Abstract
Subanesthetic doses of ketamine, an N-methyl-D-aspartic acid (NMDA) antagonist, have a rapid antidepressant effect which lasts for up to 2 weeks. However, the neurobiological mechanism regarding this effect remains unclear. In the present study, the effects of subanesthetic doses of ketamine on serotonergic systems in conscious monkey brain were investigated. Five young monkeys underwent four positron emission tomography measurements with [11C]-3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)benzonitrile ([11C]DASB) for the serotonin transporter (SERT), during and after intravenous infusion of vehicle or ketamine hydrochloride in a dose of 0.5 or 1.5 mg/kg for 40 min, and 24 h post infusion. Global reduction of [11C]DASB binding to SERT was observed during ketamine infusion in a dose-dependent manner, but not 24 h later. The effect of ketamine on the serotonin 1A receptor (5-HT1A-R) and dopamine transporter (DAT) was also investigated in the same subjects studied with [11C]DASB. No significant changes were observed in either 5-HT1A-R or DAT binding after ketamine infusion. Microdialysis analysis indicated that ketamine infusion transiently increased serotonin levels in the extracellular fluid of the prefrontal cortex. The present study demonstrates that subanesthetic ketamine selectively enhanced serotonergic transmission by inhibition of SERT activity. This action coexists with the rapid antidepressant effect of subanesthetic doses of ketamine. Further studies are needed to investigate whether the transient combination of SERT and NMDA reception inhibition enhances each other's antidepressant actions.
Keywords: subanesthetic ketamine, serotonin transporer, [11C]DASB, PET, monkey
INTRODUCTION
Prevalence estimates for major depressive disorder in an American population is ca. 16% (Kessler et al, 2003). Pharmacological therapy of depression was revolutionized in the late 1950s. Two classes of drugs were serendipitously found to be effective antidepressants. The first antidepressant was iproniazid, originally developed as an antitubercular drug, and the second the tricyclic antidepressant imipramine arose from antihistamine research (for review, see Nestler et al, 2002). The acute mechanism of action of both antidepressant medications had been identified. Iproniazid is a monoamine oxidase inhibitor, and imipramine inhibits serotonin and/or norepinephrine reuptake transporters (Frazer, 1997). Today, there is a wide range of available antidepressants. Selective serotonin reuptake inhibitors (SSRIs) and selective serotonin/noradrenalin inhibitors are the most common treatments for patients with depression (Celada et al, 2004). Because the serotonin transporter (SERT) located on the presynaptic nerve terminal has a key role in the regulation of the serotonin levels in the synaptic cleft, inhibition of SERT would be expected to result in enhanced serotonergic neurotransmission (Salomon et al, 1993). Especially, increased serotonin level in prefrontal cortex is thought to be a key step in the therapeutic mechanism of SSRIs (Bel and Artigas, 1992; Invernizzi et al, 1992, 1996). Positron emission tomography (PET) studies with [11C]-3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)benzonitrile ([11C]DASB; Wilson et al, 2000) have shown that 80% occupancy of SERT by SSRIs is needed for the improvement of depression symptom (Meyer et al, 2001, 2004; Suhara et al, 2003). However, only one-third of patients show significant mood improvement in response to an initial antidepressant treatment (Trivedi et al, 2006). Moreover, there is a time lag of several weeks before a therapeutic effect is observed (Krystal., 2010). This lengthy time to achieve remission is thought to be caused by indirect activation of the serotonin 1A receptor (5-HT1A-R) (Chaput et al, 1986; Invernizzi et al, 1996). Elevated extracellular serotonin levels in response to acute blockade of SERT engaged inhibition of the 5-HT1A-R in presynaptic neurons (autoreceptor) of the dorsal raphe. This inhibits serotonergic neural activity, resulting in reduced subsequent serotonin release in terminal brain areas such as a frontal cortex (Bel and Artigas, 1992; Gartside et al, 1995; Invernizzi et al, 1992, 1996). In contrast, after chronic administrations of SSRIs, these 5-HT1A autoreceptors are desensitized, resulting in a pronounced increase in serotonin levels in the prefrontal cortex (Invernizzi et al, 1994).
We previously investigated the clinical pharmacological effects of CI-581, now known as ketamine, a noncompetitive N-methyl-D-aspartic acid (NMDA) glutamate receptor antagonist (Domino et al, 1965). Ketamine has analgesic and dissociative anesthetic properties (Reich and Silvay, 1989). The brain distribution and kinetics of ketamine have already been studied with PET. The uptake of [11C]ketamine reflects the distribution of NMDA receptors in the brain with a rapid brain-plasma exchange rate (Hartvig et al, 1994; Kumlien et al, 1999). Ketamine in anesthetic doses affects several monoaminergic neuronal systems. In conscious monkeys and animal, PET anesthetic doses of ketamine significantly alter the synthesis rate of dopamine (Tsukada et al, 2000). Although dopamine D2 receptor binding was decreased (Ohba et al, 2009; Onoe et al, 1994; Tsukada et al, 2000), dopamine transporter (DAT) availability is increased in living monkey brains (Harada et al, 2004; Tsukada et al, 2001). Although an inhibitory effect of ketamine on SERT was reported in vitro (Martin et al, 1990; Nishimura et al, 1998; Zhao and Sun, 2008), no one has evaluated the effects of subanesthetic doses of ketamine on SERT in vivo.
The rapid antidepressant effect of ketamine at subanesthetic dose for treatment-resistant depressed patients suggests a possible new approach for its therapy, compared with the standard medications required for several weeks (Krystal, 2010). In addition to robust and rapid antidepressant effects after a single dose, ketamine has sustained antidepressant effects in depressed patients for 1–2 weeks (Berman et al, 2000; Diazgranados et al, 2010; Price et al, 2009; Zarate et al, 2006). This is surprising because of an approximate 3-h half-life of ketamine in plasma (Clements et al, 1982) and its absence in brain in 24 h. These findings suggest that the neurobiological mechanism underlying the antidepressant effects of ketamine is far more complex than simple antagonism of the NMDA receptor.
The aim of present study was to determine the effects of subanesthetic doses of ketamine on serotonergic activity in the conscious monkey brain. First, PET studies were performed with [11C]DASB for the SERT and 4-[18F]fluoro-N-[2-[1-(2-methoxyphenyl)-1 piperazinyl]ethyl-N-2-pyridinyl-benzamide ([18F]MPPF) for the 5-HT1A-R, respectively, after the administration of subanesthetic doses of ketamine. We also performed PET studies with [11C]2-β-carbomethoxy-3β-(4-flurophenyldopamine ([11C]β-CFT) for the DAT, to verify the specificity of subanesthetic ketamine effects on serotonergic systems. Next, it was determined whether subanesthetic doses of ketamine increased serotonin levels in the extracellular fluid (ECF) of prefrontal cortex by microdialysis.
