease processes,23, 24, 26, 31-33 and for mortality. 21, 22 In a retrospective cohort study involving more than 21,000 healthy individuals 20 years or older, short sleep at baseline was associated with weight gain and obesity, during a 3-year period.14 This is further supported by a 6-year prospective study of 276 adults, which showed an association of short sleep duration with weight gain, when adjusting for levels of physical activity and other relevant confounders.12 Reduced sleep is associated with fatigue and pain,33 significant adverse cardiovascular, metabolic23, 24, 26, 31-33 and, as more recently reported with respiratory outcomes,27 and increased mortality.21, 22 To our knowledge, in the general population, similarly-defined very long sleep duration was not cross-sectionally associated with obesity,12 though, it was associated with weight gain in time,12 and likewise with mortality. 21, 22 Our novel cross-sectional association may portend a more accelerated weight gain in this group of asthmatics, which remains to be investigated in future studies.
Sleep is often disturbed in duration and quality among individuals with asthma, and our data raises several important questions.10, 34 Indeed, half of our subjects were either shorter (212—35%) or longer (91—15%) sleepers. While both these groups appear to be larger than reported for the average US population,35 most clinical studies of asthma patients in stable condition report reduced sleep time by means of self-report,36 and objective measures including actigraphy37 and polysomnography:17, 18 as compared with controls, the asthmatic patient sleeps ~50 minutes less on average.17, 36 Furthermore, while asthma impairs sleep,17, 38 reduced sleep may also worsen disease control,34, 38 thereby perpetuating perhaps asthma-related mechanisms of weight gain. In this study, we did observe associations of very short sleep duration with asthma step (2.77 [1.71-4.49], p<0.0001)) and persistent disease (2.69 [1.53-4.71], p=0.0006) while no such associations were seen for longer sleep categories. Last, in general populations, sleeping 7-8h appears to be “optimal” based on studies of association with weight gain,12, 16 other important endpoints including respiratory outcomes23, 24, 26, 27, 31-33 and mortality.21, 22 However, we find that the association of long (>8 to ≤9h) sleep duration with obesity was no different than that for normal sleep duration (7-8h). In this context, our observations give rise to several intriguing clinical questions: 1) what are the distribution and determinants of sleep duration in large, unselected populations of asthmatics?, and 2) what is an “optimal” sleep duration in asthmatics, do they need longer “sleep” for optimal health?
The observed relationship of sleep duration with obesity was independent of asthma steps (Table 3), suggesting that other mechanisms of weight gain may be operational in the asthmatic individual with altered sleep. In healthy individuals, experimental sleep restriction under controlled conditions of caloric intake and physical activity, considerably increased appetite and hunger, particularly for calorie-dense foods with high carbohydrate content.39 This was attributed to the decreased concentration of leptin, an anorexigenic hormone, and to a higher level of ghrelin, which acts in an opposite direction to leptin, as an appetite stimulator.39 On the other hand, long sleep has been associated with physical inactivity and depressive symptoms,10, 35 and other adverse endpoints,23, 24, 27 but our findings are independent of psychopathology. Whether asthmatics demonstrate similar or augmented responses to sleep deprivation paradigms, or their disease or other medical comorbidity23, 24, 27 keeps them even more inactive and at risk for weight gain if their sleep is altered remains untested, but our findings call for such controlled studies.
Our data show an association of asthma step with obesity. While a causal nature of this association remains to be confirmed , these data complement previous reports of asthma (self-reported physician-diagnosis, or asthma attacks or asthma-like breathing symptoms in the preceding 12-months) leading to obesity later in life.5 Additionally, for the first time, we show that the association of asthma step with obesity follows a dose-response pattern (Figure 1, Tables 2 and 3), suggesting that asthmatics with poorer disease control may be more predisposed to weight gain and obesity. What links asthma and inflammatory processes with neuroendocrine processes in this direction remains unknown, but leptin may be one link. Leptin has a dual role, as a cytokine underlying inflammatory/immune responses,40 and a hormone. Indeed, its circulating levels are elevated in both adults and children with asthma, independent of body fat measurements,41, 42 and levels subside with ICS treatment.42 Whether these sustained levels of hyperleptinemia create in any way a state of central “leptin resistance” and interfere with its role as a regulator of appetite and energy homeostasis in asthma, remains to be elucidated.
