Abstract
Several studies have reported the coupling of dopamine signaling to phospholipase C β (PLCβ) both in vitro and in vivo. However, the precise physiological relevance of this signaling pathway in mediating dopamine behaviors is still unclear. Here we report that stimulation of dopamine receptor signaling in vivo with systemic administration of apomorphine, amphetamine, and cocaine leads to increased production of inositol triphosphate (IP3) in the mouse striatum. Using selective antagonists and dopamine D1 and D2 receptor knock-out animals, we show that the production of IP3 is mediated by the D1 receptor, but not the D2 receptor. A selective blocker of PLCβ, U73122, was used to assess the physiological relevance of D1-mediated IP3 production. We show that U73122 inhibits the locomotor-stimulating effects of apomorphine, amphetamine, cocaine, and SKF81297. Furthermore, U73122 also suppresses the spontaneous hyperactivity exhibited by dopamine transporter knock-out mice. Importantly, the effects of U73122 are selective to dopamine-mediated hyperactivity, as this compound does not affect hyperactivity induced by the glutamate NMDA receptor antagonist MK801. Finally, we present evidence showing that an imbalance of D1- and D2-mediated signaling following U73122 treatment modifies the locomotor output of animals from horizontal locomotor activity to vertical activity, further highlighting the importance of the PLCβ pathway in the regulation of forward locomotion via dopamine receptors.
Introduction
Dopamine neurotransmission regulates both motor and nonmotor behaviors (Carlsson, 2001; Greengard, 2001; Beaulieu and Gainetdinov, 2011). The role of dopamine in facilitating voluntary movement is evidenced by the locomotor-stimulant effects of dopaminergic drugs like amphetamine, and conversely by the inhibitory effects of dopamine antagonists like haloperidol (Carlsson, 2001).
Dopamine acts on the following two subclasses of receptors: the D1 class, composed of D1 and D5 receptors; and the D2 class, composed of the D2, D3, and D4 receptors (Missale et al., 1998; Beaulieu and Gainetdinov, 2011). Classically, the D1 class has been shown to be positively coupled to cAMP production through a Gs/olf heterotrimeric G-protein, while activation of the D2 class leads to reduction in cAMP levels through Gi/o proteins (Missale et al., 1998; Neve et al., 2004; Beaulieu and Gainetdinov, 2011).
Traditionally, the cAMP pathway has been thought to mediate the locomotor behaviors ascribed to dopamine. However, several published studies raise the possibility that cAMP is not the only pathway for dopamine-mediated locomotion. Indeed, some dopamine-mediated locomotor behaviors are preserved when the Gs/adenylyl cyclase/protein kinase A (PKA) pathway is perturbed by knocking out key signaling molecules. For example, Golf knock-out mice are hyperactive (Zhuang et al., 2000), and basal activity is essentially normal in mice lacking the catalytic subunit of PKA or dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32; Brandon et al., 1998; Nally et al., 2003). Alterations in the cAMP pathway are most evident in psychostimulant response, where mice deficient in DARPP-32 and Golf are less responsive to cocaine and amphetamine (Fienberg et al., 1998; Zachariou et al., 2006; Corvol et al., 2007). Furthermore, selective deletion of DARPP-32 in D1 or D2 neurons bidirectionally affects basal activity (Bateup et al., 2010). These observations highlight the complexity of D1 signaling and raise the possibility that alternate signaling pathways converge or function in parallel with cAMP signaling to elicit dopamine-mediated locomotion (Neve, 2010).
Several studies have described the coupling of a D1-like receptor to phospholipase C β (PLCβ). (Undie and Friedman, 1990; Friedman et al., 1997; Undieh, 2010). In addition, it was shown that concomitant activation of D1 and D2 receptors within a heterodimeric complex is linked to an increase in intracellular calcium levels (Lee et al., 2004; Rashid et al., 2007; Hasbi et al., 2010; Verma et al., 2010; Perreault et al., 2011), likely via multiple mechanisms (Chun et al., 2013).
Despite these studies that have convincingly demonstrated that PLCβ is a downstream effector of dopamine signaling, the precise physiological role of this dopamine-signaling pathway remains unclear. We sought to investigate in vivo the role of dopamine receptor signaling through PLCβ and inositol triphosphate (IP3) by evaluating the contribution of this pathway to the modulation of locomotor activity. Our study shows that PLCβ is a critical modulator of dopamine-mediated forward locomotor activity, and that, in vivo, direct and indirect dopamine agonists lead to stimulation of IP3 production via the D1 dopamine receptor. Furthermore, the inhibition of PLCβ signaling shifts the l-DOPA-mediated locomotor activity of animals from horizontal activity to vertical activity, stressing the importance of the PLCβ pathway for forward locomotion.
Materials and Methods
Animals.
All behavioral experiments were performed during the light cycle and used 3- to 5-month-old C57BL/6J age- and sex-matched animals of either sex. Dopamine transporter knock-out (DAT-KO) mice and wild-type littermates (WT) (Giros et al., 1996) maintained on a C57BL/6J genetic background of the same age were also used. Animals were housed four to five per cage in a humidity- and temperature-controlled room with 12 h light/dark cycle (lights on at 8:00 A.M.). Mice were provided food and water ad libitum. All mice were experimentally naïve, and a separate group of mice was used for each testing paradigm. Experiments were conducted in accordance with the National Institutes of Health or Canadian Council for Animal Care guidelines for the care and use of animals and an approved animal protocol from either the Duke University Animal Care and Use Committee or the Faculties of Medicine and Pharmacy Animal Care Committee at the University of Toronto.
Reagents.
Apomorphine, α-methyl-para-tyrosine (αMPT), raclopride, SKF81297, SCH 23390, MK801, l-DOPA, carbidopa, U73122, and U73343 were purchased from Sigma-Aldrich. Anti-phospho-GluR1-Ser-845 antibody and anti-total-GluR1 antibody were purchased from Millipore. Anti-GAPDH antibody was purchased from Sigma. Species-specific Alexa Fluor 680 antibody was purchased from Invitrogen. Species-specific IRDye 800CW antibody was purchased from LI-COR Biosciences.
Drugs.
