Summary
Although descriptions of striking diversity in animal behavior are plentiful, little is known about the mechanisms by which behaviors change and evolve between groups. To fully understand behavioral evolution, it will be necessary to identify the genetic mechanisms that mediate behavioral change in a natural context [1–3]. Genetic analysis of behavior can also reveal associations between behavior and morphological or neural phenotypes, providing insight into the proximate mechanisms that control behavior. Relatively few studies to date have successfully identified genes or genomic regions that contribute to behavioral variation among natural populations or species [2], particularly in vertebrates [4–8]. Here, we apply genetic approaches to dissect a complex social behavior that has long fascinated biologists, schooling behavior [9–13]. We performed Quantitative Trait Locus (QTL) analysis of schooling in an F2 intercross between strongly schooling marine and weakly schooling benthic sticklebacks (Gasterosteus aculeatus) and found that distinct genetic modules control different aspects of schooling behavior. Two key components of the behavior, tendency to school and body position when schooling, are uncorrelated in hybrids and map to different genomic regions. Our results further point to a genetic link between one behavioral component, schooling position, and variation in the neurosensory lateral line.
Results and Discussion
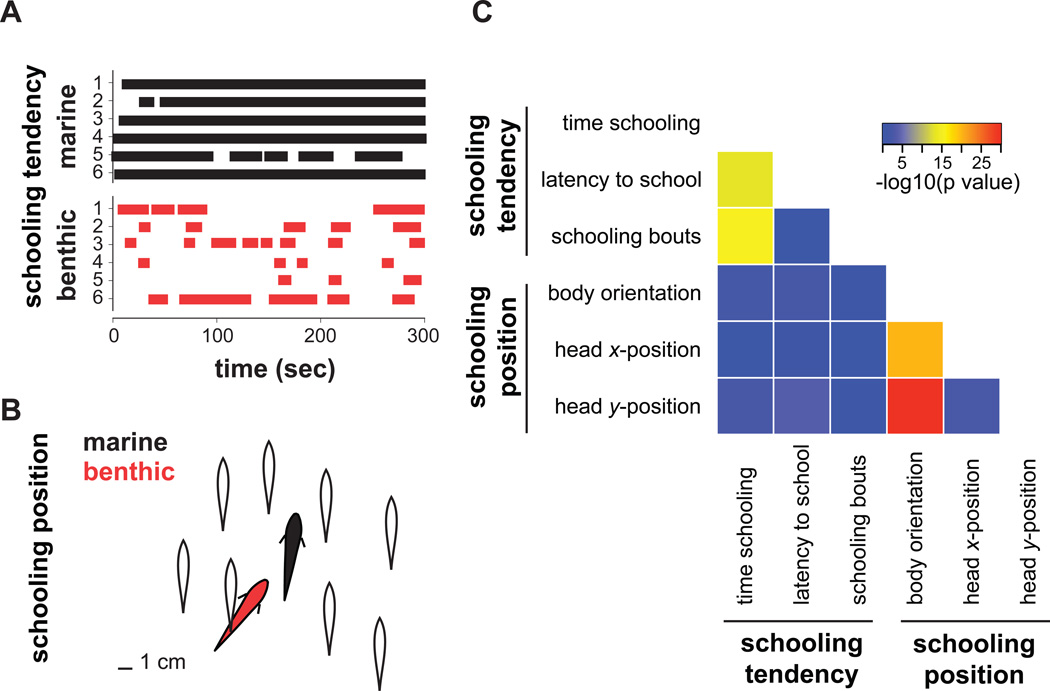
To dissect the genetic contributions to the evolution of behavior, we focused on schooling, a complex social behavior that is representative of social grouping behaviors seen throughout the animal kingdom. Social grouping provides several key benefits, but also has associated costs, so the frequency of schooling varies with ecological context [11, 13–15]. We previously developed an assay that utilizes a school of model sticklebacks to reliably elicit divergent schooling behavior between two populations of threespine sticklebacks from distinct habitats. In this assay, lab-raised marine sticklebacks from the Pacific Ocean in Hokkaido, Japan school very strongly, but lab-raised benthic sticklebacks from Paxton Lake in British Columbia, Canada show dramatically reduced schooling behavior [16] (Figure 1a,b). Differences in measures such as time spent with the models and latency to approach the models suggest that marine and benthic sticklebacks differ in their social attraction, or tendency to behave socially (Figure 1a). Social attraction is a central feature of two types of collective behavior in fish: shoaling and schooling [13, 17]. Shoals are defined exclusively by social attraction, but for a group to be recognized as a school, individuals must also maintain a coordinated body position with their schoolmates, displaying polarized orientation and synchronized movement [17, 18]. In our assay, marine fish assume a relatively parallel orientation with the models whereas benthic sticklebacks that follow the school do so with a significantly less parallel orientation (Figure 1b). Because the positions of the models are fixed relative to one another, our assay also permits measurement of the preferred schooling position of the fish amongst the models, showing that benthic and marine fish assume distinct positions (as assessed by head position) within the model school (Figure 1b). Thus, the model school assay enables quantification of the two fundamental components of schooling: schooling tendency (comprising time schooling, latency to school and number of schooling bouts) and schooling position (comprising body orientation and head position). We predicted that because shoals can exist independent of schools [13, 17], these two aspects of schooling behavior would be separable and controlled by distinct genetic modules.
