Abstract
Borrelia burgdorferi (Bb) is the causative agent of Lyme disease transmitted to humans by ticks of the Ixodes spp. Bb is a unique bacterial pathogen because it does not require iron (Fe2+) for its metabolism. Bb encodes a ferritin-like Dps homolog called NapA (also called BicA), which can bind Fe or copper (Cu2+), and a manganese (Mn2+) transport protein, Borrelia metal transporter A (BmtA); both proteins are required for colonization of the tick vector, but BmtA is also required for the murine host. This demonstrates that Bb's metal homeostasis is a critical facet of the complex enzootic life cycle between the arthropod and murine hosts. Although metals are known to influence the expression of virulence determinants during infection, it is unknown how or if metals regulate virulence in Bb. Recent evidence demonstrates that Bb modulates the intracellular Mn2+ and zinc (Zn2+) content and, in turn, these metals regulate gene expression through influencing the Ferric Uptake Regulator (Fur) homolog Borrelia Oxidative Stress Regulator (BosR). This mini-review focuses on the burgeoning study of metal-dependent gene regulation within Bb.
Keywords: Borrelia burgdorferi, Lyme disease, copper, manganese, zinc, calprotectin
Introduction
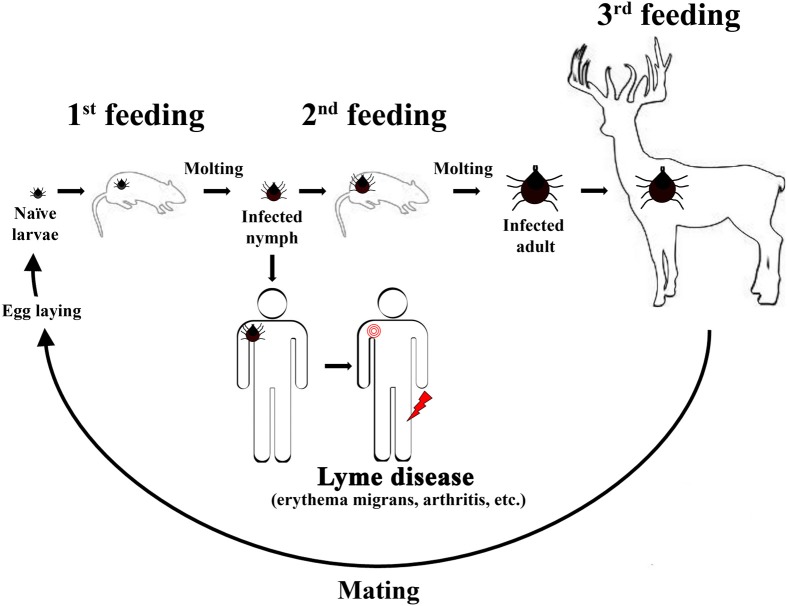
Borrelia burgdorferi (Bb) is the causative agent of a multisystem disorder known as Lyme disease. Bb persists within an enzootic cycle that includes two diverse hosts, a tick vector and a warm-blooded host, typically small rodents. “Hard ticks” of the Ixodes genus are important arthropod hosts for colonization by Bb. Ixodes ticks are slow-feeding ticks (≈48 h for a bloodmeal) that have a 2-year life cycle including three distinct stages: larvae, nymph, and adult (Figure 1). At each stage, ticks will feed once on a warm-blooded host then undergo a molting process, which precedes a period of dormancy that may last months (Figure 1). Because Bb colonization of ticks does not appear to occur through transovarial transmittance, unfed larvae ticks are naïve and acquire Bb during feeding on an infected warm-blooded host. Feeding ticks can acquire Bb at any stage of the usual 2-year life cycle and transmission of Bb can occur during feeding on an animal host at any subsequent stage of the life cycle. Small rodents (especially the white-footed mouse, Peromyscus leucopus) are the primary animal reservoirs for Bb within this enzootic cycle and are sources for the bloodmeal during the larval and nymphal stages (Figure 1). Unlike most bacterial pathogens, Bb lacks lipopolysaccharide (LPS), lipooligosaccharide (LOS), and capsule (Radolf and Samuels, 2010). Bb is highly motile due to the presence of flagella; however, Bb's flagella are contained within the periplasmic space between the outer and inner membranes. Therefore, Bb's flagella is not surface exposed and is called an endoflagella. The endoflagella are anchored at each end of the cell and provide Bb with a characteristic corkscrew movement. Despite Bb's limited metabolism and fastidious nature Bb survives within two hosts, a tick vector and a small rodent host. Other animals, such as humans, are infected by Bb, but are not considered important for persistence of Bb within the enzootic cycle. Of significant interest, Bb is one of the few pathogens that does not require iron (Fe2+) to grow (Posey and Gherardini, 2000). Given the importance of Fe2+ in the regulation of virulence within other bacteria, it is not clear which metals Bb utilize for regulating virulence factors. Recent work suggests that metals may play an important role in regulation of virulence within Bb.
Figure 1.
The usual 2-year enzootic cycle of the Lyme disease spirochete. A naïve Ixodes scapularis larvae will feed on a small rodent near the end of the Summer season or early Fall. The feeding larvae can acquire Bb at this feeding (1st feeding) and remain colonized throughout the molting process, which occurs during the Winter season. For the 2nd feeding, infected nymphs will feed late in the Spring season or early in the Summer season. The infected nymphs transmit Bb to either a small rodent host, which maintains the enzootic cycle in nature, or humans (accidental host). Infected humans develop Lyme disease and may develop erythema migrans (signified by a red bulls eye near the shoulder in the figure shown) shortly after an infected nymph feeds. Typically, if subject to late Lyme manifestations Lyme disease patients develop Lyme arthritis at one or both knee joints (signified by a red lightning bolt near the knee in the figure shown). For the final feeding (3rd feeding), nymphs will molt and emerge as adults to feed on large mammals, such as deer, during the Fall season. Deer are considered incompetent hosts for Bb, but the 3rd feeding is important in the enzootic cycle because female ticks will mate and lay eggs over the Winter season. Naïve larvae will emerge following hatching and the cycle begins anew.