Compounds or saline (0.9% NaCl) were administered intraperitoneally or subcutaneously in a volume of 10 ml/kg. For U73122 and U73343, 5 mg of compound was first dissolved in 20 μl of Tween 20 and then resuspended in saline solution for intraperitoneal injection. Apomorphine was dissolved in distilled water containing 0.1% ascorbate and injected subcutaneously.
Locomotor analysis.
Locomotor activity was measured in an automated infrared beam-break apparatus (AccuScan Instruments) during the light phase of the light/dark cycle. Animals were placed in an activity monitor chamber (20 × 20 cm), and individual activity data were collected at 5 min intervals. Forward locomotion was measured as the total distance traveled unless otherwise indicated. Vertical activity was measured as the time spent in the vertical rearing position.
IP3 level measurement.
IP3 measurement was performed using the GE Healthcare IP3 [3H] Biotrak Assay System (TRK1000) according to manufacturer notes. Briefly, mice were killed by cervical dislocation, and striatal tissue was dissected rapidly within 1 min. The tissue was immediately submerged in 1 ml of 10% perchloric acid and incubated for 10 min, after which the tissue was sonicated three times for 10 s. Following sonication, the sample was centrifuged for 15 min at 10,000 × g at 4°C. The supernatant was then transferred to a 15 ml conical tube and titrated to pH 7.5 using 1.5 m KOH containing 60 mm HEPES. The pH of each sample was verified to be 7.5 ± 0.1. The samples were then centrifuged at 2000 × g to precipitate KClO4. The supernatant protein concentration was measured using the Bradford assay. A protein sample in the supernatant of 100 μl was then assayed using the TRK1000 kit following the procedure outlined by the manufacturer.
Western blot.
Mice were pretreated with either vehicle or U73122 (10 mg/kg, i.p.) 30 min before administration of either vehicle or SKF81297 (3 mg/kg, i.p.). Fifteen minutes after the second injection, mice were killed by cervical dislocation, and striatum was rapidly dissected and snap frozen in liquid nitrogen. As described by Ghisi et al. (2009), striatum was homogenized in 1% SDS and 2 μm okadaic acid with a hand-held homogenizer, and the protein concentration determined using a BCA assay (Pierce). Fifty micrograms of striatal protein was resolved on a 10% SDS polyacrylamide gel and transferred to a PVDF membrane. The immunostaining of blots was performed overnight at 4°C with the following primary antibodies: anti-phospho-GluR1-Ser-845 (1:500) and anti-total-GluR1 (1:1500). Appropriate secondary antibodies (1:5000, Alexa Fluor 680 or IRDye 800CW) were used, and blots were developed using the LI-COR Biosciences Odyssey Imaging System. Densitometric analysis was performed with ImageJ software. Total GluR1 protein signal was used as the loading control for phospho-GluR1 protein levels.
Results
Direct and indirect dopamine agonists increase IP3 levels in vivo
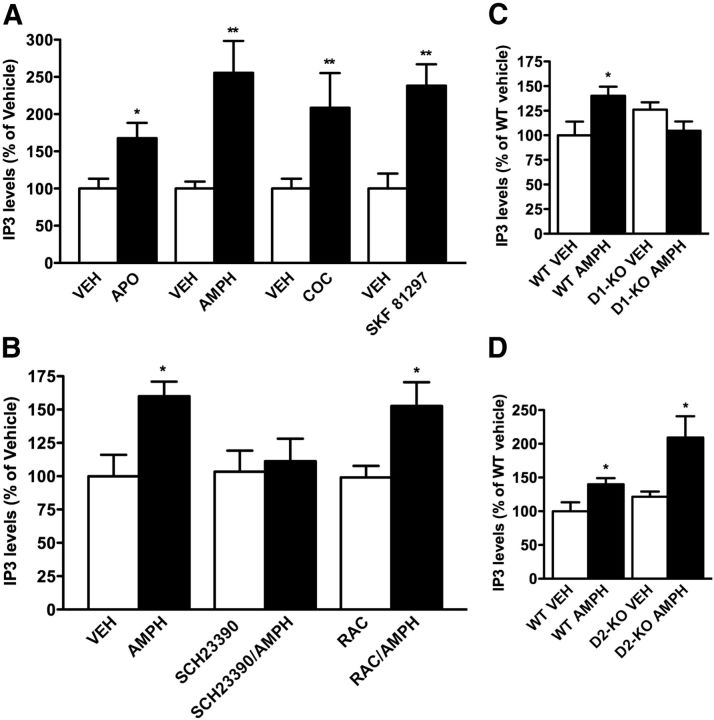
To test whether activation of dopamine receptors can lead to an activation of PLCβ in vivo, we measured levels of IP3 accumulation in the striatal tissue following systemic administration of direct and indirect dopamine receptor agonists. As shown in Figure 1A, the injection of mice with 1 mg/kg apomorphine, 3 mg/kg amphetamine, 20 mg/kg cocaine, or 10 mg/kg SKF81297 leads respectively to a 50, 150, 100, and 120% increase in IP3 levels compared with saline treatment.
Figure 1.
Direct and indirect dopamine agonists lead to accumulation of IP3 in the striatum via D1 receptors. A, C57BL/6J mice were injected with 1 mg/kg apomorphine (APO), 3 mg/kg amphetamine (AMPH), 20 mg/kg cocaine (COC), or 10 mg/kg SKF81297, and IP3 levels were quantified using radioimmunoassay. B, C57BL/6J mice were pretreated with vehicle (VEH), 0.1 mg/kg SCH23390 (D1 antagonist), or 2 mg/kg raclopride (RAC; D2 antagonist) before amphetamine administration (3 mg/kg). C, D, IP3 levels were assessed after amphetamine (3 mg/kg) or vehicle injection in D1-KO mice (C) or D2-KO mice (D) and their respective WT littermates. All data are presented as the means ± SEM. N = 5. *p < 0.05; **p < 0.01 as determined by Student's t test.
Based on the observation that SKF81297, a D1-selective agonist, increases striatal IP3 levels, we performed a series of studies to determine whether the increase of IP3 is due to the activation of a dopamine D1 or D2 receptor. Mice were injected with either SCH23390 (D1 antagonist) or raclopride (D2 antagonist) before injections with amphetamine (3 mg/kg). As shown in Figure 1B, injection of SCH23390 (0.1 mg/kg) completely abolishes production of IP3 by amphetamine, while pretreatment with raclopride (2 mg/kg) has no effect on amphetamine-induced IP3 accumulation. This indicates that stimulation of IP3 production in the mouse striatum in vivo is mediated by D1 and not D2 class receptors.