Figure 1.
Schooling tendency and schooling position are separable features of schooling behavior. A. Raster plots depicting differences in schooling tendency of typical marine and benthic sticklebacks tested in the model school assay. This assay uses a school of eight model sticklebacks that are positioned and moved to mimic schools of live fish (Supplemental Experimental Procedures). Bars represent schooling episodes (bouts) as a function of time for a random sample of six lab-raised fish from each population [16]. Marine fish have a shorter latency to school and spend more time schooling [16]. B. Schematic depicting differences in average body orientation and head position of marine and benthic fish when following the model school, based on data from [16]. Open silhouettes represent positions of the model sticklebacks. Marine fish (black silhouette) have a significantly more parallel body orientation than benthic fish (red silhouette). The scale bar represents 1 cm. C. Heatmap showing strength of correlations between schooling variables in benthic-marine F2 hybrids. Measures of schooling tendency are not strongly correlated with measures of schooling position in F2 hybrids.
Schooling behavior comprises two genetically separable behavioral components
We first investigated the modularity of schooling behavior by evaluating phenotypic correlations among 229 benthic-marine F2 hybrids that were tested in the model school assay (Supplemental Experimental Procedures). These F2 hybrids expressed a wide range of schooling behaviors (Figure S1) and revealed that the two key features of schooling behavior were indeed genetically separable. Parameters reflecting social attraction or tendency of the fish to school were correlated with one another: time spent schooling was strongly correlated with the latency to join the school and the number of schooling bouts (Figure 1c) (time and latency: Spearman’s Rho = −0.477, p < 0.0001; time and bouts: Rho = −0.519, p < 0.0001). It is important to note that some of these variables are in part definitionally correlated. For example, latency to school necessarily sets an upper limit on total time spent schooling, although it does not solely dictate the duration of schooling. Variables measuring schooling position were also correlated with one another (Figure 1c): the average body orientation was strongly correlated with the average x- and y-position of the head relative to the models (orientation and head x-position: Rho = 0.582, p < 0.0001; orientation and head y-position: Rho = 0.683, p < 0.0001). Importantly, measures of schooling position were not strongly correlated with measures of schooling tendency. Most tendency and position variables were not significantly correlated (p > 0.05), with the exception of weak correlations between head y-position and both latency (Rho = −0.254; p < 0.01) and time schooling (Rho = 0.202; p < 0.05). The lack of strong correlation in the F2 hybrids suggests a separable genetic basis for these behavioral components.
