Abstract
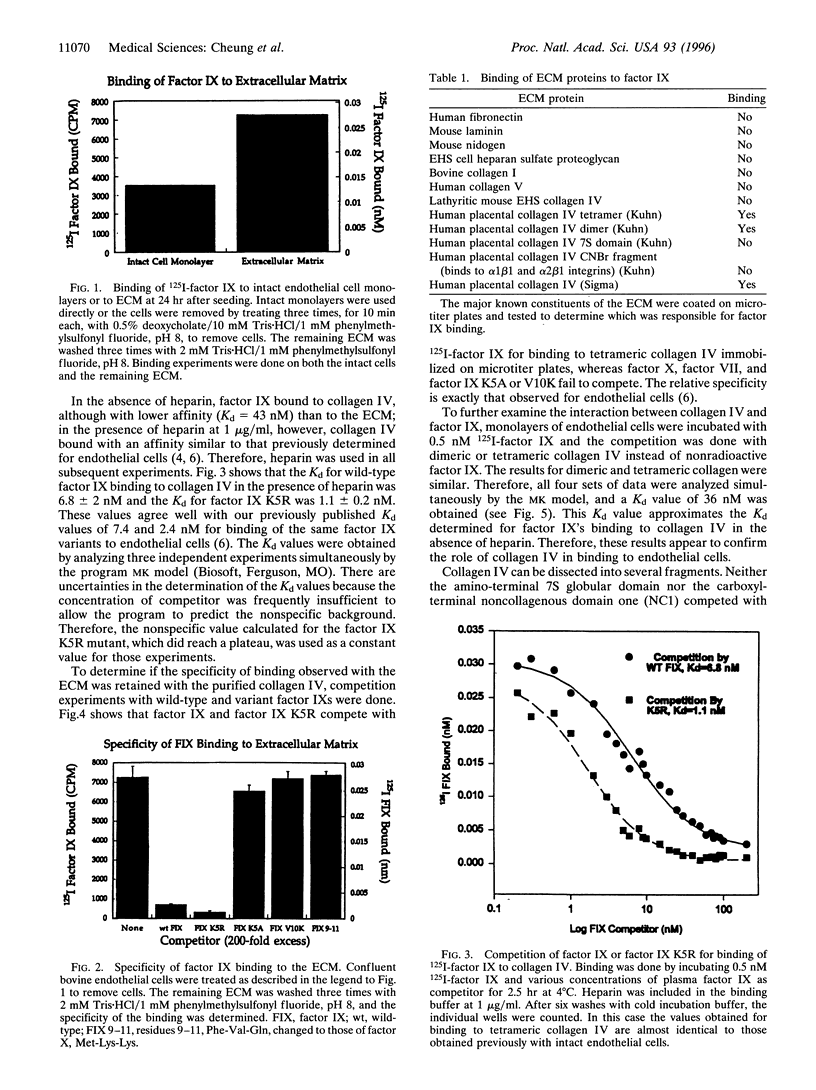
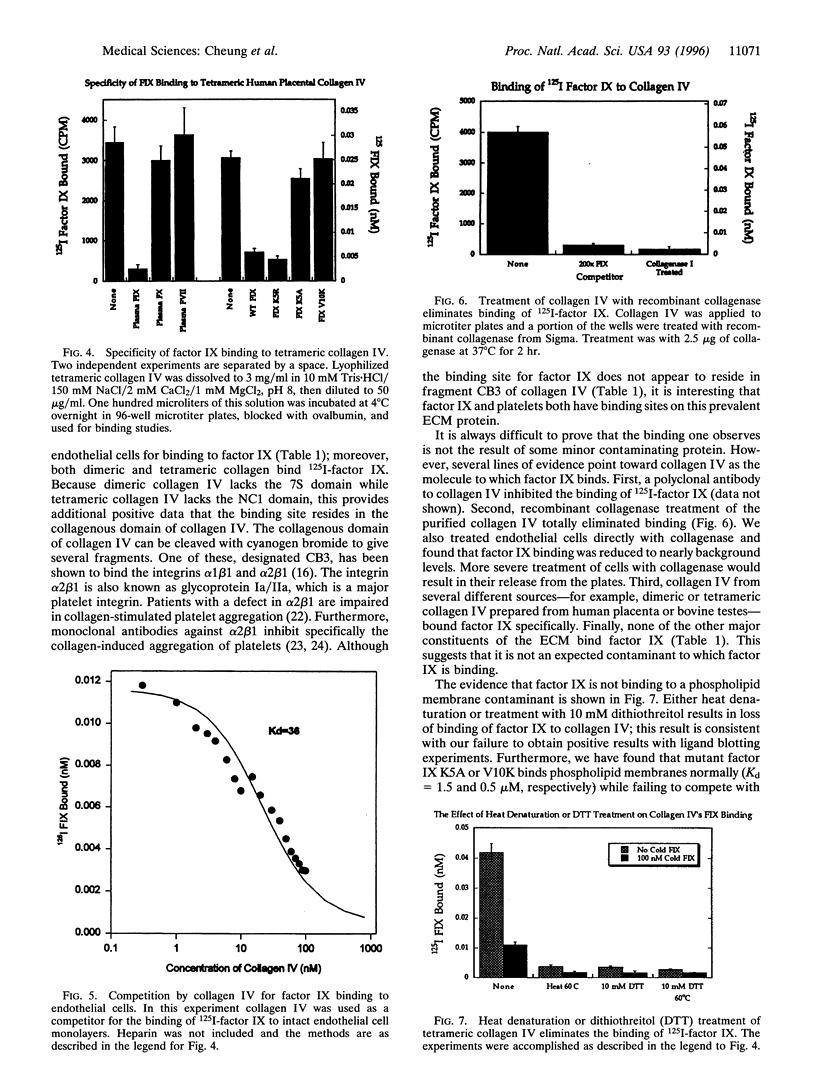
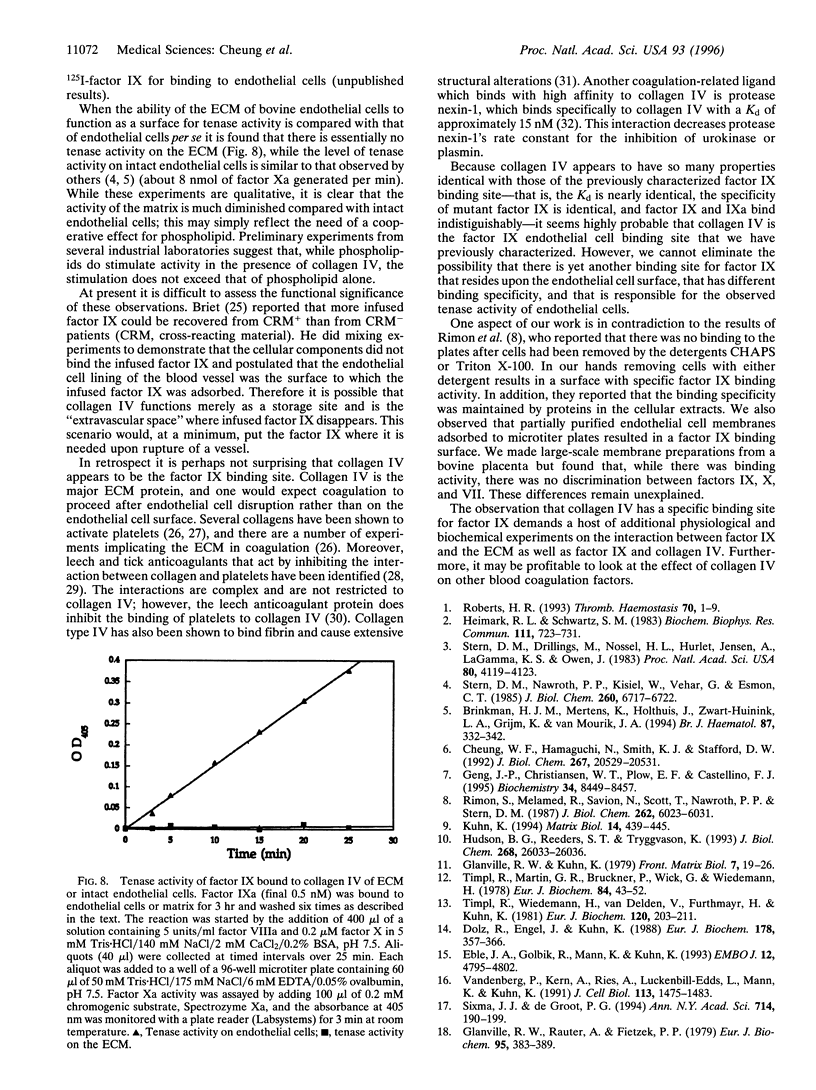
We previously demonstrated that the primary region of factor IX and IXa responsible for saturable specific binding to bovine aortic endothelial cells resides in residues 3-11 at the amino terminus of factor IX. We also demonstrated that mutations of lysine to alanine at residue 5, factor IX K5A, or valine to lysine at residue 10, factor IX V10K, resulted in a molecule unable to bind to endothelial cells. Moreover, a mutation with lysine to arginine at residue 5, factor IX K5R, resulted in a factor IX molecule with increased affinity for the endothelial cell binding site. In this paper we report that collagen IV is a strong candidate for the factor IX binding site on endothelial cells. Factor IX and factor IX K5R compete with 125I-labeled factor IX for binding to tetrameric collagen IV immobilized on microtiter plates, while factor X, factor VII, and factor IX K5A or V10K fail to compete. The Kd for wild-type factor IX binding to collagen IV in the presence of heparin was 6.8 +/- 2 nM, and the Kd for factor IX K5R was 1.1 +/- 0.2 nM, which agrees well with our previously published Kd values of 7.4 and 2.4 nM for binding of the same proteins to endothelial cells. Our working assumption is that we have identified the endothelial cell binding site and that it is collagen IV. Its physiological relevance remains to be determined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkman H. J., Mertens K., Holthuis J., Zwart-Huinink L. A., Grijm K., van Mourik J. A. The activation of human blood coagulation factor X on the surface of endothelial cells: a comparison with various vascular cells, platelets and monocytes. Br J Haematol. 1994 Jun;87(2):332–342. doi: 10.1111/j.1365-2141.1994.tb04918.x. [DOI] [PubMed] [Google Scholar]
- Cheung W. F., Hamaguchi N., Smith K. J., Stafford D. W. The binding of human factor IX to endothelial cells is mediated by residues 3-11. J Biol Chem. 1992 Oct 15;267(29):20529–20531. [PubMed] [Google Scholar]