To further validate the results in Figure 1B, we measured the stimulation of IP3 accumulation after amphetamine administration (3 mg/kg) in D1 and D2 receptor knock-out animals (D1-KO and D2-KO). As shown in Figure 1, C and D, the accumulation of IP3 levels after amphetamine injection is completely abolished in D1-KO animals, while IP3 accumulation is still observed in D2-KO mice. These results, using both pharmacological and genetic approaches, clearly demonstrate that the accumulation of IP3 after amphetamine injection in vivo is mediated by the D1 and not the D2 dopamine receptor.
The PLCβ pathway is critical for dopamine-mediated locomotor activity
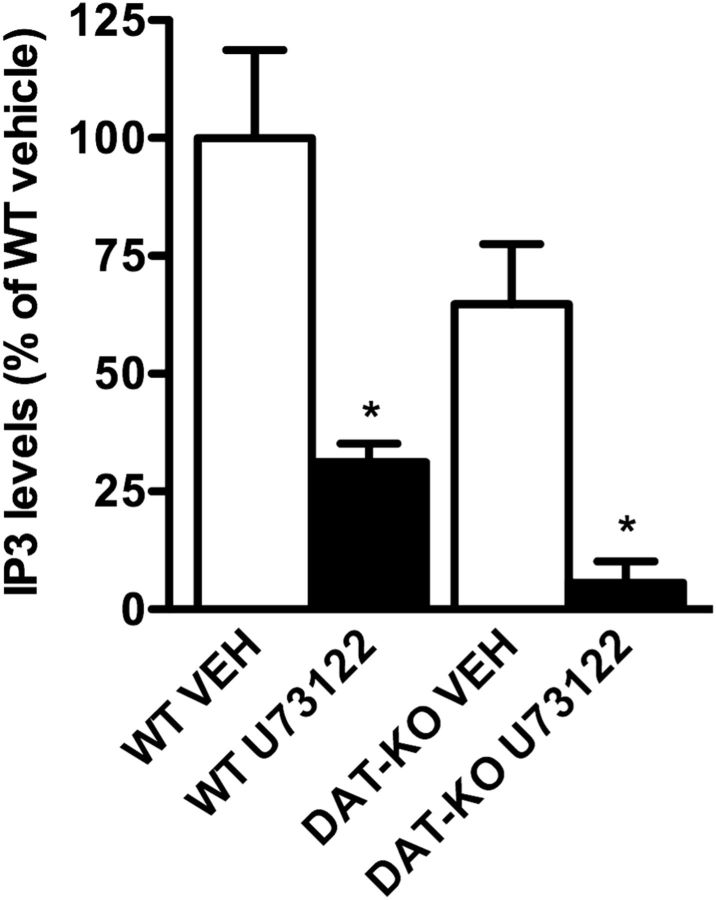
Having shown that stimulation of D1 receptors leads to an increase in striatal IP3 levels, we next investigated the role of this pathway in dopamine-mediated locomotor activity. For this, the selective PLCβ inhibitor U73122 was used. We first determined that U73122 effectively reduces IP3 levels in the brain. Peripheral injection of U73122 (10 mg/kg) reduces the measureable levels of IP3 in the striatum of both WT and DAT-KO mice, which have a fivefold increase in extracellular dopamine (Giros et al., 1996; Fig. 2). Interestingly, DAT-KO mice show a trend decrease in their basal levels of IP3, and U73122 has a greater effect in reducing IP3 levels in DAT-KO mice (Fig. 2). These results may be explained by the 50% reduction in D1 receptor levels in the DAT-KO mice (Giros et al., 1996; Ghisi et al., 2009), a compensatory adaptation to sustained hyperdopaminergia. Thus, although DAT-KO mice have high extracellular dopamine levels, signaling through the PLCβ pathway is likely desensitized through compensatory adaptations.
Figure 2.
Selective PLCβ inhibition reduces IP3 levels in the striatum of WT and DAT-KO mice. WT and DAT-KO mice were treated with vehicle (VEH) or a selective PLCβ inhibitor, U73122 (10 mg/kg). IP3 levels were assessed using radioimmunoassay and were reported as a percentage of WT vehicle-treated mice. Data are presented as the mean ± SEM. N = 5. *p < 0.05, Student's t test.
We next tested whether the PLCβ/IP3 pathway plays a role in dopamine-mediated locomotor activity. In the first set of experiments, C57BL/6J mice were pretreated with U73122 before administration of direct or indirect dopamine receptor agonists. Pretreatment with U73122 (10 mg/kg) reduces basal activity, and also reduces the locomotor responses of mice to the direct dopamine agonists apomorphine (1 mg/kg) and SKF81297 (2.5 mg/kg), as well as to indirect dopamine agonists amphetamine (3 mg/kg) and cocaine (20 mg/kg; Fig. 3). These results suggest a prominent role of the PLCβ/IP3 pathway in mediating dopamine-stimulated locomotor activity.
Figure 3.