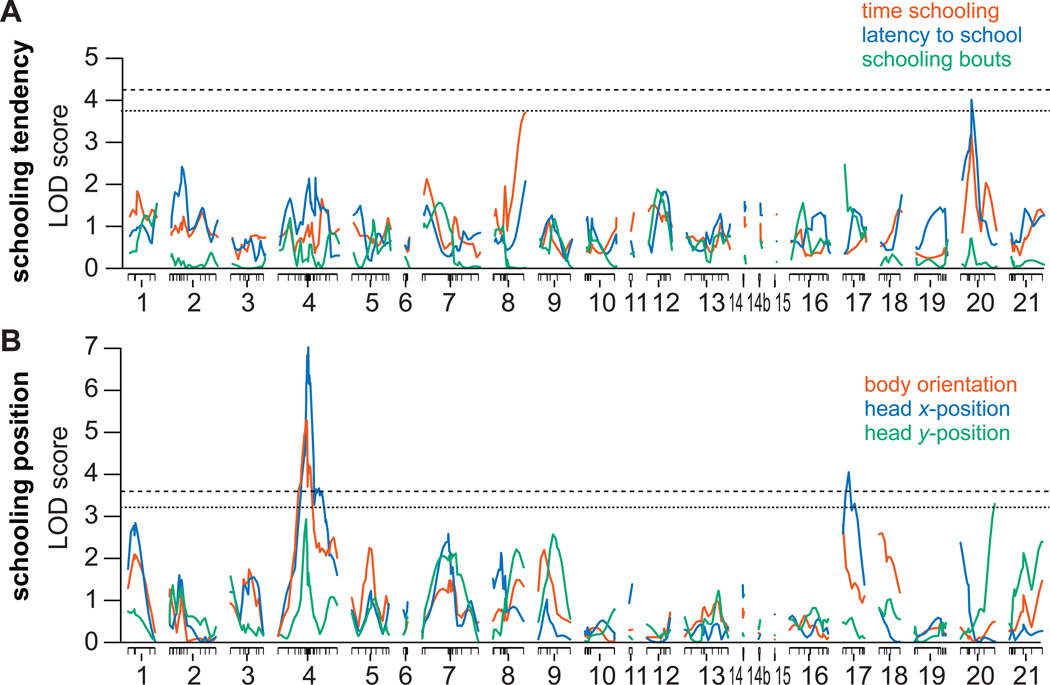
We next asked whether common or disparate genomic regions control schooling tendency vs. schooling position. To identify regions of the genome associated with different schooling parameters, we subjected the same benthic-marine F2 individuals to QTL analysis (Supplemental Experimental Procedures). Fish were genotyped using single nucleotide polymorphism (SNP) markers that spanned the genome [19, 20]. We identified two significant QTL that were associated with measures of schooling position (Figure 2; Table 1). Both body orientation and head x-position mapped to the same region on linkage group (LG) 4, and body orientation was also linked to a region on LG17 (Figure 2; Table 1). Another QTL on LG20 for y-position had a Likelihood of Odds (LOD) score that reached a genome-wide threshold of p < 0.1, and was therefore considered a suggestive QTL (Figure 2; Table 1). Measures of schooling tendency did not show any association to these QTL regions (Figure 2; Table 1). We identified a suggestive QTL for latency to school on LG20, but the confidence interval for this QTL was non-overlapping with the QTL for y-position (Figure 2; Table 1). The fact that we detected suggestive QTL for schooling tendency variables reveals that this cross would have sufficient power to identify any potentially overlapping regions of linkage between tendency and position components of schooling behavior. These data provide additional support for the modularity of schooling behavior on a genomic level.
Figure 2.
QTL for schooling behavior. A. Graph showing LOD score as a function of linkage group for measures of schooling tendency: time schooling (orange); latency to school (blue) and number of bouts (green). B. Graph showing LOD score by linkage group for measures of schooling position: body orientation (orange); head x-position (blue) and head y-position (green). Dashed line represents genome wide significance level of p < 0.05 and dotted line represents p < 0.1, highlighting suggestive QTL.
Table 1.
