Summary
Establishment and maintenance of stable muscle attachments is essential for coordinated body movement. Studies in Drosophila have pioneered a molecular understanding of the morphological events in the conserved process of muscle attachment formation, including myofiber migration, muscle–tendon signaling, and stable junctional adhesion between muscle cells and their corresponding target insertion sites. In both Drosophila and vertebrate models, integrin complexes play a key role in the biogenesis and stability of muscle attachments through the interactions of integrins with extracellular matrix (ECM) ligands. We show that Drosophila importin-7 (Dim7) is an upstream regulator of the conserved Elmo–Mbc→Rac signaling pathway in the formation of embryonic muscle attachment sites (MASs). Dim7 is encoded by the moleskin (msk) locus and was identified as an Elmo-interacting protein. Both Dim7 and Elmo localize to the ends of myofibers coincident with the timing of muscle–tendon attachment in late myogenesis. Phenotypic analysis of elmo mutants reveal muscle attachment defects similar to those previously described for integrin mutants. Furthermore, Elmo and Dim7 interact both biochemically and genetically in the developing musculature. The muscle detachment phenotype resulting from mutations in the msk locus can be rescued by components in the Elmo signaling pathway, including the Elmo–Mbc complex, an activated Elmo variant, or a constitutively active form of Rac. In larval muscles, the localization of Dim7 and activated Elmo to the sites of muscle attachment is attenuated upon RNAi knockdown of integrin heterodimer complex components. Our results show that integrins function as upstream signals to mediate Dim7–Elmo enrichment to the MASs.
Key words: Drosophila, Muscle, Cell adhesion, Elmo, Dim7, Myotendinous junction
Introduction
The Drosophila body wall musculature, which is functionally and structurally similar to vertebrate skeletal muscles, is a useful model to study conserved, complex developmental processes, including cell fusion, cell migration and cell–extracellular matrix (ECM) adhesion. The muscle–tendon attachment site, or myotendinous junction (MTJ), is a highly-specialized cell–ECM adhesion site, through which the ends of striated muscles are connected to specialized epidermal attachment cells called tendon cells (Brown, 2000; Gilsohn and Volk, 2010). Functionally, the proper establishment and maintenance of muscle attachment sites (MASs) in Drosophila embryogenesis is essential for tendon cells to withstand mechanical forces generated by contracting muscles (Volk, 1999; Schweitzer et al., 2010; Schejter and Baylies, 2010).
Structurally, integrin-mediated hemi-adherens junctions provide a crucial link in stable MAS formation. The transmembrane integrin heterodimer complex on the surface of both muscle (αPS2βPS) and tendon (αPS1βPS) cells anchors these opposing cell types to the adjacent ECM by binding to the ligands Tiggrin (Tig), Laminin (Lam) or Thrombospondin (Tsp) (Bunch et al., 1998; Graner et al., 1998; Chanana et al., 2007; Subramanian et al., 2007). Internally, a core group of proteins associate with the cytoplasmic tail of integrins to bridge the link between the extracellular milieu and the internal actin cytoskeleton, including the IPP complex [integrin-linked kinase (ILK), PINCH and Parvin], Talin and Wech (Clark et al., 2003; Löer et al., 2008; Zervas et al., 2001; Legate et al., 2006; Sebé-Pedros et al., 2010; Vakaloglou and Zervas, 2012). Focal adhesion kinase (FAK) and Git1 are two additional proteins that accumulate at the MASs in late embryonic myogenesis, the molecular function of which is unclear (Bahri et al., 2009; Grabbe et al., 2004). We recently showed that mutations in the msk locus result in the detachment of muscles from their corresponding tendon cells in late embryogenesis (Liu and Geisbrecht, 2011).
The Drosophila Engulfment and Cell Motility (Elmo)–Myoblast city (Mbc)→Rac signaling pathway is evolutionarily conserved from Caenorhabditis elegans to vertebrates (ELMO–DOCK180) and is essential for many developmental processes, including phagocytosis and cell migration (Katoh and Negishi, 2003; Côté and Vuori, 2007). A diverse array of receptors across multiple organisms have been identified upstream of Elmo–Dock complexes that regulate Rac activation. Integrins, growth factor receptors and phagocytic receptors are responsible for relaying extracellular signals to internal ELMO–DOCK signaling complexes to promote cell migration, phagocytosis or neurite outgrowth in mammalian systems (Côté and Vuori, 2007; Kinchen and Ravichandran, 2007; Miyamoto and Yamauchi, 2010; Park and Ravichandran, 2010). Studies in C. elegans have revealed the INA-1 integrin α-subunit, transmembrane protein CED-1, and the phosphatidylserine receptor function as engulfment receptors in apoptotic cell clearance (Hsu and Wu, 2010; Kinchen et al., 2005; Wang et al., 2003). However, in Dictyostelium discoideum, ElmoE has been identified as an essential component to mediate G-protein-coupled receptor (GPCR) signaling to a Dock–RacB complex (Yan et al., 2012). The functional conservation of the Elmo–Mbc→Rac signaling pathway in Drosophila is well-established in multiple biological processes, including ommatidial development, myoblast fusion and cell migration; although little is known about the upstream signals that regulate the Elmo→Rac complex (Luo et al., 1994; Duchek et al., 2001; Hakeda-Suzuki et al., 2002; Geisbrecht et al., 2008; Bianco et al., 2007; Nolan et al., 1998). Platelet-derived growth factor- and vascular endothelial growth factor-receptor related (PVR) is known to act upstream of Rac in border cell migration and thorax closure, but the upstream receptor in eye and muscle cells remain elusive (Ishimaru et al., 2004; Duchek et al., 2001; Bianco et al., 2007). The existence of a constitutive interaction between endogenous Dock180 and Elmo is observed in CHO cells, regardless of the presence of extracellular stimuli (Patel et al., 2011), suggesting that there are yet, unidentified signals or proteins that regulate the activity of this complex.
