Summary
The correct formation of primary cilia is central to the development and function of nearly all cells and tissues. Cilia grow from the mother centriole by extension of a microtubule core, the axoneme, which is then surrounded with a specialized ciliary membrane that is continuous with the plasma membrane. Intraflagellar transport moves particles along the length of the axoneme to direct assembly of the cilium and is also required for proper cilia function. The microtubule motor, cytoplasmic dynein-2 mediates retrograde transport along the axoneme from the tip to the base; dynein-2 is also required for some aspects of cilia formation. In most cells, the Golgi lies adjacent to the centrioles and key components of the cilia machinery localize to this organelle. Golgi-localized proteins have also been implicated in ciliogenesis and in intraflagellar transport. Here, we show that the transmembrane Golgi matrix protein giantin (GOLGB1) is required for ciliogenesis. We show that giantin is not required for the Rab11–Rabin8–Rab8 pathway that has been implicated in the early stages of ciliary membrane formation. Instead we find that suppression of giantin results in mis-localization of WDR34, the intermediate chain of dynein-2. Highly effective depletion of giantin or WDR34 leads to an inability of cells to form primary cilia. Partial depletion of giantin or of WDR34 leads to an increase in cilia length consistent with the concept that giantin acts through dynein-2. Our data implicate giantin in ciliogenesis through control of dynein-2 localization.
Key words: Golgi, Cilia, Dynein
Introduction
Primary cilia are required for many aspects of development (Drummond, 2012) and aberrant cilia formation or function underlies many disease states (Tobin and Beales, 2009). The formation and function of primary cilia are entirely dependent on a coordination of membrane and cytoskeletal dynamics. The microtubule motor protein cytoplasmic dynein-2 is one of the principal drivers of intraflagellar transport (IFT) (Ishikawa and Marshall, 2011). Dynein-2 is built around the specific dynein heavy chain DHC2, which is also called DHC1b (gene name DYNC2H1). DHC2 is widely expressed in ciliated epithelial cells and its expression level increases during ciliogenesis (Criswell et al., 1996). In Chlamydomonas, knockout of dynein-2 leads to the formation of very short flagella that fail to extend (Pazour et al., 1998; Pazour et al., 1999; Porter et al., 1999; Hou et al., 2004). In Tetrahymena, dynein-2 is not required for ciliogenesis per se but for proper ciliary length control (Asai et al., 2009; Rajagopalan et al., 2009). Knockout of dynein-2 in several other organisms, including mice, causes major defects in cilia formation (Rana et al., 2004; Huangfu and Anderson, 2005; May et al., 2005). Additional subunits co-assemble with DHC2 to produce a functional dynein-2 motor. These include the intermediate chain FAP133 (known as WDR34 in humans) (Rompolas et al., 2007; Ishikawa and Marshall, 2011), a specific light intermediate chain, D2LIC or LIC3 (Grissom et al., 2002; Perrone et al., 2003; Hou et al., 2004), and the dynein light chains LC8 (Rompolas et al., 2007) and Tctex-1 (Palmer et al., 2011). Dynein-2 has been shown to localize to the Golgi (Vaisberg et al., 1996), but is not required to maintain normal Golgi morphology (Pazour et al., 1999; Hou et al., 2004; Palmer et al., 2009).
Other components of the ciliary machinery have also been localized to the Golgi. IFT20 localizes to the Golgi, basal body and cilium (Follit et al., 2006); it is targeted there through an interaction with GMAP210 (also called TRIP11) (Follit et al., 2008). GMAP210/TRIP11 itself is not directly required for cilium formation in vitro (Yoshimura et al., 2007) or in vivo (Follit et al., 2008; Smits et al., 2010); however, recent data (published while this manuscript was in revision) have implicated the Caenorhabditis elegans ortholog of GMAP210/TRIP11 (called SQL-1) in the process of intraflagellar transport (Broekhuis et al., 2013). GMAP210/TRIP11 is a member of the golgin family of proteins that act in functional organization of the Golgi complex (Cardenas et al., 2009; Ramirez and Lowe, 2009). The structure of the Golgi complex is highly ordered and is maintained in most cells by the action of a series of Golgi matrix proteins that includes the golgins. One such golgin, giantin, is a 300 kDa tail-anchored membrane protein. Little is known about its function in cells but it appears to act in maintenance of normal Golgi structure (Nizak et al., 2003). Its large rod-like structure makes it an obvious candidate to form part of the ‘tentacular network’, which probably functions in docking of incoming vesicles from other compartments (Sinka et al., 2008).
Given the links between GMAP210/TRIP11 and ciliary function, we sought to explore how the structure and function of the early secretory pathway, including the Golgi, was linked to ciliogenesis. Using RNA interference we found that the transmembrane Golgi matrix protein giantin (GOLGB1) is required for ciliogenesis. By contrast, the functionally related golgin GM130 was not required for cilia formation. We show that giantin is required to maintain the pericentrosomal location of WDR34. Loss of either giantin or WDR34 results in a defect in ciliary length control and ultimately in ciliogenesis, probably because of defective retrograde intraflagellar transport.