Locations and effects of schooling QTL. PVE = percent variance explained.
| Trait | LG | cM | Marker | LOD | p value | PVE | MM | MB | BB |
|---|---|---|---|---|---|---|---|---|---|
| Body orientation (°) | 4 | 48 | chrIV:10812344 | 5.29 | p < 0.001 | 10.4 | 81 ±1.4 | 73 ± 1.3 | 69 ± 1.8 |
| Head x-position (cm) | 4 | 51.6 | chrIV:13850026 | 7.02 | p < 0.001 | 13.5 | 4.1 ± 0.5 | 0.9 ± 0.4 | 0.3 ± 0.5 |
| 17 | 10 | chrUn:2632376 | 4.05 | p < 0.03 | 7.1 | 3.9 ± 0.7 | 1.1 ±0.3 | 1.0 ± 0.6 | |
| Head y-position (cm) | 20 | 58.5 | chrXX:232763 | 3.29 | p = 0.073 | 6.6 | −2.0 ± 0.4 | −0.8 ± 0.2 | 0.02 ± 0.3 |
| Latency (s) | 20 | 15.5 | chrXX:14411783 | 4.01 | p = 0.084 | 6.3 | 23 ± 6 | 19 ± 3 | 48 ± 10 |
The QTL we detected for schooling position explain a modest amount of the phenotypic variance in these traits. For example, the two QTL for head x-position account for just over 20% of the variance (Table 1). This suggests additional undetected QTL and/or an environmental component to this trait. Similarly, the lack of significant QTL for schooling tendency might be due to higher environmental variance and/or a genetic architecture composed of small effect QTL for these traits. Although QTL of large effect have been detected for several morphological traits in sticklebacks [e.g. 21], many other morphological and behavioral traits appear to exhibit a more complex genetic basis [e.g. 5, 20]. Furthermore, our studies are consistent other behavioral genetic studies: although single genes can have large impacts on behavior [2, 22], many complex behaviors, particularly in vertebrates, are associated with multiple genetic regions of small to modest effect [4, 6, 23, 24].
Genetic association between schooling behavior and neurosensory phenotypes
Schooling is a highly precise behavior, requiring sophisticated motor and sensory capabilities. When fish school, they must maintain a characteristic body position and orientation within a highly dynamic group of conspecifics, a task that requires accurately assessing sensory information to quickly respond to changes in the composition of the school. Two sensory systems have previously been implicated in controlling body position when schooling: vision and the lateral line [10, 25, 26]. Vision likely plays a role in both the tendency to school as well as influencing body position and orientation when schooling [10, 25, 26]. No work has directly compared the visual ability of the marine and benthic sticklebacks that we study here. However, it is known that there are no differences in relative eye size between these populations [27], and laboratory behavioral studies have revealed that individuals from both populations readily respond to the visual stimulus of conspecifics [5, 28–30]. Despite a lack of evidence for differences in visual capacity between marine and benthic sticklebacks, it remains possible that unexplored differences in visual processing between these populations could contribute to divergent schooling behavior.