Metal homeostasis is important to maintain the metabolism of bacterial pathogens. This is accomplished through the combined action of metal transporters, both importers and exporters, which control the abundance of specific metals and the ratio of the transition metals within the cell. Although some metal transporters are highly specific for a cognate metal, others are capable of importing several metals with different affinity of each metal. In addition to the importance of metals in bacterial physiology, metals play a critical role in the control of gene regulation within pathogens. The role of metals within Bb is not fully understood. Only a single protein, Borrelia metal transporter A (BmtA) is known to participate in metal transport. Analysis of the intracellular metal content with in vitro grown Bb suggests that BmtA transports Mn2+ since this metal is nearly undetectable in ΔbmtA strains (Ouyang et al., 2009a; Troxell et al., 2013). BmtA may also be involved in the import/export of other metals since deletion of bmtA alters the intracellular concentrations of Fe2+, Cu2+, and Zn2+ (Wang et al., 2012). The mechanism of BmtA-dependent metal transport is still unknown, but recent evidence indicates that BmtA and Mn2+ are involved in regulation of virulence through a Ferric uptake regulator (Fur) homolog named Borrelia Oxidative Stress Regulator (BosR). BosR is redox sensing DNA binding protein that utilizes Zn2+ as a cofactor (Boylan et al., 2003; Katona et al., 2004). Discussed here is the role of metals in Bb physiology and gene expression as it relates to virulence factors required in vivo.
Bb: a non-combatant in the war for Fe2+
Just as a siege limits the influx of food and supplies to an enemy's stronghold, during infection the host transports metals away from the locale of pathogens and synthesizes copious amounts of metal-chelating proteins to limit access of these essential micronutrients. The hosts' ability to produce metal-chelating proteins is important for defending against pathogens since deletion of the chelating protein calprotectin enhances virulence of Acinetobacter baumannii, Staphylococcus aureus, and the opportunistic yeast pathogen Candida albicans (Corbin et al., 2008; Kehl-Fie et al., 2011; Damo et al., 2013). Calprotectin can bind Mn2+ and Zn2+ and is an abundant protein present in neutrophils (Yui et al., 2003), which are an early host defender against invading pathogens. Some bacterial pathogens are capable of overcoming the growth inhibition exerted by calprotectin; Salmonella enterica serovar Typhimurium (S. Typhimurium) expresses a high affinity Zn2+ ATP-binding cassette (ABC) transport system that outcompetes Zn2+ chelation by calprotectin (Liu et al., 2012). Calprotectin is known to inhibit in vitro growth of Bb through Zn2+ sequestration (Lusitani et al., 2003). The contribution of calprotectin to Bb growth in vivo is unknown, but Bb encodes several putative uncharacterized ABC transporters that could be involved in metal transport during infection. In addition, whether calprotectin inhibits Bb growth through Mn2+ chelation is unknown. The fierce war between the pathogen and host for accessibility of Fe2+ poses a problem to pathogens; however, Bb has evolved a novel solution by becoming a non-combatant in the war for Fe2+. Bb does not appear to transport Fe2+, lacks many biosynthetic and catabolic pathways that require Fe2+, and exhibits no defect in growth in the absence of detectable Fe2+ (Posey and Gherardini, 2000). Although a recent study indicates there is detectable Fe2+ within Bb, the physiological relevance of this finding remains uncertain (Wang et al., 2012). Another study did not detect intracellular Fe2+ following in vitro cultivation of Bb (Aguirre et al., 2013). Therefore, additional experiments are required to address these discrepancies. At this point, how Fe2+ is transported within Bb is unknown. Future work is required to determine the contribution of intracellular Fe2+ to Bb gene regulation and metabolism. Instead, because calprotectin inhibits Bb growth by Zn2+ sequestration, the existing data suggests that Zn2+ is an important metal within the metabolism of Bb. This is supported by the Zn2+-dependent enzymatic activity of peptide deformylase (Nguyen et al., 2007) and the glycolytic enzyme fructose-1,6-bisphosphate aldolase (Bourret et al., 2011). Furthermore, peptide deformylase may be an essential enzyme (Jain et al., 2005) and, since glycolysis is the sole mechanism for the generation of ATP within Bb, Zn2+ may be a critical metal for Bb.
BicA and BmtA: two proteins with novel function within Bb
Bacteria encode metal binding proteins (ferritins or ferritin-like proteins) that store metals and serve as a facile source of essential metals when encountering a metal-depleted environment. Bb encodes a metal binding protein (NapA or BicA) that exhibits homology to the ferritin-like Dps present in other bacteria. Purified BicA is capable of binding Fe2+ or Cu2+, but lacks either metal when isolated (Li et al., 2007; Wang et al., 2012). The majority of studies have focused on the role of Fe2+ chelation by the host in nutritional immunity, but recent evidence demonstrates the importance of chelating Zn2+ and Mn2+ in thwarting bacterial infections (Kehl-Fie and Skaar, 2010). However, as part of the antimicrobial defense present within ticks, an antimicrobial peptide, known as microplusin, inhibits bacterial growth by Cu2+ chelation (Silva et al., 2009, 2011). Microplusin is expressed within the hemocele of ticks (Esteves et al., 2009), implying that this locale is a Cu2+ limited environment. Bb does appear to regulate its intracellular Cu2+, but the relevance or need for Cu2+ is unknown (Wang et al., 2012). The importance of BicA to the enzootic cycle is restricted to residence within the tick vector (Li et al., 2007), implying that Zn2+ and Cu2+ are limiting within this host.