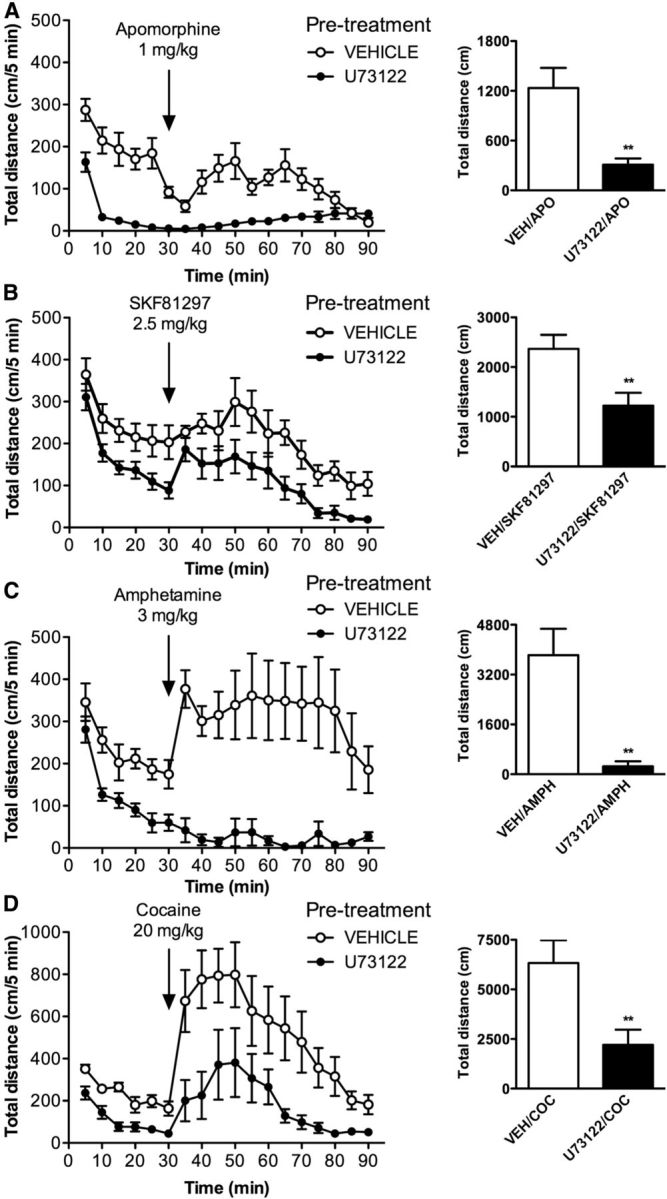
Inhibition of PLCβ reduces the locomotor response of mice to direct and indirect dopamine agonists. Mice were pretreated with vehicle (VEH) or U73122 (10 mg/kg) and placed in locomotor activity chambers. A–D, After 30 min, mice were injected with 1 mg/kg apomorphine (APO; A), 2.5 mg/kg SKF81297 (B), 3 mg/kg amphetamine (AMPH; C), or 20 mg/kg cocaine (COC; D), and locomotor activity was monitored for another 60 min. Distance traveled (in centimeters) was measured over 5 min intervals, and total distance is shown as a sum of 60 min. Data are presented as the mean ± SEM. N = 8. **p < 0.01, Student's t test.
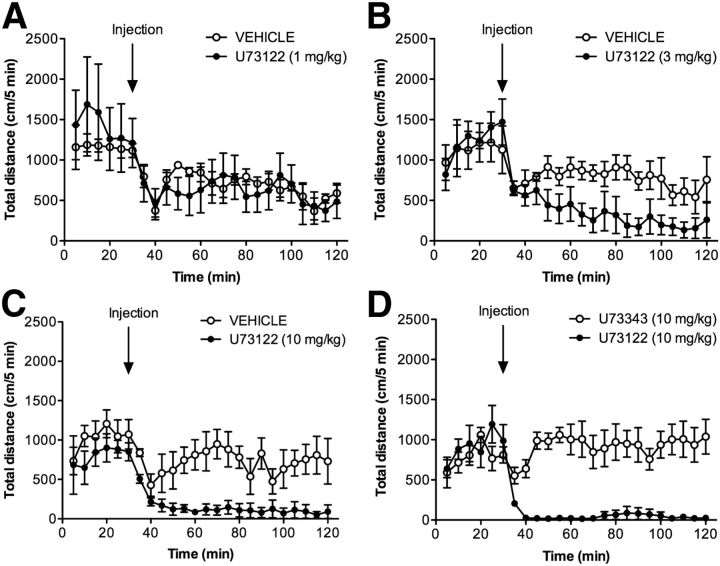
To corroborate these pharmacological results, we used a genetic model of enhanced dopamine transmission. For this, U73122 was used to reduce IP3 levels in DAT-KO animals. DAT-KO mice have increased extracellular dopamine levels, and display hyperactivity and impaired habituation in a novel environment (Giros et al., 1996; Jones et al., 1998). The administration of U73122 to DAT-KO mice dose dependently reduces their hyperlocomotor activity (Fig. 4). Importantly, the injection of DAT-KO animals with U73343 (10 mg/kg), the inactive analog of U73122, has no major effect on locomotor hyperactivity, indicating that the effects of U73122 (10 mg/kg) are mediated through its inhibition of PLCβ (Fig. 4D).
Figure 4.
Selective inhibition of PLCβ dose-dependently reduces hyperactivity in DAT-KO mice. A–C, DAT-KO mice were habituated to the activity monitor chambers for 30 min before injection with vehicle or increasing doses of U73122 (1 mg/kg, A; 3 mg/kg, B; 10 mg/kg, C; N = 4). The hyperactivity of DAT-KO animals was dose-dependently reduced by U73122 treatment. D, Mice were treated with either U73122 (10 mg/kg) or U73343 (10 mg/kg), the inactive analog of U73122 (N = 8). Distance traveled (in centimeters) was measured in 5 min intervals for 30 min before injection and for 90 min after injection. Data are presented as the mean ± SEM.