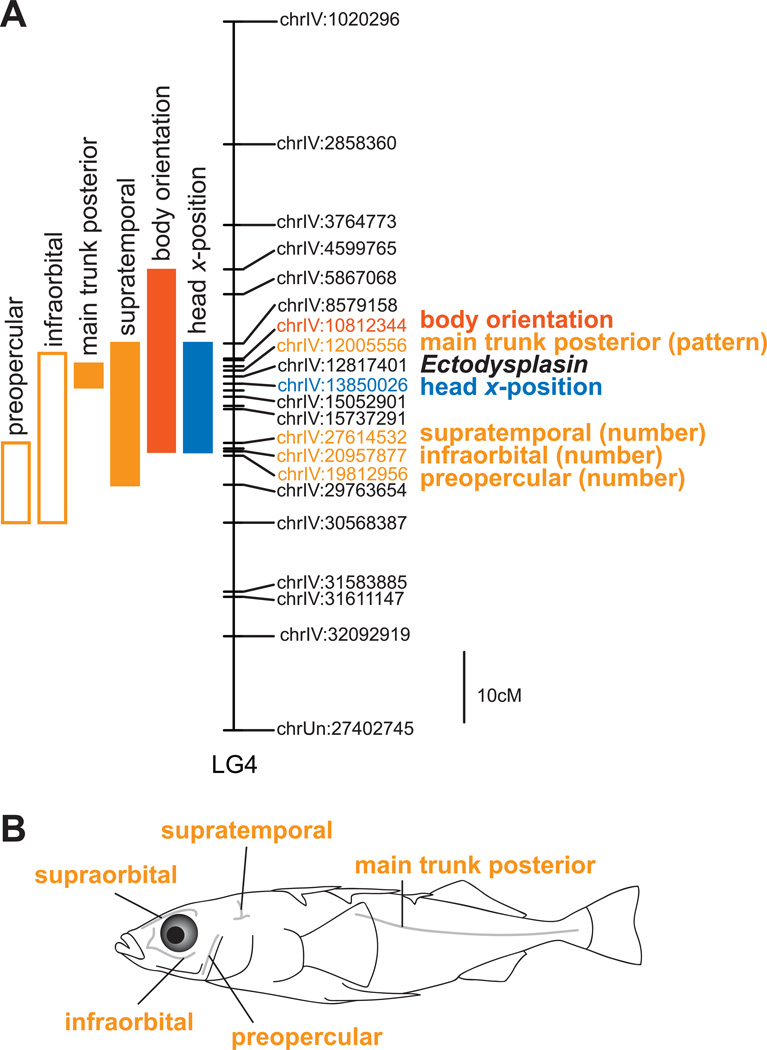
The lateral line has been implicated in influencing position and orientation during social grouping in other fish species [25, 26, 31, 32]. The lateral line is a peripheral, mechanoreceptive sensory system consisting of hair cells that respond to water movement and are grouped into structures called neuromasts [33]. Sticklebacks only have superficial neuromasts, which are found on the surface of the skin [34]. We have shown striking differences in both the number and arrangement of neuromasts across the bodies of marine and benthic sticklebacks [20], and we hypothesized that this variation might contribute to their differences in schooling position. In a previous QTL mapping study, we uncovered a modular genetic basis for lateral line anatomy, highlighting multiple genomic regions that control different anatomical portions of the lateral line [20]. This QTL analysis was performed in the same F2 individuals used in the current study, enabling us to ask whether common genomic regions underlie both neurosensory and behavioral variation. Indeed, the schooling position QTL on LG4 also contains QTL for several aspects of lateral line anatomy (Figure 3) [20]. This region is associated with differences in the number of neuromasts in the supratemporal portion of the lateral line [20], and also contains suggestive QTL for neuromast number in the infraorbital and preopercular lines (LOD = 3.14, p = 0.095 and LOD = 3.25, p = 0.084, respectively). In addition, this region harbors a major QTL for the pattern of neuromasts in the posterior portion of the main trunk line [20]. Marine alleles at this locus are associated with a paired dorso-ventral arrangement of neuromasts, whereas fish with benthic alleles possess a single row of neuromasts [20]. This variant pattern is tightly linked to the presence of bony plates [20], a conspicuous morphological difference between marine and benthic fish that is controlled by the gene Ectodysplasin (Eda), located within this QTL [21]. Body segments that have bony plates contain dorso-ventral pairs of neuromasts, whereas neuromasts on unplated segments are found in a single row. Ongoing work in our laboratory is manipulating Eda expression through transgenesis to ask whether expression of ectopic plates alters neuromast patterning and/or schooling position. However, there are several additional candidate genes in this region that have been implicated in lateral line development or in social behavior, which are also viable candidates for future functional analysis (Table S1).
Figure 3.