MATERIALS AND METHODS
Subjects and Drug
Experiments were conducted in accordance with the recommendations of the US National Institutes of Health and the guidelines of the Central Research Laboratory, Hamamatsu Photonics. Eight male rhesus monkeys (Macaca mulatta; 7.8±0.8 years old, weighing 6.4±1.4 kg) were studied. Five monkeys participated in the PET experiments, and another three were used for the microdialysis experiment. The doses of ketamine hydrochloride were based on human clinical studies (Diazgranados et al, 2010). Saline or each dose of ketamine was infused intravenously for 40 min. PET scans were started after the end of ketamine infusion. Vital signs including heart rate, respiration rate, systolic and diastolic blood pressure, and body temperature were monitored throughout the ketamine infusion.
PET Experiments
A high-resolution animal PET scanner (SHR-7700; Hamamatsu Photonics, Hamamatsu, Japan) with a transaxial resolution of 2.6 mm full-width half-maximum in the enhanced 2D mode and a center-to-center distance of 3.6 mm (Watanabe et al, 1997) was used. PET images were reconstructed by a filtered backprojection method with a 4.5-mm Hanning filter, resulting in an in-plane reconstructed resolution of 4.5 mm. PET scans with [11C]DASB and [18F]MPPF were performed with arterial blood sampling. To avoid excessive arterial blood sampling, PET scans with [11C]β-CFT were performed without sampling. A saphenous venous cannula in an inferior limb and another cannula in the femoral artery of the other leg were inserted. The trained animal's head was rigidly fixed to the upper frame of a monkey chair using an acrylic head-restraining device. The animal sitting in a restraining chair was placed at a fixed position in the PET gantry with stereotactic coordinates aligned parallel to the orbitomeatal line. Transmission data with a 68Ge–68Ga pin source were obtained for an attenuation correction. After i.v. bolus injection of each radiotracer, PET scans were acquired for 91 min. The injected dose of [11C]DASB, [18F]MPPF, and [11C]β-CFT was 212.1±37.0, 98.3±13.2, and 199.4±65.3 MBq/kg (mean±SD, n=5), respectively. A summation image from 28–40 min postinjection was obtained. The brain MRI was automatically coregistered to the PET images by pixel-wise kinetic modeling (PMOD) software (PMOD Technologies, Zurich, Switzerland). For each monkey, the following regions of interest (ROIs) were obtained on the basis of the registered MRI: midbrain, thalamus, striatum, prefrontal cortex, and cerebellum.
Arterial blood samples were obtained every 8 s from 10 to 66 s, followed by 96, 156, 246, and 336 s, then 20, 30, 45, 60, 75, and 90 min after tracer injection. Blood samples were centrifuged to separate plasma, weighed, and radioactivity was measured. For metabolite analysis, methanol was added to some plasma samples (sample/methanol=1/1), centrifuged, and the supernatants were developed with a thin-layer chromatography plate (AL SIL G/UV; Whatman, Kent, UK) using a mobile phase of dichloromethane:diethyl ether:triethylamine=20 : 15 : 1 for [11C]DASB and chloroform:methanol:triethylamine=38 : 2 : 1 for [18F]MPPF. At each sampling time point for analysis, the ratio of radioactivity in the unmetabolized fraction to that in total plasma was determined using a phosphoimaging plate (FLA-7000; Fuji Film, Tokyo, Japan). Time-activity curves of radioactivity in metabolite-corrected arterial plasma were used as the arterial input function.
PET Data Analysis
For [11C]DASB and [18F]MPPF analysis, the Logan plot with an arterial input function was performed in PMOD software (PMOD Technologies). The Logan arterial input method determines the total distribution volume (Vt) using the following equation (Logan et al, 1990):
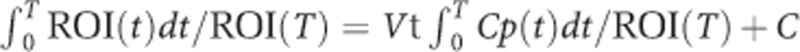
where ROI(T) and Cp(T) represent tissue and arterial plasma radioactivities, respectively, at time t=T, Vt is the slope, and C is the intercept on the y-axis. In reversibly labeled compounds, the Logan plot becomes linear after some time with a slope that is equal to Vt. The ratio of Vt in target ROI (Vttar) to Vt in the reference (Vtref) minus one (Vttar/Vtref−1) was calculated as binding potential nondisplaceable (BPND), which is the ratio at equilibrium of specifically bound radioligand to that of nondisplaceble (ND) radioligand in tissue (Innis et al, 2007). In Logan arterial input method, t* was fixed at 15 min, and Vt value of the cerebellum was used as Vtref. Parametric images were also generated of the BPND by the Logan plot method with an arterial input function based on PXMOD. [11C]β-CFT binding was quantified by the Simplified Reference Tissue Model with the cerebellum as a reference region (Sasaki et al, 2012).
Microdialysis Experiments
Three monkeys were used for the microdialysis experiment. Determination of serotonin and dopamine in the ECF of prefrontal cortex was done by reverse-phase high-performance liquid chromatography with electrochemical detection as described previously (Tsukada et al, 2004; Yamamoto et al, 2007). The guide cannula was implanted into the prefrontal cortex (anterior, 35 mm; lateral, 10 mm; and depth, 1 mm) according to the MRI with reference to the stereotactic brain atlas during the procedure for the attachment of the acrylic plate. Each monkey had two microdialysis sessions (saline and ketamine at 1.5 mg/kg). A microdialysis probe with a membrane region 250 μm in diameter and 3 mm in length (Eicom A-I-8-03; Eicom, Tokyo, Japan) was inserted into the prefrontal cortex via the guide cannula. The probe was initially perfused with Ringer's solution at a rate of 2 μl/min for 120 min to remove serotonin and dopamine overflow from the damaged tissues. Then, 30 μl samples were collected every 15 min. The averaged data obtained from 0 to 120 min before the administration of ketamine were used as baseline data. Saline or ketamine at dose of 1.5 mg/kg in 10 ml saline was i.v. infused in 40 min, and the changes in serotonin and dopamine levels in the ECF of prefrontal cortex were measured. The serotonin and dopamine levels were expressed as percentages (%) of corresponding baselines. To verify the exact positioning of the inserted probe, 5 μl of China ink was injected via the guide cannula at the end of measurements. Animals were anesthetized with sodium pentobarbital and decapitated. The brains were quickly removed, coronal sections were cut on a cryostat, and the location of probe implantation site was determined visually.
Statistics
The regional BPND values obtained with vehicle, 0.5 mg/kg, and 1.5 mg/kg were compared with one-way repeated-measures ANOVA, followed by Bonferroni correction. The regional BPND values between baseline and 24 h were compared with paired t-tests. The level of significance was P<0.05.