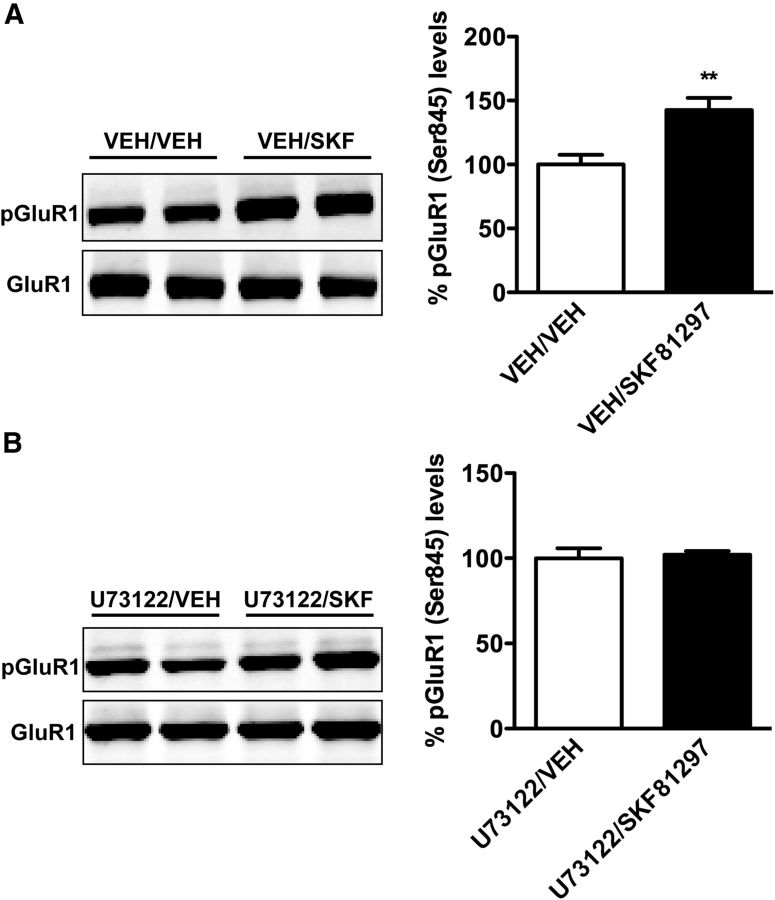
Because D1 receptors are known to signal through Gs/olf coupling to elevate cAMP, we next performed studies to examine potential cross talk between cAMP and IP3 pathways. One of the established indicators of D1-Gs/olf signaling is the dopamine-mediated phosphorylation of the AMPA GluR1 subunit. Residue Ser845 is selectively phosphorylated by PKA in a dopamine-dependent manner (Roche et al., 1996; Corvol et al., 2007). We examined whether PLCβ inhibition would alter phosphorylation of GluR1 by comparing the levels of phospho-GluR1 after SKF81297 injection in the presence or absence of U73122. Interestingly, we discovered that U73122 pretreatment prevented the SKF-induced phosphorylation of GluR1 (Fig. 5), suggesting the existence of some level of cross talk between the two pathways.
Figure 5.
PLCβ inhibition blocks SKF81297-induced phosphorylation (Ser845) of GluR1. A, B, Mice were pretreated with vehicle (VEH; A) or U73122 (10 mg/kg; B) and then injected with vehicle or SKF81297 (3 mg/kg). Phosphorylated GluR1 (Ser845) and total GluR1 protein levels in the striatum were assessed by Western blot 15 min after SKF injection. Phosphorylated GluR1 levels were corrected to total GluR1 levels and relative to vehicle in each pretreatment group. Data are presented as the mean ± SEM. N = 4–5. **p < 0.01, Student's t test.
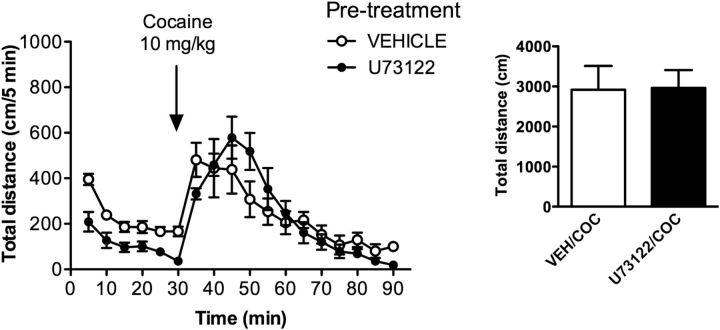
While U73122 administration fully inhibited locomotor responses to amphetamine, apomorphine, and SKF81297, it did not fully inhibit the effect of cocaine on locomotion at a 20 mg/kg dose (Fig. 3D). To examine this discrepancy further, we evaluated the effect of U73122 on the locomotor activity elicited by a lower (10 mg/kg) dose of cocaine. To our surprise, while the U73122 pretreatment had a clear effect on basal activity, there was no effect on the cocaine-stimulated locomotor activity at 10 mg/kg (Fig. 6). Furthermore, the level of activity induced by cocaine at this dose was within the same range as the response observed following the administration of 20 mg/kg cocaine along with a U73122 pretreatment. This suggests that at least two complementary mechanisms may underlie locomotor responsiveness to cocaine. It is noteworthy that previous findings have shown that DARPP-32 knock-out mice are insensitive to 10 mg/kg doses of cocaine, but respond normally to higher doses (20 mg/kg; Fienberg et al., 1998). Together, these observations hint that perhaps at low doses, it is the cAMP/DARPP32 pathway that mediates the locomotor-stimulating effects of cocaine (Corvol et al., 2007), while activation of the PLC pathway would contribute to enhanced responses at higher cocaine doses.
Figure 6.
Inhibition of PLCβ does not affect hyperactivity induced by a low dose of cocaine (COC; 10 mg/kg). Mice were pretreated with vehicle (VEH) or U73122 (10 mg/kg). After 30 min, mice were injected with cocaine (10 mg/kg), and locomotor activity was monitored for another 60 min. Distance traveled (in centimeters) was measured over 5 min intervals, and the total distance is shown as a sum of 60 min. Data are the mean ± SEM. N = 8.
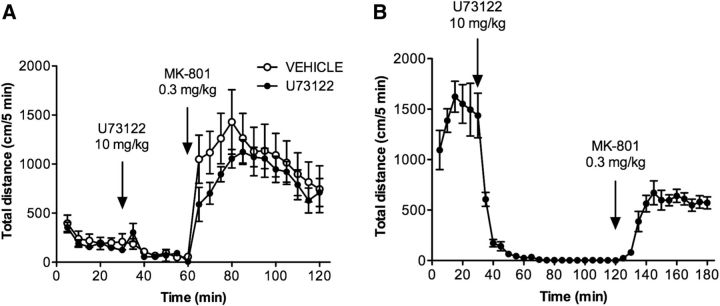
The question of whether PLCβ inhibition selectively affects dopamine-mediated activity was addressed using MK-801, an NMDA receptor antagonist that increases locomotor activity independent of dopamine (Gainetdinov et al., 2001; Chartoff et al., 2005). As shown in Figure 7A, the injection of WT mice with MK-801 (0.3 mg/kg) results in a robust increase in locomotor activity that is unaffected by U73122 pretreatment (10 mg/kg). MK-801 treatment also stimulates locomotor activity in DAT-KO mice pretreated with U73122 (Fig. 7B). These studies demonstrate that U73122 selectively inhibits dopamine-mediated activity, and that the locomotor effects of the inhibitor are not due to general sedation, neuromuscular block, or muscular weakness.
