Abstract
Objective. The objective of this study was to determine if associations between pain distribution (unilateral vs bilateral) and measures of function (self-report vs performance-based) were influenced by knee pain intensity of the painful knee(s) in persons with moderate to severe symptomatic knee OA.
Methods. Data from persons in the Osteoarthritis Initiative (OAI) dataset (n = 852) with symptomatic knee OA were studied. Key dependent variables were the WOMAC physical function, Knee Injury and Osteoarthritis Outcome Score (KOOS) quality of life, the repeated chair stand test and the 20-m walk test. In addition to covariates, the independent variables were the presence of unilateral or bilateral OA involvement (either Kellgren and Lawrence grade 3 or 4 or a grade <3) and pain category (mild, moderate or severe).
Results. WOMAC physical function scores consistently showed the strongest association with pain intensity for persons with unilateral vs bilateral knee pain. For example, in persons with unilateral severe knee pain, WOMAC scores averaged 19.9 (s.d. = 12.0) points while persons with bilateral knee pain with at least one knee rated as severe had WOMAC scores ranging from 25.3 to 28.9, depending on pain severity of the contralateral knee. These differences were statistically significant (P < 0.001) as was the test for trend (P = 0.001). Self-report measures generally showed larger effect sizes than performance-based measures.
Conclusion. Knee pain intensity influences self-report and performance-based tests differently depending on whether knee pain is unilateral or bilateral. WOMAC scores are most strongly associated with pain intensity in persons with unilateral vs bilateral pain while walking tests are least influenced by pain intensity.
Keywords: osteoarthritis, knee, pain, quality of life, performance, function, association, self-reported function, walking, validity
Introduction
Osteoarthritic knee pain occurs in 7–17% [1, 2] of adults age ≥45 years in the USA. Greater knee pain severity is associated with both a greater self-reported functional loss [3] and worse physical performance [4]. Self-reported functional status measures capture a person’s perception of his/her ability to do activities, while performance measures indicate a person’s actual ability to perform activities.
Quantifying pain in patients with knee OA is standard clinical practice, but the impact of unilateral vs bilateral knee pain severity on functional status or performance measures have received surprisingly little attention by researchers. As noted by White et al. [5], when examining knee OA epidemiology, researchers have measured knee pain during daily activities without considering whether the pain was unilateral or bilateral [6–8]. Not accounting for unilateral vs bilateral pain and pain intensity of both limbs in studies of persons with knee OA may influence study results.
Many daily activities such as stair climbing, walking and rising from a chair require bilateral limb movement. Therefore one might logically conclude that a person with bilateral knee pain would have greater difficulty with daily activities than a person with unilateral knee pain. For example, a person with bilateral knee pain of a given intensity would experience a greater pain load as compared with a person with unilateral pain of the same intensity. This greater pain load could theoretically compound functional deficits, particularly for activities like walking that require bilateral knee movements. Research to date on this issue, however, has come to divergent conclusions, with some suggesting that the presence of unilateral vs bilateral knee pain has no association with functional status [9], while others have found that persons with bilateral knee pain display greater functional loss or greater worsening than persons with unilateral knee pain [5, 10, 11]. None of this prior evidence accounted for pain severity of both limbs, which we suspect may help to explain the divergent findings. Given that pain and functional status are associated in persons with knee OA, pain severity of both limbs may need to be accounted for when studying persons with bilateral vs unilateral knee pain. This issue is also important for clinical practice, given that both self-report and performance-based functional status measure are in common use. Self-reported functional status is substantially driven by pain [12–14] and it is unknown if self-report and performance-based functional measures equally capture functional loss that may be associated with bilateral vs unilateral knee pain.
The main purpose of our study was to determine if associations between pain distribution (unilateral vs bilateral) and measures of function were influenced by differing levels of knee pain intensity in persons with moderate to severe symptomatic knee OA. The potential interaction between pain intensity and distribution was examined for both self-report functional status measures and performance-based measures. We hypothesized that when pain intensity of both limbs was taken into account, functional status limitations would be greater for persons with bilateral vs unilateral knee pain. In addition, because self-report and physical performance measures are influenced by different phenomena [15, 16] and are only moderately correlated [4, 16–18], we hypothesized that associations between unilateral/bilateral pain severity and function would be greater for self-report measures vs performance-based measures. The WOMAC physical function scale, for example, has been shown to be highly correlated with pain [16, 19] and unable to differentiate among pain and function in construct validation studies [20, 21].
Materials and methods
The Osteoarthritis Initiative
The Osteoarthritis Initiative (OAI) is a National Institutes of Health (NIH) and privately funded longitudinal cohort study or persons with or at risk of developing knee OA. A total of three subcohorts (i.e. control, incidence and progression) have been defined in the OAI with each subcohort having racially and ethnically diverse mixes of persons between the ages of 45 and 79 years at baseline. Subjects were recruited to four clinic sites using a variety of approaches, including mailings to clinical populations in the four recruitment sites, advertisements in local newspapers, presentations to churches, community and civic organizations and via a website. Subjects were recruited from (i) the University of Maryland School of Medicine in Baltimore, Maryland, (ii) Ohio State University in Columbus, Ohio, (iii) the University of Pittsburgh in Pittsburgh, Pennsylvania and (iv) Memorial Hospital of Rhode Island, in Pawtucket, Rhode Island. The study was approved by the institutional review board at the OAI coordinating centre, the University of California at San Francisco, in accordance with the Declaration of Helsinki. Data are available for public use at http://oai.epi-ucsf.org/datarelease/About.asp.