We found that high dose inhaled steroids were significantly associated with obesity in the univariate analysis (Figure 3 and Table 2), and though it was reduced to a trend in the multivariate models, this association strengthened with the addition of relevant covariates (Table 3). It has been previously proposed that weight may increase following pharmacological asthma treatment.5 Weight gain was one of the most frequently documented side-effects of corticosteroid treatment for patients with asthma during the 1950s and 1960s.43 While use of more modern asthma medications was not found to relate to obesity, no separation more specifically of ICS use, and furthermore of ICS doses was reported.8 ICS, particularly fluorinated compounds, are highly lipophilic and in a dose-dependent fashion are systemically absorbed;44, 45 in one study, 5 days of treatment with high dose of fluticasone caused significant ablation of the 24-hour plasma cortisol.44 Through their systemic absorption, ICS could long-term result in weight gain and obesity, as they do with other steroid-related systemic side-effects.46 Our data corroborated with this biological plausibility calls for longitudinal studies to clarify this relationship.
We observed an independent association of psychopathology (depression, anxiety, panic or bipolar disorders) with obesity (Table 3), suggesting this comorbidity we found with similar prevalence as in another recent report,47 may predispose asthmatics to weight gain. Our finding in a clinic-based sample complements reports from epidemiological studies. In the Zurich Cohort Study, depressive traits in young adulthood were related to increased weight gain and being overweight 20 years later.48 Childhood depressive symptoms were a risk factor for obesity y and asthma 20 years later, during adulthood, partially explaining the asthma-obesity comorbidity.5 Some of these observations arose independent of physical activity levels, suggesting mechanisms related to the neurobiology of the disease or its related dysfunctions in the HPA and catecholaminergic systems, and systemic inflammation, may be at play in this relationship.5, 48
The strengths of this study stem from its large sample size acquired from two large regional institutions, from two states which rank among the highest in the nation with respect to obesity prevalence.30 Secondly, the multidimensional assessments with objective data and self-administered questionnaires, allowed a comprehensive set of relevant variables, not concomitantly included in prior reports, to be tested for relationships of interest.
We recognize our study has a cross-sectional design which precludes assessment of causality. Relationships between sleep, asthma, comorbidities, and obesity are likely bidirectional and our cross-sectional study cannot tease them out fully; nevertheless, these data are intriguing enough to warrant further prospective studies, to better understand these interacting pathways. A second limitation relates to use of self-reported rather than objectively quantified sleep, which lends itself to the possibility of measurement error. However, our methodology has been previously validated against the average time spent sleeping recorded on 1-week diaries (Spearman correlation coefficient rho=0.79, p<0.0001)21 and has been extensively used in sleep research.21-27 In population studies, relative to objectively recorded sleep, individuals particularly short sleepers, typically overestimate their sleep time.49, 50 If so, by relying on self-report in our study, the potential contribution of shorter sleep may be in fact underestimated. Asthmatics consistently report more sleep disturbance and worse sleep quality,10, 17, 34, 38 in relationship to the disease severity.34 Furthermore, their perceived sleep quality improves with effective asthma treatment.38 Thus, the possibility that asthmatics, who often suffer nocturnal disruptions, ons, are better perceivers of their sleep duration also exists and remains to be tested in future studies, as does the relationship between self-reported and objectively recorded sleep among these patients. Third, our participants had been treated for their asthma (Table 1) and our step classification was solely based on symptoms and FEV1% predicted, which may explain the heavier representation of milder asthma steps in our population. Additionally, comorbidities such as psychopathology may still have been missed in some subjects, as our study design did not include their prospective evaluation and conversely, those recognized in clinical settings were often treated, potentially reducing their contribution to the associations of interest. Thus, the observed associations may actually underestimate their true relationships with obesity. Last, our study is among the first to explore the potential roles of sleep, asthma and related factors in promoting obesity. A wider range of traditional factors for weight gain, such as physical activity, socio-economic status, diabetes, thyroid disease and family history of obesity, were not included in the design. Future investigations to expand on these initial observations should include assessments of these additional factors.
In summary, this large study of asthma patients finds that shorter and very long sleep duration, worst asthma severity, psychopathology, and high dose ICS are associated with obesity each independent of each other. We believe current data adds to the literature suggesting that the relationship between asthma and obesity is bidirectional. In view of the obesity epidemic and the resulting distinct clinical phenotype,51 it is important to understand which are the risk factors for this phenotype, particularly those modifiable ones. Our study offers a unique perspective on this relationship, as it suggests that sleep-related factors, the disease and related characteristics, and mood disorders may each play a role in this interaction. Optimal control of asthma may well require an understanding of the asthmatic individual during the 24-hour period, including his/her sleep-wake, activity-rest and energy balance cycles.
Acknowledgments
Funding support: This study was supported by the University of Michigan General Clinical Research Center (MO1 RR00042) and Neurology Department Training Grant 5T32NS007222; University of Wisconsin School of Medicine and Public Health, Department of Medicine, and Medical Education and Research Committee - New Investigator Award; 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health; and additional resources from William S. Middleton Memorial Hospital, Madison, Wisconsin.