Schooling position QTL on LG4 overlap with lateral line QTL. A. Map of LG4 indicating positions of QTL; some markers have been omitted for clarity. Bars on the left side represent 95% confidence intervals for individual QTL. Significant QTL are shown with filled bars; suggestive QTL with open bars. For each trait, the marker at the QTL peak is labeled on the right side. The position of the candidate gene Ectodysplasin is indicated on the right side. B. Schematic depicting anatomical positions of the lateral line segments that map to LG4 and LG17.
In addition to the overlap in neural and behavioral QTL on LG4, we found that the schooling position QTL region on LG17 also contains a lateral line QTL [20]. In particular, this region has a suggestive QTL (LOD = 3.66, p = 0.063) for the number of neuromasts in the supraorbital l ine, which is located on the dorsal surface of the head (Figure 3). The suggestive QTL that we detected for measures of schooling tendency did not overlap with any lateral line QTL [20], further reinforcing the separate genetic and neural control of these two components of schooling behavior.
The overlap between QTL for lateral line and schooling position phenotypes suggests one of two scenarios. First, these neural and behavioral traits could be mechanistically independent, arising from closely linked genes or from a single gene that has independent pleiotropic effects on both schooling behavior and the lateral line. Alternatively, differences in the lateral line might themselves drive the observed behavioral differences. Because the lateral line has previously been implicated in schooling behavior, we favor the hypothesis that the genetic effects we observe on schooling behavior are likely to act at least in part through the lateral line sensory system. Testing this hypothesis will require targeted ablations of the specific regions of the lateral line that are genetically linked to variation in schooling behavior.
Conclusions
Our work reveals that schooling in sticklebacks is composed of separable behavioral components with distinct genetic architectures. Because shoaling and schooling are distinct behaviors that share the component of social attraction, the neural and genetic mechanisms that control the tendency to school vs. the ability to maintain a coordinated body position during schooling are likely to be distinct. Consistent with this prediction, these behavioral features appear to be controlled by separate genetic factors. In addition to genetic modularity, our study reveals putative neural modularity in the control of schooling behavior. Within a single sensory system, the lateral line, some anatomical regions are linked to behavioral variation whereas others are not associated. This finding is consistent with previous work suggesting that different portions of the lateral line may be important for specific behaviors [6, 35].
Our work joins a growing number of studies aimed at dissecting the genetic basis for complex behaviors in natural populations, including those that have used association mapping and/or linkage analysis to identify genomic regions that contribute to behavioral evolution in vertebrates [2, 4–7]. In particular, recent work used a complementary approach to ours to dissect the genetic and sensory basis for differences in schooling behavior in the tetra Astyanax [36]. Astyanax inhabit both river and cave environments, and those from surface environments school strongly, but cavefish, which are also blind, do not school. Kowalko et al [36] show that vision plays a major role in the evolution of schooling tendency in Astyanax, but also identify vision-independent aspects of divergent schooling. Studies like these—linking genetic, neural and behavioral variation in natural populations—will continue to provide significant new insights into the proximate mechanisms that underlie behavioral evolution.
Supplementary Material
Highlights.