Persons were excluded from the OAI if the following were present: RA, bilateral knee arthroplasty or pre-existing plans to undergo bilateral (not unilateral) knee arthroplasty in the next 3 years, bilateral Osteoarthritis Research Society International (OARSI) stage 3 (severe) knee OA [22], positive pregnancy test, inability to provide a blood sample, use of ambulatory aids other than a single straight cane for >50% of the time, comorbid conditions that might interfere with 4-year participation, unlikely to reside in the clinic area for at least 3 years, current participation in a double-blind randomized controlled trial and being unwilling to sign an informed consent. Because of MRI requirements, men weighing >130 kg and women weighing >114 kg were excluded because they were unable to undergo 3.0 T MRI. In total, 27% (n = 4796) of those screened (n = 17 457) were admitted to the study. The study design protocol is available at http://www.oai.ucsf.edu/datarelease/docs/StudyDesignProtocol.pdf
Sample for current study
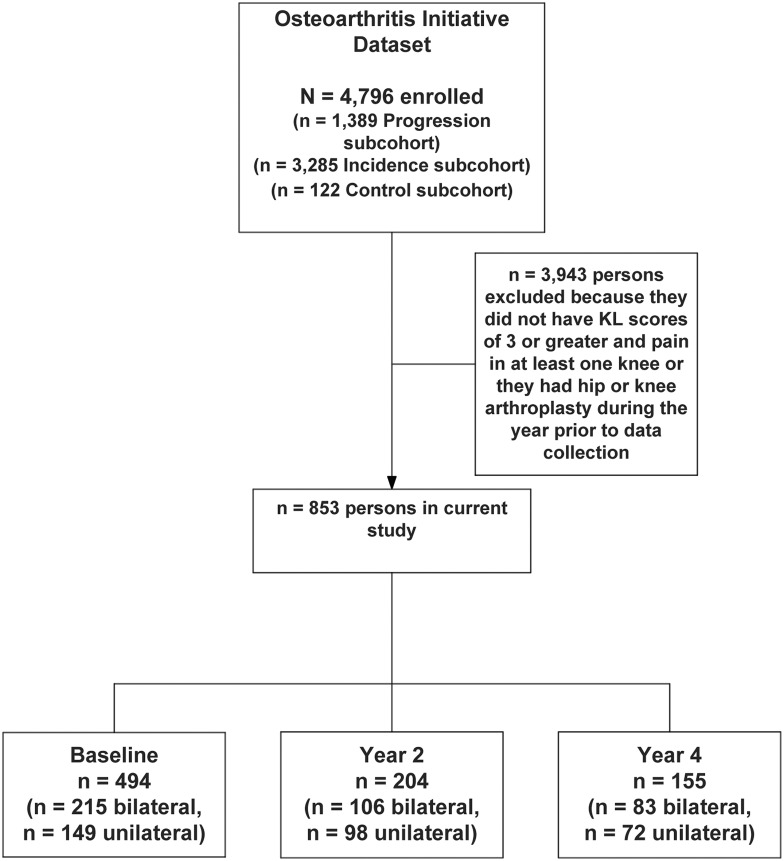
To be included in the current study, persons had to have either unilateral or bilateral knee pain and a Kellgren and Lawrence (KL) centrally adjudicated grade of 3 or 4 on at least one knee. We chose these criteria for two reasons. First, persons with KL grades of 3 or 4 are much more likely to report more severe and more frequent pain [23], and thus to seek medical care, than persons with either no or mild knee OA. Second, a recently published study by Marmon et al. [9] used similar criteria and we were interested in making comparisons with their findings. Because the OAI study is longitudinal, we also recruited persons from the 2- and 4-year follow-up sessions that met the inclusion criteria but were not included in the baseline dataset. The study sample comprised 852 unique persons, 404 of whom had bilateral knee pain and KL grades of 3 or 4 in both knees, 247 of whom had a right-sided knee OA grade of 3 or 4 and right knee pain and 202 of whom had left knee OA and pain (see Fig. 1). A total of 494 persons were recruited from the baseline dataset, 204 were recruited from the 2-year follow-up dataset and 155 were recruited from the 4-year follow-up dataset. Data from release versions 0.2.2, 3.2.1 and 6.2.1 were used in our study.
Fig. 1.
Flow of patients through the study.
Variables of interest
For the function measures we chose the following self-report functional status measures: WOMAC physical function [24], Knee Injury and Osteoarthritis Outcome Score (KOOS) function, sports and recreational activities scale and KOOS quality of life (QOL) scale [25]. We chose these three self-report scales to capture the full depth and breadth of self-reported function from typical everyday activities (WOMAC) to higher-order activities (KOOS function and sports) to more global judgments of knee function (KOOS QOL). The self-report scales are commonly used in research and practice and have undergone extensive validation [24–30]. Physical performance measures were the five repetitions of the chair stand test, measured in stands per second, the 20-m walk test, measured in metres per second, and the 400-m walk test, measured in seconds. The performance-based tests were chosen to reflect the variety of physical activities performed by persons with knee OA and are highly reliable [14, 31, 32]. These performance tests also require differing amounts of endurance, strength and balance. All self-report and performance measurements are thoroughly described in the OAI operations manuals, which are available online (http://www.oai.ucsf.edu/datarelease/OperationsManuals.asp).