Abbreviations list
- BMI
Body Mass Index (in kilograms per meter squared)
- CI
Confidence Interval
- ICS
Inhaled Corticosteroids
- HPA
Hypothalamic Pituitary Axis
- LABA
Long Acting Beta Agonist
- LTM
Leukotriene Modifiers
- FEV1
Forced Expiratory Volume in first second of the Forced Vital Capacity maneuver
- FVC
Forced Vital Capacity
- GERD
Gastroesophageal Reflux Disease
- NAEPP
National Asthma Education and Prevention Program
- SAS
Statistical Analysis Software
- UM
University of Michigan
- UW
University of Wisconsin
References
- 1.Ford ES, Mannino DM. Time trends in obesity among adults with asthma in the United States: findings from three national surveys. J Asthma. 2005;42:91–5. [PubMed] [Google Scholar]
- 2.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174:112–9. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–35. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 4.Fida NG, Enquobahrie DA, Gelaye B, Qiu C, Williams MA. Associations of asthma with body mass index and adult weight change among reproductive age women. J Asthma. 48:701–6. doi: 10.3109/02770903.2011.604885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasler G, Gergen PJ, Ajdacic V, Gamma A, Eich D, Rossler W, et al. Asthma and body weight change: a 20-year prospective community study of young adults. Int J Obes (Lond) 2006;30:1111–8. doi: 10.1038/sj.ijo.0803215. [DOI] [PubMed] [Google Scholar]
- 6.Goldey DH, Mansmann HC, Jr, Rasmussen AI. Zinc status of asthmatic, prednisone-treated asthmatic, and non-asthmatic children. J Am Diet Assoc. 1984;84:157–63. [PubMed] [Google Scholar]
- 7.Zeitlin SR, Bond S, Wootton S, Gregson RK, Radford M. Increased resting energy expenditure in childhood asthma: does this contribute towards growth failure? Arch Dis Child. 1992;67:1366–9. doi: 10.1136/adc.67.11.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedberg A, Rossner S. Body weight characteristics of subjects on asthma medication. Int J Obes Relat Metab Disord. 2000;24:1217–25. doi: 10.1038/sj.ijo.0801382. [DOI] [PubMed] [Google Scholar]
- 9.Minas M, Papaioannou AI, Tsaroucha A, Daniil Z, Hatzoglou C, Sgantzos M, et al. Body composition in severe refractory asthma: comparison with COPD patients and healthy smokers. PLoS One. 5(10):e13233. doi: 10.1371/journal.pone.0013233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goral A, Lipsitz JD, Muhsen K, Gross R. Depressive symptoms, risk factors and sleep in asthma: results from a national Israeli health survey. Gen Hosp Psychiatry. 2012;34:17–23. doi: 10.1016/j.genhosppsych.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Anderson SE, Cohen P, Naumova EN, Must A. Association of depression and anxiety disorders with weight change in a prospective community-based study of children followed up into adulthood. Archives of pediatrics & adolescent medicine. 2006;160:285–91. doi: 10.1001/archpedi.160.3.285. [DOI] [PubMed] [Google Scholar]
- 12.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–23. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T. Association between weight gain, obesity, and sleep duration: a large-scale 3-year cohort study. Sleep Breath. 2012;16:829–33. doi: 10.1007/s11325-011-0583-0. [DOI] [PubMed] [Google Scholar]
- 15.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzpatrick MF, Engleman H, Whyte KF, Deary IJ, Shapiro CM, Douglas NJ. Morbidity in nocturnal asthma: sleep quality and daytime cognitive performance. Thorax. 1991;46:569–73. doi: 10.1136/thx.46.8.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krouse HJ, Krouse JH. Diurnal variability of lung function and its association with sleep among patients with asthma. J Asthma. 2007;44:759–63. doi: 10.1080/02770900701645686. [DOI] [PubMed] [Google Scholar]
- 19.Teodorescu M, Holland AS, Bria WF, Consens FB, Coffey MJ, Chervin RD. Factors associated with obesity in patients with asthma. Proc Am Thorac Soc. 2006;3:A792. [Google Scholar]
- 20.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987;136:225–44. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 21.Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 22.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 23.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 24.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 25.Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29:881–9. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel SR, Malhotra A, Gao X, Hu FB, Neuman MI, Fawzi WW. A prospective study of sleep duration and pneumonia risk in women. Sleep. 2012;35:97–101. doi: 10.5665/sleep.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Department of Health and Human Services. National Institute of Health; National Heart, Lung and Blood Institute. National Asthma Education and Prevention: Practical guide for the diagnosis and management of asthma. Expert Panel Report 2. 1997 [Google Scholar]
- 29.US Department of Health and Human Services. National Institute of Health; National Heart, Lung and Blood Institute. National Asthma Education and Prevention Program Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma—Update on Selected Topics. 2002 [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. [August 23, 2012];Adult Obesity Facts: Obesity prevalence in 2011 varies across states and regions. Available at http://www.cdc.gov/obesity/data/adult.html.