Figure 7.
The PLCβ inhibitor U73122 does not suppress locomotor hyperactivity induced by MK801, an NMDA receptor antagonist. A, B, C57BL/6J (A) and DAT-KO (B) mice were habituated to the locomotor activity chamber for 30 min and then treated with vehicle (only C57BL/6J mice) or U73122 (10 mg/kg, i.p.; both C57BL/6J and DAT-KO mice). After 30 min (for C57BL/6J mice) or 90 min (for DAT-KO mice), animals were injected with MK-801 (0.3 mg/kg, i.p.), and locomotor activity was assessed for 60 min. Distance traveled (in centimeters) was measured over 5 min intervals. Data are presented as the mean ± SEM. N = 8.
Last, we evaluated the effect of the PLCβ/IP3 pathway on the behavior elicited by dopamine itself. The previous experiments used direct or indirect agonists of the dopamine system or a genetic model of hyperdopaminergia. However, a unique feature of DAT-KO mice allows the assessment of the effect of dopamine depletion and dopamine restoration on motor activity. In DAT-KO animals, all available dopamine results from de novo synthesis by tyrosine hydroxylase (TH). Inhibition of TH by αMPT leads to complete depletion of the brain dopamine content in these animals, resulting in complete akinesia and immobility (Sotnikova et al., 2005; Costa et al., 2006; Dzirasa et al., 2006; Managò et al., 2012). These animals are termed dopamine-deficient DAT-KO mice (DDD mice). Importantly, dopamine can be restored in DDD mice by injecting them with l-DOPA, which bypasses the inhibition of TH and restores locomotor activity. This system thus allows the assessment of dopamine signaling directly, without the use of direct or indirect ligands. We therefore investigated the functional role of the PLCβ/IP3 pathway on dopamine motor activity using DDD mice.
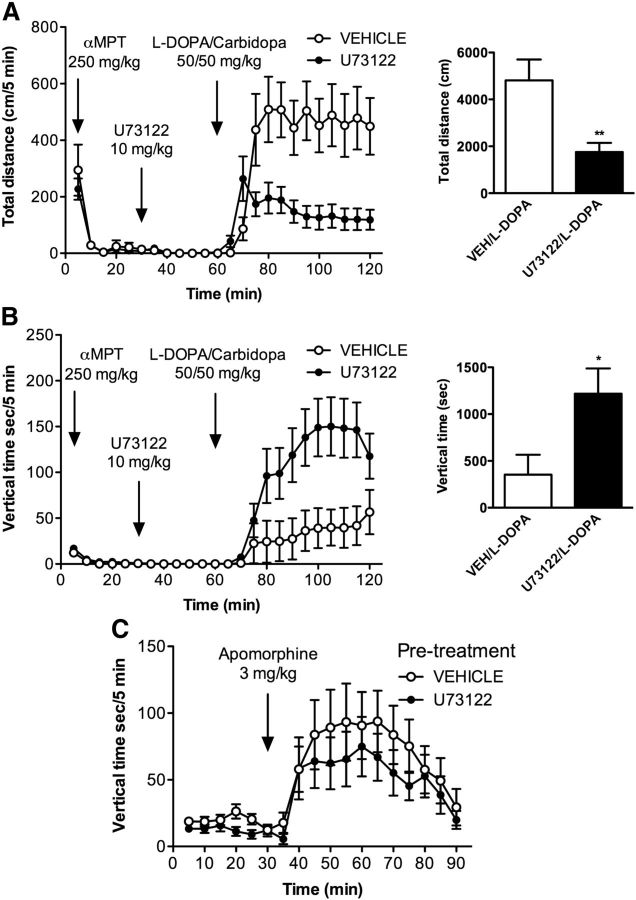
DAT-KO mice were first injected with αMPT (250 mg/kg), and their locomotor activity was monitored for 30 min. This produced complete immobility, as expected. Mice were subsequently injected with U73122 (10 mg/kg) or vehicle. Thirty minutes after the U73122 injection, animals received an injection of l-DOPA/carbidopa (50/50 mg/kg), which normally restores locomotor activity (Sotnikova et al., 2005; Costa et al., 2006; Dzirasa et al., 2006; Managò et al., 2012).
The administration of U73122 prevents the restoration of locomotor activity by l-DOPA as measured by the total distance traveled (Fig. 8A). Interestingly, however, there is a striking switch in the locomotor behavior of the mice injected with U73122. As shown in Figure 8B, the animals that were injected with U73122 display a vertical activity phenotype after l-DOPA injection instead of horizontal activity. This result suggests that the PLCβ/IP3 pathway is primarily important for regulating forward locomotor activity in mice, and that the inhibition of this pathway can switch the behavioral locomotor output of the dopamine system from horizontal to vertical (climbing) activity.
Figure 8.
Vertical activity is preserved following inhibition of PLCβ. DAT-KO mice were made dopamine deficient by treatment with αMPT (250 mg/kg, i.p.) and were placed in locomotor activity chambers. After 30 min, mice were treated with vehicle (VEH; N = 13) or U73122 (10 mg/kg, i.p.; N = 16). After another 30 min, all mice were injected with a combination of l-DOPA/carbidopa (50/50 mg/kg, i.p.). A, Locomotor activity was measured as the distance traveled (in centimeters) over 5 min intervals (left). Total distance traveled in the 60 min after l-DOPA/carbidopa injection is shown in the right panel. B, During the same treatment regimen, time (in seconds) spent in vertical activity was quantified in 5 min intervals (left). The total time spent in vertical activity over the 60 min after l-DOPA/carbidopa injection is shown in the right panel. C, C57BL/6J mice were pretreated with vehicle or U73122 (10 mg/kg, i.p.) and placed in locomotor activity chambers. After 30 min, mice were injected with apomorphine (3 mg/kg, s.c.), and locomotor activity was recorded for another 60 min. The time (in seconds) spent in vertical activity is shown in 5 min intervals. N = 8. All data are presented as the mean ± SEM. *p < 0.05, **p < 0.01 as determined by Student's t test.