Elmo comprises N-terminal Armadillo repeats, an internally conserved ‘ELMO’ domain with unknown function, and C-terminal pleckstrin homology (PH) and proline-rich (PxxP) motifs that directly bind to Dock family members (Patel et al., 2010). A combination of bioinformatics approaches and in vitro validation has revealed structural domains that regulate Elmo activity through an intracellular autoinhibitory switch (Patel et al., 2010). An N-terminal Elmo inhibitory domain (EID), composed of HEAT and Armadillo repeats, physically interacts with an Elmo autoregulatory domain (EAD) residing between the PH and PxxP motifs in the C-terminus. Mutations in ELMO1 that disrupt the direct EAD–EID binding result in an active, open conformation. When expressed in integrin-activated cells, activated Elmo mutants (ELMO1 I204D or ELMO1 M692A/E693A) accumulate at the cell periphery to promote cell elongation and cell motility (Patel et al., 2010). The association of active RhoG with ELMO1 competes with the endogenous EID–EAD interaction, or closed conformation, within ELMO1. Thus, after binding to ELMO1, RhoG may unleash the open, active form of Elmo or recruit the closed conformation of Elmo to the membrane. Drosophila does not have a RhoG ortholog and the GTPase Rac exhibits the highest homology by primary sequence comparison. Thus, the identification of extracellular triggers and upstream molecules that mediate Elmo–Dock mutual autoinhibition and Rac activation in vivo remains unclear.
In this manuscript, we demonstrate a novel role for Elmo in the formation and maintenance of MASs. Furthermore, we place Dim7 as an upstream component of the canonical Elmo–Mbc→Rac signaling pathway and we show that integrins regulate the subcellular distribution of Dim7 and activated Elmo to the ends of actively contracting muscles. We postulate that the intracellular temporal and spatial regulation of Rac activity at the muscle membrane serves to locally remodel the actin cytoskeleton to modulate the stability of the muscle–tendon junctions during muscle growth or in response to changes in force transmission in active muscle contraction.
Results
We previously identified the Dock family members Mbc and Sponge (Spg) in an in vivo proteomics approach aimed at uncovering proteins that physically interact with Elmo in Drosophila embryogenesis (Geisbrecht et al., 2008; Biersmith et al., 2011). Herein, we present an additional Elmo-interacting protein that emerged from this mass spectrometry (MS) screen (Geisbrecht et al., 2008). The Drosophila Importin-7 ortholog (Dim7), encoded by the moleskin (msk) locus, was present in seven Elmo HA-tagged immunoprecipitates versus one untagged Elmo run. MS analysis after isolation of Mbc–HA complexes also revealed Dim7 peptides in six individual Mbc-tagged runs versus none in untagged controls.
Mutations in elmo and the rac genes exhibit muscle detachment phenotypes
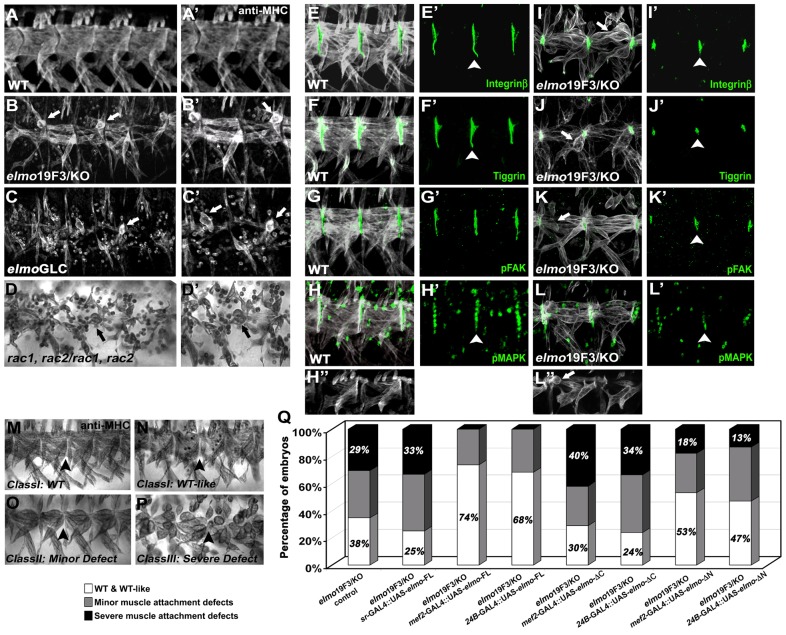
Based upon the identification of Dim7 as a potential Elmo-binding candidate and our previous characterization of msk in muscle attachment (Liu and Geisbrecht, 2011), we began our analysis by examining elmo mutant embryos for myogenic defects after the completion of myoblast fusion. In all experiments except where noted, we focused on the ventral musculature, where the muscles have a stereotypical rectangular shape and the attachment sites are relatively large in wild-type (WT) embryos (Fig. 1A,A′). Next, we analyzed embryos trans-heterozygous for a particular combination of elmo alleles (elmo19F3/KO; Fig. 1B,B′) or elmo germline clone (GLC) embryos (Fig. 1C,C′), in which the maternal and zygotic contribution of a hypomorphic elmo allele (elmoPB[c06760]) was depleted. Although the myoblast fusion defects varied in severity depending upon the genotype (Geisbrecht et al., 2008), the number of detached muscles was comparable in elmo zygotic or GLC embryos. Furthermore, a decrease in Elmo protein levels was not biased towards any particular muscle groups, suggesting that Elmo is required in all muscles for proper muscle attachment.