The role of Mn2+ in Bb metabolism is not understood. The gene bmtA, encoding a Mn2+ transport protein BmtA, is not essential for in vitro growth within virulent Bb strains from the B31 (tick isolated) and 297 (human isolated) lineages despite reducing cellular Mn2+ to near undetectable concentrations (Troxell et al., 2013). Bb cultivation in vitro requires a complex growth medium called BSK (Barbour, 1984). Treatment of BSK medium with a chelating resin, called Chelex, results in significant changes of the concentrations of metals. Chelex treatment of BSK reduces Zn2+, but Mn2+ becomes undetectable in the medium. Despite the undetectable Mn2+ in Chelex-treated BSK growth medium, no growth defects are observed for wild-type or ΔbmtA strains during cultivation in this medium (Troxell et al., 2013). BmtA has homology to the GufA family of metal transporters (Guerinot, 2000). BmtA has 8 membrane spanning domains and is predicted to transport cations through a novel mechanism (Ouyang et al., 2009a). To date, only a single protein within Bb is characterized as being Mn2+-dependent; specifically, the superoxide dismutase (SOD) encoded by sodA (Troxell et al., 2012; Aguirre et al., 2013). The expression of bmtA and the intracellular concentration of Mn2+ are enhanced during cultivation at 25°C, suggesting there may be a requirement for Mn2+ at cooler temperatures (Ojaimi et al., 2003; Troxell et al., 2013). The physiological need for more Mn2+ at 25°C is unknown, but this may be due to the need for defense against reactive oxygen species (ROS) because Bb encodes a Mn-dependent SOD and lower temperatures contain increased concentrations of dissolved O2 that could lead to enhanced formation of superoxide radical (O−2) (Troxell et al., 2012; Aguirre et al., 2013). However, Bb may encode additional proteins that require Mn2+.
Mn2+ is considered an essential trace element within biology. In bacteria, Mn2+ is critical for defense against several stresses such as oxidative stress, bile stress, and resistance to antibiotics (Anjem et al., 2009; Srinivasan et al., 2012). In addition, Mn2+ is involved in gene regulation through indirect mechanisms. For instance, the alarmone guanosine tetraphosphate (ppGpp) is synthesized and degraded by SpoT/RelA homolog proteins. During conditions of nutrient deprivation, ppGpp is synthesized and binds to the RNA polymerase (RNAP) in order to enhance transcription of genes important for survival or virulence while reducing transcription of genes involved in growth and cell division (Magnusson et al., 2005). SpoT/RelA homologs contain a highly conserved Mn2+ binding site and require Mn2+ as a cofactor for the enzymatic degradation of ppGpp (Sy, 1977; Sun et al., 2010). Bb encodes a SpoT/RelA homolog, bb0198, that is induced during serum starvation and is responsible for both synthesis and degradation of ppGpp (Concepcion and Nelson, 2003; Bugrysheva et al., 2005). This suggests that Bb may require Mn2+ in order to initiate cell growth. Recently, Bb's peptide deformylase was isolated with bound Mn2+ (Aguirre et al., 2013); however, an enzymatic assay of the Mn-bound enzyme was not conducted. Whether peptide deformylase functions with Mn2+ is unknown, but this enzyme is active with Zn2+ as a cofactor (Nguyen et al., 2007). Future work is needed to determine the metal specificity of BB0198 and the peptide deformylase and to identify Bb proteins that require Mn2+.
Surprisingly, some enhancement in the intracellular concentration of Zn2+ for ΔbmtA has been noted (Wang et al., 2012). It has been hypothesized that within ΔbmtA there may be compensation for the reduction of Mn2+ by enhancing the transport of Zn2+ and thereby replacing the requirement of Mn2+ with Zn2+. Although future work is required to fully test this hypothesis, the replacement of Mn2+ for Zn2+ in Mn2+-dependent enzymes causes a pronounced reduction in catalytic efficiency or abrogates enzymatic activity altogether (Ose and Fridovich, 1976; Sobota and Imlay, 2011; Gu and Imlay, 2013). Metal-dependent transcription factors can utilize a variety of metals for function, i.e., Mn2+ or Fe2+ in the case of Fur (Privalle and Fridovich, 1993), and host metal-sequestering proteins exhibit promiscuity in metal binding, which is demonstrated by the Mn2+ or Zn2+ binding site (S1 site) in calprotectin (Damo et al., 2013). This is in contrast to metal-dependent enzymes, which exhibit stringent metal specificity for activity, as is the case for SpoT/RelA homologs and Bb's SodA (Sy, 1977; Troxell et al., 2012; Aguirre et al., 2013). However, because many of Bb's putative metalloenzymes are uncharacterized, the possibility exists that a significant number of these proteins can utilize either Mn2+ or Zn2+ within the cell.
Bb's metal requirement within the tick
The unfed tick is presumed to be a nutrient deprived environment for Bb. Starvation conditions may mimic oxidative stress conditions and factors responsible for defense against ROS are also important for survival during starvation (Jenkins et al., 1988; Nystrom et al., 1996). Bb may require Mn2+ in order to defend against ROS that occurs during onset of the bloodmeal. Although Mn2+ complexed with other biological compounds, such as bicarbonate, are capable of degrading ROS, this requires large concentrations of intracellular Mn2+ that occurs within Lactobacillus plantarum (Archibald and Fridovich, 1981, 1982; Stadtman et al., 1990). In the only report to compare directly the intracellular Mn2+ content of L. plantarum with Bb, it was observed that Bb contains 20 to 100-fold lower intracellular Mn2+ compared to L. plantarum, indicating this is an unlikely mechanism for ROS defense within Bb (Posey and Gherardini, 2000). However, Bb's intracellular Mn2+ can fluctuate during in vitro growth conditions (Troxell et al., 2013), suggesting that environmental conditions within the tick-mouse life cycle may exist whereby Bb could contain sufficient intracellular Mn2+ to degrade ROS in a manner similar to L. plantarum. Although sodA is required for infection of the murine host (Esteve-Gassent et al., 2009), the contribution of sodA within the tick vector is unknown. It is currently unclear if Bb contains a high intracellular Mn2+ within the unfed tick or is starved for metals. Because of the involvement of BicA in Cu2+ and Zn2+ homeostasis and since ΔbicA exhibits a defect within the unfed tick (Li et al., 2007), the results support the notion that these two metals are limiting. In addition, the contribution of BmtA to the unfed tick is unknown.