Results
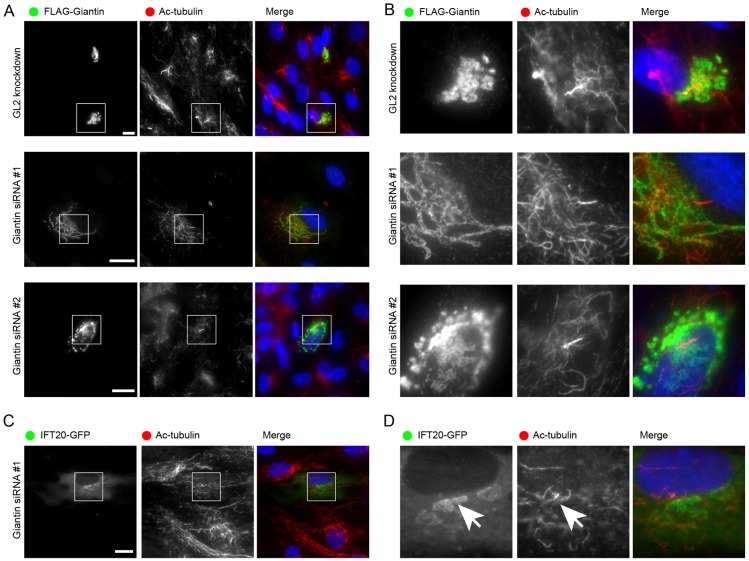
We used RNA interference to suppress expression of the transmembrane Golgi matrix protein giantin in cultured cells. We found that depletion of giantin from cells resulted in a dramatic failure of human telomerase immortalized retinal pigment epithelial (hTERT-RPE1) cells to form primary cilia upon serum starvation (Fig. 1A,B). Validation of the efficacy of suppression was demonstrated by immunoblotting (Fig. 1C) and by immunofluorescence (Fig. 1D). Note that giantin siRNA #2 was much less effective than #1. This difference was recapitulated in terms of the percentage of ciliated cells (Fig. 1E). It is important to note that in our hands, our most effective siRNA targeting giantin was as effective as one against the Golgi-localized IFT component IFT20, which is itself known to be required for ciliogenesis (Fig. 1E). Validation of IFT20 suppression is shown in Fig. 1F. The high variability of results using siRNA #2 led us to test further siRNAs to validate this finding. Fig. 1G shows immunoblots showing efficacy of two further siRNA duplexes targeting giantin (#2 and #4) and the effects on cilia formation are shown in Fig. 1H. In addition, we validated the specificity of the giantin depletion phenotype by simultaneous suppression and re-introduction of a siRNA-resistant cDNA (Fig. 2A; enlarged in Fig. 2B). Here, transient expression of FLAG-giantin only resulted in robust Golgi localization in ∼5% of transfected cells. However, in all cells with rim-like Golgi labelling of FLAG-giantin, we observed the formation of primary cilia in response to serum starvation. These data show that giantin is required for ciliogenesis. By contrast, expression of IFT20-GFP was not able to rescue the ciliogenesis defect in giantin-depleted cells (Fig. 2C; enlarged in Fig. 2D). Immunoblots showing overexpression of IFT20-GFP as well as siRNA-mediated knockdown of IFT20-GFP using an anti-GFP antibody are shown in Fig. 1F.
Fig. 1.
Giantin is required for ciliogenesis. (A,B) Cells were transfected with either control (A, GL2) or giantin (B, Giantin siRNA #1) siRNAs to suppress expression of giantin and serum starved for 24 hours. Cells were immunolabelled for giantin and acetylated tubulin and images acquired at the same settings for each slide. Boxed regions are enlarged in the lower panels; the lower box in the Gi #1 suppression panels shows a contrast enhanced version to highlight the remaining giantin. (C) Immunoblots showing effective suppression of giantin and lamin A/C as indicated. α-tubulin serves as a loading control. (D) Immunofluorescence showing effective suppression of giantin using siRNA #1 and #2. (E) Quantification of the percentage of ciliated cells following depletion of giantin or IFT20. (F) Immunoblotting with an anti-GFP antibody shows overexpression of IFT20-GFP and its effective suppression by siRNA. (G) Validation of suppression of giantin using siRNA transfection with additional independent siRNAs. (H) All four giantin siRNA duplexes result in statistically detectable reduction in ciliogenesis (n = 3 independent labelling experiments on the same population of labelled cells; *P<0.01 using ANOVA with Dunnett's post-hoc test). Note that results in panels C–E come from the same set of experiments. Results in E and F are from a distinct set of three independent experiments. Giantin siRNA #2 gives a more variable level of suppression yet on average still results in a decrease in ciliogenesis. Error bars represent s.d. Scale bars: 10 µm.
Fig. 2.
Expression of FLAG-giantin but not IFT20-GFP rescues the ability of giantin-depleted cells to form primary cilia. (A) The defect in ciliogenesis (acetylated tubulin in red) is rescued by expression of FLAG-giantin (green). (B) Enlargements from the boxed regions in A. Note the clearly defined rim-like labelling of the Golgi with FLAG-giantin and the presence of primary cilia in these cells. (C,D) IFT20-GFP expression does not rescue the ability of giantin-depleted cells to form primary cilia. 50 cells were examined that showed localization of IFT20-GFP to the Golgi and none contained a primary cilium. Arrow indicates Golgi-localized IFT20-GFP (left) and centrosomal acetylated tubulin (right). Scale bars: 10 µm.
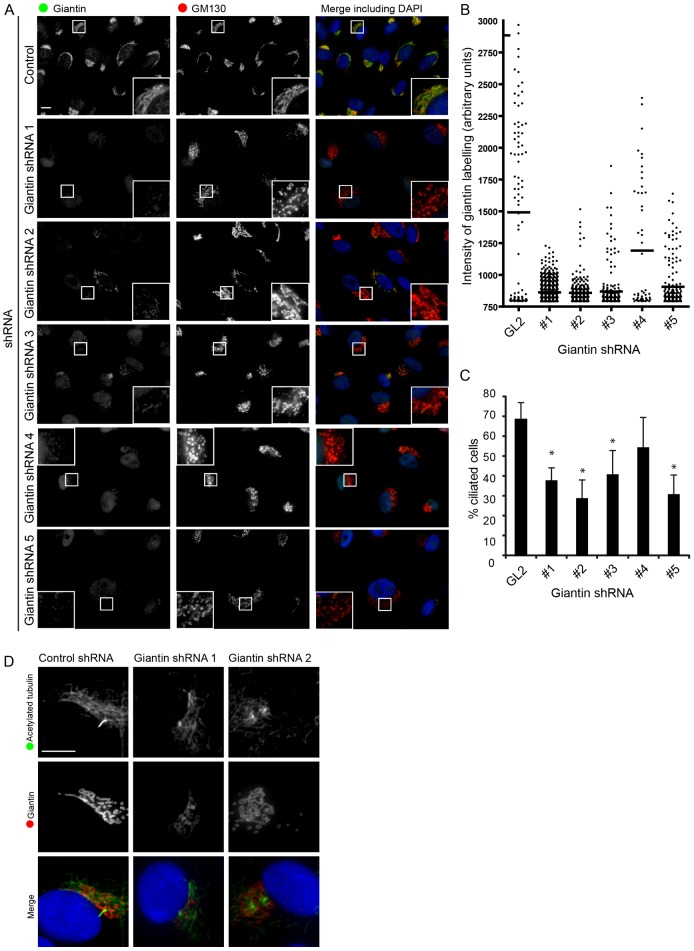
We further validated this role for giantin in ciliogenesis with five different shRNAs (Fig. 3). Effective suppression was demonstrated by immunofluorescence (Fig. 3A; quantified in Fig. 3B). These data also demonstrate that targeting of GM130 to the Golgi was not affected by giantin depletion (Fig. 3A). Four of five shRNAs caused a significant reduction in giantin expression and a concomitant inhibition of ciliogenesis. It is important to note that these data come from scoring ciliogenesis across a whole experiment. On a cell-by-cell basis, this correlation between efficacy of knockdown and failure of cells to generate cilia was much higher. For example, Fig. 3D shows cells with a robust reduction in giantin expression that failed to extend an axoneme. We also repeated these experiments in pig kidney epithelial cells (LLC-PK1), which generate primary cilia on reaching confluence and do not require serum starvation (supplementary material Fig. S1). Here, four siRNA duplexes targeting Sus scrofa giantin were all effective, as determined by immunoblotting and as shown for two of these, result in a strong inhibition of ciliogenesis (supplementary material Fig. S1C).