The pain measure used in the OAI was a verbal pain rating scale. The scale was the following: ‘Please rate the pain that you’ve had in your right (or left) knee during the past 7 days that best describes the pain at its worst. “0” means “No pain” and “10” means “Pain as bad as you can imagine”.’ Persons reporting unilateral pain ratings from 1 to 3 were labelled mild, 4–6 moderate and 7–10 severe. Using the same pain classification categories, persons with bilateral involvement were labelled mild–mild, moderate–mild, moderate–moderate, severe–mild, severe–moderate and severe–severe. For example, a person with bilateral knee OA reporting moderate pain on the more involved limb and mild pain on the less involved limb was labelled moderate–mild. Pautex et al. [33] examined persons with primarily arthritic pain and reported high reliability [Intraclass Correlation Coefficient (ICC) = 0.97] and strong associations between a similar verbal rating scale to that used in the OAI study and more traditional visual analogue scales of pain measurement. Jensen [34] reported strong associations with very similar scales to those used in the OAI and more traditional pain intensity scales as well as responsiveness to changes following treatment.
Covariates
We adjusted for age [35], gender [36], comorbidity [37, 38] and BMI. The modified Charlson comorbidity index asks patients a series of questions regarding the presence and impact of 13 diseases, such as heart attack, diabetes and cancer. Scores in our OAI ranged from 0 to 10, with 70% of the sample scoring a 0. We therefore dichotomized the score to either 0 or ≥1. The covariates were chosen because they have been shown to be related to knee OA incidence or progression in persons with knee pain and arthritis [39–43].
Statistical analysis
We calculated descriptive statistics and these are summarized in Table 1. The dependent variables were the self-report functional status measures (WOMAC and KOOS measures) and the physical performance measures (20-m walk test, 400-m walk test and repeated chair stand test). The independent variable was the level of OA involvement (either grade 3 or 4 KL or KL grade <3) and pain. Nine combinations or levels of involvement were created. These levels were unilateral mild, unilateral moderate, unilateral severe, bilateral mild–mild, bilateral moderate–mild, bilateral moderate–moderate, bilateral severe–mild, bilateral severe–moderate and bilateral severe–severe. We included the following covariates in all analyses: sex, age, BMI and number of comorbidities. For each dependent variable we performed three analyses of variance (ANOVAs) that included the following independent variable levels: (i) severe unilateral, severe–mild bilateral, severe–moderate bilateral, severe–severe bilateral; (ii) moderate unilateral, moderate–mild bilateral, moderate–moderate bilateral and (iii) mild unilateral, mild–mild bilateral. For the moderate and severe involvement categories, two non-orthogonal contrasts were performed. The first contrast tested for a difference in the dependent variable’s mean score for the unilateral and weighted mean of the comparable bilateral involvement group. For example, the severe unilateral mean score was compared to the weighted mean of the severe–mild, severe–moderate and severe–severe scores. The second contrast tested for a linear trend specific to involvement status. Using the severe category as an example, we used the following hierarchy of involvement: bilateral severe–severe > bilateral severe–moderate > bilateral severe–mild > unilateral severe. Given that we performed five analyses for each dependent variable, we controlled for these multiple comparisons by dividing our chosen family-wise error rate of 0.05 by 5. Accordingly, an effect was considered statistically significant if P < 0.01. Data were analysed using STATA release 12 (StataCorp, College Station, TX, USA).
Table 1.
Characteristics of the 853 persons in the study
| Variable | Mean (s.d., min, max) or n (%) | Missing data, n |
|---|---|---|
| Demographic | ||
| Age in years, mean (s.d.) | 64.7 (8.9, 45, 83) | 0 |
| Female, total (%) | 433 (50.8) | 0 |
| Race, total (%) | 0 | |
| Other non-white | 20 (2.3) | |
| White or Caucasian | 660 (77.4) | |
| Black or African American | 167 (19.6) | |
| Asian | 6 (0.7) | |
| Comorbidity score, mean (s.d.) | 0.49 (1.0, 0, 12) | 12 |
| BMI, mean (s.d.) | 29.9 (4.7, 18.2, 48.7) | |
| Sub-cohort at baseline, total (%) | ||
| Incidence | 353 (41.4) | |
| Progression | 500 (58.6) | |
| Self-reported function and performance | ||
| WOMAC Physical Function Score, mean | 15.6 (12.3, 0, 67) | 11 |
| KOOS QOL Score, mean (s.d.) | 55.4 (20.0, 0, 100) | |
| KOOS Function, Sports and Recreational Activities | 59.0 (25.3, 0, 100) | 322 |
| 20-m walk pace, m/s | 1.28 (0.22, 0.32, 2.02) | 18 |
| 400-m walk, s | 317.6 (62.3, 57.9, 685.9) | 52 |
| Repeated chair stand, stands/s | 0.48 (0.14, 0.14, 1.14) | 66 |
Results
Tables 2–5 summarize the results for the chair stand test, the 20-m walk test, the WOMAC physical function test and the KOOS QOL scale. Reported in these tables are the unadjusted means and means adjusted for sex, age, BMI and number of covariates. These tables also report the ANOVA contrast results. For the unilateral mild pain comparison there is only one possible bilateral comparison group (mild–mild), while there are two comparisons for the moderate pain groups (e.g. unilateral mild to bilateral mild and moderate) and three comparisons for the severe pain groups.