Fig. 1.
Mutations affecting components of the Elmo→Rac signaling pathway are defective in somatic muscle attachment. (A–P) The ventral musculature in stage 16–17 embryos stained with anti-myosin heavy chain (MHC; A–D′, M–P) or anti-tropomyosin (TM; E–L″). (A,A′) The stereotypical muscle pattern in WT embryos. (B–C′) Detached muscle fibers (arrows) are present in elmo19F3/KO zygotic (B,B′) or elmo GLC embryos (C,C′). (D,D′) Embryos mutant for rac1, rac2 exhibit muscle attachment defects (arrows). (E–K′) The localization of attachment site proteins (green; arrowhead) in two hemisegments of the ventral musculature (white). (E–F′, I–J′) βPS-integrin and Tig are properly localized to MASs in both WT (E,F′) and elmo mutant embryos (I–J′). (G–K′) Phosphorylated FAK (pFAK) is present at the ends of muscle fibers in both WT (G,G′) and elmo mutants (K,K′). (H–H‴,L–L‴) pMAPK normally accumulates in tendon cell nuclei. Loss of Elmo results in a decrease in pMAPK-positive tendon cells (L,L″) compared with WT embryos (H,H′). (M–P) Representative images of ventral muscle attachment phenotypes scored in cell-specific rescue experiments: WT (M); WT-like normal muscle pattern with a few unfused myoblasts (N); minor muscle attachment defects (O); or severe muscle attachment defects (P). (Q) Graph quantifying the extent of muscle or tendon cell-specific rescue in elmo19F3/KO mutants.
All available alleles of mbc result in severe myoblast fusion defects (Erickson et al., 1997; Balagopalan et al., 2006). This technical limitation precluded phenotypic analysis of Mbc function in later myogenic events, including MAS formation. To further test whether other components of the Elmo–Mbc→Rac pathway are essential in muscle–tendon attachment, we took two approaches to reduce Rac activity in the developing muscle. Even though both Rac1 and Rac2 in Drosophila are essential for actin-mediated myoblast fusion (Hakeda-Suzuki et al., 2002), small amounts of muscle fibers are able to form in rac1rac2 mutant embryos because of the perdurance of maternally loaded gene product (Fig. 1D,D′). These myofibers round up upon muscle contraction, similar to the muscle detachment phenotype observed in elmo or msk mutants. Furthermore, expression of dominant-negative Rac (RacN17) in ventral longitudinal muscle 1 (VL1, also known as muscle 12) using the 5053-GAL4 driver resulted in detached muscles (supplementary material Fig. S1). This data, taken together, demonstrate that both Elmo and the Rac proteins are essential for embryonic muscle attachment.
To determine whether the Elmo–Dim7 complex is required for maintaining attachment in mature contractile muscles, we utilized RNAi approaches to examine the requirement for Dim7, Elmo and Mbc in the larval musculature. Expression of UAS-RNAi constructs to knockdown elmo, msk or mbc levels with mef2-GAL4 resulted in pupal lethality (data not shown). Fillets of third larval (L3) instar control animals (mef2-GAL4/+) exhibited a normal muscle pattern. Upon a reduction in Elmo, Dim7 or Mbc levels, dissected L3 individuals revealed occasional muscle detachment accompanied by frequent missing muscles and muscle fiber thinning (supplementary material Fig. S1). Thus, Dim7 and the Elmo–Mbc complex all play essential roles in the MAS maintenance from late embryonic myogenesis through to larval stages.
The inability of the somatic muscles to remain attached in elmo mutant embryos raised the possibility that abnormal integrin-mediated adhesion is an underlying cause of muscle detachment. To further elucidate the role of Elmo in embryonic muscle attachment, the protein distribution of known MAS and tendon cell markers were examined. In WT embryos, both βPS integrin (Fig. 1E,E′) and the ECM ligand Tig (Fig. 1F,F′) accumulated at the interface between the muscle and tendon cells. In zygotic elmo mutants, βPS integrin (Fig. 1I,I′) or Tig (Fig. 1J,J′) were correctly localized to the hemi-adherens junctions at the segment borders, although the MASs were reduced in size. Owing to the phenotypic similarities of the muscle attachment defects in both elmo and msk mutants (Liu and Geisbrecht, 2011), we chose to further examine whether other proteins known to be affected in msk mutants were similarly aberrant upon loss of Elmo.
Previous cell-specific rescue experiments revealed that Dim7 exhibits both muscle-cell autonomous and non-autonomous roles, where Dim7 is responsible for the localization of phosphorylated FAK (pFAK) in the muscle cell and regulates the nuclear translocation of phosphorylated MAPK (pMAPK) and Sr for the maintenance of tendon cell identity through the secreted Vein–Egfr signaling pathway (Liu and Geisbrecht, 2011). To determine whether Elmo similarly affects pFAK and/or pMAPK proteins, both WT and elmo mutant embryos were immunostained to examine the subcellular localization and levels of these proteins. In contrast to msk mutants where pFAK protein is not detectable (Liu and Geisbrecht, 2011), this phosphoprotein was found to accumulate at the MASs in both WT (Fig. 1G,G′) and elmo (Fig. 1K,K′) mutant embryos. pMAPK is normally localized to the tendon cell nuclei in WT embryos (ref; Fig. 1H,H′) but this accumulation was reduced in elmo mutants (Fig. 1L,L′). Thus, a reduction in both elmo and msk levels results in loss of pMAPK, although it is less obvious in elmo mutants, consistent with the weaker muscle detachment phenotypes observed in zygotic elmo mutants. In summary, loss of Elmo or Dim7 result in similar muscle detachment phenotypes and a decrease in the number of mature tendon cells as determined by pMAPK staining.