Fig. 3.
Stable suppression of giantin in hTERT-RPE1 cells using short hairpin RNAs. (A) Immunofluorescence shows loss of giantin (green) whereas GM130 (red) remains localized to the enlarged and more dispersed Golgi. (B) Quantification of loss of giantin in stably depleted cells. All five shRNAs show a statistically detectable reduction in giantin labelling. (C) Stable suppression of giantin expression results in defects in ciliogenesis (examples are shown only from shRNAs #1 and #2). There is no statistically detectable difference between shRNA #4 and control. Error bars represent s.d. (D) Cells showing the most effective depletion of giantin fail to generate primary cilia in response to serum withdrawal. Scale bars: 10 µm.
To determine whether the role for giantin in ciliogenesis was specific, we depleted cells of GM130 (supplementary material Fig. S2), another golgin that acts in combination with giantin in docking and fusion of transport vesicles at the early Golgi (Sönnichsen et al., 1998; Seemann et al., 2000; Alvarez et al., 2001). Supplementary material Fig. S2A,B shows that depletion of GM130 using two independent siRNA duplexes was effective. GM130 depletion did not affect expression or the Golgi localization of giantin. Golgi structure was perturbed, as shown by GalT and giantin labelling (supplementary material Fig. S2B,C), but ciliogenesis was not affected (supplementary material Fig. S2D, quantified in Fig. S3E). Cilia length was also unaffected (supplementary material Fig. S2F).
Previous work has characterized a Rab11–Rabin8–Rab8 pathway that acts at these earliest stages of ciliogenesis (Westlake et al., 2011). We sought to determine whether giantin was involved upstream of this endosomal network. Fig. 4A shows that giantin depletion affected the early phase of ciliogenesis detectable following only 1–2 hours of serum withdrawal. Fig. 4B,C shows that despite a robust effect on ciliogenesis, depletion of giantin did not affect the accumulation of Rabin8 around the centrosome. Rab11A and Rab11B were depleted as a positive control and, as expected, effectively inhibited the centrosomal localization of Rabin8. Cells in this experiment showed lower levels of cilia formation because these cells, which stably express GFP-Rabin8, were grown at 50–70% confluence and starved for only 1 hour to visualize the earliest stages of cilia formation.
Fig. 4.
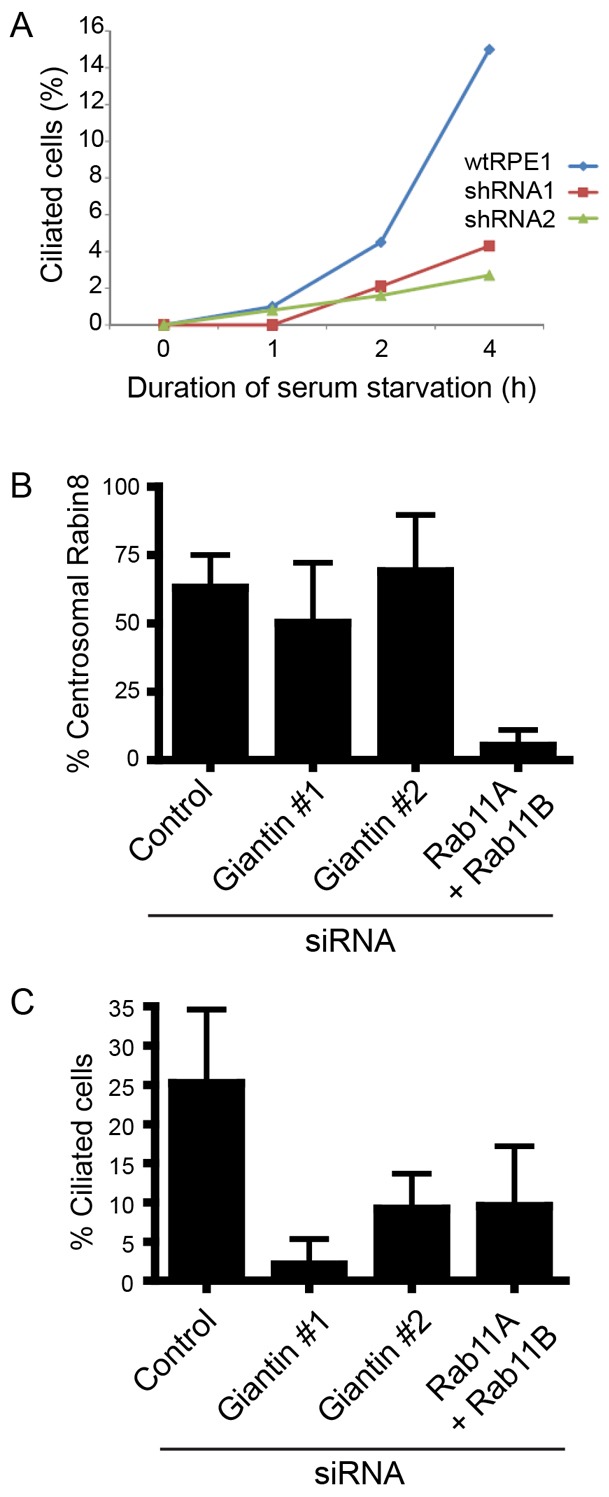
Giantin is required during the early stages of ciliogenesis independently of the Rab11–Rabin8–Rab8 pathway. (A) Wild-type or giantin depleted cells were serum starved and the emergence of the cilium measured using acetylated tubulin. (B) Depletion of giantin, unlike that of Rab11A and Rab11B, does not disrupt the centrosomal accumulation of Rabin8. (C) Validation of inhibition of ciliogenesis in those cells evaluated in B. Error bars represent s.d.