RESULTS
Although no significant changes of heart rate, systolic, and diastolic blood pressure were observed between baseline and ketamine or saline challenge, there was a slight tendency to be decreased after ketamine administration (Table 1).
Table 1. Vital Signs During PET Measurements.
|
Variable |
Vehicle |
0.5 mg/kg, 40 min |
1.5 mg/kg, 40 min |
1.5 mg/kg, 24 h |
| Heart rate, mean±SD | 177.5±17.1 | 167.5±39.5 | 155.0±34.2 | 185.0±17.3 |
| Systolic blood pressure | 127.5±9.6 | 115.0±19.1 | 117.5±12.6 | 125.0± 26.5 |
| Diastolic blood pressure | 85.0±12.9 | 70.0±14.1 | 72.5±9.6 | 87.5±27.5 |
Data are show as mean±SD for five animals.
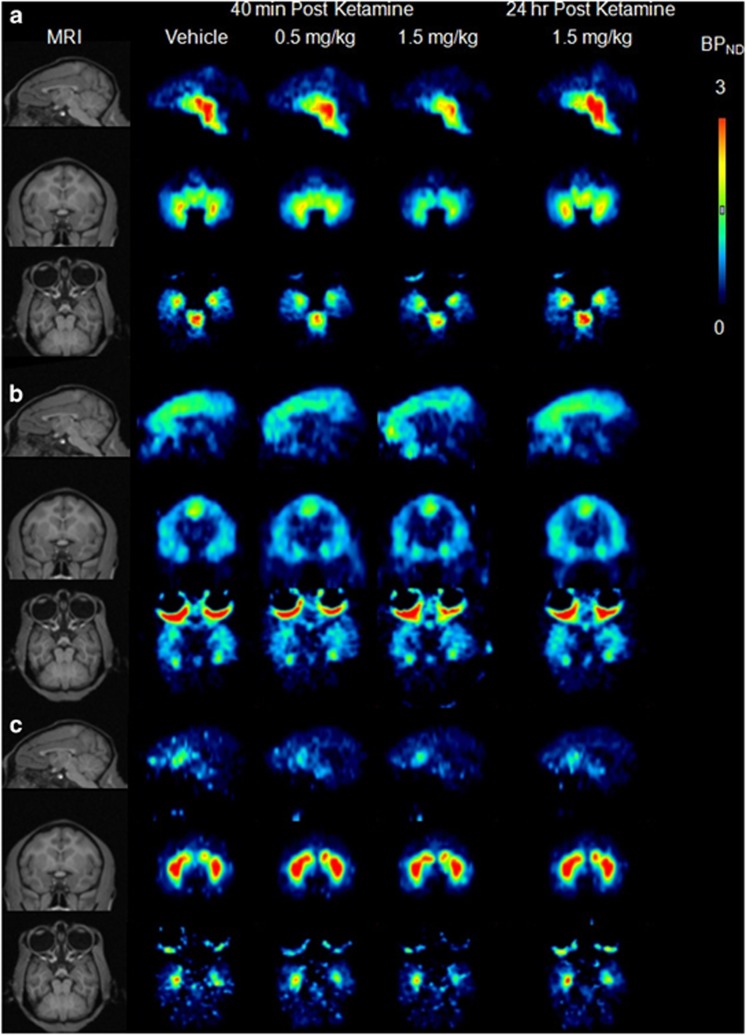
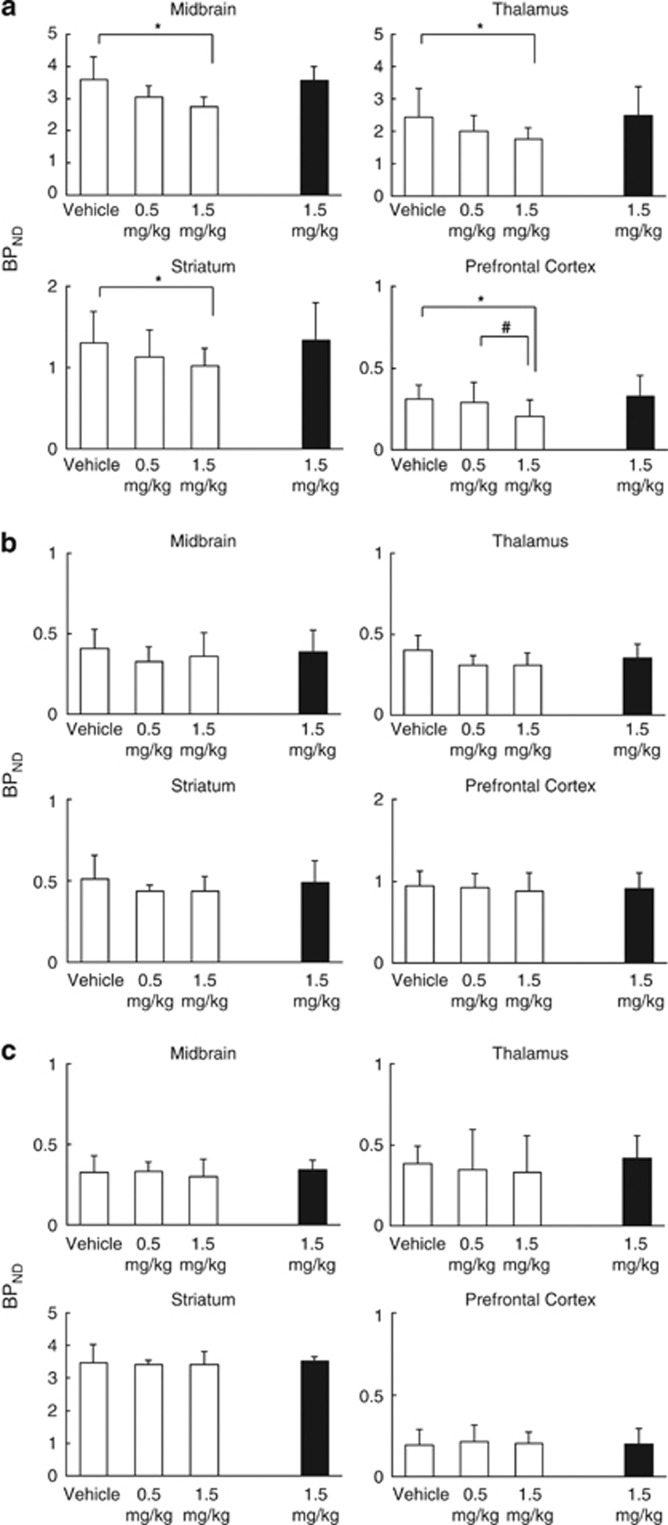
Representative BPND images of [11C]DASB to the SERT are illustrated in Figure 1a. Consistent with the known distribution of the SERT in monkey, as previously reported (Yamamoto et al, 2007; Yokoyama et al, 2010), the BPND of [11C]DASB in the baseline scan was the highest in the midbrain and thalamus, followed by the striatum, and lowest in the cortices. Although [11C]DASB binding in the prefrontal cortex was lower than that in midbrain, [11C]DASB can be used for the evaluation of SERT-specific binding in the prefrontal cortex (Szabo et al, 2002). The mean BPND values of [11C]DASB from all five animals are illustrated in Figure 2a. The BPND values of [11C]DASB 40 min post ketamine at 1.5 mg/kg in the midbrain, thalamus, striatum, and prefrontal cortex were significantly lower than those in baseline (Figure 2a). The reduction in BPND from baseline to 40 min post ketamine at 1.5 mg/kg was 24% in the midbrain, 28% in the thalamus, 22% in the striatum, and 34% in the prefrontal cortex. There were no significant differences in the BPND of [11C]DASB between baseline and 24 h post ketamine at 1.5 mg/kg in any brain regions. The cerebellar Vt values of each experimental condition (vehicle, 40 min post ketamine at 0.5 mg/kg, 40 min post ketamine at 1.5 mg/kg, and 24 h post ketamine at 1.5 mg/kg) for [11C]DASB were 14.7±3.5, 15.4±2.7, 13.3±2.3, and 14.1±1.4, respectively, showing no significant differences among conditions (P=0.62).