Based upon the final muscle morphology observed in homozygous elmo mutant embryos, we categorized the detached muscles into three phenotypic groups. Class I embryos (WT or WT-like) had rectangular muscles connected to MASs with a large interface area between muscles and their target tendon cells (Fig. 1M). WT-like embryos retained normal muscle attachment with occasional unfused myoblasts (Fig. 1N). Moderate defects were observed in elmo mutants designated as Class II, in which spindle-shaped muscles with pointed ends exhibited small MASs and weakened muscle attachment (Fig. 1O). Mutants in Class III exhibited severe defects, with rounded muscles and a complete loss of muscle attachment to the epidermis (Fig. 1P). Severe muscle attachment defects occurred in 30.3% of elmo19F3/KO mutant embryos (Fig. 1Q; supplementary material Table S1).
To confirm whether the muscle detachment phenotype was due to a requirement for Elmo in the muscle or tendon cells, we performed tissue-specific rescue experiments by expressing a full-length elmo cDNA (UAS-elmo-FL) with the appropriate GAL4 drivers. The extent of muscle detachment rescue for each embryo was categorized according to the classes in Fig. 1M–P. In the zygotic elmo-null genetic background, sole expression of Elmo-FL in the tendon cells using sr-GAL4 did not improve muscle attachment compared with elmo mutants alone. In contrast, the muscle detachment phenotypes were efficiently rescued by the muscle-specific expression of Elmo using either the muscle-specific mef2-GAL4 or mesoderm-specific 24B-GAL4 drivers. Although the elmo mutant embryos were not completely rescued to WT using either of the muscle GAL4 drivers, no severe muscle defects were observed, accompanied by the ∼40% increase in embryos with WT muscle shape. These results indicate that Elmo, like Dim7, functions within muscle cells to mediate muscle attachment.
Elmo constructs that removed regions of the protein predicted to be important for function were introduced into elmo mutants to reveal the relative contribution of each domain in fly viability. The ubiquitous expression of Elmo using the actin-GAL4 driver fully rescued lethal elmo mutations to adult viability in two heterozygous elmo mutant backgrounds, elmo9F4/19F3 or elmoPB/19F3 (Geisbrecht et al., 2008). The expression of deleted versions of Elmo in elmo mutants (ElmoΔN, ElmoΔC, or ElmoΔPH) failed to rescue lethality, indicating indispensable roles of either the N- or C-terminal regions in Elmo for fly survival. In contrast, a small portion (7–12%) of adult flies eclosed when the ElmoΔPxxP truncation was expressed in an elmo mutant background (Fig. 1Q; supplementary material Table S1).
We extended these structure/function studies into the musculature with the expectation that we could uncover the domains of Elmo essential in myogenesis. The functional contribution of the N- or C-terminal regions of Elmo was determined by expressing either UAS-elmoΔN or UAS-elmoΔC under control of mef2-GAL4 or 24B-GAL4 in elmo mutants (Fig. 1Q; supplementary material Table S1). Muscle-specific overexpression of any of the indicated Elmo deletion constructs did not reveal obvious embryonic muscle patterning defects, suggesting that Elmo did not induce dominant effects in the musculature (data not shown). Expression of the N-terminal region of Elmo only (ElmoΔC) had no effect on rescuing the attachment defects, whereas reintroduction of the Elmo C-terminal region (ElmoΔN) provided partial rescue. Using the same experimental assay to evaluate the role of Elmo in myoblast fusion, we observed that the ElmoΔN-truncated version provided partial rescue of elmo-triggered myoblast fusion defects, whereas the ElmoΔC did not (supplementary material Fig. S2; Table S2). Thus, it appears that the C-terminal portion of Elmo contains information to direct both early myoblast fusion events (Balagopalan et al., 2006), and subsequent muscle attachment events in myogenesis.
Dim7 genetically and physically interacts with Elmo both in vitro and in vivo
The identification of Dim7 as a possible Elmo-binding partner in our in vivo proteomics approach and the similarities in the msk and elmo embryonic phenotypes raise the possibility that a Dim7–Elmo complex could function in the musculature. To confirm a biochemical interaction between Elmo and Dim7, we first transfected S2 cells with a FLAG-tagged version of Elmo-FL and subjected the resulting lysates to a GST pulldown assay using a bacterially expressed GST–Dim7 fusion protein. Elmo protein was detected in a complex with GST–Dim7, but not GST alone (Fig. 2A). Our rescue results in Fig. 1 suggest that the C-terminal region of Elmo is essential to mediate muscle attachment. Consistent with this, S2-transfected ElmoΔC–FLAG could not be found in a complex with GST–Dim7 (Fig. 2B).
Fig. 2.
Elmo and Dim7 biochemical and genetic interactions. (A) GST pulldowns of S2 cells transfected with ElmoFL–FLAG or ElmoΔC–FLAG constructs. Anti-FLAG detects ElmoFL-FLAG, but not ElmoΔC-FLAG in a complex with GST–Dim7. (B) Loading control for A. (C,D) Lysates of larval expressing Elmo–YFP or ElmoEDE–YFP were incubated with either GST alone or GST–Dim7 beads (C) or anti-Elmo antibodies for immunoisolation (D). Western blotting using anti-Elmo (C) or anti-Dim7 (D) antibodies reveal that both endogenous Elmo and YFP-tagged Elmo proteins are associated with Dim7. (E) Loading controls for C and D. (F–I) Classification of muscle detachment in stage 16–17 embryos. (F) Class I: WT and WT-like; (G) Class II: minor attachment defects; (H) Class III: severe attachment defects. (I) Quantification of embryonic muscle attachment defects shows that embryos heterozygous for msk (msk/+) enhance the attachment defects in homozygous elmo mutants, whereas loss of one copy of elmo or mbc enhances muscle detachment in msk/msk mutants.