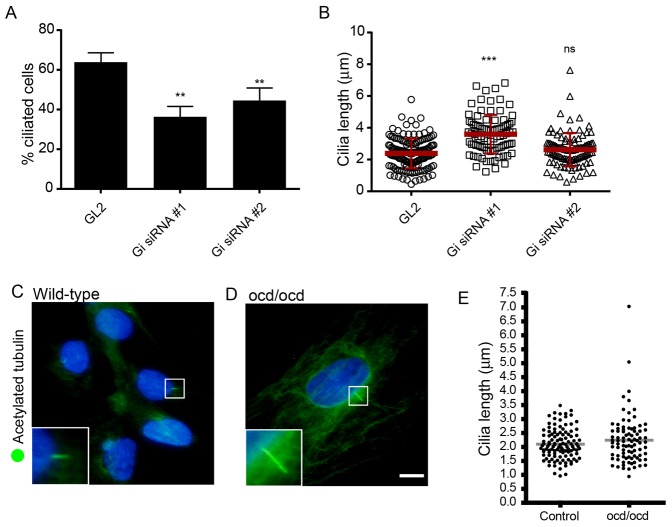
Overall, in our hands, depletion of giantin caused a reproducible loss of cilia, but typically 30–40% of cells remained ciliated. In this population, notably those transfected with siRNA duplex #1, we consistently noted an elongation of remaining cilia. Quantification (Fig. 5A,B) shows that depletion of giantin with siRNA #1 resulted in a statistically detectable elongation of cilia compared with controls. Fig. 5A shows the proportion of ciliated cells from the same data set used for Fig. 5B. This was not detected in cells transfected with siRNA #2 which was less effective (Fig. 1), but is consistent with a partial depletion of giantin, resulting in elongated cilia. A whole-animal knockout for giantin exists in the form of the ocd/ocd rat. This rat has an insertional mutation in the GOLGB1 (giantin) gene (Katayama et al., 2011) that results in premature termination and loss of the majority of the protein coding region including the C-terminal membrane anchor sequence. The ocd/ocd rat has severe skeletal abnormalities associated with osteochondrodysplasia (Suzuki et al., 1988; Katayama et al., 2011) as well as severe kidney abnormalities (Kikukawa et al., 1989), systemic oedema, and gross defects in extracellular matrix secretion and assembly (Kikukawa et al., 1990; Kikukawa et al., 1991a; Kikukawa et al., 1991b; Kikukawa and Suzuki, 1992; Katayama et al., 2011). Fig. 5C,D shows that fibroblasts from ocd/ocd rats generated cilia following serum starvation to the same degree as controls (29% ciliated cells). Cilia >3.5 µm are only seen in ocd/ocd cells and these show an increase in mean cilia length (2.24±0.86 µm) compared with controls (2.10±0.54 µm); however, there was no statistically detectable difference overall (Fig. 3E). Although these data suggest that giantin might not be essential for ciliogenesis in vivo, they are consistent with a role in the control of ciliary length.
Fig. 5.
Giantin is required to maintain cilia length but is not required for ciliogenesis in vivo. (A) Giantin depletion results in a partial reduction in the number of ciliated cells. Error bars represent s.d. **P<0.005. (B) The length of the remaining cilia from those experiments in A was measured. Note the statistically significant increase in cilia length with giantin siRNA #1. ***P<0.001. (C) Fibroblasts from wild-type rats or (D) ocd/ocd rats were serum starved for 24 hours and immunolabelled to detect acetylated tubulin. (E) Quantification of cilia length in wild-type versus ocd/ocd fibroblasts. No statistical difference was detected using a Mann–Whitney test but cilia >3.5 µm are only seen in ocd/ocd rat cells. Scale bar: 10 µm.
In our search for a mechanistic explanation for the role of giantin in cilia function, we examined its role in a number of processes at the Golgi and centrosome (supplementary material Fig. S2). As seen following shRNA depletion of giantin (Fig. 3A), siRNA-mediated suppression of giantin did not affect localization of GM130 (supplementary material Fig. S3A). Similarly, expression and localization of GMAP210/TRIP11 (supplementary material Fig. S3B) or galactosyltransferase T (not shown) to the Golgi was unaffected. Giantin was also not required for localization of ODF2 to the mother centriole (supplementary material Fig. S3C), its depletion did not affect steady-state actin organization (supplementary material Fig. S3D) or polarization of the centrosome and Golgi following scratch wounding (supplementary material Fig. S3E).
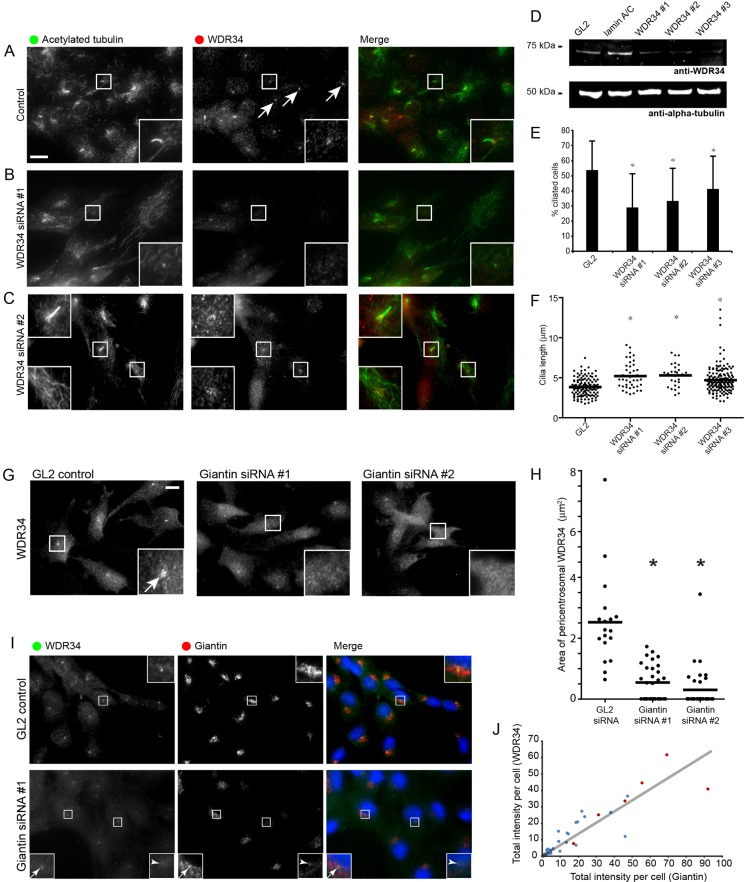
Obvious candidates for control of cilia length are the microtubule-based motors that act in intraflagellar transport. Indeed, our previous work has shown that siRNA depletion of dynein-2 components results in an increase in cilia length (Palmer et al., 2011), similar to that seen here (Fig. 5B). Therefore, we examined the localization of WDR34, the intermediate chain subunit of dynein-2. Consistent with the known role for dynein-2 in other systems, WDR34 localizes at steady state to pericentriolar material (Fig. 6A, arrows). We then depleted cells of WDR34 using siRNA transfection. Depletion of WDR34 was verified by immunofluorescence (Fig. 6A–C) and immunoblotting (Fig. 6D). As we observed following giantin depletion, the ciliogenesis defect in WDR34-depleted cells was not complete (Fig. 6E), but was very similar to depletion of giantin, in that remaining cilia were significantly longer than in control cells (Fig. 6F). As shown in Fig. 6C, the level of depletion of WDR34 correlated with the ability of cells to form primary cilia (compare insets). We then found that giantin was required to maintain the localization of the dynein-2 intermediate chain subunit WDR34 to the pericentrosomal region (Fig. 6G, quantified in H). We noted here that the level of giantin suppression correlated well with pericentrosomal localization of WDR34 (compare the two insets in the lower panels of Fig. 6I). Quantification of these data confirmed this correlation (Fig. 6J). Despite extensive efforts, we have not been able to detect any interaction between giantin and WDR34 by co-immunoprecipitation.
