Abstract
Background
The International Liaison Committee on Resuscitation (ILCOR) and UK Resuscitation Council (UKRC) updated guidance on newborn resuscitation in late 2010.
Objectives
To describe delivery room (DR) practice in stabilisation following very preterm birth (<32 weeks gestation) in the UK.
Methods
We emailed a national survey of current DR stabilisation practice of very preterm infants to all UK delivery units and conducted telephone follow-up calls.
Results
We obtained 197 responses from 199 units (99%) and complete data from 186 units. Tertiary units administered surfactant in the DR (93% vs. 78%, P = 0.01), instituted DR CPAP (77% vs. 50%, P = 0.0007), provided PEEP in the delivery room (91% vs. 69%, P = 0.0008), and started resuscitation in air or blended oxygen (91% vs. 78%, P = 0.04) more often than non-tertiary units. Routine out of hours consultant attendance at very preterm birth was more common in tertiary units (82% vs. 55%, P = 0.0005).
Conclusions
Marked variation in DR stabilisation practice of very preterm infants persisted one year after the publication of revised UKRC guidance. Delivery room care provided in non-tertiary units was less consistent with current international guidance.
Abbreviations: NICU, neonatal intensive care unit; DR, delivery room; CPAP, continuous positive airway pressure; PEEP, positive end expiratory pressure; NDAU, neonatal data analysis unit; NPEU, national perinatal epidemiology unit
Keywords: Stabilisation practice in preterm infants, Neonatal resuscitation, Survey, Practice variation
1. Introduction
Approaches to delivery room (DR) stabilisation of preterm infants should reflect International Consensus on Cardiopulmonary Resuscitation (ILCOR) and UK Resuscitation Council (UKRC) guidelines. These were recently updated.1,2
While only around 10% of term infants need additional support in perinatal transition2 many very preterm infants benefit from assisted stabilisation in the delivery room. Only a few studies have examined the consistency in clinical practice in DR resuscitation and data from other developed countries on standard clinical practices in DR resuscitation showed inconsistency and discordance from current clinical evidence.3–8 A recent study by Mann et al. showed marked variations in resuscitation practices of term infants among UK neonatal units.3
Few data describe current clinical practices in delivery room stabilisation of preterm infants although clinical opinion suggests effective stabilisation is important for good outcome.6,7
The aims of our study were:
-
1.
To describe current DR stabilisation practices for very preterm infants (<32 weeks) of gestation at UK neonatal units.
-
2.
To identify differences in clinical practice by unit level.
2. Methods
We sent a structured questionnaire about usual DR management after very preterm birth in the UK.
In September 2011, we sent a previously piloted structured web based questionnaire to neonatal contacts on the central database of British Association of Perinatal Medicine (BAPM) and National Perinatal Epidemiology Unit (NPEU) asking about usual delivery room management following very preterm birth at the 199 delivery centres. Between October and November 2011 we resent the questionnaire. Finally, we contacted non responding units by telephone between December 2011 and January 2012, accepting a response from a consultant, senior trainee or senior nursing sister.
The data were analysed using Fisher's exact test for categorical variables and Mann–Whitney U test for non-parametric numerical variables. In all the domains a P < 0.05 was considered statistically significant. In analysing data NICUs were considered as “tertiary units” while local neonatal units (LNUs) and special care units (SCUs) were classified as “non-tertiary” units.
Our study was approved by local research and development (R&D) department at Bradford Teaching Hospitals NHS Foundation Trust but did not require ethics advisory committee approval.
3. Results
We obtained 197 responses from 199 hospitals (99%), with 186/197 (94%) questionnaires fully completed. Completion rates were similar between tertiary (n = 55, 92%) and non-tertiary (131, 95%) units. Of the total 197 responses, 39% (n = 78) responders were consultants, 14% (n = 28) senior trainees, and 46% (n = 91) senior neonatal sisters.