Regulation of σS by Zn2+ and Mn2+ within Bb
Bb is capable of surviving within two diverse hosts through changes in gene expression, specifically outer surface lipoproteins that modulate adaptation within each host. Outer Surface Proteins A (OspA) and C (OspC) are a lipoproteins produced by Bb within the tick and animal host, respectively. Bb contains a limited genome that contains a relatively small number of transcription factors and sigma factors: Bb encodes only three sigma factors the housekeeping σ70, and two alternative sigma factors, RpoN (σ54) and RpoS (σS) (Fraser et al., 1997; Samuels, 2011; Radolf et al., 2012). In addition, Bb genome encodes only one bacterial enhancer binding protein (bEBP), known as Rrp2, which is involved in σ54 activation. The requirement of the Rrp2-RpoN-RpoS pathway (or Rrp2-σ54−σS sigma factor cascade) in the regulation of ospA and ospC demonstrates the importance of this regulatory network (Hubner et al., 2001; Yang et al., 2003; Caimano et al., 2004; Fisher et al., 2005; Gilbert et al., 2007). Rrp2 and σ54 directly activates transcription of rpoS (Smith et al., 2007; Blevins et al., 2009). σS then activates transcription of ospC by direct binding to the promoter of ospC (Yang et al., 2005) and also represses expression of ospA (Caimano et al., 2007). In addition, BosR, a Fur/PerR-like family transcription factor and a Zn2+-dependent DNA binding protein, has been shown to be essential for transcription of rpoS (Ouyang et al., 2009b, 2011). More recently, Wang et al. demonstrated that BosR may also directly repress ospA (Wang et al., 2013). Because RpoS regulates many genes important for Bb transmission and mammalian infection such as ospC, this pathway is essential for the enzootic cycle of Bb (Caimano et al., 2004; Grimm et al., 2004; Pal et al., 2004; Boardman et al., 2008; Ouyang et al., 2008). Moreover, bosR is required for transmission from the tick vector and infection of the mammalian host (Hyde et al., 2009; Ouyang et al., 2009b). Thus, Bb has evolved to utilize the transcription factor BosR for virulence.
Bb is a highly fastidious pathogen. The cultivation of Bb requires a complex medium that is analogous to cell culture media for eukaryotic cells (Barbour, 1984). Comparisons of Bb replication within a feeding tick and during in vitro growth at 35–37°C demonstrate that both conditions support growth with a generation time of ≈8–10 h (De Silva and Fikrig, 1995). Metal analysis of the cultivation medium for Bb indicates there is ≈5 μ M Zn2+, ≈4 μM Cu2+, and ≈0.1 μM Mn2+ (Wang et al., 2012; Troxell et al., 2013). Besides Fe2+, other transition metals, such as Zn2+ and Mn2+ are known to influence gene regulation within bacterial pathogens (Corbin et al., 2008). Based on the Zn2+-dependent nature of BosR (Boylan et al., 2003; Katona et al., 2004), and because BosR regulates rpoS, Zn2+ could regulate rpoS within Bb.
Metal analysis indicates that while intracellular Zn2+ remained relatively constant under different conditions, Mn2+ was subject to temperature-dependent regulation within Bb (Troxell et al., 2013). Moreover, the intracellular Mn2+ can fluctuate 20-fold during in vitro growth conditions and the temperature-dependent inverse concentration of intracellular of Mn2+ is reminiscent of the inverse regulation of ospA and ospC within Bb (Stevenson et al., 1995; Obonyo et al., 1999; Yang et al., 2000; Alverson et al., 2003). To test if Mn2+ could suppress regulation by σS, MnCl2 was added to cultures growing under conditions of σS activation. The addition of MnCl2 increases intracellular Mn2+ and reduces the expression of rpoS and σS-activated ospC (Troxell et al., 2013). The addition of excess ZnSO4 increases the intracellular Zn2+, increases the level of BosR protein, and abrogates the repression of rpoS by Mn2+. Surprisingly, MnCl2 did not influence transcription of bosR, but reduced the level of the BosR protein (Troxell et al., 2013). In addition, deletion of bmtA in two infectious strains does not alter bosR transcription, but enhances temperature-dependent activation in the level of BosR, which results in increased transcription of rpoS and ospC. As an earlier study shows, the BosR protein level is increased by CO2 despite the inability of dissolved CO2 to regulate transcription of bosR (Hyde et al., 2007). These combined results suggest that either metals or CO2 may control the level of the BosR protein, which activates transcription of rpoS. Correlation of the intracellular Mn2+:Zn2+ indicates that the ratio between these two metals play an important role in the level of BosR protein and rpoS regulation. Collectively, these results support the hypothesis that a combined reduction in intracellular Mn2+ while increasing Zn2+ regulates σS by dramatically enhancing the level of BosR protein.