Fig. 6.
WDR34 is required for ciliogenesis. Cells were transfected with siRNA duplexes to suppress WDR34. (A–C) Silencing of WDR34 (red) causes a defect in ciliogenesis (acetylated tubulin labelling, green). Boxed regions show enlargements. Note that in C, the lower inset illustrates that a robust loss of WDR34 correlates with a failure to form cilia. The upper box shows that those cells that are less effectively suppressed still retain the ability to form primary cilia and that these remaining cilia are longer than controls (compare with A). (D) Immunoblotting of WDR34-depleted cells and lamin-A/C-depleted control, α-tubulin is used as a loading control. (E) Quantification of the ciliogenesis defect on WDR34 suppression. *P<0.01 using ANOVA with Dunnett's post-hoc test. Error bars represent s.d. (F) As in C, remaining cilia in WDR34-depleted cells were significantly longer than in control cells. (G) The typical pericentrosomal accumulation of WDR34 seen in control cells (arrows) is lost on suppression of giantin. (H) Quantification of loss of pericentrosomal WDR34 in giantin-depleted cells. *P<0.01 from ANOVA with Dunnett's post-hoc test comparing samples to the GL2 control. (I) Images show that the level of giantin remaining in cells correlates well with the pericentrosomal accumulation of WDR34. Insets to the lower panels illustrate how very low effective suppression of giantin results in a near complete loss of detectable WDR34 (arrowhead), whereas a less-effective suppression of giantin correlates with some faint but detectable pericentrosomal WDR34 labelling (arrow). (J) Quantification shows that the level of expression of giantin correlates well with the pericentrosomal accumulation of WDR34 (red dots indicate control; blue dots, giantin-depleted cells). The correlation (grey line) is significant (Spearman's correlation, P = 0.016; Pearson's correlation P = 0.036). Scale bars: 10 µm.
Discussion
Our findings are consistent with known functions of dynein-2 in the formation of primary cilia and in controlling axoneme length through IFT (Criswell et al., 1996; Pazour et al., 1999; Porter et al., 1999; Signor et al., 1999; Grissom et al., 2002). Length control in primary cilia has been linked to several developmental disorders, for example mutations in MKS3 (Tammachote et al., 2009) or NEK8 (Sohara et al., 2008) lead to cilium elongation and polycystic kidney disease. Severe ciliopathy phenotypes without overt morphological defects in cilia have been seen in both animal models [including deletion of IFT-B component IFT80 (Rix et al., 2011)] and patient groups [such as those with mutations in DYNC2H1 (Schmidts et al., 2013)].
GMAP210/TRIP11 is required to maintain the localization of IFT20 to the Golgi (Follit et al., 2008), thereby implicating GMAP210/TRIP11 in ciliogenesis. More recent data have implicated GMAP210/TRIP11 in the control of cilia length, perhaps through stabilization of IFT complexes (Broekhuis et al., 2013). A second mouse model has been characterized with severe skeletal defects resulting (at least in part) from an intracellular accumulation of perlecan (Smits et al., 2010). Significantly, this work also showed that achondrogenesis type 1A in humans was also caused by mutation of GMAP210/TRIP11. The key difference here is that although animal models with defects in either giantin or GMAP210/TRIP11 show skeletal defects, only giantin appears to be required for ciliogenesis in vitro (Yoshimura et al., 2007). GMAP210/TRIP11 is also not required for localization of giantin to the Golgi (Follit et al., 2008). Further work will be essential to dissect the contributions of giantin to extracellular matrix deposition, skeletogenesis and ciliary trafficking: processes that are known to be linked (Huber and Cormier-Daire, 2012).
In our experiments, robust knockdown of giantin leads to a failure to produce cilia (at least as defined by immunofluorescence). Incomplete suppression of giantin, or indeed of WDR34, leads to elongated cilia, suggesting a failure of length control. Parallels can be drawn here to animal knockouts of dynein-2 subunits. In mice, knockout of dynein-2 light intermediate chain results in both loss of cilia and defects in those that remain (Rana et al., 2004). Knockout of DHC2 leads to deformed cilia rather than a defect in their formation per se (Huangfu and Anderson, 2005; May et al., 2005). These data suggest complex roles for dynein-2 (and by inference giantin) in ciliogenesis that could differ depending on species, or tissue context. It is therefore perhaps less surprising that we do not see a gross ciliogenesis defect in the ocd/ocd rat cells.
Giantin depletion causes a moderate dispersion of Golgi membranes concomitant with the defect in ciliogenesis. Other perturbations that disrupt Golgi structure do not result in ciliogenesis defects (such as depletion of dynein-1 subunits (see Palmer et al., 2009; Palmer et al., 2011) and so we do not infer that Golgi structure is linked to ciliogenesis per se. Others have suggested that the connection between the Golgi and centrosome is required for ciliogenesis (Hurtado et al., 2011) but in this case, disruption of Golgi structure through expression of AKAP450 constructs does not always correlate with a defect in ciliogenesis. Indeed, suppression of giantin does not significantly disrupt the connection between Golgi and centrosome. Nor is giantin required for cell polarization in response to scratch wounding, which is also linked to Golgi–centrosome coupling (Hurtado et al., 2011). Furthermore, we show here that the disruption in Golgi structure caused by depletion of GM130 has no effect on the ability of cells to form or control the length of primary cilia.
Controlling dynein-2 stability and/or localization at the Golgi complex could provide a mechanism to link axoneme initiation to membrane dynamics at the Golgi. Alternatively, giantin could simply serve as a platform for assembly of dynein-2 or of IFT particles prior to delivery to the cilium. As an alternative to this more passive role it remains possible that giantin, as both a transmembrane component of the Golgi and also one that localizes to the cis and medial cisterna, could link ‘early’ membranes of the Golgi to ciliogenesis through selective delivery of cargo. Other data have suggested such specific transport routes for at least some cargo to the cilium including polycystin-2 (Hoffmeister et al., 2011). Whether giantin plays a direct role in vectorial membrane trafficking to the newly forming cilium, tethers membranes to centrioles via dynein-2, or acts in further as yet undefined ways remains to be defined.