Figure 1.
Typical MRI (left) and parametric PET images of [11C]DASB (a), [18F]MPPF (b), and [11C]β-CFT (c) binding in conscious monkey brain. Parametric maps of BPND of [11C]DASB and [18F]MPPF were calculated by Logan plot with an arterial input method. Parametric maps of the BPND of [11C]β-CFT were calculated by the Simplified Reference Tissue Model.
Figure 2.
BPND values of [11C]DASB (a), [18F]MPPF (b), and [11C]β-CFT (c) in conscious monkey brain. Statistical differences were obtained using one-way repeated-measures ANOVA (P<0.05) with dose as factors. Bonferroni tests confirmed a significant difference at *P<0.05 compared with baseline scan. Bonferroni tests also confirmed significant differences at each time point at #P<0.05 values, mean±SD.
Representative BPND images of [18F]MPPF to the 5-HT1A-R are illustrated in Figure 1b. The BPND of [18F]MPPF in the baseline scan was the highest in the hippocampus, followed by the cortices and midbrain. The BPND pattern of [11C]MPPF was consistent with the previously reported 5-HT1A-R distribution determined in monkey brain with PET (Udo de Haes et al, 2006). There were no significant differences in the BPND values of [11C]MPPF between baseline and ketamine administration in any brain region (Figure 2b). The cerebellar VT values of each experimental condition for [18F]MPPF were 2.1±0.6, 1.8±0.4, 1.7±0.2, and 1.7±0.2, respectively, indicating no significant differences among conditions (P=0.45).
Representative BPND images of [11C]β-CFT to DAT are presented in Figure 1c. As expected, the BPND of [11C]β-CFT in the baseline scan was the highest in the striatum, followed by the hippocampus, and lowest in the cortices. The distribution pattern of [11C]β-CFT was consistent with the previously reported DAT distribution determined in monkey brain with PET (Harada et al, 2004; Tsukada et al, 2001). There were no significant differences in the BPND values of [11C]β-CFT between baseline and ketamine administration in any brain regions (Figure 2c).
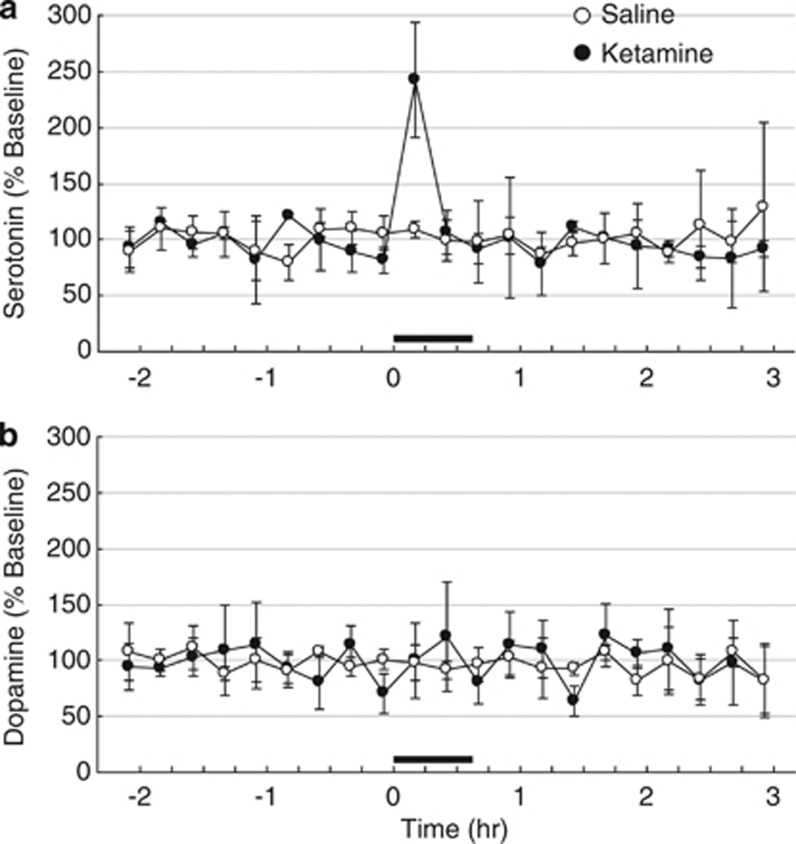
As described in the Materials and methods, the microdialysis probes were inserted with coordinates relevant to the prefrontal cortex. Just after the start of the intravenous infusion of ketamine in a dose of 1.5 mg/kg, the serotonin level in the ECF of prefrontal cortex transiently increased up to 2.4 times greater than baseline, followed by a decrease to control (Figure 3a). In contrast, no significant changes were observed after saline infusion in the ECF serotonin release. Dopamine levels in the ECF of prefrontal cortex remained at constant level without any significant changes after ketamine or saline infusion (Figure 3b).
Figure 3.
Effects of subanesthetic ketamine on extracellular serotonin (a) and dopamine (b) levels in the prefrontal cortex in the conscious monkey brain. Open and filled circles represent the time courses of neurotransmitter change, following saline and ketamine infusion, respectively. A microdialysis probe was inserted into the prefrontal cortex according to MRI via a guide cannula. Ketamine was administrated in a dose of 1.5 mg/kg at time 0. Samples were collected every 15 min. Serotonin- and dopamine-release concentrations were measured using high-performance liquid chromatography with electrochemical detection. The serotonin and dopamine levels were expressed as percentage of the baseline preketamine infusion.