- 31.Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 32.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 33.Ranjbaran Z, Keefer L, Stepanski E, Farhadi A, Keshavarzian A. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm Res. 2007;56:51–7. doi: 10.1007/s00011-006-6067-1. [DOI] [PubMed] [Google Scholar]
- 34.Luyster FS, Teodorescu M, Bleecker E, Busse W, Calhoun W, Castro M, et al. Sleep quality and asthma control and quality of life in non-severe and severe asthma. Sleep & breathing = Schlaf & Atmung. 2011 doi: 10.1007/s11325-011-0616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009;169:1052–63. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vir R, Bhagat R, Shah A. Sleep disturbances in clinically stable young asthmatic adults. Ann Allergy Asthma Immunol. 1997;79:251–5. doi: 10.1016/S1081-1206(10)63010-4. [DOI] [PubMed] [Google Scholar]
- 37.Krouse HJ, Yarandi H, McIntosh J, Cowen C, Selim V. Assessing sleep quality and daytime wakefulness in asthma using wrist actigraphy. J Asthma. 2008;45:389–95. doi: 10.1080/02770900801971800. [DOI] [PubMed] [Google Scholar]
- 38.Mastronarde JG, Wise RA, Shade DM, Olopade CO, Scharf SM. Sleep quality in asthma: results of a large prospective clinical trial. J Asthma. 2008;45:183–9. doi: 10.1080/02770900801890224. [DOI] [PubMed] [Google Scholar]
- 39.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 40.Lago R, Gomez R, Lago F, Gomez-Reino J, Gualillo O. Leptin beyond body weight regulation--current concepts concerning its role in immune function and inflammation. Cell Immunol. 2008;252:139–45. doi: 10.1016/j.cellimm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Sood A, Ford ES, Camargo CA., Jr Association between leptin and asthma in adults. Thorax. 2006;61:300–5. doi: 10.1136/thx.2004.031468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gurkan F, Atamer Y, Ece A, Kocyigit Y, Tuzun H, Mete N. Serum leptin levels in asthmatic children treated with an inhaled corticosteroid. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2004;93:277–80. doi: 10.1016/S1081-1206(10)61501-3. [DOI] [PubMed] [Google Scholar]
- 43.Rosenhall L, Skoogh B-E. Inhaled glucocorticoids in the long-term treatment of Asthma. In: Strandberg K, Beermann B, LoÈnnerholm G, editors. Workshop, Pharmacological Treatment of Bronchial Asthma II, Report no 1993 I. Medical Products Agency; Uppsala: Sep, 1992. [Google Scholar]
- 44.Donnelly R, Williams KM, Baker AB, Badcock CA, Day RO, Seale JP. Effects of budesonide and fluticasone on 24-hour plasma cortisol. A dose-response study. Am J Respir Crit Care Med. 1997;156:1746–51. doi: 10.1164/ajrccm.156.6.9703003. [DOI] [PubMed] [Google Scholar]
- 45.Martin RJ, Szefler SJ, Chinchilli VM, Kraft M, Dolovich M, Boushey HA, et al. Systemic effect comparisons of six inhaled corticosteroid preparations. Am J Respir Crit Care Med. 2002;165:1377–83. doi: 10.1164/rccm.2105013. [DOI] [PubMed] [Google Scholar]
- 46.Ernst P, Baltzan M, Deschenes J, Suissa S. Low-dose inhaled and nasal corticosteroid use and the risk of cataracts. Eur Respir J. 2006;27:1168–74. doi: 10.1183/09031936.06.00043005. [DOI] [PubMed] [Google Scholar]
- 47.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 48.Hasler G, Pine DS, Gamma A, Milos G, Ajdacic V, Eich D, et al. The associations between psychopathology and being overweight: a 20-year prospective study. Psychol Med. 2004;34:1047–57. doi: 10.1017/s0033291703001697. [DOI] [PubMed] [Google Scholar]
- 49.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva GE, Goodwin JL, Sherrill DL, Arnold JL, Bootzin RR, Smith T, et al. Relationship between reported and measured sleep times: the sleep heart health study (SHHS) J Clin Sleep Med. 2007;3:622–30. [PMC free article] [PubMed] [Google Scholar]
- 51.Kent BD, Lane SJ. Twin Epidemics: Asthma and Obesity. Int Arch Allergy Immunol. 157:213–4. doi: 10.1159/000329874. [DOI] [PubMed] [Google Scholar]