If so, the induction of vertical (climbing) activity in mice should be relatively insensitive to U73122 inhibition. It is well described that, at doses of 2–4 mg/kg, apomorphine induces vertical activity (climbing) in mice (Chow and Beck, 1984). We therefore determined whether U73122 would affect apomorphine-induced climbing. As shown in Figure 8C, injection of U73122 (10 mg/kg) had no significant effect on apomorphine (3 mg/kg)-induced vertical activity, highlighting the importance of the PLCβ/IP3 pathway in the selective modulation of horizontal locomotor activity.
Discussion
Here we report in vivo evidence that dopamine stimulation of the PLCβ/IP3 pathway is an important contributor to locomotor activity in mice. Over the years, there have been numerous studies that have reported the ability of the dopamine system to modulate the PLCβ/IP3 pathway; however, to date, a clear physiological role for this pathway has not been established (Undie and Friedman, 1990; Hasbi et al., 2010). We show that blockade of this pathway leads to the inhibition of both basal and psychostimulant-mediated locomotor activity. Importantly, the PLCβ/IP3 pathway is selective for dopamine-mediated behavior, as the stimulant effects of MK-801 were not blocked by U73122 treatment.
Additionally, using a high dose of apomorphine, we demonstrate that the PLCβ/IP3 pathway selectively modulates “forward” locomotor activity and does not inhibit vertical activity (climbing). Furthermore, in DDD mice treated with U73122, restoring locomotor activity with l-DOPA led to vertical rather than the horizontal activity. These observations clearly indicate that the PLCβ/IP3 pathway is more important for forward locomotion than vertical activity. Intriguingly, vertical activity induced by dopamine agonists has been linked by some to l-DOPA-induced dyskinesas (Johnston et al., 2005). Although the signaling mechanisms underlying these diverse stereotypic behaviors are poorly understood (Undie et al., 2000), our study may provide evidence for the role of D2 receptors in vertical activity. Alternately, our studies could be interpreted to indicate that D1 signaling through other pathways besides PLCβ/IP3 is responsible for dopamine-mediated vertical activity.
Evidence that G-protein-coupled dopamine receptors signal through multiple pathways has been demonstrated in several systems (Kotecha et al., 2002; Beaulieu et al., 2005; Masri et al., 2008; Rajagopal et al., 2010; Chun et al., 2013). Our studies lend support to the notion that D1 receptors can signal through both PKA and PLCβ, and that these two pathways can exhibit cross talk in modulating Ser845, a PKA-dependent AMPA receptor phosphorylation site. In line with this, it has previously been shown that the activation of PKC, the downstream effector of PLCβ, can modulate PKA activity through a CDK5/DARPP32 signaling pathway (Bibb et al., 1999; Sahin et al., 2008). This raises the possibility that activation of PLCβ signaling by D1 receptor may also contribute to the maintenance of PKA activity following its activation by this same receptor.
It has long been known that a subpopulation of D1-like receptors are able to stimulate IP3 production through a PLCβ mechanism (Undieh, 2010). These studies have been performed mainly on brain slices ex vivo. Sahu et al. (2009) showed that dopamine-induced IP3 accumulation was attenuated in striatal slices from D5-KO animals. In another study, Friedman et al. (1997) were still able to detect IP3 production following dopamine stimulation in cortical slices from D1-KO mice. However, it is important to note that Friedman et al. (1997) conducted IP3 measurements on cortical slices, while our studies were performed in the striatum. Therefore, it is possible that different receptor subtypes may be mediating dopamine-induced IP3 production in different brain structures.
In addition to reports that D1-like receptors mediate PLCβ/IP3 production, a series of studies have also highlighted the ability of the D1/D2 receptor heteromer to mobilize calcium through a Gq/PLCβ-mediated pathway (Hasbi et al., 2009, 2010; Perreault et al., 2011). However, a recent study reports that dopamine receptor-mediated calcium signaling can occur through multiple pathways, including those that are independent of D1/D2 heteromers or Gq (Chun et al., 2013). It should also be noted that the population of medium spiny neurons coexpressing D1 and D2 receptors are mainly located in the nucleus accumbens (Perreault et al., 2010), while locomotor activity is mainly regulated by the dorsal striatum (Hnasko et al., 2006). Consequently, the modulation of the PLCβ/IP3 pathway by dopamine receptors may be different in ventral and dorsal striatum. Thus, while activation of the D1/PLCβ/IP3 pathway in the dorsal striatum may be required to modulate locomotor activity, stimulation of the PLCβ/IP3 pathway in the ventral striatum may be required to mediate limbic responses.
In conclusion, we report the first demonstration of the involvement of a PLCβ/IP3 pathway in selectively regulating dopamine-mediated locomotor activity. Our results show that this pathway is crucial for horizontal activity and does not influence stereotypical vertical activity displayed by mice after apomorphine injection. Interestingly, our in vivo studies indicate that direct or indirect dopamine agonists stimulate IP3 production exclusively though D1 dopamine receptor. This signaling cascade can potentially be targeted for the development of novel therapies for movement disorders including Parkinson's disease.
Footnotes
This work was partially supported by National Institutes of Health K99 Grant 1K99ES016816-01, and Canadian Institutes of Health Research Operating Grants 210296 (to A.S.) and 258294 (to A.J.R.). We thank Marc G. Caron for support and critical reading of the manuscript.
The authors declare no competing financial interests.