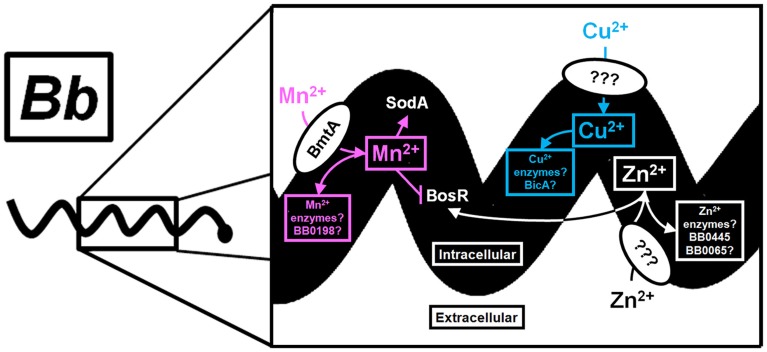
Why does Bb require bmtA for the enzootic cycle? The presence of excess Mn2+ suggests there is a collection of unknown targets that require Mn2+ for activity. One function may be to control σS activation during the enzootic cycle. Precise regulation of ospC and other outer surface proteins, such as vlsE, is required for infection of the murine host; constitutive activation of either surface protein results in rapid elimination of Bb by either innate cells or the humoral response of the host (Liang et al., 2002; Xu et al., 2006, 2008a,b). In the absence of any defined metabolic requirement for Mn2+ within Bb, the importance of Mn2+ to the enzootic cycle could be to control regulation of highly immunogenic outer surface proteins. Nevertheless, the limited genome of Bb encodes several homologs that may require Mn2+ for activity. Furthermore, BmtA appears to influence not only the intracellular Mn2+ concentration, but also the concentrations of Cu2+ and Zn2+. How Zn2+ and Cu2+ are transported within Bb is unknown, but it is likely that these metals are required for proper regulation of virulence genes and unknown metabolic genes. Future work will no doubt shed light on the importance of these metals as cofactors and their influence on gene regulation. This is summarized in Figure 2, which depicts the known and putative roles of Mn2+, Cu2+, and Zn2+ within Bb.
Figure 2.
Known and putative roles of Mn2+, Cu2+, and Zn2+ in gene regulation and metabolism of Bb. A schematic of the importance of transition metals within Bb is shown with a magnification of a section from a single Bb cell. Extracellular Mn2+ is transported through BmtA and supplies the appropriate cofactor for the Mn-SOD and possibly the SpoT/RelA homolog BB0198 (designated by a pink arrow). In addition, Mn2+ reduces the level of BosR protein (designated by a pink blunted line), which controls transcription of the alternative sigma factor, rpoS (not shown). The putative role of Mn2+ as a cofactor for additional unknown enzymes is shown with a pink box. Zn2+ transport is uncharacterized in Bb, but is presumed to be transported by a membrane bound protein. The requirement for Zn2+ within Bb is likely to include enzymes within glycolysis, such as fructose 1,6-bisphosphatase (BB0445), and the peptide deformylase (BB0065) shown in the white box. Zn2+ is a known cofactor for the DNA binding protein BosR. Therefore, the intracellular Mn2+:Zn2+ can modulate the level of BosR protein. The transporter for Cu2+ and the role of Cu2+ within Bb is unknown, but BicA may be involved in transport and homeostasis (blue box). Moreover, the contribution of Cu2+ to gene regulation within Bb is unknown, but is predicted to involve redox sensing transcription factors (Changela et al., 2003; Gomez-Santos et al., 2011). Future work is required to elucidate the complete role of these metals in gene regulation and physiology of this important vector borne pathogen.
Conclusions
Unlike most bacterial pathogens, Bb does not require Fe2+ for growth, which presents a unique model system to study metal-dependent gene regulation and stress responses. The bloodmeal is rich in Zn2+/Cu2+ and relatively poor in Mn2+, which suggests that Bb's intracellular Zn2+/Cu2+ content may increase through unidentified transporters (Figure 2). Because Mn2+ regulates the BosR protein level, but not bosR transcription (Troxell et al., 2013), the low Mn2+ content in blood may further enhance expression rpoS, which is required for Bb to exit the tick midgut and reach the salivary glands during tick feeding (Fisher et al., 2005; Dunham-Ems et al., 2012). How does Bb coordinate the regulation of transport of Mn2+, Cu2+, and Zn2+ during the enzootic cycle? What is apparent from in vitro work is that the intracellular Mn2+:Zn2+ ratio regulates transcription of the alternative sigma factor, rpoS, which controls activation of genes required for infection of mammals. A caveat to these studies is the heavy reliance on in vitro experiments due to the difficulties of measuring intracellular metal content while detecting changes in gene expression during in vivo studies. Infection studies with ΔbicA and ΔbmtA demonstrate the importance of these genes within the enzootic cycle, but the mechanism for why Bb requires them is unknown. Although the contribution to the unfed tick is known for bicA, the contribution of bmtA to survival within the dormant tick is unknown. How BicA and BmtA control metal homeostasis or gene expression in vivo would greatly improve our understanding of their importance in infection. Moreover, the identification of a dedicated Zn transport system and Cu transport system within Bb would provide additional and much needed clarity. It is clear that we are only beginning to understand the importance of metals in the metabolism and gene regulation within the Lyme disease spirochete
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Bryan Troxell was supported by NIH T32 AI060519. Funding for this work was partially provided by NIH grants AI083640 and AI085242, Indiana INGEN and METACyt grants of Indiana University, funded by the Lilly Endowment, Inc (to X. Frank Yang).