To confirm an in vivo physical interaction between Elmo and Dim7, third instar larval lysates expressing an Elmo–YFP fusion protein in the musculature were incubated with GST alone or GST–Dim7 beads. As shown in Fig. 2C, both endogenous Elmo and the higher molecular mass Elmo–YFP protein were detected in GST–Dim7 pulldown experiments. Using these same Elmo–YFP lysates, western blotting confirmed the presence of Dim7 in anti-Elmo immunoisolated complexes (Fig. 2D). To verify this Dim7–Elmo interaction without overexpression of Elmo proteins, we performed immunoprecipitations (IPs) with endogenous Elmo or Dim7 protein lysates from WT embryos or larvae. Dim7 was detected in anti-Elmo immunoisolated complexes from both embryos and larval lysates. Elmo protein was also detected upon GST pulldown of Dim7 (supplementary material Fig. S3).
In vitro studies with human ELMO1 reveal that mutations in the EAD domain (ELMO1L202E/I204D/L205E) unleash an autoinhibitory interaction in the native protein, similar to the conformational changes observed in Elmo upon Dock180 binding (Patel et al., 2010). We used site-directed mutagenesis to recapitulate mutations that prevent the autoinhibitory interaction between the EID and EAD (hereafter referred to as ElmoEDE) domains confirmed in ELMO1 (supplementary material Fig. S1). After the creation of transgenic flies expressing UAS–ElmoEDE–YFP, we tested whether this ‘activated’ version of Elmo binds Dim7. Larval lysates expressing ElmoEDE in the musculature were subjected to GST–Dim7 pulldown assays (Fig. 2C) or anti-Elmo IPs (Fig. 2D) and in all experiments, a Dim7–ElmoEDE interaction was detected. These data suggest that Dim7 can bind Elmo in either the open or closed conformation.
We took advantage of genetic interaction analyses to determine whether Dim7 functions with the Elmo–Mbc complex for stable muscle attachment. Similar to the quantification method used in the experiments in Fig. 1Q, each genotype was categorized into three phenotypic classes on the basis of the severity of the muscle detachment phenotype: WT (Fig. 2F), minor attachment defects (Fig. 2G), or severe attachment defects (Fig. 2H). In elmo19F3/KO mutant embryos, only 21% of embryos had either minor or severe muscle attachment defects (Fig. 2I; supplementary material Table S1). However, the number of affected individuals increased to 54% in elmo19F3/KO embryos that also lacked one copy of the msk allele. In the converse experiment, removal of one copy of elmo in a homozygous msk4/msk5 mutant background (74% detached muscles) moderately enhanced the muscle detachment phenotype compared to msk mutants alone (55% detached muscles). An increase in the number of embryos with detached muscles was also observed upon reduction of mbc in a msk mutant background (73% compared with 55% with detached muscles). This data strongly suggests that msk genetically interacts with the Elmo–Mbc complex in the processes of muscle attachment.
MAS localization of Dim7, Elmo and Mbc during embryonic and larval myogenesis
We utilized previously generated anti-Elmo antibodies (Geisbrecht, et al., 2008) to examine the subcellular distribution of Elmo protein in embryogenesis. These immunostainings revealed a cytoplasmic distribution and faint muscle attachment site accumulation of Elmo in developing myofibers (supplementary material Fig. S4). In larval muscles, this same Elmo staining pattern persisted and we additionally detected Elmo protein in the nucleus. All anti-Elmo staining in the musculature appeared specific as this signal was lost in elmoKO mutants (supplementary material Fig. S4). Therefore, we examined the full-length (Elmo–YFP) and activated YFP versions of Elmo (ElmoEDE–YFP) to discern whether we could observe a change in the spatiotemporal localization of Elmo in myogenesis. The ElmoEDE–YFP fusion protein was mainly in the myoplasm and weakly observed at the ends of a few myofibers in stage 14 embryos (Fig. 3A–A′). Activated Elmo began to accumulate at the ends of the ventral muscle fibers in stage 15, when muscle migration is complete and the establishment of adhesion junctions between the muscle and tendon cells is initiated (Fig. 3B). After stage 16, ElmoEDE–YFP overlapped with βPS integrin at the MASs (Fig. 3C). Analysis of Elmo–YFP revealed a similar distribution except that basal Elmo was not apparent until after stage 15 (supplementary material Fig. S4). Since this pattern of Elmo localization mirrors that of Dim7 localization in myogenesis (Liu and Geisbrecht, 2011), we sought to extend our immunolocalization analysis in the larval musculature to determine whether Elmo and Dim7 still accumulate at the MASs in actively contracting muscles. Continual MAS localization of Dim7 (Fig. 3D–E′) and Elmo (Fig. 3F–G′) persisted in all larval stages. Examination of Dim7, Elmo or Mbc distribution within the body wall muscles of larvae revealed they were all enriched at the Z-disc also (supplementary material Fig. S5).
Fig. 3.