Table 2.
Analysis for the unilateral–bilateral pain comparisons for the repeated chair stand test (stands/s)a
| Severe | Unilateral, severe | Bilateral, severe–mild | Bilateral, severe–moderate | Bilateral, severe–severe | Unilateral–bilateral contrast P* | Trend P | ||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | 0.50, 0.15, 88 | 0.43, 0.11, 42 | 0.44, 0.13, 60 | 0.39, 0.12, 39 | ||||
| Adjusted | 0.49, 87 | 0.43, 42 | 0.44, 60 | 0.40, 39 | <0.001 | 0.003 | ||
| Moderate | Unilateral, moderate | Bilateral, moderate–mild | Bilateral, moderate–moderate | Unilateral–bilateral contrast P* | Trend P | |||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | 0.48, 0.14, 130 | 0.49, 0.14, 67 | 0.43, 0.11, 71 | |||||
| Adjusted | 0.47, 126 | 0.49, 65 | 0.45, 70 | 0.27, 0.61 | 0.13 | |||
| Mild | Unilateral, mild | Bilateral, mild–mild | Unilateral–bilateral contrast P* | Trend P | ||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | 0.52, 0.13, 201 | 0.48, 0.14, 89 | ||||||
| Adjusted | 0.52, 199 | 0.49, 89 | 0.21 | |||||
*All P-values are adjusted for age, gender, comorbidity and BMI.
aAll data are reported in the following order: mean, s.d. and sample size for unadjusted analyses and mean and sample size for the adjusted analyses.
Table 3.
Analysis for the unilateral–bilateral comparisons for the 20-m walk (m/s)a
| Severe | Unilateral, severe | Bilateral, severe–mild | Bilateral, severe–moderate | Bilateral, severe–severe | Unilateral–bilateral contrast P* | Trend P | ||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | 1.25, 0.21, 93 | 1.24, 0.20, 49 | 1.20, 0.20, 65 | 1.17, 0.21, 43 | ||||
| Adjusted | 1.23, 93 | 1.25, 49 | 1.21, 64 | 1.19, 43 | 0.21 | 0.47 | ||
| Moderate | Unilateral, moderate | Bilateral, moderate–mild | Bilateral, moderate–moderate | Unilateral–bilateral contrast P* | Trend P | |||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | 1.28, 0.20, 138 | 1.27, 0.23, 73 | 1.22, 0.21, 75 | |||||
| Adjusted | 1.27, 133 | 1.27, 71 | 1.25, 74 | 0.78 | 0.47 | |||
| Mild | Unilateral, mild | Bilateral, mild–mild | Unilateral–bilateral contrast P* | Trend P | |||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | 1.36, 0.21, 209 | 1.32, 0.22, 90 | |||||||
| Adjusted | 1.35, 207 | 1.35, 90 | 0.98 | ||||||
*All P-values are adjusted for age, gender, comorbidity and BMI.
aAll data are reported in the following order: mean, s.d. and sample size for unadjusted analyses and mean and sample size for the adjusted analyses.
Table 4.
Analysis for the unilateral–bilateral comparisons for the WOMAC physical function scalea
| Severe | Unilateral, severe | Bilateral, severe–mild | Bilateral, severe–moderate | Bilateral, severe–severe | Unilateral–bilateral contrast P* | Trend P |
|---|---|---|---|---|---|---|
| Unadjusted | 19.9, 12.0, 93 | 25.3, 11.5, 48 | 28.7, 12.0, 67 | 28.9, 13.4, 41 | ||
| Adjusted | 20.7, 92 | 25.6, 48 | 28.1, 67 | 28.1, 41 | <0.001 | 0.001 |
| Moderate | Unilateral, moderate | Bilateral, moderate–mild | Bilateral, moderate–moderate | Unilateral–bilateral contrast P* | Trend P | |
|---|---|---|---|---|---|---|
| Unadjusted | 13.3, 9.9, 139 | 17.5, 9.3, 74 | 20.2, 9.6, 78 | |||
| Adjusted | 13.6, 134 | 17.3, 72 | 19.9, 77 | <0.001 | <0.001 |
| Mild | Unilateral, mild | Bilateral, mild–mild | Unilateral–bilateral contrast P* | Trend P | ||
|---|---|---|---|---|---|---|
| Unadjusted | 5.7, 6.0, 211 | 10.9, 8.5, 91 | ||||
| Adjusted | 6.0, 209 | 10.4, 91 | <0.001 |
*All P-values are adjusted for age, gender, comorbidity and BMI.
aAll data are reported in the following order: mean, s.d. and sample size for unadjusted analyses and mean and sample size for the adjusted analyses.