References
- Aguirre J. D., Clark H. M., McIlvin M., Vazquez C., Palmere S. L., Grab D. J., et al. (2013). A manganese-rich environment supports superoxide dismutase activity in a Lyme disease pathogen, Borrelia burgdorferi. J. Biol. Chem. 288, 8468–8478 10.1074/jbc.M112.433540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alverson J., Bundle S. F., Sohaskey C. D., Lybecker M. C., Samuels D. S. (2003). Transcriptional regulation of the ospAB and ospC promoters from Borrelia burgdorferi. Mol. Microbiol. 48, 1665–1677 10.1046/j.1365-2958.2003.03537.x [DOI] [PubMed] [Google Scholar]
- Anjem A., Varghese S., Imlay J. A. (2009). Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol. Microbiol. 72, 844–858 10.1111/j.1365-2958.2009.06699.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. (1981). Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J. Bacteriol. 145, 442–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. (1982). The scavenging of superoxide radical by manganous complexes: in vitro. Arch. Biochem. Biophys. 214, 452–463 10.1016/0003-9861(82)90049-2 [DOI] [PubMed] [Google Scholar]
- Barbour A. G. (1984). Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57, 521–525 [PMC free article] [PubMed] [Google Scholar]
- Blevins J. S., Xu H., He M., Norgard M. V., Reitzer L., Yang X. F. (2009). Rrp2, a sigma54-dependent transcriptional activator of Borrelia burgdorferi, activates rpoS in an enhancer-independent manner. J. Bacteriol. 191, 2902–2905 10.1128/JB.01721-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman B. K., He M., Ouyang Z., Xu H., Pang X., Yang X. F. (2008). Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect. Immun. 76, 3844–3853 10.1128/IAI.00467-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourret T. J., Boylan J. A., Lawrence K. A., Gherardini F. C. (2011). Nitrosative damage to free and zinc-bound cysteine thiols underlies nitric oxide toxicity in wild-type Borrelia burgdorferi. Mol. Microbiol. 81, 259–273 10.1111/j.1365-2958.2011.07691.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan J. A., Posey J. E., Gherardini F. C. (2003). Borrelia oxidative stress response regulator, BosR: a distinctive Zn-dependent transcriptional activator. Proc. Natl. Acad. Sci. U.S.A. 100, 11684–11689 10.1073/pnas.2032956100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrysheva J. V., Bryksin A. V., Godfrey H. P., Cabello F. C. (2005). Borrelia burgdorferi rel is responsible for generation of guanosine-3'-diphosphate-5'-triphosphate and growth control. Infect. Immun. 73, 4972–4981 10.1128/IAI.73.8.4972-4981.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano M. J., Eggers C. H., Hazlett K. R., Radolf J. D. (2004). RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 72, 6433–6445 10.1128/IAI.72.11.6433-6445.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano M. J., Iyer R., Eggers C. H., Gonzalez C., Morton E. A., Gilbert M. A., et al. (2007). Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 65, 1193–1217 10.1111/j.1365-2958.2007.05860.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changela A., Chen K., Xue Y., Holschen J., Outten C. E., O'Halloran T. V., et al. (2003). Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 301, 1383–1387 10.1126/science.1085950 [DOI] [PubMed] [Google Scholar]
- Concepcion M. B., Nelson D. R. (2003). Expression of spoT in Borrelia burgdorferi during serum starvation. J. Bacteriol. 185, 444–452 10.1128/JB.185.2.444-452.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin B. D., Seeley E. H., Raab A., Feldmann J., Miller M. R., Torres V. J., et al. (2008). Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319, 962–965 10.1126/science.1152449 [DOI] [PubMed] [Google Scholar]
- Damo S. M., Kehl-Fie T. E., Sugitani N., Holt M. E., Rathi S., Murphy W. J., et al. (2013). Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc. Natl. Acad. Sci. U.S.A. 110, 3841–3846 10.1073/pnas.1220341110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva A. M., Fikrig E. (1995). Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am. J. Trop. Med. Hyg. 53, 397–404 [DOI] [PubMed] [Google Scholar]
- Dunham-Ems S. M., Caimano M. J., Eggers C. H., Radolf J. D. (2012). Borrelia burgdorferi requires the alternative sigma factor RpoS for dissemination within the vector during tick-to-mammal transmission. PLoS Pathog. 8:e1002532 10.1371/journal.ppat.1002532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve-Gassent M. D., Elliott N. L., Seshu J. (2009). sodA is essential for virulence of Borrelia burgdorferi in the murine model of Lyme disease. Mol. Microbiol. 71, 594–612 10.1111/j.1365-2958.2008.06549.x [DOI] [PubMed] [Google Scholar]
- Esteves E., Fogaca A. C., Maldonado R., Silva F. D., Manso P. P., Pelajo-Machado M., et al. (2009). Antimicrobial activity in the tick Rhipicephalus (Boophilus) microplus eggs: cellular localization and temporal expression of microplusin during oogenesis and embryogenesis. Dev. Comp. Immunol. 33, 913–919 10.1016/j.dci.2009.02.009 [DOI] [PubMed] [Google Scholar]
- Fisher M. A., Grimm D., Henion A. K., Elias A. F., Stewart P. E., Rosa P. A., et al. (2005). Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. U.S.A. 102, 5162–5167 10.1073/pnas.0408536102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C. M., Casjens S., Huang W. M., Sutton G. G., Clayton R., Lathigra R., et al. (1997). Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390, 580–586 10.1038/37551 [DOI] [PubMed] [Google Scholar]
- Gilbert M. A., Morton E. A., Bundle S. F., Samuels D. S. (2007). Artificial regulation of ospC expression in Borrelia burgdorferi. Mol. Microbiol. 63, 1259–1273 10.1111/j.1365-2958.2007.05593.x [DOI] [PubMed] [Google Scholar]