- Coller B. S., Beer J. H., Scudder L. E., Steinberg M. H. Collagen-platelet interactions: evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood. 1989 Jul;74(1):182–192. [PubMed] [Google Scholar]
- Connolly T. M., Jacobs J. W., Condra C. An inhibitor of collagen-stimulated platelet activation from the salivary glands of the Haementeria officinalis leech. I. Identification, isolation, and characterization. J Biol Chem. 1992 Apr 5;267(10):6893–6898. [PubMed] [Google Scholar]
- Donovan F. M., Vaughan P. J., Cunningham D. D. Regulation of protease nexin-1 target protease specificity by collagen type IV. J Biol Chem. 1994 Jun 24;269(25):17199–17205. [PubMed] [Google Scholar]
- Dölz R., Engel J., Kühn K. Folding of collagen IV. Eur J Biochem. 1988 Dec 15;178(2):357–366. doi: 10.1111/j.1432-1033.1988.tb14458.x. [DOI] [PubMed] [Google Scholar]
- Eble J. A., Golbik R., Mann K., Kühn K. The alpha 1 beta 1 integrin recognition site of the basement membrane collagen molecule [alpha 1(IV)]2 alpha 2(IV). EMBO J. 1993 Dec;12(12):4795–4802. doi: 10.1002/j.1460-2075.1993.tb06168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J. P., Christiansen W. T., Plow E. F., Castellino F. J. Transfer of specific endothelial cell-binding properties from the procoagulant protein human factor IX into the anticoagulant protein human protein C. Biochemistry. 1995 Jul 4;34(26):8449–8457. doi: 10.1021/bi00026a028. [DOI] [PubMed] [Google Scholar]
- Glanville R. W., Rauter A., Fietzek P. P. Isolation and characterization of a native placental basement-membrane collagen and its component alpha chains. Eur J Biochem. 1979 Apr 2;95(2):383–389. doi: 10.1111/j.1432-1033.1979.tb12976.x. [DOI] [PubMed] [Google Scholar]
- Gunwar S., Noelken M. E., Hudson B. G. Properties of the collagenous domain of the alpha 3(IV) chain, the Goodpasture antigen, of lens basement membrane collagen. Selective cleavage of alpha (IV) chains with retention of their triple helical structure and noncollagenous domain. J Biol Chem. 1991 Jul 25;266(21):14088–14094. [PubMed] [Google Scholar]