Table 5.
Analysis for the unilateral–bilateral comparisons for the KOOS QOL scalea
| Severe | Unilateral, severe | Bilateral, severe–mild | Bilateral, severe–moderate | Bilateral, severe–severe | Unilateral–bilateral contrast P* | Trend P | |||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | 47.3, 19.6, 96 | 43.4, 17.7, 49 | 40.0, 15.7, 68 | 37.4, 20.7, 44 | |||||
| Adjusted | 46.5, 95 | 42.4, 49 | 41.7, 67 | 37.9, 44 | 0.07 | 0.014 | |||
| Moderate | Unilateral, moderate | Bilateral, moderate–mild | Bilateral, moderate–moderate | Unilateral–bilateral contrast P* | Trend P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | 57.9, 19.4, 141 | 54.1, 16.8, 74 | 51.5, 13.8, 78 | ||||||
| Adjusted | 57.9, 136 | 54.3, 72 | 51.4, 77 | 0.035 | 0.011 | ||||
| Mild | Unilateral, mild | Bilateral, mild–mild | Unilateral–bilateral contrast P* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | 68.3, 16.5, 212 | 61.2, 17.0, 91 | |||||||
| Adjusted | 68.4, 210 | 61.0, 91 | 0.005 | ||||||
*All P-values are adjusted for age, gender, comorbidity and BMI.
aAll data in the table are reported in the following order: mean, s.d. and sample size for unadjusted analyses and mean and sample size for the adjusted analyses.
Chair stand test
Table 2 presents the results for the chair stand test. Inspection of the unadjusted and adjusted means reveals they are similar. The contrast analyses were statistically significant for the severe group only. The interpretations of these contrasts are that the unilateral severe group performed more stands per second than the severe bilateral groups combined and there was a trend towards fewer chair stands per second as the level of involvement increased.
20-m walk test
Table 3 presents the results for the 20-m walk test. None of the contrast analyses were statistically significant for these performance tests. The 400-m walk test also had no statistically significant differences among the contrasts (data not shown).
WOMAC physical function
Table 4 summarizes results for the WOMAC physical function scale. All comparisons were statistically significant for all unilateral–bilateral contrast comparisons. For example, patients with bilateral severe pain had WOMAC physical function scores that were ∼7 points higher than persons with unilateral severe pain after adjustment for covariates.
KOOS QOL scale
For the KOOS QOL scale, all comparisons with the exception of the severe unilateral–bilateral comparisons were significant (see Table 5). The results for the KOOS function, sports and recreational activities scale (data not shown) were very similar to those found for the KOOS QOL scale. For example, KOOS QOL scores for persons with unilateral moderate pain were ∼6 points higher (better QOL) than persons with bilateral moderate pain after covariate adjustment.
Based on the results for the performance-based and self-report measures, we calculated the following effect sizes [44] for comparisons of the subgroups with unilateral vs bilateral symptomatic knee OA: 0.10–0.26 for the chair stand, 0.02–0.10 for the 20-m walk test, 0.26–0.28 for the WOMAC physical function scale and 0.16–0.20 for the KOOS QOL measure.
Discussion
Our hypothesis that pain intensity is associated with measures of function when symptomatic knee OA pain severity in one knee vs in both knees is accounted for was generally supported. Differences in effect sizes in the hypothesized direction also were found for the self-report measures compared with the performance measures. This was particularly true for the WOMAC physical function scale, but was also true for the KOOS QOL and function and sports scales, relative to most of the performance measures.
We speculate that one explanation for this more noticeable effect of pain on self-report vs performance measures is that persons have a limited capacity to distinguish between the ability to complete activities such as walking and stair climbing and what they experience (e.g. pain) when doing these types of activities. This has been well substantiated for the WOMAC scale, with multiple studies indicating strong associations with pain and an inability to differentiate between pain and function constructs [16, 19–21]. Our data would suggest that the KOOS QOL and sports scales are less vulnerable to this limitation.
A substantial literature also exists to demonstrate that associations between self-report and performance measures are weakly to moderately correlated [4, 15, 45–47]. For example, after accounting for the repeated chair stand test score, variations in self-reports of rising from a chair were explained by age, BMI, greater comorbidity and serum albumin levels in persons age ≥60 years, 44% of whom were diagnosed with arthritis [15]. This body of literature suggests that while self-report and performance measures are related, a person’s mental and physical health characteristics (including pain) and idiosyncratic views of their abilities likely influence self-report measures. This evidence indicates that self-report and performance-based measures capture different phenomena, and our study supports this assertion.
Persons with bilateral vs unilateral symptomatic knee OA of varying pain intensities perform essentially equally during walking tests when controlling for pain severity. For the repeated chair stand, a more demanding performance test requiring substantial quadriceps and hip extensor muscle forces, we found statistically significant differences among persons with unilateral vs bilateral pain that was severe in at least one limb. Pain reported as severe in at least one limb impacts repeated chair stand performance and the extent of impact varies depending on whether the contralateral limb pain is mild, moderate or severe. Evidence of a dose-response also was found (test for trend P < 0.003). However, bilateral pain that is moderate or mild at its worst does not appear to influence repeated chair stand performance relative to unilateral moderate or mild pain. These data suggest that the knee pain–chair stand relationship is complex and likely non-linear when considering the effects of unilateral vs bilateral knee pain on chair stand performance.