Innate differences in schooling comprise separable behavioral components
Schooling tendency and schooling position map to distinct genetic loci
Schooling position is genetically linked to regions of the neurosensory lateral line
Acknowledgments
We thank Shaun McCann for assistance with fish husbandry, Barry Wark of Physion Consulting for development of custom tracking software, David Kingsley for comments on the manuscript, and Johanna Kowalko and Cliff Tabin for sharing data prior to publication. This work was supported by a National Institutes of Health Center of Excellence in Genomic Science Grant (P50 HG002568) to CLP and a National Science Foundation Grant (IOS 1145866) to AKG and CLP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boake CRB, editor. Quantitative Genetic Studies of Behavioral Evolution. Chicago: University of Chicago Press; 1994. [Google Scholar]
- 2.Bendesky A, Bargmann CI. Genetic contributions to behavioural diversity at the gene-environment interface. Nat Rev Genet. 2011;12:809–820. doi: 10.1038/nrg3065. [DOI] [PubMed] [Google Scholar]
- 3.Robinson GE, Fernald RD, Clayton DF. Genes and social behavior. Science. 2008;322:896–900. doi: 10.1126/science.1159277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber JN, Peterson BK, Hoekstra HE. Discrete genetic modules are responsible for complex burrow evolution inPeromyscusmice. Nature. 2013;493:402–405. doi: 10.1038/nature11816. [DOI] [PubMed] [Google Scholar]
- 5.Kitano J, Ross JA, Mori S, Kume M, Jones FC, Chan YF, Absher DM, Grimwood J, Schmutz J, Myers RM, et al. A role for a neo-sex chromosome in stickleback speciation. Nature. 2009;461:1079–1083. doi: 10.1038/nature08441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshizawa M, Yamamoto Y, O'Quin KE, Jeffery WR. Evolution of an adaptive behavior and its sensory receptors promotes eye regression in blind cavefish. BMC Biology. 2012;10:108. doi: 10.1186/1741-7007-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright D, Nakamichi R, Krause J, Butlin RK. QTL analysis of behavioral and morphological differentiation between wild and laboratory zebrafish (Danio rerio) Behav Genet. 2006;36:1–14. doi: 10.1007/s10519-005-9029-4. [DOI] [PubMed] [Google Scholar]
- 8.Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- 9.Pitcher TJ, Parrish JK. Functions of shoaling behavior in teleosts. In: Pitcher TJ, editor. Behaviour of Teleost Fishes. 2nd Edition. New York: Chapman & Hall; 1993. pp. 363–439. [Google Scholar]
- 10.Shaw E. Schooling fishes. Am Sci. 1978;66:166–175. [Google Scholar]
- 11.Berdahl A, Torney CJ, Ioannou CC, Faria JJ, Couzin ID. Emergent sensing of complex environments by mobile animal groups. Science. 2013;339:574–576. doi: 10.1126/science.1225883. [DOI] [PubMed] [Google Scholar]
- 12.Herbert-Read JE, Perna A, Mann RP, Schaerf TM, Sumpter DJ, Ward AJ. Inferring the rules of interaction of shoaling fish. Proc Natl Acad Sci U S A. 2011;108:18726–18731. doi: 10.1073/pnas.1109355108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause J, Ruxton GD. Living in Groups. New York: Oxford University Press; 2002. [Google Scholar]
- 14.Hoare DJ, Couzin ID, Godin JGJ, Krause J. Context-dependent group size choice in fish. Anim Behav. 2004;67:155–164. [Google Scholar]
- 15.Ioannou CC, Guttal V, Couzin ID. Predatory fish select for coordinated collective motion in virtual prey. Science. 2012;337:1212–1215. doi: 10.1126/science.1218919. [DOI] [PubMed] [Google Scholar]
- 16.Wark AR, Greenwood AK, Taylor EM, Yoshida K, Peichel CL. Heritable differences in schooling behavior between marine and freshwater sticklebacks revealed by a novel assay. PLoS ONE. 2011;6:e18316. doi: 10.1371/journal.pone.0018316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitcher TJ. Heuristic definitions of fish shoaling behavior. Anim Behav. 1983;31:611–613. [Google Scholar]
- 18.Delcourt J, Poncin P. Shoals and schools: back to the heuristic definitions and quantitative references. Rev Fish Biol Fisher. 2012;22:595–619. [Google Scholar]