- Hedman K., Kurkinen M., Alitalo K., Vaheri A., Johansson S., Hök M. Isolation of the pericellular matrix of human fibroblast cultures. J Cell Biol. 1979 Apr;81(1):83–91. doi: 10.1083/jcb.81.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimark R. L., Schwartz S. M. Binding of coagulation factors IX and X to the endothelial cell surface. Biochem Biophys Res Commun. 1983 Mar 16;111(2):723–731. doi: 10.1016/0006-291x(83)90365-0. [DOI] [PubMed] [Google Scholar]
- Hudson B. G., Reeders S. T., Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem. 1993 Dec 15;268(35):26033–26036. [PubMed] [Google Scholar]
- Jones M., Gabriel D. A. Influence of the subendothelial basement membrane components on fibrin assembly. Evidence for a fibrin binding site on type IV collagen. J Biol Chem. 1988 May 25;263(15):7043–7048. [PubMed] [Google Scholar]
- Karczewski J., Waxman L., Endris R. G., Connolly T. M. An inhibitor from the argasid tick Ornithodoros moubata of cell adhesion to collagen. Biochem Biophys Res Commun. 1995 Mar 17;208(2):532–541. doi: 10.1006/bbrc.1995.1371. [DOI] [PubMed] [Google Scholar]
- Kehrel B. Platelet-collagen interactions. Semin Thromb Hemost. 1995;21(2):123–129. doi: 10.1055/s-2007-1000386. [DOI] [PubMed] [Google Scholar]
- Kühn K. Basement membrane (type IV) collagen. Matrix Biol. 1995 Feb;14(6):439–445. doi: 10.1016/0945-053x(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis H. K., Sakariassen K. S., Houdijk W. P., Nievelstein P. F., Sixma J. J. Deficiency of platelet membrane glycoprotein Ia associated with a decreased platelet adhesion to subendothelium: a defect in platelet spreading. Blood. 1986 Sep;68(3):692–695. [PubMed] [Google Scholar]
- Rimon S., Melamed R., Savion N., Scott T., Nawroth P. P., Stern D. M. Identification of a factor IX/IXa binding protein on the endothelial cell surface. J Biol Chem. 1987 May 5;262(13):6023–6031. [PubMed] [Google Scholar]
- Risteli J., Bächinger H. P., Engel J., Furthmayr H., Timpl R. 7-S collagen: characterization of an unusual basement membrane structure. Eur J Biochem. 1980;108(1):239–250. doi: 10.1111/j.1432-1033.1980.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Santoro S. A. Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell. 1986 Sep 12;46(6):913–920. doi: 10.1016/0092-8674(86)90073-5. [DOI] [PubMed] [Google Scholar]