Our study is consistent with previously published evidence [5, 10, 11] but contradicts a recent paper published by Marmon et al. [9]. We suspect that because our study had a substantially larger sample than that of the Marmon et al. [9] study (n = 853 in our study vs n = 152) and because we stratified by pain severity levels of both limbs, we found potentially meaningful differences in persons with unilateral vs bilateral pain.
According to Cohen’s interpretive guidelines [44], the effect sizes observed for our self-report and repeated chair stand data would be considered small. Some interpretation of the meaningfulness of these effect sizes would provide some context. With the exception of the WOMAC physical function scale, there is a paucity of information concerning important between group (level) differences for the outcome measures reported in our study. A substantial amount of evidence suggests that a within-person change of 6–9 WOMAC physical function scale points is a meaningful difference [48–51]. Goldsmith et al. [52], in a novel study that was not specific to the WOMAC, reported that an important between-group difference (i.e. the difference between a drug and placebo) is ∼50% of an important within-patient change. Applying this recommendation from Goldsmith et al., a between-group difference of ∼3 or 4 points would be considered important for the WOMAC physical function scale. This standard was met for our data. More limited data are available to judge the magnitude of the effect for the repeated chair stand test results. French et al. [53] reported effect sizes for performance-based measures including the repeated chair stand test. A total of 39 patients with knee OA completed a course of physical therapy emphasizing exercise, an intervention known to be at least mildly effective for patients with knee OA [54]. The effect size reported by French et al. [53] for the repeated chair stand was 0.33. The French et al. effect size is similar to what we estimated for the severe group comparison (0.26), but was larger compared with our moderate (0.11) and mild (0.10) pain groups.
Our study has some important limitations. We examined associations between pain severity in one or both limbs and function using a cross-sectional design. Further study using follow-up data would provide additional important information about the potential influence of pain severity and unilateral vs bilateral pain distribution on function over time. In addition, it is known that self-report measures are strongly influenced by pain [12–14, 16], and while we adjusted for pain severity for both limbs, it is possible that the differences we found in function between persons with unilateral vs bilateral pain were simply a reflection of differences in pain severity in the contralateral limb and not an actual difference in function. The data indicate consistent trends of worsening with increasing pain of the contralateral limb for both self-report and performance-based measures, suggesting that function was actually worse, but the exact role of pain in our study is not entirely clear.
In conclusion, we found that pain severity in the contralateral limb of persons with symptomatic knee OA is associated with the extent of functional loss. Self-report measures are susceptible to influences of a contralateral painful knee and the WOMAC physical function scale showed the strongest associations. The only performance-based measure that was influenced by contralateral knee pain was the repeated chair stand test and for this test only persons with severe knee pain had potentially meaningful differences in performance depending on the severity of pain in the contralateral limb. Clinicians should be aware that self-report measures and performance measures are affected by pain differently. Self-report measures are prone to being influenced by the severity of pain in the contralateral limb. It is likely that these variations most often reflect idiosyncratic differences in persons’ mental and physical health profiles and not actual performance-based differences. Future research should explore the bases for differences in self-report and performance-based measures in samples of persons with symptomatic knee OA.
Rheumatology key messages.
Function with symptomatic knee OA is influenced by whether the pain is unilateral or bilateral.
In knee OA, self-report measures are influenced more by unilateral vs bilateral knee pain distribution than performance-based measures.
Acknowledgements
The OAI is a public–private partnership comprised of five contracts (N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, N01-AR-2-2262) funded by the National Institutes of Health, a branch of the U.S. Department of Health and Human Services, and conducted by the OAI study investigators. Private funding partners include Merck Research Laboratories, Novartis Pharmaceuticals, GlaxoSmithKline and Pfizer. Private-sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use dataset and does not necessarily reflect the opinions or views of the OAI investigators, the NIH or the private funding partners.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–80. [PubMed] [Google Scholar]
- 3.Perrot S, Poiraudeau S, Kabir-Ahmadi M, et al. Correlates of pain intensity in men and women with hip and knee osteoarthritis. Results of a national survey: the French ARTHRIX study. Clin J Pain. 2009;25:767–72. doi: 10.1097/AJP.0b013e3181b43d4f. [DOI] [PubMed] [Google Scholar]