Materials and Methods
All reagents were purchased from Sigma-Aldrich (Poole, UK), unless otherwise stated. Antibodies used were rabbit polyclonal anti-giantin (PRB-114C), mouse monoclonal anti-GFP (MMS-118P) and rabbit polyclonal anti-pericentrin (PRB-432C) (all from Covance, CA); mouse monoclonal anti-giantin (ab37266, Abcam, Cambridge, UK); mouse monoclonal anti-GM130 (610822; BD Biosciences, Oxford, UK); rabbit polyclonal anti-lamin A/C (2032; Cell Signaling Technologies, Hitchin, UK) and mouse monoclonal anti-α-tubulin (MS-581-PCL; Neomarkers, Fremont, CA). Rabbit polyclonal anti-FLAG (F7425), rabbit polyclonal anti-ODF2 (HPA001874), mouse monoclonal anti-acetylated tubulin and rabbit polyclonal anti-WDR34 (HPA040764, used for immunofluorescence) were purchased from Sigma. Rabbit polyclonal anti-WDR34 (NBP1-88805, used for immunoblotting) was obtained from Novus Biologicals. Cy2-conjugated donkey anti-mouse (715-225-151) and Cy3-conjugated donkey anti-rabbit (711-165-152) were from Jackson ImmunoResearch (Baltimore, MD). Peroxidase-conjugated donkey anti-mouse IgG (715-035-150) and peroxidase-conjugated donkey anti-rabbit IgG (711-035-150) were supplied by Jackson ImmunoResearch Laboratories Inc. IRDye 800CW donkey anti-mouse (926-32212), IRDye 680 donkey anti-rabbit (926-32223) were from Li-Cor (Cambridge, UK). Alexa-Fluor-568-phalloidin was from Molecular Probes (Paisley, UK). Transfections, immunoblotting, immunofluorescence and cell imaging were performed as described previously (Townley et al., 2008). Immunblots in Fig. 1 were developed using ECL (GE Healthcare, Cardiff, UK) and those in Fig. 4 using an Odyssey Sa imager (Li-Cor, Cambridge, UK).
RNAi-mediated gene suppression
siRNA duplexes were purchased from MWG-Eurofins (Ebersberg, Germany) or Sigma-Aldrich (Poole, UK). Targeted depletion of genes of interest was performed with the following oligonucleotide duplexes. Giantin was depleted in RPE1 cells with giantin siRNA #1 (ACUUCAUGCGAAGGCCAAATT), Giantin siRNA #2 (AGAGAGGCUUAUGAAUCAATT), Giantin siRNA #3 (GGAAGAGAGAAAAGCUGCU (Kim et al., 2010) and Giantin siRNA #4 (ACUUCAUGCGAAGGCCAAAUU (Nizak et al., 2003). Giantin was depleted from pig kidney epithelial cells (LLC-PK1) using the following siRNA duplexes: Ss-GolgB1-a (GAAGCUACAGGAAGUAUUA); Ss-GolgB1-b (AGAGAGGCUUAUGAAUCAA); Ss-GolgB1-c (GUUCAGUGAUGCUAUUCAA); and Ss-GolgB1 d (UCACAUGUGUACCGAGGUA). GMAP210/TRIP11 was targeted with GCCAGAGACAAUCUAGCAC and IFT20 with GGAAGAGUGCAAAGACUUU (Yoshimura et al., 2007). GM130 was depleted using siRNA duplexes #1 (AAGUUAGAGAUGACGGAACUC) and #2 (AUGAGAACAUGGAGAUCACC). Duplexes for suppressing WDR34 were (#1) SASI_Hs01_00038845 GAUGGUGUCUUGUCUGUAU, (#2) SASI_Hs02_00361525 GCUGUUUGAUCUCCAGAAA and (#3) UCCGAGAGCUGAACAAGAA. GL2 (CGUACGCGGAAUACUUCGAUU) and lamin A/C (CUGGACUUCCAGAAGAACA) were used as negative and positive controls, respectively. Giantin was stably suppressed in RPE-1 cells using lentiviral particles (Mission shRNA) from Sigma-Aldrich with clone identifiers of (TRCN0000) 146702, 147571, 147752, 147837 and 147838. For siRNA, cells were transfected using a modified calcium phosphate protocol (Watson and Stephens, 2006). For ciliogenesis experiments, cells were serum starved for the last 24 hours of this 72 hour period.
Ciliogenesis assays
Confluent hTERT-RPE1 cells were washed twice with PBS to remove serum and incubated at 37°C with 5% CO2 for 24–48 hours in serum-free medium to induce cell cycle exit and subsequent cilium assembly. LLC-PK1 cells were grown to confluence and then cultured a further 24–48 hours without serum starvation to induce ciliogenesis. Cells were fixed and typically labelled with anti-acetylated tubulin to mark primary cilia. Cilia lengths were measured using ImageJ.
Rabin8 trafficking
RPE cells stably expressing GFP-Rabin8 (Westlake et al., 2011) were starved for 1 hour followed by live epifluorescence imaging using a 63× oil objective. GFP-Rabin8 accumulation was quantified from time-lapse image series (1 frame/second for 5 seconds) to help visualize vesicular pools of Rabin8 accumulating at the pericentriolar region of cells. Data are representative of three separate experiments. Error bars indicate s.d. Statistical analysis was by one-way ANOVA with Bonferroni's post-hoc test.
Supplementary Material
Acknowledgments
We would like to thank Greg Pazour (IFT20-GFP) and Martin Lowe (FLAG-giantin) for plasmids. We are grateful to the members of the Bristol Cell Biology Laboratories, as well as Franck Perez, Cheryll Tickle, Fiona Bangs, and Helen Dawe for helpful comments and ideas through this project.
Footnotes
Author contributions
D.J.S. designed the study, performed experiments, analyzed the data and wrote the manuscript. D.A., L.M.M., A.K.T. and K.J.P. performed experiments and analyzed the data; K.K. and H.S. designed, performed, and analyzed experiments using the ocd/ocd rat; M.A.W. and C.J.W. designed, performed, and analyzed experiments on Rab11 and Rabin8.