3.1. CPAP provision
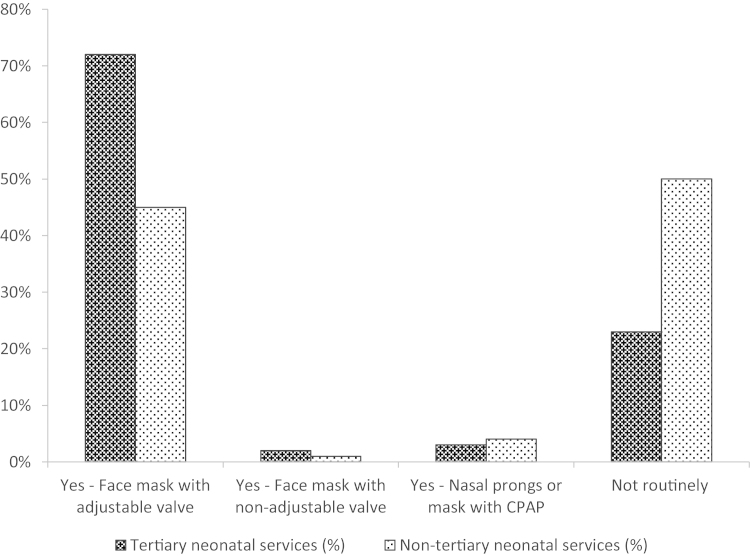
Overall 60% units (112 of 186 units) provide CPAP routinely during stabilisation following very preterm birth. Tertiary neonatal units provided CPAP following very preterm birth as part of the stabilisation process in DR more frequently than non-tertiary neonatal units (77% vs. 50%, P = 0.0007) (Fig. 1).
Fig. 1.
Mode of CPAP or PEEP provision during DR stabilisation among tertiary and nontertiary neonatal services UK 2011–2012.
Out of those centres routinely giving CPAP, 11/44 (25%) tertiary units only do this routinely for babies with gestation <28 weeks compared to 4/68 (6%) of non-tertiary units (P = 0.008).
There was marked variation among both tertiary and non-tertiary neonatal units in clinical practice under what gestation they provide CPAP to non-ventilated infants routinely (range under 26–32 weeks of gestation).
For ventilated infants, 76% units (142 units) provide positive end expiratory pressure (PEEP). There was a significant difference between tertiary and non-tertiary centres (tertiary 91%, non-tertiary 69%, P = 0.0008).
3.2. Surfactant administration
Administration of surfactant in DR, regardless of infant condition, was reportedly part of their standard resuscitation practice in 157 units (82%) while in 34 units (18%) this surfactant administration in DR was not a routine practice. Tertiary units were more likely to do so (93% vs. 78%, P = 0.01).
Out of those centres routinely giving surfactants, 41/52 (79%) tertiary units only do this routinely for babies with gestation <28 weeks compared to 54/105 (51%) of non-tertiary units (P = 0.001).
There was wide variation in gestational age (range under 27–32 weeks; median for tertiary units <28 weeks while for non-tertiary units was <29 weeks) under which different neonatal units routinely administered surfactant in DR as part of standard resuscitation practice.
3.3. Oxygen therapy
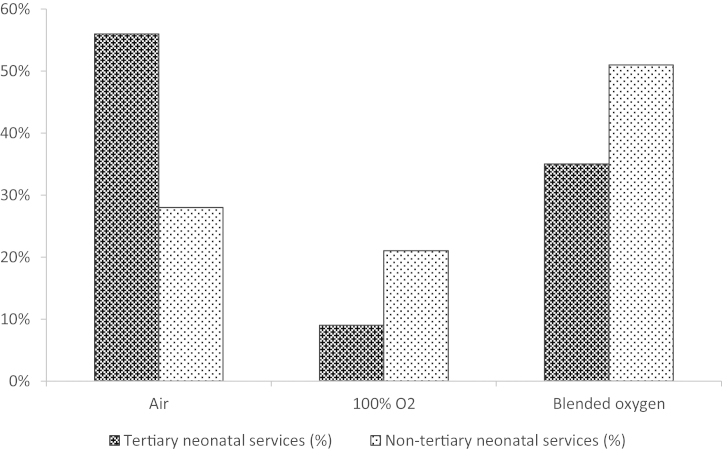
Of the 186 units providing data, 69 units (37%) commence stabilisation in air, 83 units (45%) in blended oxygen and 34 units (18%) in 100% oxygen. Tertiary units started stabilisation in air or blended gas more frequently than non-tertiary units (91% vs. 78%, P = 0.04) (Fig. 2).
Fig. 2.
Proportion of neonatal services using air, oxygen or blended oxygen for DR stabilisation following very preterm birth.
3.4. Use of pulse oximeter
Pulse oximetry to monitor heart rate or titrate oxygen delivery was used routinely in 30% units (n = 56). For 10 units (5%) this data was not provided. There was insufficient evidence of a difference in pulse oximetry between tertiary and non-tertiary units (79% vs. 66%, P = 0.12).