DISCUSSION
To our knowledge, this is the first study to demonstrate the rapid effects of subanesthetic doses of ketamine on the SERT in the brain of conscious monkey. Infusion of ketamine at subanesthetic doses results in the decreased [11C]DASB binding to the SERT in the midbrain and prefrontal cortex in a dose-dependent manner. About a 30% decrease in [11C]DASB binding was observed during ketamine infusion, but the lowered [11C]DASB binding was not present at 24 h. The 5-HT1A-R binding measured with [18F]MPPF was not affected by subanesthetic doses of ketamine in conscious monkey brain.
One of the possible mechanisms of lowered [11C]DASB binding by subanesthetic doses of ketamine was direct competitive inhibition on the SERT. In vitro studies have reported that ketamine in concentrations of >10−6 M inhibited the uptake of [3H]5-HT by the SERT transfected in human embryonic kidney 293 cells in a dose-dependent manner (Nishimura et al, 1998; Zhao and Sun, 2008). Another in vitro study also showed that ketamine in concentrations of >10−5 M inhibited the uptake of [3H]5-HT in the rat brain (Martin et al, 1990). It is unknown whether administration of ketamine in the doses of 0.5 and 1.5 mg/kg used in the present study reaches 10−6 M concentrations in the living brain. However, ketamine in concentrations of >10−6 M inhibits DAT availability transfected in human embryonic kidney 293 cells (Nishimura et al, 1998). Our present data demonstrate that subanesthetic doses of ketamine affect SERT availability and the extracellular serotonin level in the prefrontal cortex, but not DAT availability and extracellular dopamine level.
The effect of extracellular serotonin concentration on the SERT expression is another concern. Our previous PET study showed that the changes in the extracellular serotonin level did not affect the [11C]DASB binding to SERT, when the increase in extracellular serotonin was <10 times compared with baseline (Yamamoto et al, 2007). The microdialysis data indicate that the serotonin level in the ECF of the prefrontal cortex increased only 2.4 times greater than the baseline during ketamine infusion. This result was consistent with previous microdialysis studies showing that a noncompetitive NMDA antagonist, dizocilpine (MK-801), in doses of 0.25–1 mg/kg, transiently increased serotonin levels about two times in the rat brain (López-Gil et al, 2007; Whitton et al, 1992; Yan et al, 1997). There is little literature on the effects of subanesthetic or low doses of ketamine on serotonin release in the brain. Nikiforuk et al (2010) reported that 10 mg/kg of ketamine did not affect serotonin concentration in the rat prefrontal cortex. It is unlikely that the slightly increased serotonin level by ketamine infusion affected the [11C]DASB binding in the present study. Milak et al (2005) reported that [11C]DASB binding is decreased when the extracellular serotonin level was ‘decreased' in the living brain of nonhuman primate. They explained that the decreased [11C]DASB binding was due to an internalization of SERT, probably by protein kinase C (PKC)-dependent phosphorylation and sequestration (Ramamoorthy and Blakely, 1999). Serotonin prevents PKC-dependent phosphorylation and sequestration of SERT. A decreased synaptic serotonin level promotes the internalization of SERT. It is also unlikely that the decreased [11C]DASB binding by ketamine infusion was induced by an internalization of the SERT in the present study, because ketamine infusion induced ‘increased', not decreased, levels of serotonin in the ECF.
The present study indicates that subanesthetic doses of ketamine decreased SERT activity and increased prefrontal serotonin release for only a short time. In clinical settings, ketamine brings about both rapid and long-lasting antidepressant effect (Berman et al, 2000; Diazgranados et al, 2010; Price et al, 2009; Zarate et al, 2006). Probably, there is a mechanism to switch the initial transient effects to long-lasting antidepressant effect. Among the multiple serotonin receptor subtypes, 5-HT1A-R is highly enriched in the prefrontal cortex (Feng et al, 2001; Kia et al, 1996a). Postsynaptic 5-HT1A-Rs are found only in the dendritic compartment and associated with dendritic spines (Kia et al, 1996b) in which glutamate receptors are concentrated. Intriguingly, physical interactions between 5-HT1A-R and NMDA receptors in the prefrontal cortex have been reported. Although some contradictory results are reported (Purkayastha et al, 2012; Yuen et al, 2005), activation of 5-HT1A-R by enhanced serotonin transmission suppresses NMDA receptors in the prefrontal cortex (Yuen et al, 2005). Poleszak et al (2011) suggested a possible contribution of the serotonergic system to the antidepressive effect of glycine/NMDA receptor antagonists. When animals were pretreated with an inhibitor of serotonin synthesis, the antidepressant effects of glycine/NMDA receptor antagonists were abolished. Li et al (2010) reported that activation of mammalian target of rapamycin (mTOR) signaling by ketamine elevated the expression of synapse-associated proteins and spine numbers in the prefrontal cortex of rat. In addition, these effects resulted in enhanced serotonergic neurotransmission observed at 24 h post ketamine injection, which represented a mechanism for the rapid antidepressant actions of ketamine (Li et al, 2010). Recently, a basic study of liver disease showed that serotonin activated mTOR signaling in mice fed with a high fat and high fructose diet (Osawa et al, 2011). Enhanced serotonergic neurotransmission during ketamine infusion initiates an activation cascade of the mTOR pathway. This interaction between the serotonergic system and NMDA receptor might provide the switching mechanism of the transient effects to long-lasting antidepressant effects of subanesthetic doses of ketamine even after the rapid ketamine disappearance from the brain.
A major clinical limitation of SSRI treatment is the markedly delayed onset of therapeutic efficacy. This is thought to be caused by indirect activation of 5-HT1A-R (Chaput et al, 1986; Invernizzi et al, 1996). [18F]MPPF for the 5-HT1A-R could be used for the evaluation of 5-HT1A autoreceptor internalization (Zimmer et al, 2004). It has been demonstrated that decreased [18F]MPPF binding in the dorsal raphe occurs after an acute SSRI administration in both an animal study (Riad et al, 2004) and a clinical PET study (Sibon et al, 2008). Hence, the 5-HT1A autoreceptor internalization in response to acute blockade of SERT and a subsequent rise in extracellular 5-HT level in the dorsal raphe (Bel and Artigas, 1992; Gartside et al, 1995; Invernizzi et al, 1992) appears to be the most feasible interpretation for the decreased [18F]MPPF binding in the dorsal raphe after SSRIs.