In vivo detection of a Dim7–Elmo complex at MASs in developing and mature muscles. (A–C′) Muscle-specific expression of ElmoEDE–YFP in two hemisegments of the embryonic ventral muscles. ElmoEDE protein, barely detectable in stage 14 embryos (A,A′), accumulates at the ends of myofibers in stage 15 (B,B′) or stage 16 (C,C′) embryos. (D–G′) Filleted L3 individuals showing Dim7 accumulation at MASs, revealed using an antibody against Dim7 (D,D′) or by expressing a YFP–Dim7 fusion protein (E,E′). Elmo protein persists at MASs into late larval stages (F–G′). (H–Q″) Duolink in situ PLA in WT embryos or a third instar larva reveal that Elmo and Dim7 proteins are found in close proximity at the sites of muscle attachment. Virtually no signal is observed in control samples (H,K,N,O). (H–M′) Three segments of the embryonic ventral muscles are shown either in lateral (H,H′,I,I′,J,J′) or dorsal views (K,K′,L,L′,M,M′). Weak duolink signal between Elmo and Dim7 is observed at stage 15 (I,I′,L,L′), and the signal intensifies from stage 16 onwards (J,J′, M,M′). (N–Q″) Phase-contrast and fluorescence images are shown for L3 muscle fillets where intense Duolink signal is observed between Elmo and Dim7 at larval MASs (P–Q″). Arrowheads indicate MAS.
To confirm an in vivo association between Elmo and Dim7 within muscle tissue, we took advantage of the in situ proximity ligation assay (PLA) technology, or Duolink assay. The PLA recognizes the potential interaction of endogenous proteins using antibodies to detect proteins in close proximity to one another (<40 nm). As this technique was new in our hands, a series of control experiments was performed to ensure signal specificity. First, omission of either or both primary antibodies and exposure to secondary antibodies conjugated with complementary PLA probes and amplification reagents resulted in non-specific signal in embryos (Fig. 3H,H′,K,K′) or larval tissue (Fig. 3N–O″; supplementary material Fig. S6). Incubation of an anti-Elmo with an antibody generated against the transcription factor Mef2 resulted in faint signal in a few nuclei (supplementary material Fig. S6). In contrast, the in vivo Dim7–Elmo Duolink signal was weakly detected at the embryonic MASs at stage 15 (Fig. 3I,I′,L,L′) and remained strong after the completion of muscle attachment (Fig. 3J,J′,M,M′). In actively contracting larval muscles, Elmo–Dim7 staining was apparent at the MTJs (Fig. 3P–Q″; supplementary material Fig. S6). These data are consistent with a Dim7–Elmo complex that functions at the MASs to maintain strong and cohesive MTJ.
Dim7 functions upstream of the Elmo–Mbc complex for muscle attachment
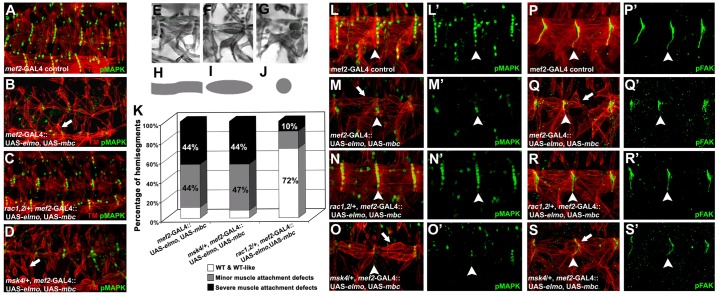
To determine where Dim7 resides within the Elmo–Mbc→Rac pathway in the attachment of embryonic muscles, we took advantage of a previous strategy for analyzing this tripartite complex in the Drosophila eye (Geisbrecht et al., 2008). A rough eye phenotype induced by Elmo–Mbc overexpression is suppressed upon removal of rac1J11 and rac2Δ, resulting in a WT eye morphology, and is consistent with Rac functioning downstream of Elmo–Mbc. To confirm this signaling paradigm in the somatic musculature and extend this strategy to determine where Dim7 functions, we overexpressed both Elmo and Mbc together in muscle and simultaneously reduced one gene copy of endogenous rac1, rac2 or msk. Overexpression of either Elmo alone or Mbc alone resulted in a WT-like muscle pattern (Geisbrecht et al., 2008), similar to mef2-GAL4/+ (Fig. 4A). Both myoblast fusion defects and muscle detachment phenotypes were prevalent upon muscle-specific overexpression of the Elmo–Mbc complex (Fig. 4B). Muscle detachment was largely rescued to WT in a Δrac1, rac2/+ genetic background (Fig. 4C). In contrast, there was no suppression of muscle attachment defects as a result of expression of this RacGEF complex upon removal of one copy of the msk4 allele (Fig. 4D,K). These data suggest that Dim7 functions either upstream or parallel to the Elmo–Mbc complex.
Fig. 4.
The muscle-specific Elmo–Mbc→Rac signaling module non-autonomously signals to maintain tendon cell identity through pMAPK. (A–D) Representative embryos detailing the lateral musculature (TM; red) and tendon cell nuclei (pMAPK; green) in stage 16–17 embryos. Muscle-specific coexpression of Mbc and Elmo result in severe muscle attachment defects with smaller MASs (B; arrows) than mef2-GAL4/+ control embryos (A). Removal of one copy of endogenous rac1J1rac2Δ(C), but not msk4 (D), restores normal muscle attachment. (E–K) For quantification (K), the muscles in hemisegments A1–4 in each embryo (n = 200) were scored as WT (E,H); minor (F,I); or severe muscle detachment (G,J). (L–S′) High magnification of two hemisegments of the embryonic ventral musculature (TM; red) co-stained with the muscle or tendon cell markers, pFAK and pMAPK, respectively (green). In mef2-GAL4 controls, pFAK properly localizes to MASs (P,P′) with 6–7 pMAPK(+) tendon cells at the segmental border (L,L′). An excess of Elmo and Mbc in muscles results in smaller MASs, indicated by pFAK staining (Q,Q′) and a decrease in the number of pMAPK(+) cells (M,M′). The pFAK and pMAPK expression patterns are rescued to WT with the decrease of endogenous rac1 and rac2 (N,N′, R,R′), whereas they remain unaltered upon loss of one copy of msk (O,O′, S,S′).