3.5. Thermoregulation
Almost all neonatal units (190 of 192; 99%) use plastic wrapping to enhance thermoregulation in the DR and there was no statistical difference between use of plastic bag in tertiary and non-tertiary units (P = 0.477). Median gestation under what both tertiary and non-tertiary neonatal units used occlusive wrapping was 30 weeks).
3.6. Cord clamping
Deferred cord clamping (DCC) was reported as usual practice at 52 units (28%). There was marked variation in duration of DCC (30 s–3 min) with most common practice between 31 and 60 s. 4 units reported practicing cord milking. There was no significant difference in the percentage practicing DCC between tertiary and non-tertiary units (35% vs. 24%, P = 0.16).
3.7. Routine consultant attendance at delivery of very preterm infants
119 units (63%) reported routine out of hours consultant attendance at very preterm birth and there was wide variation between units under what gestation consultants routinely attend very preterm birth delivery. A higher proportion of tertiary units practiced routine consultant attendance at very preterm birth (82% vs. 55%, P = 0.0005).
4. Discussion
We found that some aspects of recommended stabilisation practice have penetrated well into current UK practice, such as use of occlusive plastic wrapping. The utilisation of other techniques, such as delivery room CPAP, delivery room pulse oximetry and provision of mixed ventilation gases has improved in a short time frame, although marked variation in practice persists [3]. Our data show that tertiary units appear to have adopted recommended practices more quickly than other units.
Our study has significant strengths – particularly its very high response rate, which means it is likely we are describing practices neonatal professionals intend to deploy one year after the revised ILCOR guidelines. However, our questionnaire approach precludes describing actual clinical practice.
Meta-analysis of studies comparing outcome following resuscitation in air and oxygen demonstrated increased mortality in infants started in 100% oxygen.9 The ILCOR 2010 guidelines recommend starting resuscitation for term infants in air rather than 100% oxygen and administration of supplementary oxygen should be regulated by blending oxygen and air and the amount delivered to be guided by oximetry.1
Studies on preterm infants comparing initiating resuscitation in higher oxygen (90–100%) to lower (21–30%) oxygen concentrations showed that preterm infants starting in low oxygen concentrations frequently need supplemental oxygen to achieve SpO2 targets initially.10–12
However in the absence of evidence from large trials measuring clinically important outcomes, it is reasonable to start resuscitation in air or lower oxygen concentrations, which may be increased or reduced with a blender, guided by pulse oximetry.13–15 82% of UK units initiate stabilisation with either air or a mixture of air and oxygen, with initial use of air being common practice and consistent with teaching materials from the Resuscitation Council (UK).16 The use of oximetry has become more common, and the use of 100% oxygen less common, in association with the publication of the recent guidance.3
Respiratory support, particularly the provision of CPAP in delivery suite is of significant and increasing interest. The SUPPORT and COIN trials supported consideration of CPAP as an alternative to elective DR intubation and surfactant in preterm infants.17,18 Our data suggest that provision of CPAP in the DR has become progressively more common as compared to national survey reported by Mann et al. survey done in 2009–2010.3 Our data suggest that many units aim to provide this, and it may be that commercially available variable PEEP valves facilitate this. We were surprised to find this was the predominant means of delivering CPAP in the DR, given the practical challenges of maintaining mask seal and transferring a baby while maintaining such a seal.19,20
Deferred cord clamping in preterm infants has been reported to be associated with decreased incidence of intraventricular haemorrhage (IVH), decreased need of blood transfusion, better haemodynamic stability and lower risk of necrotising enterocolitis, but it is uptake in clinical practice remains quite low, perhaps on account of the small numbers of infants studied. DCC was far from universally practiced, with little consistency in the duration of deferral.
Anecdotally, consultant presence at very preterm birth delivery appears to improve DR management, increased chances of DCC and better outcome in extremely preterm infants. More tertiary units seem to have adopted DCC and routine out of hours consultants presence but wide variation in practice persists among both tertiary and non-tertiary neonatal units which could reflect lack of robust evidence in these areas.
Conflict of interest statement
The authors declare no financial or other conflicts of interest.