- Gomez-Santos N., Perez J., Sanchez-Sutil M. C., Moraleda-Munoz A., Munoz-Dorado J. (2011). CorE from Myxococcus xanthus is a copper-dependent RNA polymerase sigma factor. PLoS Genet. 7:e1002106 10.1371/journal.pgen.1002106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D., Tilly K., Byram R., Stewart P. E., Krum J. G., Bueschel D. M., et al. (2004). Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U.S.A. 101, 3142–3147 10.1073/pnas.0306845101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M., Imlay J. A. (2013). Superoxide poisons mononuclear iron enzymes by causing mismetallation. Mol. Microbiol. 89, 123–134 10.1111/mmi.12263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot M. L. (2000). The ZIP family of metal transporters. Biochim. Biophys. Acta 1465, 190–198 10.1016/S0005-2736(00)00138-3 [DOI] [PubMed] [Google Scholar]
- Hubner A., Yang X., Nolen D. M., Popova T. G., Cabello F. C., Norgard M. V. (2001). Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. U.S.A. 98, 12724–12729 10.1073/pnas.231442498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde J. A., Shaw D. K., Smith Iii R., Trzeciakowski J. P., Skare J. T. (2009). The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol. Microbiol. 74, 1344–1355 10.1111/j.1365-2958.2009.06951.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde J. A., Trzeciakowski J. P., Skare J. T. (2007). Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J. Bacteriol. 189, 437–445 10.1128/JB.01109-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R., Chen D., White R. J., Patel D. V., Yuan Z. (2005). Bacterial Peptide deformylase inhibitors: a new class of antibacterial agents. Curr. Med. Chem. 12, 1607–1621 10.2174/0929867054367194 [DOI] [PubMed] [Google Scholar]
- Jenkins D. E., Schultz J. E., Matin A. (1988). Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J. Bacteriol. 170, 3910–3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona L. I., Tokarz R., Kuhlow C. J., Benach J., Benach J. L. (2004). The fur homologue in Borrelia burgdorferi. J. Bacteriol. 186, 6443–6456 10.1128/JB.186.19.6443-6456.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie T. E., Chitayat S., Hood M. I., Damo S., Restrepo N., Garcia C., et al. (2011). Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 10, 158–164 10.1016/j.chom.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie T. E., Skaar E. P. (2010). Nutritional immunity beyond iron: a role for manganese and zinc. Curr. Opin. Chem. Biol. 14, 218–224 10.1016/j.cbpa.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Pal U., Ramamoorthi N., Liu X., Desrosiers D. C., Eggers C. H., et al. (2007). The Lyme disease agent Borrelia burgdorferi requires BB0690, a Dps homologue, to persist within ticks. Mol. Microbiol. 63, 694–710 10.1111/j.1365-2958.2006.05550.x [DOI] [PubMed] [Google Scholar]
- Liang F. T., Jacobs M. B., Bowers L. C., Philipp M. T. (2002). An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195, 415–422 10.1084/jem.20011870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Z., Jellbauer S., Poe A. J., Ton V., Pesciaroli M., Kehl-Fie T. E., et al. (2012). Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 11, 227–239 10.1016/j.chom.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusitani D., Malawista S. E., Montgomery R. R. (2003). Calprotectin, an abundant cytosolic protein from human polymorphonuclear leukocytes, inhibits the growth of Borrelia burgdorferi. Infect. Immun. 71, 4711–4716 10.1128/IAI.71.8.4711-4716.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson L. U., Farewell A., Nystrom T. (2005). ppGpp: a global regulator in Escherichia coli. Trends Microbiol. 13, 236–242 10.1016/j.tim.2005.03.008 [DOI] [PubMed] [Google Scholar]
- Nguyen K. T., Wu J. C., Boylan J. A., Gherardini F. C., Pei D. (2007). Zinc is the metal cofactor of Borrelia burgdorferi peptide deformylase. Arch. Biochem. Biophys. 468, 217–225 10.1016/j.abb.2007.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom T., Larsson C., Gustafsson L. (1996). Bacterial defense against aging: role of the Escherichia coli ArcA regulator in gene expression, readjusted energy flux and survival during stasis. EMBO J. 15, 3219–3228 [PMC free article] [PubMed] [Google Scholar]
- Obonyo M., Munderloh U. G., Fingerle V., Wilske B., Kurtti T. J. (1999). Borrelia burgdorferi in tick cell culture modulates expression of outer surface proteins A and C in response to temperature. J. Clin. Microbiol. 37, 2137–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojaimi C., Brooks C., Casjens S., Rosa P., Elias A., Barbour A., et al. (2003). Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71, 1689–1705 10.1128/IAI.71.4.1689-1705.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ose D. E., Fridovich I. (1976). Superoxide dismutase. Reversible removal of manganese and its substitution by cobalt, nickel or zinc. J. Biol. Chem. 251, 1217–1218 [PubMed] [Google Scholar]
- Ouyang Z., Blevins J. S., Norgard M. V. (2008). Transcriptional interplay among the regulators Rrp2, RpoN, and RpoS in Borrelia burgdorferi. Microbiology 154, 2641–2658 10.1099/mic.0.2008/019992-0 [DOI] [PubMed] [Google Scholar]
- Ouyang Z., Deka R. K., Norgard M. V. 2011. Bos R (BB0647)controls the RpoN-RpoS regulatory pathway and virulence expression in Borrelia burgdorferi by a novel DNA-binding mechanism. PLoS. Pathog 7, e1001272 10.1371/journal.ppat.1001272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z., He M., Oman T., Yang X. F., Norgard M. V. (2009a). A manganese transporter, BB0219 (BmtA), is required for virulence by the Lyme disease spirochete, Borrelia burgdorferi. Proc. Natl. Acad. Sci. U.S.A. 106, 3449–3454 10.1073/pnas.0812999106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z., Kumar M., Kariu T., Haq S., Goldberg M., Pal U., et al. (2009b). BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol. Microbiol. 74, 1331–1343 10.1111/j.1365-2958.2009.06945.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal U., Yang X., Chen M., Bockenstedt L. K., Anderson J. F., Flavell R. A., et al. (2004). OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Invest. 113, 220–230 10.1172/JCI19894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey J. E., Gherardini F. C. (2000). Lack of a role for iron in the Lyme disease pathogen. Science 288, 1651–1653 10.1126/science.288.5471.1651 [DOI] [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. (1993). Iron specificity of the Fur-dependent regulation of the biosynthesis of the manganese-containing superoxide dismutase in Escherichia coli. J. Biol. Chem. 268, 5178–5181 [PubMed] [Google Scholar]