We next tested whether the distribution of pMAPK (Fig. 4L,L′) or pFAK protein (Fig. 4P,P′) was altered upon activated-Rac-induced muscle detachment. In embryos with an excess of both Elmo and Mbc in the musculature, the number of pMAPK-positive tendon cells was reduced (Fig. 4M,M′), while pFAK was still properly localized (Fig. 4Q,Q′). Embryos of the genotype mef2:UAS-elmo,UAS-mbc; rac1J11, rac2−/+ exhibited WT pMAPK and pFAK expression patterns with six or seven aligned pMAPK(+) tendon cell nuclei in each hemisegment border (Fig. 4N,N′), and larger, extended pFAK-enriched MASs (Fig. 4R,R′), demonstrating that rescue of the muscle attachment defects also rescued tendon cell identity. However, a reduction in Dim7 levels (mef2:UAS-elmo,UAS-mbc; msk4/+) was not capable of suppressing aberrant pMAPK accumulation (Fig. 4O,O′) or pFAK localization (Fig. 4S,S′). Activation of Rac through the Elmo–Mbc complex phenocopies the loss of pMAPK in the tendon cells observed upon overexpression of Dim7 in the musculature (Liu and Geisbrecht, 2011), suggesting that the Elmo–Mbc complex may function with Dim7 to mediate tendon cell identity.
The above genetic experiments suggested that Dim7 is not downstream of Elmo, so we tested whether Dim7 functions upstream of the Elmo–Mbc→Rac signaling pathway by attempting to answer two independent questions: (1) is the muscle detachment phenotype associated with the muscle-specific overexpression of Dim7 suppressed by reducing endogenous levels of Mbc?; (2) can the msk muscle detachment phenotype be rescued by overexpression of putative downstream components? Consistent with our previously published results (Liu and Geisbrecht, 2011), excess Dim7 in the musculature, from the early mesoderm driver twist-GAL4, perturbs the ability of the muscles to firmly adhere to the tendon cells (Fig. 5A). However, further removing one copy of mbc largely suppressed the muscle attachment defects, revealed by a decrease in embryos with severe muscle attachment defects from ∼30% to 3% (Fig. 5B,C). These data suggest that Dim7 may act upstream of the Elmo–Mbc→Rac signaling pathway. Further support for this linear model was revealed by rescue experiments in which we expressed putative downstream proteins in an attempt to ameliorate muscle detachment in msk4/4 mutants (Fig. 5D). The introduction of exogenous Elmo (Fig. 5E) or the Elmo–Mbc complex (Fig. 5F) partially rescued the muscle detachment phenotype associated with mutations in msk, although more complete rescue was observed with the latter GEF complex (Fig. 5H; supplementary material Fig. S7). If the Elmo–Mbc complex upregulates Rac activity within the musculature, expression of activated Rac would be expected to also rescue msk mutants. To circumvent the complete block in myoblast fusion upon excess Rac activation using mef2-GAL4, we reduced the levels of activated Rac being expressed in early myogenesis using the weaker sticks and stones (sns)-GAL4 driver that is expressed in a subset of myoblasts (Kocherlakota et al., 2008). Although occasional missing muscle fibers were present in embryos of the genotype msk4/4, sns-GAL4::UAS-RacV12, presumably due to incomplete fusion events, the muscle attachment defects were largely rescued (Fig. 5G,H). We did not observe complete rescue of the muscle detachment phenotype in msk4/4 mutants using this assay. Excess levels of Elmo–Mbc, RacV12 or Dim7 alone resulted in detached muscles, suggesting that protein levels or regulation of protein activity is precisely controlled in establishment of the muscle–tendon junction. These data, taken together, indicate a linear pathway in which Dim7 acts upstream of the Elmo–Mbc→Rac signaling to mediate muscle attachment.
Fig. 5.
Genetic manipulation places Dim7 upstream of the Elmo–Mbc complex. (A,B,D–G) MHC staining of stage 16–17 embryos. (A–C) Excess Dim7 expression in myofibers results in the separation of muscles from smaller MASs (A). This muscle detachment phenotype is suppressed by removal of one copy of mbcD11.2 (B,C). (D–H) Expression of Elmo–Mbc→Rac signaling components rescues the muscle defects in msk mutants. The severe attachment defects observed in msk4/msk4 embryos (D) are partially restored upon muscle-specific expression of Elmo (E). Improved restoration of muscle attachment is observed by introduction of the Elmo–Mbc complex (F) or RacV12 (G). (H) Bar graph quantifying the rescue of muscle attachment defects in msk4/4 mutant embryos upon muscle-specific expression of Elmo, the Elmo–Mbc complex, the ElmoEDE variant or RacV12.