References
- Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, Fisone G, Nestler EJ, Greengard P. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci U S A. 2010;107:14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Brandon EP, Logue SF, Adams MR, Qi M, Sullivan SP, Matsumoto AM, Dorsa DM, Wehner JM, McKnight GS, Idzerda RL. Defective motor behavior and neural gene expression in RIIβ-protein kinase A mutant mice. J Neurosci. 1998;18:3639–3649. doi: 10.1523/JNEUROSCI.18-10-03639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A. A paradigm shift in brain research. Science. 2001;294:1021–1024. doi: 10.1126/science.1066969. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Heusner CL, Palmiter RD. Dopamine is not required for the hyperlocomotor response to NMDA receptor antagonists. Neuropsychopharmacology. 2005;30:1324–1333. doi: 10.1038/sj.npp.1300678. [DOI] [PubMed] [Google Scholar]
- Chow HL, Beck HM. The effect of apomorphine on the open-field behavior of rats: alone and in pairs. Pharmacol Biochem Behav. 1984;21:85–88. doi: 10.1016/0091-3057(84)90135-7. [DOI] [PubMed] [Google Scholar]
- Chun LS, Free RB, Doyle TB, Huang XP, Rankin ML, Sibley DR. D1–D2 dopamine receptor synergy promotes calcium signaling via multiple mechanisms. Mol Pharmacol. 2013;84:190–200. doi: 10.1124/mol.113.085175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol JC, Valjent E, Pascoli V, Robin A, Stipanovich A, Luedtke RR, Belluscio L, Girault JA, Hervé D. Quantitative changes in Galphaolf protein levels, but not D1 receptor, alter specifically acute responses to psychostimulants. Neuropsychopharmacology. 2007;32:1109–1121. doi: 10.1038/sj.npp.1301230. [DOI] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, Nicolelis MA. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52:359–369. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Ribeiro S, Costa R, Santos LM, Lin SC, Grosmark A, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA. Dopaminergic control of sleep-wake states. J Neurosci. 2006;26:10577–10589. doi: 10.1523/JNEUROSCI.1767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault J, et al. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Friedman E, Jin LQ, Cai GP, Hollon TR, Drago J, Sibley DR, Wang HY. D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: evidence from D1A knockout mice. Mol Pharmacol. 1997;51:6–11. doi: 10.1124/mol.51.1.6. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Mohn AR, Bohn LM, Caron MG. Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc Natl Acad Sci U S A. 2001;98:11047–11054. doi: 10.1073/pnas.191353298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisi V, Ramsey AJ, Masri B, Gainetdinov RR, Caron MG, Salahpour A. Reduced D2-mediated signaling activity and trans-synaptic upregulation of D1 and D2 dopamine receptors in mice overexpressing the dopamine transporter. Cell Signal. 2009;21:87–94. doi: 10.1016/j.cellsig.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O'Dowd BF, George SR. Calcium signaling cascade links dopamine D1–D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci U S A. 2009;106:21377–21382. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, O'Dowd BF, George SR. Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms. Curr Opin Pharmacol. 2010;10:93–99. doi: 10.1016/j.coph.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Perez FA, Scouras AD, Stoll EA, Gale SD, Luquet S, Phillips PE, Kremer EJ, Palmiter RD. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc Natl Acad Sci U S A. 2006;103:8858–8863. doi: 10.1073/pnas.0603081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston TH, Lee J, Gomez-Ramirez J, Fox SH, Brotchie JM. A simple rodent assay for the in vivo identification of agents with potential to reduce levodopa-induced dyskinesia in Parkinson's disease. Exp Neurol. 2005;191:243–250. doi: 10.1016/j.expneurol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, MacDonald JF. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron. 2002;35:1111–1122. doi: 10.1016/S0896-6273(02)00859-0. [DOI] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lança AJ, O'Dowd BF, George SR. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Managò F, Espinoza S, Salahpour A, Sotnikova TD, Caron MG, Premont RT, Gainetdinov RR. The role of GRK6 in animal models of Parkinson's disease and L-DOPA treatment. Sci Rep. 2012;2:301. doi: 10.1038/srep00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR, Caron MG. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci U S A. 2008;105:13656–13661. doi: 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Nally RE, McNamara FN, Clifford JJ, Kinsella A, Tighe O, Croke DT, Fienberg AA, Greengard P, Waddington JL. Topographical assessment of ethological and dopamine receptor agonist-induced behavioral phenotype in mutants with congenic DARPP-32 “knockout.”. Neuropsychopharmacology. 2003;28:2055–2063. doi: 10.1038/sj.npp.1300259. [DOI] [PubMed] [Google Scholar]
- Neve KA. The dopamine receptors. New York: Humana; 2010. [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/RRS-200029981. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O'Dowd BF, George SR. The dopamine D1–D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem. 2010;285:36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, O'Dowd BF, George SR. The dopamine d1–d2 receptor heteromer in striatal medium spiny neurons: evidence for a third distinct neuronal pathway in basal ganglia. Front Neuroanat. 2011;5:31. doi: 10.3389/fnana.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O'Dowd BF, George SR. D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/S0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Sahin B, Hawasli AH, Greene RW, Molkentin JD, Bibb JA. Negative regulation of cyclin-dependent kinase 5 targets by protein kinase C. Eur J Pharmacol. 2008;581:270–275. doi: 10.1016/j.ejphar.2007.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A, Tyeryar KR, Vongtau HO, Sibley DR, Undieh AS. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol Pharmacol. 2009;75:447–453. doi: 10.1124/mol.108.053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotnikova TD, Beaulieu JM, Barak LS, Wetsel WC, Caron MG, Gainetdinov RR. Dopamine-independent locomotor actions of amphetamines in a novel acute mouse model of Parkinson disease. PLoS Biol. 2005;3:e271. doi: 10.1371/journal.pbio.0030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253:987–992. [PubMed] [Google Scholar]
- Undie AS, Berki AC, Beardsley K. Dopaminergic behaviors and signal transduction mediated through adenylate cyclase and phospholipase C pathways. Neuropharmacology. 2000;39:75–87. doi: 10.1016/S0028-3908(99)00106-9. [DOI] [PubMed] [Google Scholar]
- Undieh AS. Pharmacology of signaling induced by dopamine D(1)-like receptor activation. Pharmacol Ther. 2010;128:37–60. doi: 10.1016/j.pharmthera.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V, Hasbi A, O'Dowd BF, George SR. Dopamine D1–D2 receptor Heteromer-mediated calcium release is desensitized by D1 receptor occupancy with or without signal activation: dual functional regulation by G protein-coupled receptor kinase 2. J Biol Chem. 2010;285:35092–35103. doi: 10.1074/jbc.M109.088625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Sgambato-Faure V, Sasaki T, Svenningsson P, Berton O, Fienberg AA, Nairn AC, Greengard P, Nestler EJ. Phosphorylation of DARPP-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacology. 2006;31:555–562. doi: 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Belluscio L, Hen R. GOLFα mediates dopamine D1 receptor signaling. J Neurosci. 2000;20:RC91. doi: 10.1523/JNEUROSCI.20-16-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]