- 4.Stratford PW, Kennedy D, Pagura SM, et al. The relationship between self-report and performance-related measures: questioning the content validity of timed tests. Arthritis Rheum. 2003;49:535–40. doi: 10.1002/art.11196. [DOI] [PubMed] [Google Scholar]
- 5.White DK, Zhang Y, Felson DT, et al. The independent effect of pain in one versus two knees on the presence of low physical function in a multicenter knee osteoarthritis study. Arthritis Care Res. 2010;62:938–43. doi: 10.1002/acr.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller ME, Rejeski WJ, Messier SP, et al. Modifiers of change in physical functioning in older adults with knee pain: the Observational Arthritis Study in Seniors (OASIS) Arthritis Rheum. 2001;45:331–9. doi: 10.1002/1529-0131(200108)45:4<331::AID-ART345>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Ling SM, Xue QL, Simonsick EM, et al. Transitions to mobility difficulty associated with lower extremity osteoarthritis in high functioning older women: longitudinal data from the Women’s Health and Aging Study II. Arthritis Rheum. 2006;55:256–63. doi: 10.1002/art.21858. [DOI] [PubMed] [Google Scholar]
- 8.Williams DA, Farrell MJ, Cunningham J, et al. Knee pain and radiographic osteoarthritis interact in the prediction of levels of self-reported disability. Arthritis Rheum. 2004;51:558–61. doi: 10.1002/art.20537. [DOI] [PubMed] [Google Scholar]
- 9.Marmon AR, Zeni JA, Jr, Snyder-Mackler L. Perception and presentation of function in patients with unilateral versus bilateral knee osteoarthritis. Arthritis Care Res. 2012;65:406–13. doi: 10.1002/acr.21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jinks C, Jordan KP, Blagojevic M, et al. Predictors of onset and progression of knee pain in adults living in the community. A prospective study. Rheumatology. 2008;47:368–74. doi: 10.1093/rheumatology/kem374. [DOI] [PubMed] [Google Scholar]
- 11.Keenan AM, Tennant A, Fear J, et al. Impact of multiple joint problems on daily living tasks in people in the community over age fifty-five. Arthritis Rheum. 2006;55:757–64. doi: 10.1002/art.22239. [DOI] [PubMed] [Google Scholar]
- 12.Maly MR, Costigan PA, Olney SJ. Determinants of self-report outcome measures in people with knee osteoarthritis. Arch Phys Med Rehabil. 2006;87:96–104. doi: 10.1016/j.apmr.2005.08.110. [DOI] [PubMed] [Google Scholar]
- 13.Stratford PW, Kennedy DM. Does parallel item content on WOMAC’s pain and function subscales limit its ability to detect change in functional status? BMC Musculoskelet Disord. 2004;5:17. doi: 10.1186/1471-2474-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stratford PW, Kennedy DM, Woodhouse LJ. Performance measures provide assessments of pain and function in people with advanced osteoarthritis of the hip or knee. Phys Ther. 2006;86:1489–96. doi: 10.2522/ptj.20060002. [DOI] [PubMed] [Google Scholar]
- 15.Louie GH, Ward MM. Association of measured physical performance and demographic and health characteristics with self-reported physical function: implications for the interpretation of self-reported limitations. Health Qual Life Outcomes. 2010;8:84. doi: 10.1186/1477-7525-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stratford PW, Kennedy DM. Performance measures were necessary to obtain a complete picture of osteoarthritic patients. J Clin Epidemiol. 2006;59:160–7. doi: 10.1016/j.jclinepi.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy CJ, Oldham JA. The reliability, validity and responsiveness of an aggregated locomotor function (ALF) score in patients with osteoarthritis of the knee. Rheumatology. 2004;43:514–7. doi: 10.1093/rheumatology/keh081. [DOI] [PubMed] [Google Scholar]
- 18.Steultjens MP, Dekker J, van Baar ME, et al. Internal consistency and validity of an observational method for assessing disability in mobility in patients with osteoarthritis. Arthritis Care Res. 1999;12:19–25. doi: 10.1002/1529-0131(199902)12:1<19::aid-art4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Parent E, Moffet H. Comparative responsiveness of locomotor tests and questionnaires used to follow early recovery after total knee arthroplasty. Arch Phys Med Rehabil. 2002;83:70–80. doi: 10.1053/apmr.2002.27337. [DOI] [PubMed] [Google Scholar]
- 20.Thumboo J, Chew LH, Soh CH. Validation of the Western Ontario and McMaster University osteoarthritis index in Asians with osteoarthritis in Singapore. Osteoarthritis Cartilage. 2001;9:440–6. doi: 10.1053/joca.2000.0410. [DOI] [PubMed] [Google Scholar]
- 21.Faucher M, Poiraudeau S, Lefevre-Colau MM, et al. Algo-functional assessment of knee osteoarthritis: comparison of the test-retest reliability and construct validity of the WOMAC and Lequesne indexes. Osteoarthritis Cartilage. 2002;10:602–10. doi: 10.1053/joca.2002.0533. [DOI] [PubMed] [Google Scholar]
- 22.Altman RD, Hochberg M, Murphy WA, Jr, et al. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(Suppl A):3–70. [PubMed] [Google Scholar]
- 23.Neogi T, Felson D, Niu J, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986;5:231–41. doi: 10.1007/BF02032362. [DOI] [PubMed] [Google Scholar]
- 25.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 27.Bellamy N, Kirwan J, Boers M, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. Consensus development at OMERACT III. J Rheumatol. 1997;24:799–802. [PubMed] [Google Scholar]
- 28.Bellamy N. The WOMAC Knee and Hip Osteoarthritis Indices: development, validation, globalization and influence on the development of the AUSCAN Hand Osteoarthritis Indices. Clin Exp Rheumatol. 2005;23:S148–53. [PubMed] [Google Scholar]