Financial disclosure
This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research funding scheme (RP-PG-0609-10107). The views expressed in this paper are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Acknowledgements
We would like to thank all the neonatal units and staff who responded to our survey. We would also like to thank Mr. Richard A. Parker from the Centre for Applied Medical Statistics, University of Cambridge for statistical support.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Perlman J.M., Wyllie J., Kattwinkel J. Part 11: neonatal resuscitation: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2010;122:S516–S538. doi: 10.1161/CIRCULATIONAHA.110.971127. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Richmond S., Wyllie J. Newborn life support. In: Nolan J.P., editor. The resuscitation guidelines 2010. The Resuscitation Council (UK); London: 2010. (Chapter 11) [Google Scholar]
- 3.Mann C., Grubb M., Hayes-Gill B., Crowe J., Marlow N., Sharkey D. Marked variations in newborn resuscitation practice. Resuscitation. 2012;83:607–611. doi: 10.1016/j.resuscitation.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donnell C.P., Davis P.G., Morley C.J. Neonatal resuscitation: review of ventilation equipment and survey of practice in Australia and New Zealand. J Paediatr Child Health. 2004;40:208–212. doi: 10.1111/j.1440-1754.2004.00339.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Saenz P., Brugada M., de Jongh B. A survey of intravenous sodium bicarbonate in neonatal asphyxia among European neonatologists: gaps between scientific evidence and clinical practice. Neonatology. 2011;99:170–176. doi: 10.1159/000313780. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Iriondo M., Thio M., Buron E., Salguero E., Aguayo J., Vento M. A survey of neonatal resuscitation in Spain: gaps between guidelines and practice. Acta Paediatr. 2009;98:786–791. doi: 10.1111/j.1651-2227.2009.01233.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Acolet D., Elbourne D., McIntosh N. Project 27/28: inquiry into quality of neonatal care and its effect on the survival of infants who were born at 27 and 28 weeks in England, Wales, and Northern Ireland. Pediatrics. 2005:1457–1465. doi: 10.1542/peds.2004-2691. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Polglase G.R., Hillman N.H., Pillow J.J. Positive end-expiratory pressure and tidal volume during initial ventilation of preterm lambs. Pediatr Res. 2008;64:517–522. doi: 10.1203/PDR.0b013e3181841363. [PMC free article] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis P.G., Tan A., O’Donnell C.P., Schulze A. Resuscitation of newborn infants with 100% oxygen or air: a systematic review and meta-analysis. Lancet. 2004;364:1329–1333. doi: 10.1016/S0140-6736(04)17189-4. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Dinger J., Topfer A., Schaller P., Schwarze R. Effect of positive end expiratory pressure on functional residual capacity and compliance in surfactant-treated preterm infants. J Perinat Med. 2001;29:137–143. doi: 10.1515/JPM.2001.018. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Sweet D.G., Carnielli V., Greisen G., Hallman M., Ozek E., Plavka R. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants – 2010 update. Neonatology. 2010;97:402–417. doi: 10.1159/000297773. [DOI] [PubMed] [Google Scholar]
- 12.Leone T.A., Rich W., Finer N.N. A survey of delivery room resuscitation practices in the United States. Pediatrics. 2006;117:e164–e175. doi: 10.1542/peds.2005-0936. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Richmond S., Wyllie J. European Resuscitation Council Guidelines for Resuscitation 2010 Section 7. Resuscitation of babies at birth. Resuscitation. 2010;81:1389–1399. doi: 10.1016/j.resuscitation.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Finer N., Saugstad O., Vento M. Use of oxygen for resuscitation of the extremely low birth weight infant. Pediatrics. 2010;125:389–391. doi: 10.1542/peds.2009-1247. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell C.P.F. Turn and face the strange – ch.ch.ch.changes to neonatal resuscitation guidelines in the past decade. J Paediatr Child Health. 2012;48:735–739. doi: 10.1111/j.1440-1754.2012.02531.x. [DOI] [PubMed] [Google Scholar]
- 16.Richmond S., Russell A.B., Coleman A. 2nd ed. Published by Resuscitation Council (UK); London: 2006. Newborn life support. [Google Scholar]
- 17.Finer N.N., Carlo W.A., Walsh M.C. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–1979. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morley C.J., Davis P.G., Doyle L.W. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358:700–708. doi: 10.1056/NEJMoa072788. [DOI] [PubMed] [Google Scholar]
- 19.Wood F.E., Morley C.J., Dawson J.A. Assessing the effectiveness of two round neonatal resuscitation masks: study 1. Arch Dis Child Fetal Neonatal Ed. 2008;93:F235–F237. doi: 10.1136/adc.2007.117713. [DOI] [PubMed] [Google Scholar]
- 20.Wood F.E., Morley C.J., Dawson J.A., Kamlin C.O.F., Owen L.S., Donath S. Improved techniques reduce face mask leak during simulated neonatal resuscitation: study 2. Arch Dis Child Fetal Neonatal Ed. 2008;93:F230–F234. doi: 10.1136/adc.2007.117788. [DOI] [PubMed] [Google Scholar]