Synergies associated with combining NMDA antagonists and other treatments have been proposed by Krystal (2010). Artigas (1993) has proposed that the administration of 5-HT1A-R antagonists could accelerate the clinical therapeutic efficacy of SSRIs. The 5-HT1A-R antagonists could prevent the internalization of 5-HT1A autoreceptors in the dorsal raphe, and this mimic the 5-HT1A-R desensitization produced by the prolonged administration of SSRIs. Animal studies (Beyer et al, 2002; Ceglia et al, 2004; Dawson and Nguyen, 1998; Gartside et al, 1995; Hjorth et al, 1997; Sharp et al, 1997), and a clinical trial (Artigas et al, 1994), partially support this hypothesis. However, one important concern is the lack of selectivity of 5-HT1A-R antagonists for presynaptic vs postsynaptic 5-HT1A-R (Rabiner et al, 2002). Full blockade of postsynaptic 5-HT1A-Rs may cancel the increased serotonergic transmission. In the present study, the rapid inhibition of SERT by subanesthetic doses of ketamine, without affecting 5-HT1A-R, may contribute to the quick antidepressant effect of ketamine. This interpretation is supported by the microdialysis results that extracellular serotonin levels in the prefrontal cortex increase rapidly after subanesthetic doses of ketamine.
It is known that ketamine at doses of 25–30 mg/kg induces dopamine release ca. 2–5 times in the rat prefrontal cortex (Lindefors et al, 1997; Verma and Moghaddam 1996). Ketamine at dose of 30 mg/kg also induced dopamine release in the striatum, although small amount of increase (ca. 25%) was observed (Moghaddam et al, 1997). In the several previous studies, [11C]raclopride, a PET probe for dopamine D2 receptor, has been used to monitor the synaptic dopamine level following administration of subanesthetic ketamine, showing conflicting results. Thus, some reports demonstrated that the subanesthetic ketamine significantly decreased [11C]raclopride binding in the striatum of human brain (Breier et al, 1998; Smith et al, 1998). Other reports, in contrast, showed no significant effect of ketamine on the striatal [11C]raclopride binding in human brain (Aalto et al, 2002; Kegeles et al, 2002). At anesthetic doses of ketamine, we previously reported a dose-dependent decrease in [11C]raclopride binding and increase in [11C]β-CFT binding in the striatum of monkey brain (Tsukada et al, 2000). We interpreted that dynamic turnover of endogenous dopamine, accompanied by increased dopamine synthesis/release and facilitated DAT availability, resulted in the decreased [11C]raclopride binding at the anesthetic doses of ketamine. As our present data showed no significant changes in DAT availability and extracellular dopamine level after subanesthetic dose of ketamine, we speculate that subanesthetic doses of ketamine might not affect [11C]raclopride binding in the striatum of monkey brain.
A limitation in interpreting the results of the present study is that the changes in SERT availability, measured by PET, as well as the serotonin levels in the prefrontal cortex, as determined by microdialysis, were small. These alterations occurred using normal animals. Animal models of depression should be used with the same experimental protocol. It may be possible to detect greater changes in serotonergic transmission by low-dose ketamine more clearly, especially the mTOR signaling pathway, brain-derived neurotrophic factor release, and so on.
FUNDING AND DISCLOSURE
This research was funded primarily by Hamamatsu Photonics, Hamamatsu, Japan, as part of the Central Research Laboratory support, and the University of Michigan Psychopharmacology Research Fund 361024. The authors declare no conflict of interest.
References
- Aalto S, Hirvonen J, Kajander J, Scheinin H, Någren K, Vilkman H, et al. Ketamine does not decrease striatal dopamine D2 receptor binding in man. Psychopharmacology. 2002;164:401–406. doi: 10.1007/s00213-002-1236-6. [DOI] [PubMed] [Google Scholar]
- Artigas F. 5-HT and antidepressants: new views from microdialysis studies. Trends Pharmacol Sci. 1993;14:262. doi: 10.1016/0165-6147(93)90125-4. [DOI] [PubMed] [Google Scholar]
- Artigas F, Perez V, Alvarez E. Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch Gen Psychiatry. 1994;51:248–251. doi: 10.1001/archpsyc.1994.03950030084009. [DOI] [PubMed] [Google Scholar]
- Bel N, Artigas F. Fluvoxamine preferentially increases extracellular 5-hydroxytryptamine in the raphe nuclei: an in vivo microdialysis study. Eur J Pharmacol. 1992;229:101–103. doi: 10.1016/0014-2999(92)90292-c. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Boikess S, Luo B, Dawson LA. Comparison of the effects of antidepressants on norepinephrine and serotonin concentrations in the rat frontal cortex: an in-vivo microdialysis study. J Psychopharmacol. 2002;16:297–304. doi: 10.1177/026988110201600403. [DOI] [PubMed] [Google Scholar]
- Breier A, Adler CM, Weisenfeld N, Su TP, Elman I, Picken L, et al. Effects of NMDA antagonism on striatal dopamine release in healthy subjects: application of a novel PET approach. Synapse. 1998;29:142–147. doi: 10.1002/(SICI)1098-2396(199806)29:2<142::AID-SYN5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ceglia I, Acconcia S, Fracasso C, Colovic M, Caccia S, Invernizzi RW. Effects of chronic treatment with escitalopram or citalopram on extracellular 5-HT in the prefrontal cortex of rats: role of 5-HT1A receptors. Br J Pharmacol. 2004;142:469–478. doi: 10.1038/sj.bjp.0705800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- Clements JA, Nimmo WS, Grant IS. Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. J Pharm Sci. 1982;71:539–542. doi: 10.1002/jps.2600710516. [DOI] [PubMed] [Google Scholar]
- Dawson LA, Nguyen HQ. Effects of 5-HT1A receptor antagonists on fluoxetine-induced changes in extracellular serotonin concentrations in rat frontal cortex. Eur J Pharmacol. 1998;345:41–46. doi: 10.1016/s0014-2999(97)01580-x. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF, Chodoff P, Corssen G. Pharmacologic effects of CI-581, a new dissociative anesthetic, in man. Clin Pharmacol Ther. 1965;6:279–291. doi: 10.1002/cpt196563279. [DOI] [PubMed] [Google Scholar]