Funding
This work was funded by the UK Medical Research Council [grant numbers G0801848 and J000604/1] and a doctoral training studentship (to D.J.S.). M.A.W. and C.J.W. are funded by the U.S. National Institutes of Health. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.131664/-/DC1
References
- Alvarez C., Garcia-Mata R., Hauri H. P., Sztul E. (2001). The p115-interactive proteins GM130 and giantin participate in endoplasmic reticulum-Golgi traffic. J. Biol. Chem. 276, 2693–2700 10.1074/jbc.M007957200 [DOI] [PubMed] [Google Scholar]
- Asai D. J., Rajagopalan V., Wilkes D. E. (2009). Dynein-2 and ciliogenesis in Tetrahymena. Cell Motil. Cytoskeleton 66, 673–677 10.1002/cm.20397 [DOI] [PubMed] [Google Scholar]
- Broekhuis J. R., Rademakers S., Burghoorn J., Jansen G. (2013). SQL-1, homologue of the Golgi protein GMAP210, modulates intraflagellar transport in C. elegans. J. Cell Sci. 126, 1785–1795 10.1242/jcs.116640 [DOI] [PubMed] [Google Scholar]
- Cardenas J., Rivero S., Goud B., Bornens M., Rios R. M. (2009). Golgi localisation of GMAP210 requires two distinct cis-membrane binding mechanisms. BMC Biol. 7, 56 10.1186/1741-7007-7-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell P. S., Ostrowski L. E., Asai D. J. (1996). A novel cytoplasmic dynein heavy chain: expression of DHC1b in mammalian ciliated epithelial cells. J. Cell Sci. 109, 1891–1898 [DOI] [PubMed] [Google Scholar]
- Drummond I. A. (2012). Cilia functions in development. Curr. Opin. Cell Biol. 24, 24–30 10.1016/j.ceb.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit J. A., Tuft R. A., Fogarty K. E., Pazour G. J. (2006). The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell 17, 3781–3792 10.1091/mbc.E06-02-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit J. A., San Agustin J. T., Xu F., Jonassen J. A., Samtani R., Lo C. W., Pazour G. J. (2008). The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet. 4, e1000315 10.1371/journal.pgen.1000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom P. M., Vaisberg E. A., McIntosh J. R. (2002). Identification of a novel light intermediate chain (D2LIC) for mammalian cytoplasmic dynein 2. Mol. Biol. Cell 13, 817–829 10.1091/mbc.01-08-0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister H., Babinger K., Gürster S., Cedzich A., Meese C., Schadendorf K., Osten L., de Vries U., Rascle A., Witzgall R. (2011). Polycystin-2 takes different routes to the somatic and ciliary plasma membrane. J. Cell Biol. 192, 631–645 10.1083/jcb.201007050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Pazour G. J., Witman G. B. (2004). A dynein light intermediate chain, D1bLIC, is required for retrograde intraflagellar transport. Mol. Biol. Cell 15, 4382–4394 10.1091/mbc.E04-05-0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D., Anderson K. V. (2005). Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA 102, 11325–11330 10.1073/pnas.0505328102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber C., Cormier-Daire V. (2012). Ciliary disorder of the skeleton. Am. J. Med. Genet. 160C, 165–174 10.1002/ajmg.c.31336 [DOI] [PubMed] [Google Scholar]
- Hurtado L., Caballero C., Gavilan M. P., Cardenas J., Bornens M., Rios R. M. (2011). Disconnecting the Golgi ribbon from the centrosome prevents directional cell migration and ciliogenesis. J. Cell Biol. 193, 917–933 10.1083/jcb.201011014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Marshall W. F. (2011). Ciliogenesis: building the cell's antenna. Nat. Rev. Mol. Cell Biol. 12, 222–234 10.1038/nrm3085 [DOI] [PubMed] [Google Scholar]
- Katayama K., Sasaki T., Goto S., Ogasawara K., Maru H., Suzuki K., Suzuki H. (2011). Insertional mutation in the Golgb1 gene is associated with osteochondrodysplasia and systemic edema in the OCD rat. Bone 49, 1027–1036 10.1016/j.bone.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Kikukawa K., Suzuki K. (1992). Histochemical and immunohistochemical distribution of glycosaminoglycans, type II collagen, and fibronectin in developing fetal cartilage of congenital osteochondrodysplasia rat (ocd/ocd). Teratology 46, 509–523 10.1002/tera.1420460515 [DOI] [PubMed] [Google Scholar]
- Kikukawa K., Kamei T., Suzuki K. (1989). Gross abnormalities of kidney in congenital osteochondrodysplasia rat (ocd/ocd). Nippon Juigaku Zasshi 51, 1029–1031 10.1292/jvms1939.51.1029 [DOI] [PubMed] [Google Scholar]
- Kikukawa K., Kamei T., Suzuki K., Maita K. (1990). Electron microscopic observations and electrophoresis of the glycosaminoglycans in the epiphyseal cartilage of the congenital osteochondrodysplasia rat (ocd/ocd). Matrix 10, 378–387 10.1016/S0934-8832(11)80145-9 [DOI] [PubMed] [Google Scholar]
- Kikukawa K., Kamei T., Suzuki K. (1991a). Chromatographic analysis of glycosaminoglycans in epiphyseal cartilage of congenital osteochondrodysplasia (ocd/ocd) rat. J. Vet. Med. Sci. 53, 1091–1092 [DOI] [PubMed] [Google Scholar]
- Kikukawa K., Kamei T., Suzuki K. (1991b). A histological and histochemical study on glycosaminoglycans in epiphysial cartilage of osteochondrodysplasia rat (OCD/OCD). Connect. Tissue Res. 25, 301–309 10.3109/03008209109029165 [DOI] [PubMed] [Google Scholar]
- Kim J., Lee J. E., Heynen-Genel S., Suyama E., Ono K., Lee K., Ideker T., Aza-Blanc P., Gleeson J. G. (2010). Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464, 1048–1051 10.1038/nature08895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May S. R., Ashique A. M., Karlen M., Wang B., Shen Y., Zarbalis K., Reiter J., Ericson J., Peterson A. S. (2005). Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev. Biol. 287, 378–389 10.1016/j.ydbio.2005.08.050 [DOI] [PubMed] [Google Scholar]
- Nizak C., Martin-Lluesma S., Moutel S., Roux A., Kreis T. E., Goud B., Perez F. (2003). Recombinant antibodies against subcellular fractions used to track endogenous Golgi protein dynamics in vivo. Traffic 4, 739–753 10.1034/j.1600-0854.2003.00132.x [DOI] [PubMed] [Google Scholar]