- Radolf J. D., Caimano M. J., Stevenson B., Hu L. T. (2012). Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10, 87–99 10.1038/nrmicro2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Samuels D. S. (eds.). (2010). Borrelia: Molecular Biology, Host Interaction, and Pathogenesis. Norfolk: Caister Academic Press [Google Scholar]
- Samuels D. S. (2011). Gene regulation in Borrelia burgdorferi. Annu. Rev. Microbiol. 65, 479–499 10.1146/annurev.micro.112408.134040 [DOI] [PubMed] [Google Scholar]
- Silva F. D., Rezende C. A., Rossi D. C., Esteves E., Dyszy F. H., Schreier S., et al. (2009). Structure and mode of action of microplusin, a copper II-chelating antimicrobial peptide from the cattle tick Rhipicephalus (Boophilus) microplus. J. Biol. Chem. 284, 34735–34746 10.1074/jbc.M109.016410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva F. D., Rossi D. C., Martinez L. R., Frases S., Fonseca F. L., Campos C. B., et al. (2011). Effects of microplusin, a copper-chelating antimicrobial peptide, against Cryptococcus neoformans. FEMS Microbiol. Lett. 324, 64–72 10.1111/j.1574-6968.2011.02386.x [DOI] [PubMed] [Google Scholar]
- Smith A. H., Blevins J. S., Bachlani G. N., Yang X. F., Norgard M. V. (2007). Evidence that RpoS (sigmaS) in Borrelia burgdorferi is controlled directly by RpoN (sigma54/sigmaN). J. Bacteriol. 189, 2139–2144 10.1128/JB.01653-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobota J. M., Imlay J. A. (2011). Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc. Natl. Acad. Sci. U.S.A. 108, 5402–5407 10.1073/pnas.1100410108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan V. B., Vaidyanathan V., Mondal A., Venkataramaiah M., Rajamohan G. (2012). Functional characterization of a novel Mn2+ dependent protein serine/threonine kinase KpnK, produced by Klebsiella pneumoniae strain MGH78578. FEBS Lett. 586, 3778–3786 10.1016/j.febslet.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Berlett B. S., Chock P. B. (1990). Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc. Natl. Acad. Sci. U.S.A. 87, 384–388 10.1073/pnas.87.1.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B., Schwan T. G., Rosa P. A. (1995). Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63, 4535–4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Lee G., Lee J. H., Kim H. Y., Rhee H. W., Park S. Y., et al. (2010). A metazoan ortholog of SpoT hydrolyzes ppGpp and functions in starvation responses. Nat. Struct. Mol. Biol. 17, 1188–1194 10.1038/nsmb.1906 [DOI] [PubMed] [Google Scholar]
- Sy J. (1977). In vitro degradation of guanosine 5'-diphosphate, 3'-diphosphate. Proc. Natl. Acad. Sci. U.S.A. 74, 5529–5533 10.1073/pnas.74.12.5529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxell B., Xu H., Yang X. F. (2012). Borrelia burgdorferi, a pathogen that lacks iron, encodes manganese-dependent superoxide dismutase essential for resistance to streptonigrin. J. Biol. Chem. 287, 19284–19293 10.1074/jbc.M112.344903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxell B., Ye M., Yang Y., Carrasco S. E., Lou Y., Yang X. F. (2013). Manganese and zinc regulate virulence determinants in Borrelia burgdorferi. Infect. Immun. 81, 2743–2752 10.1128/IAI.00507-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Dadhwal P., Cheng Z., Zianni M. R., Rikihisa Y., Liang F. T., et al. (2013). Borrelia burgdorferi oxidative stress regulator BosR directly represses lipoproteins primarily expressed in the tick during mammalian infection. Mol. Microbiol. 89, 1140–1153 10.1111/mmi.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Lutton A., Olesik J., Vali H., Li X. (2012). A novel iron- and copper-binding protein in the Lyme disease spirochaete. Mol. Microbiol. 86, 1441–1451 10.1111/mmi.12068 [DOI] [PubMed] [Google Scholar]
- Xu Q., McShan K., Liang F. T. (2008a). Essential protective role attributed to the surface lipoproteins of Borrelia burgdorferi against innate defences. Mol. Microbiol. 69, 15–29 10.1111/j.1365-2958.2008.06264.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., McShan K., Liang F. T. (2008b). Modification of Borrelia burgdorferi to overproduce OspA or VlsE alters its infectious behaviour. Microbiology 154, 3420–3429 10.1099/mic.0.2008/019737-0 [DOI] [PubMed] [Google Scholar]
- Xu Q., Seemanapalli S. V., McShan K., Liang F. T. (2006). Constitutive expression of outer surface protein C diminishes the ability of Borrelia burgdorferi to evade specific humoral immunity. Infect. Immun. 74, 5177–5184 10.1128/IAI.00713-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Goldberg M. S., Popova T. G., Schoeler G. B., Wikel S. K., Hagman K. E., et al. (2000). Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37, 1470–1479 10.1046/j.1365-2958.2000.02104.x [DOI] [PubMed] [Google Scholar]
- Yang X. F., Alani S. M., Norgard M. V. (2003). The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U.S.A. 100, 11001–11006 10.1073/pnas.1834315100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. F., Lybecker M. C., Pal U., Alani S. M., Blevins J., Revel A. T., et al. (2005). Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J. Bacteriol. 187, 4822–4829 10.1128/JB.187.14.4822-4829.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui S., Nakatani Y., Mikami M. (2003). Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol. Pharm. Bull. 26, 753–760 10.1248/bpb.26.753 [DOI] [PubMed] [Google Scholar]