- Feng J, Cai X, Zhao J, Yan Z. Serotonin receptors modulate GABA(A) receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci. 2001;21:6502–6511. doi: 10.1523/JNEUROSCI.21-17-06502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. Pharmacology of antidepressants. J Clin Psychopharmacol. 1997;1997;17 (Suppl 1:2S–18S. doi: 10.1097/00004714-199704001-00002. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Umbers V, Hajós M, Sharp T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Ohba H, Fukumoto D, Kakiuchi T, Tsukada H. Potential of [18F]β-CFT-FE (2β-Carbomethoxy-3β-(4-fluorophenyl)-8-(2-[18F]fluoro- ethyl)nortropane) as a dopamine transporter ligand: a PET study in the conscious monkey brain. Synapse. 2004;54:37–45. doi: 10.1002/syn.20059. [DOI] [PubMed] [Google Scholar]
- Hartvig P, Valtysson J, Antoni G, Westerberg G, Långström B, Ratti Moberg E, et al. Brain kinetics of (R)- and (S)-[N-methyl-11C]ketamine in the rhesus monkey studied by positron emission tomography (PET) Nucl Med Biol. 1994;21:927–934. doi: 10.1016/0969-8051(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Westlin D, Bengtsson HJ. WAY100635-induced augmentation of the 5-HT-elevating action of citalopram: relative importance of the dose of the 5-HT1A (auto)receptor blocker versus that of the 5-HT reuptake inhibitor. Neuropharmacology. 1997;36:461–465. doi: 10.1016/s0028-3908(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Belli S, Samanin R. Citalopram's ability to increase the extracellular concentrations of serotonin in the dorsal raphe prevents the drug's effect in the frontal cortex. Brain Res. 1992;584:322–324. doi: 10.1016/0006-8993(92)90914-u. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Bramante M, Samanin R. Chronic treatment with citalopram facilitates the effect of a challenge dose on cortical serotonin output: role of presynaptic 5-HT1A receptors. Eur J Pharmacol. 1994;260:243–246. doi: 10.1016/0014-2999(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Bramante M, Samanin R. Role of 5-HT1A receptors in the effects of acute chronic fluoxetine on extracellular serotonin in the frontal cortex. Pharmacol Biochem Behav. 1996;54:143–147. doi: 10.1016/0091-3057(95)02159-0. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Martinez D, Kochan LD, Hwang DR, Huang Y, Mawlawi O, et al. NMDA antagonist effects on striatal dopamine release: positron emission tomography studies in humans. Synapse. 2002;43:19–29. doi: 10.1002/syn.10010. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kia HK, Brisorgueil MJ, Hamon M, Calas A, Vergé D. Ultrastructural localization of 5-hydroxytryptamine1A receptors in the rat brain. J Neurosci Res. 1996a;46:697–708. doi: 10.1002/(SICI)1097-4547(19961215)46:6<697::AID-JNR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, et al. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol. 1996b;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Krystal JH. N-methyl-D-aspartate glutamate receptor antagonists and the promise of rapid-acting antidepressants. Arch Gen Psychiatry. 2010;67:1110–1111. doi: 10.1001/archgenpsychiatry.2010.138. [DOI] [PubMed] [Google Scholar]
- Kumlien E, Hartvig P, Valind S, Oye I, Tedroff J, Långström B. NMDA-receptor activity visualized with (S)-[N-methyl-11C]ketamine and positron emission tomography in patients with medial temporal lobe epilepsy. Epilepsia. 1999;40:30–37. doi: 10.1111/j.1528-1157.1999.tb01985.x. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindefors N, Barati S, O'Connor WT. Differential effects of single and repeated ketamine administration on dopamine, serotonin and GABA transmission in rat medial prefrontal cortex. Brain Res. 1997;759:205–212. doi: 10.1016/s0006-8993(97)00255-2. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer D, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- López-Gil X, Babot Z, Amargós-Bosch M, Suñol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32:2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- Martin DC, Introna RP, Aronstam RS. Inhibition of neuronal 5-HT uptake by ketamine, but not halothane, involves disruption of substrate recognition by the transporter. Neurosci Lett. 1990;112:99–103. doi: 10.1016/0304-3940(90)90329-8. [DOI] [PubMed] [Google Scholar]
- Milak MS, Ogden RT, Vinocur DN, Van Heertum RL, Cooper TB, Mann JJ, et al. Effects of tryptophan depletion on the binding of [11C]-DASB to the serotonin transporter in baboons: response to acute serotonin deficiency. Biol Psychiatry. 2005;57:102–106. doi: 10.1016/j.biopsych.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry. 2001;158:1843–1849. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161:826–835. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Gołembiowska K, Popik P. Mazindol attenuates ketamine-induced cognitive deficit in the attentional set shifting task in rats. Eur Neuropsychopharmacol. 2010;20:37–48. doi: 10.1016/j.euroneuro.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, et al. Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology. 1998;88:768–774. doi: 10.1097/00000542-199803000-00029. [DOI] [PubMed] [Google Scholar]
- Ohba H, Harada N, Nishiyama S, Kakiuchi T, Tsukada H. Ketamine/xylazine anesthesia alters [11C]MNPA binding to dopamine D2 receptors and response to methamphetamine challenge in monkey brain. Synapse. 2009;63:534–537. doi: 10.1002/syn.20632. [DOI] [PubMed] [Google Scholar]
- Onoe H, Inoue O, Suzuki K, Tsukada H, Itoh T, Mataga N, et al. Ketamine increases the striatal N-11C-methylspiperone binding in vivo: positron emission tomography study using conscious rhesus monkey. Brain Res. 1994;663:191–198. doi: 10.1016/0006-8993(94)91263-7. [DOI] [PubMed] [Google Scholar]
- Osawa Y, Kanamori H, Seki E, Hoshi M, Ohtaki H, Yasuda Y, et al. L-tryptophan-mediated enhancement of susceptibility to nonalcoholic fatty liver disease is dependent on the mammalian target of rapamycin. J Biol Chem. 2011;286:34800–34808. doi: 10.1074/jbc.M111.235473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleszak E, Wlaź P, Szewczyk B, Wlaź A, Kasperek R, Wróbel A, et al. A complex interaction between glycine/NMDA receptors and serotonergic/noradrenergic antidepressants in the forced swim test in mice. J Neural Transm. 2011;118:1535–1546. doi: 10.1007/s00702-011-0630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha S, Ford J, Kanjilal B, Diallo S, Del Rosario Inigo J, Neuwirth L, et al. Clozapine functions through the prefrontal cortex serotonin 1A receptor to heighten neuronal activity via calmodulin kinase II-NMDA receptor interactions. J Neurochem. 2012;120:396–407. doi: 10.1111/j.1471-4159.2011.07565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiner EA, Wilkins MR, Turkheimer F, Gunn RN, Udo de Haes J, de Vries M, et al. 5-Hydroxytryptamine1A receptor occupancy by novel full antagonist 2-[4-[4-(7-chloro-2,3-dihydro-1,4-benzdioxyn-5-yl)-1-piperazinyl]butyl]-1,2-benzisothiazol-3-(2H)-one-1,1-dioxide: a[11C][O-methyl-3H]-N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl)cyclohexanecarboxamide trihydrochloride (WAY-100635) positron emission tomography study in humans. J Pharmacol Exp Ther. 2002;301:1144–1150. doi: 10.1124/jpet.301.3.1144. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1989;36:186–197. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]