- Palmer K. J., Hughes H., Stephens D. J. (2009). Specificity of cytoplasmic dynein subunits in discrete membrane-trafficking steps. Mol. Biol. Cell 20, 2885–2899 10.1091/mbc.E08-12-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer K. J., Maccarthy-Morrogh L., Smyllie N., Stephens D. J. (2011). A role for Tctex-1 (DYNLT1) in controlling primary cilium length. Eur. J. Cell Biol. 90, 865–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., Wilkerson C. G., Witman G. B. (1998). A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT). J. Cell Biol. 141, 979–992 10.1083/jcb.141.4.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., Dickert B. L., Witman G. B. (1999). The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 144, 473–481 10.1083/jcb.144.3.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone C. A., Tritschler D., Taulman P., Bower R., Yoder B. K., Porter M. E. (2003). A novel dynein light intermediate chain colocalizes with the retrograde motor for intraflagellar transport at sites of axoneme assembly in chlamydomonas and Mammalian cells. Mol. Biol. Cell 14, 2041–2056 10.1091/mbc.E02-10-0682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M. E., Bower R., Knott J. A., Byrd P., Dentler W. (1999). Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol. Biol. Cell 10, 693–712 10.1091/mbc.10.3.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan V., Subramanian A., Wilkes D. E., Pennock D. G., Asai D. J. (2009). Dynein-2 affects the regulation of ciliary length but is not required for ciliogenesis in Tetrahymena thermophila. Mol. Biol. Cell 20, 708–720 10.1091/mbc.E08-07-0746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez I. B., Lowe M. (2009). Golgins and GRASPs: holding the Golgi together. Semin. Cell Dev. Biol. 20, 770–779 10.1016/j.semcdb.2009.03.011 [DOI] [PubMed] [Google Scholar]
- Rana A. A., Barbera J. P., Rodriguez T. A., Lynch D., Hirst E., Smith J. C., Beddington R. S. (2004). Targeted deletion of the novel cytoplasmic dynein mD2LIC disrupts the embryonic organiser, formation of the body axes and specification of ventral cell fates. Development 131, 4999–5007 10.1242/dev.01389 [DOI] [PubMed] [Google Scholar]
- Rix S., Calmont A., Scambler P. J., Beales P. L. (2011). An Ift80 mouse model of short rib polydactyly syndromes shows defects in hedgehog signalling without loss or malformation of cilia. Hum. Mol. Genet. 20, 1306–1314 10.1093/hmg/ddr013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P., Pedersen L. B., Patel-King R. S., King S. M. (2007). Chlamydomonas FAP133 is a dynein intermediate chain associated with the retrograde intraflagellar transport motor. J. Cell Sci. 120, 3653–3665 10.1242/jcs.012773 [DOI] [PubMed] [Google Scholar]
- Schmidts M., Arts H. H., Bongers E. M., Yap Z., Oud M. M., Antony D., Duijkers L., Emes R. D., Stalker J., Yntema J. B. et al. UK10K(2013). Exome sequencing identifies DYNC2H1 mutations as a common cause of asphyxiating thoracic dystrophy (Jeune syndrome) without major polydactyly, renal or retinal involvement. J. Med. Genet. 50, 309–323 10.1136/jmedgenet-2012-101284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J., Jokitalo E. J., Warren G. (2000). The role of the tethering proteins p115 and GM130 in transport through the Golgi apparatus in vivo. Mol. Biol. Cell 11, 635–645 10.1091/mbc.11.2.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signor D., Wedaman K. P., Orozco J. T., Dwyer N. D., Bargmann C. I., Rose L. S., Scholey J. M. (1999). Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J. Cell Biol. 147, 519–530 10.1083/jcb.147.3.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka R., Gillingham A. K., Kondylis V., Munro S. (2008). Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins. J. Cell Biol. 183, 607–615 10.1083/jcb.200808018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P., Bolton A. D., Funari V., Hong M., Boyden E. D., Lu L., Manning D. K., Dwyer N. D., Moran J. L., Prysak M. et al. (2010). Lethal skeletal dysplasia in mice and humans lacking the golgin GMAP-210. N. Engl. J. Med. 362, 206–216 10.1056/NEJMoa0900158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohara E., Luo Y., Zhang J., Manning D. K., Beier D. R., Zhou J. (2008). Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J. Am. Soc. Nephrol. 19, 469–476 10.1681/ASN.2006090985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen B., Lowe M., Levine T., Jämsä E., Dirac-Svejstrup B., Warren G. (1998). A role for giantin in docking COPI vesicles to Golgi membranes. J. Cell Biol. 140, 1013–1021 10.1083/jcb.140.5.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kikukawa K., Hakamata Y., Kamei T., Imamichi T. (1988). Congenital osteochondrodysplasia with systemic subcutaneous edema (ocd/ocd): a new lethal autosomal recessive mutant of the rat. J. Hered. 79, 48–50 [DOI] [PubMed] [Google Scholar]
- Tammachote R., Hommerding C. J., Sinders R. M., Miller C. A., Czarnecki P. G., Leightner A. C., Salisbury J. L., Ward C. J., Torres V. E., Gattone V. H., 2nd et al. (2009). Ciliary and centrosomal defects associated with mutation and depletion of the Meckel syndrome genes MKS1 and MKS3. Hum. Mol. Genet. 18, 3311–3323 10.1093/hmg/ddp272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin J. L., Beales P. L. (2009). The nonmotile ciliopathies. Genet. Med. 11, 386–402 10.1097/GIM.0b013e3181a02882 [DOI] [PubMed] [Google Scholar]
- Townley A. K., Feng Y., Schmidt K., Carter D. A., Porter R., Verkade P., Stephens D. J. (2008). Efficient coupling of Sec23-Sec24 to Sec13-Sec31 drives COPII-dependent collagen secretion and is essential for normal craniofacial development. J. Cell Sci. 121, 3025–3034 10.1242/jcs.031070 [DOI] [PubMed] [Google Scholar]
- Vaisberg E. A., Grissom P. M., McIntosh J. R. (1996). Mammalian cells express three distinct dynein heavy chains that are localized to different cytoplasmic organelles. J. Cell Biol. 133, 831–842 10.1083/jcb.133.4.831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P., Stephens D. J. (2006). Microtubule plus-end loading of p150(Glued) is mediated by EB1 and CLIP-170 but is not required for intracellular membrane traffic in mammalian cells. J. Cell Sci. 119, 2758–2767 10.1242/jcs.02999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake C. J., Baye L. M., Nachury M. V., Wright K. J., Ervin K. E., Phu L., Chalouni C., Beck J. S., Kirkpatrick D. S., Slusarski D. C. et al. (2011). Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc. Natl. Acad. Sci. USA 108, 2759–2764 10.1073/pnas.1018823108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S., Egerer J., Fuchs E., Haas A. K., Barr F. A. (2007). Functional dissection of Rab GTPases involved in primary cilium formation. J. Cell Biol. 178, 363–369 10.1083/jcb.200703047 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.