- Sixma J. J., de Groot P. G. Regulation of platelet adhesion to the vessel wall. Ann N Y Acad Sci. 1994 Apr 18;714:190–199. doi: 10.1111/j.1749-6632.1994.tb12044.x. [DOI] [PubMed] [Google Scholar]
- Sixma J. J., van Zanten G. H., Banga J. D., Nieuwenhuls H. K., de Groot P. G. Platelet adhesion. Semin Hematol. 1995 Apr;32(2):89–98. [PubMed] [Google Scholar]
- Stern D. M., Drillings M., Nossel H. L., Hurlet-Jensen A., LaGamma K. S., Owen J. Binding of factors IX and IXa to cultured vascular endothelial cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4119–4123. doi: 10.1073/pnas.80.13.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. M., Nawroth P. P., Kisiel W., Vehar G., Esmon C. T. The binding of factor IXa to cultured bovine aortic endothelial cells. Induction of a specific site in the presence of factors VIII and X. J Biol Chem. 1985 Jun 10;260(11):6717–6722. [PubMed] [Google Scholar]
- Timpl R., Martin G. R., Bruckner P., Wick G., Wiedemann H. Nature of the collagenous protein in a tumor basement membrane. Eur J Biochem. 1978 Mar;84(1):43–52. doi: 10.1111/j.1432-1033.1978.tb12139.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Wiedemann H., van Delden V., Furthmayr H., Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981 Nov;120(2):203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Vandenberg P., Kern A., Ries A., Luckenbill-Edds L., Mann K., Kühn K. Characterization of a type IV collagen major cell binding site with affinity to the alpha 1 beta 1 and the alpha 2 beta 1 integrins. J Cell Biol. 1991 Jun;113(6):1475–1483. doi: 10.1083/jcb.113.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zanten G. H., Connolly T. M., Schiphorst M. E., de Graaf S., Slootweg P. J., Sixma J. J. Recombinant leech antiplatelet protein specifically blocks platelet deposition on collagen surfaces under flow conditions. Arterioscler Thromb Vasc Biol. 1995 Sep;15(9):1424–1431. doi: 10.1161/01.atv.15.9.1424. [DOI] [PubMed] [Google Scholar]