- 19.Jones FC, Chan YF, Schmutz J, Grimwood J, Brady SD, Southwick AM, Absher DM, Myers RM, Reimchen TE, Deagle BE, et al. A Genome-wide SNP genotyping array reveals patterns of global and repeated species-pair divergence in sticklebacks. Curr Biol. 2012;22:83–90. doi: 10.1016/j.cub.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wark AR, Mills MG, Dang L-H, Jones FC, Chan YF, Brady SD, Absher DM, Grimwood J, Schmutz J, Myers RM, et al. Genetic architecture of variation in the lateral line sensory system of threespine sticklebacks. G3. 2012;2:1047–1056. doi: 10.1534/g3.112.003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colosimo PF, Hosemann KE, Balabhadra S, Villarreal G, Jr, Dickson M, Grimwood J, Schmutz J, Myers RM, Schluter D, Kingsley DM. Widespread parallel evolution in sticklebacks by repeated fixation ofEctodysplasinalleles. Science. 2005;307:1928–1933. doi: 10.1126/science.1107239. [DOI] [PubMed] [Google Scholar]
- 22.Fitzpatrick MJ, Ben-Shahar Y, Smid HM, Vet LE, Robinson GE, Sokolowski MB. Candidate genes for behavioural ecology. Trends Ecol Evol. 2005;20:96–104. doi: 10.1016/j.tree.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Mackay TF, Stone EA, Ayroles JF. The genetics of quantitative traits: challenges and prospects. Nat Rev Genet. 2009;10:565–577. doi: 10.1038/nrg2612. [DOI] [PubMed] [Google Scholar]
- 24.Flint J. Analysis of quantitative trait loci that influence animal behavior. J Neurobiol. 2003;54:46–77. doi: 10.1002/neu.10161. [DOI] [PubMed] [Google Scholar]
- 25.Partridge BL, Pitcher TJ. The sensory basis of fish schools: Relative roles of lateral line and vision. J Comp Physiol. 1980;135:315–325. [Google Scholar]
- 26.Pitcher T. Sensory information and the organization of behavior in a shoaling cyprinid fish. Anim Behav. 1979;27:126–149. [Google Scholar]
- 27.Albert AY, Sawaya S, Vines TH, Knecht AK, Miller CT, Summers BR, Balabhadra S, Kingsley DM, Schluter D. The genetics of adaptive shape shift in stickleback: pleiotropy and effect size. Evolution. 2008;62:76–85. doi: 10.1111/j.1558-5646.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 28.Boughman JW. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature. 2001;411:944–948. doi: 10.1038/35082064. [DOI] [PubMed] [Google Scholar]
- 29.Vamosi SM. Predation sharpens the adaptive peaks: survival trade-offs in sympatric sticklebacks. Ann Zool Fennici. 2002;39:237–248. [Google Scholar]
- 30.Wark AR, Wark BJ, Lageson TJ, Peichel CL. Novel methods for discriminating behavioral differences between stickleback individuals and populations in a laboratory shoaling assay. Behav Ecol Sociobiol. 2011;65:1147–1157. doi: 10.1007/s00265-010-1130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitcher TJ, Partridge BL, Wardle CS. A blind fish can school. Science. 1976;194:963–965. doi: 10.1126/science.982056. [DOI] [PubMed] [Google Scholar]
- 32.Faucher K, Parmentier E, Becco C, Vandewalle N, Vandewalle P. Fish lateral system is required for accurate control of shoaling behaviour. Anim Behav. 2010;79:679–687. [Google Scholar]
- 33.Coombs S, Van Netten S. The hydrodynamics and structural mechanics of the lateral line system. In: Shadwick RE, Lauder GV, editors. Fish Biomechanics. Volume 23. New York: Elsevier; 2005. pp. 103–139. [Google Scholar]
- 34.Wark AR, Peichel CL. Lateral line diversity among ecologically divergent threespine stickleback populations. J Exp Biol. 2010;213:108–117. doi: 10.1242/jeb.031625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coombs S, Janssen J, Webb JF. Diversity of lateral line systems: evolutionary and functional considerations. In: Atema J, Fay RR, Popper AN, Tavolga WN, editors. Sensory Biology of Aquatic Animals. New York: Springer-Verlag; 1988. pp. 553–593. [Google Scholar]
- 36.Kowalko JE, Rohner N, Rompani SB, Peterson BK, Linden T, Yoshizawa M, Kay EH, Hoekstra H, Jeffery WR, Borowsky R, et al. Genetic analysis of the loss of schooling behavior in cavefish reveals both sight-dependent and independent mechanisms. doi: 10.1016/j.cub.2013.07.056. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.