- Riad M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe dorsalis of rat. J Neurosci. 2004;24:5420–5426. doi: 10.1523/JNEUROSCI.0950-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon RM, Miller HL, Delgado PL, Charney D. The use of tryptophan depletion to evaluate central serotonin function in depression and other neuropsychiatric disorders. Int Clin Psychopharmacol. 1993;Suppl 2:41–46. doi: 10.1097/00004850-199311002-00006. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Ito H, Kimura Y, Arakawa R, Takano H, Seki C, et al. Quantification of dopamine transporter in human brain using PET with 18F-FE-PE2I. J Nucl Med. 2012;53:1065–1073. doi: 10.2967/jnumed.111.101626. [DOI] [PubMed] [Google Scholar]
- Sharp T, Umbers V, Gartside SE. Effect of a selective 5-HT reuptake inhibitor in combination with 5-HT1A and 5-HT1B receptor antagonists on extracellular 5-HT in rat frontal cortex in vivo. Br J Pharmacol. 1997;121:941–946. doi: 10.1038/sj.bjp.0701235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibon I, Benkelfat C, Gravel P, Aznavour N, Costes N, Mzengeza S, et al. Decreased [18F]MPPF binding potential in the dorsal raphe nucleus after a single oral dose of fluoxetine: a positron-emission tomography study in healthy volunteers. Neuroimage. 2008;63:1135–1140. doi: 10.1016/j.biopsych.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Smith GS, Schloesser R, Brodie JD, Dewey SL, Logan J, Vitkun SA, et al. Glutamate modulation of dopamine measured in vivo with positron emission tomography (PET) and 11C-raclopride in normal human subjects. Neuropsychopharmacology. 1998;18:18–25. doi: 10.1016/S0893-133X(97)00092-4. [DOI] [PubMed] [Google Scholar]
- Suhara T, Takano A, Sudo Y, Ichimiya T, Inoue M, Yasuno F, et al. High levels of serotonin transporter occupancy with low-dose clomipramine in comparative occupancy study with fluvoxamine using positron emission tomography. Arch Gen Psychiatry. 2003;60:386–391. doi: 10.1001/archpsyc.60.4.386. [DOI] [PubMed] [Google Scholar]
- Szabo Z, McCann UD, Wilson AA, Scheffel U, Owonikoko T, Mathews WB, et al. Comparison of (+)-11C-McN5652 and 11C-DASB as serotonin transporter radioligands under various experimental conditions. J Nucl Med. 2002;43:678–692. [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Fukumoto D, Nishiyama S, Sato K, Kakiuchi T. Transient focal ischemia affects the cAMP second messenger system and coupled dopamine D1 and 5-HT1A receptors in the living monkey brain: a positron emission tomography study using microdialysis. J Cereb Blood Flow Metab. 2004;24:898–906. doi: 10.1097/01.WCB.0000126974.07553.86. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Harada N, Nishiyama S, Ohba H, Sato K, Fukumoto D, et al. Ketamine decreased striatal [11C]raclopride binding with no alteration in static dopamine concentrations in the striatal extracellular fluid in the monkey brain: Multi-parametric PET studies combined with microdialysis analysis. Synapse. 2000;37:95–103. doi: 10.1002/1098-2396(200008)37:2<95::AID-SYN3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Nishiyama S, Kakiuchi T, Ohba H, Sato K, Harada N. Ketamine alters the availability of striatal dopamine transporter as measured by [(11)C]β-CFT and [(11)C]β-CIT-FE in the monkey brain. Synapse. 2001;42:273–280. doi: 10.1002/syn.10012. [DOI] [PubMed] [Google Scholar]
- Udo de Haes JI, Harada N, Elsinga PH, Maguire RP, Tsukada H. Effect of fenfluramine-induced increases in serotonin release on [18F]MPPF binding: a continuous infusion PET study in conscious monkeys. Synapse. 2006;59:18–26. doi: 10.1002/syn.20209. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Okada H, Shimizu K, Omura T, Yoshikawa E, Kosugi T, et al. A high resolution animal PET scanner using compact PS-PMT detectors. IEEE Trans Nucl Sci. 1997;44:1277–1282. [Google Scholar]
- Whitton PS, Biggs CS, Pearce BR, Fowler LJ. MK-801 increases extracellular 5-hydroxytryptamine in rat hippocampus and striatum in vivo. J Neurochem. 1992;58:1573–1575. doi: 10.1111/j.1471-4159.1992.tb11381.x. [DOI] [PubMed] [Google Scholar]
- Wilson AA, Ginovart N, Schmidt M, Meyer JH, Threlkeld PG, Houle S. Novel radiotracers for imaging the serotonin transporter by positron emission tomography: synthesis, radiosynthesis, and in vitro and ex vivo evaluation of (11)C-labeled 2-(phenylthio)araalkylamines. J Med Chem. 2000;43:3103–3110. doi: 10.1021/jm000079i. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Onoe H, Tsukada H, Watanabe Y. Effects of increased endogenous serotonin on the in vivo binding of [11C]DASB to serotonin transporters in conscious monkey brain. Synapse. 2007;61:724–731. doi: 10.1002/syn.20422. [DOI] [PubMed] [Google Scholar]
- Yan QS, Reith ME, Jobe PC, Dailey JW. Dizocilpine (MK-801) increases not only dopamine but also serotonin and norepinephrine transmissions in the nucleus accumbens as measured by microdialysis in freely moving rats. Brain Res. 1997;765:149–158. doi: 10.1016/s0006-8993(97)00568-4. [DOI] [PubMed] [Google Scholar]
- Yokoyama C, Yamanaka H, Onoe K, Kawasaki A, Nagata H, Shirakami K, et al. Mapping of serotonin transporters by positron emission tomography with [11C]DASB in conscious common marmosets: comparison with rhesus monkeys. Synapse. 2010;64:594–601. doi: 10.1002/syn.20766. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Sun L. Antidepressants modulate the in vitro inhibitory effects of propofol and ketamine on norepinephrine and serotonin transporter function. J Clin Neurosci. 2008;15:1264–1269. doi: 10.1016/j.jocn.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer L, Riad M, Rbah L, Belkacem-Kahlouli A, Le Bars D, Renaud B, et al. Toward brain imaging of serotonin 5-HT1A autoreceptor internalization. Neuroimage. 2004;22:1421–1426. doi: 10.1016/j.neuroimage.2004.03.020. [DOI] [PubMed] [Google Scholar]