- 29.Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17. doi: 10.1186/1477-7525-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roos EM, Bremander AB, Englund M, et al. Change in self-reported outcomes and objective physical function over 7 years in middle-aged subjects with or at high risk of knee osteoarthritis. Ann Rheum Dis. 2008;67:505–10. doi: 10.1136/ard.2007.074088. [DOI] [PubMed] [Google Scholar]
- 31.Curb JD, Ceria-Ulep CD, Rodriguez BL, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54:737–42. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 32.Gill S, McBurney H. Reliability of performance-based measures in people awaiting joint replacement surgery of the hip or knee. Physiother Res Int. 2008;13:141–52. doi: 10.1002/pri.411. [DOI] [PubMed] [Google Scholar]
- 33.Pautex S, Herrmann F, Le LP, et al. Feasibility and reliability of four pain self-assessment scales and correlation with an observational rating scale in hospitalized elderly demented patients. J Gerontol A Biol Sci Med Sci. 2005;60:524–9. doi: 10.1093/gerona/60.4.524. [DOI] [PubMed] [Google Scholar]
- 34.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4:2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 35.Low Choy NL, Brauer SG, Nitz JC. Age-related changes in strength and somatosensation during midlife: rationale for targeted preventive intervention programs. Ann N Y Acad Sci. 2007;1114:180–93. doi: 10.1196/annals.1396.014. [DOI] [PubMed] [Google Scholar]
- 36.Petterson SC, Raisis L, Bodenstab A, et al. Disease-specific gender differences among total knee arthroplasty candidates. J Bone Joint Surg Am. 2007;89:2327–33. doi: 10.2106/JBJS.F.01144. [DOI] [PubMed] [Google Scholar]
- 37.van Dijk GM, Veenhof C, Lankhorst GJ, et al. Limitations in activities in patients with osteoarthritis of the hip or knee: the relationship with body functions, comorbidity and cognitive functioning. Disabil Rehabil. 2009;31:1685–91. doi: 10.1080/09638280902736809. [DOI] [PubMed] [Google Scholar]
- 38.Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Belo JN, Berger MY, Reijman M, et al. Prognostic factors of progression of osteoarthritis of the knee: a systematic review of observational studies. Arthritis Rheum. 2007;57:13–26. doi: 10.1002/art.22475. [DOI] [PubMed] [Google Scholar]
- 40.Dunlop DD, Semanik P, Song J, et al. Risk factors for functional decline in older adults with arthritis. Arthritis Rheum. 2005;52:1274–82. doi: 10.1002/art.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mallen CD, Peat G, Thomas E, et al. Predicting poor functional outcome in community-dwelling older adults with knee pain: prognostic value of generic indicators. Ann Rheum Dis. 2007;66:1456–61. doi: 10.1136/ard.2006.067975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niu J, Zhang YQ, Torner J, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329–35. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters TJ, Sanders C, Dieppe P, et al. Factors associated with change in pain and disability over time: a community-based prospective observational study of hip and knee osteoarthritis. Br J Gen Pract. 2005;55:205–11. [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen J. Analysis for the Behavioural Sciences. New York: Academic Press; 1977. Statistical Power. [Google Scholar]
- 45.Kempen GI, Steverink N, Ormel J, et al. The assessment of ADL among frail elderly in an interview survey: self-report versus performance-based tests and determinants of discrepancies. J Gerontol B Psychol Sci Soc Sci. 1996;51:254–60. doi: 10.1093/geronb/51b.5.p254. [DOI] [PubMed] [Google Scholar]
- 46.Kempen GI, van Heuvelen MJ, van den Brink RH, et al. Factors affecting contrasting results between self-reported and performance-based levels of physical limitation. Age Ageing. 1996;25:458–64. doi: 10.1093/ageing/25.6.458. [DOI] [PubMed] [Google Scholar]
- 47.Myers AM, Holliday PJ, Harvey KA, et al. Functional performance measures: are they superior to self-assessments? J Gerontol. 1993;48:M196–206. doi: 10.1093/geronj/48.5.m196. [DOI] [PubMed] [Google Scholar]
- 48.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384–91. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 49.Ehrich EW, Davies GM, Watson DJ, et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635–41. [PubMed] [Google Scholar]
- 50.Pua YH, Cowan SM, Wrigley TV, et al. The Lower Extremity Functional Scale could be an alternative to the Western Ontario and McMaster Universities Osteoarthritis Index physical function scale. J Clin Epidemiol. 2009;62:1103–11. doi: 10.1016/j.jclinepi.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64:29–33. doi: 10.1136/ard.2004.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldsmith CH, Boers M, Bombardier C, et al. Criteria for clinically important changes in outcomes: development, scoring and evaluation of rheumatoid arthritis patient and trial profiles. OMERACT Committee. J Rheumatol. 1993;20:561–5. [PubMed] [Google Scholar]
- 53.French HP, Fitzpatrick M, FitzGerald O. Responsiveness of physical function outcomes following physiotherapy intervention for osteoarthritis of the knee: an outcome comparison study. Physiotherapy. 2011;97:302–8. doi: 10.1016/j.physio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Wang SY, Olson-Kellogg B, Shamliyan TA, et al. Physical therapy interventions for knee pain secondary to osteoarthritis: a systematic review. Ann Intern Med. 2012;157:632–44. doi: 10.7326/0003-4819-157-9-201211060-00007. [DOI] [PubMed] [Google Scholar]