The recruitment of activated Elmo to the MASs requires Dim7
The open, activated version of human Elmo (ELMO1L202E/I204D/L205E, or ElmoEDE) promotes cell elongation in cultured cells (Patel et al., 2010). To extend these studies in vivo and reveal how uninhibited Elmo may influence muscle attachment, we expressed this activated version of Drosophila Elmo using mef2-GAL4. Strikingly, muscle-specific expression of ElmoEDE–YFP showed high levels of accumulation at larval MASs (Fig. 6B–B″), whereas native ElmoFL–YFP retained a broader cytoplasmic distribution within the myofiber (Fig. 6A–A″). Our hypothesis that ElmoEDE functions as an activated Elmo variant is supported by two lines of experimentation. First, 70% of L3 larvae with excess ElmoEDE had at least a few detached muscle fibers. Furthermore, ElmoEDE mimicked rescue of the muscle detachment phenotype in msk mutants to the same extent as the Elmo–Mbc complex and better than ElmoFL alone (Fig. 5H). This is the first in vivo evidence in any tissue that Elmo localization and/or activity could be regulated as a result of an autoinhibitory switch.
Fig. 6.
MAS localization of ElmoEDE is Dim7 dependent in the larval musculature. (A–D‴) F-actin (phalloidin; red) labels muscles present in one-half of a larval fillet, while the localization of Elmo protein is detected by YFP fusion proteins driven by mef2-GAL4. Expression of C-terminally fused ElmoFL–YFP is broadly distributed in the cytoplasm of muscle fibers (A–A‴), whereas an ElmoEDE–YFP fusion protein is largely enriched at the MASs (B–B‴). Accumulation of ElmoEDE at the MASs is reduced in mef2-GAL4::UAS-elmoEDE–YFP, UAS-mskRNAi animals (C–C‴), but unaffected in mef2-GAL4::UAS-elmoEDE–YFP, UAS-zaspRNAi (D–D‴). (E) Quantification of ElmoEDE–YFP accumulation at the ends of attached muscles in controls (mef2-GAL4::UAS-elmoEDE–YFP) and Dim7 or Zasp RNAi knockdown larvae. The localization of ElmoEDE was determined at the ends of the same ten muscles (see Materials and Methods) in every hemisegment of dissected and photographed fillets and displayed as the percentage of overall ElmoEDE accumulation. (F,G) Western blots and quantification of representative blots of total Elmo protein from control and knockdown third instar larvae. Detected by anti-YFP (F) or anti-Elmo antibody (G), ElmoEDE levels in Dim7 knockdown larvae is significantly reduced compared with control or Zasp52 knockdown larvae. Anti-α-tubulin staining was used as protein loading control.
We next sought to determine whether the upstream Dim7 is responsible for the enrichment of activated Elmo to larval MASs. Upon loss of Dim7 by RNAi, the amount of ElmoEDE–YFP was decreased at the ends of muscle fibers, independent of their attachment state (Fig. 6C–C″). The reduction in activated Elmo appeared to be specific to a decrease in Dim7 as depletion of the MAS protein Zasp52 had no effect on ElmoEDE–YFP localization at the ends of muscles (Fig. 6D–D″). To control for differences in data acquisition, we took two approaches. First, confocal micrographs of control and msk RNAi knockdown larvae were acquired simultaneously using the same microscope settings for direct comparison (supplementary material Fig. S8). Subsequent quantification of muscles in individual larvae revealed a 40% decrease in the amount of ElmoEDE at the ends of the muscles (Fig. 6E).
We examined global ElmoEDE protein levels by western blot analysis using anti-YFP or anti-Elmo antibodies after boiling whole larvae directly in SDS-loading buffer to eliminate the potential loss of ElmoEDE localization with membrane-anchored proteins. The mef2-GAL4/+ control and Zasp knockdown animals exhibited similar levels of ElmoEDE (Fig. 6F,G), whereas overall ElmoEDE levels were largely reduced in larvae with decreased Dim7 protein levels. These results suggest that Dim7 is essential for the localization of activated Elmo and/or ElmoEDE protein that is not anchored to the MASs, which may get targeted for degradation in the myoplasm.
Integrins function upstream of Dim7–Elmo
Three pieces of evidence triggered us to investigate the possibility that the Dim7–Elmo complex could be downstream of integrins in MAS maintenance. First, msk was uncovered in a genetic screen as a suppressor of a gain-of-function wing blistering phenotype caused by mutations in inflated (if), which encodes the αPS2 integrin subunit (Baker et al., 2002). Second, integrins are well characterized as the major receptor complex essential for both adhesion and signaling in muscle attachment (Carmignac and Durbeej, 2012; Bozyczko et al., 1989). Finally, both Dim7 and ElmoEDE become enriched at the sites of integrin localization in the MASs and this localization persists throughout active larval muscle contraction (Fig. 3) (Liu and Geisbrecht, 2011).
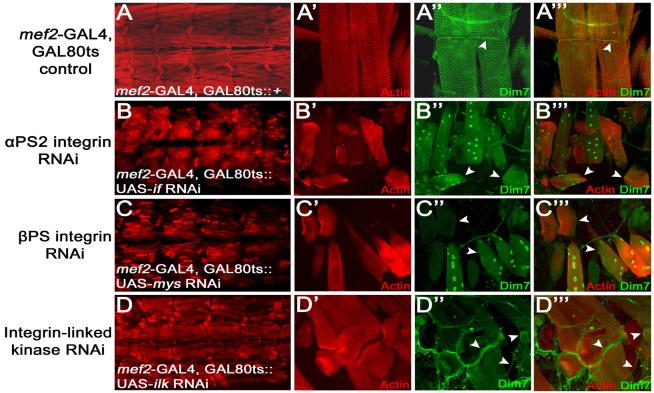
We first tested whether the localization of Dim7 protein was altered upon disruption of the integrin adhesome, using a muscle-specific RNAi knockdown strategy. Loss of αPS2, βPS, or ILK all resulted in embryonic or first instar larval lethality using the mef2 or 24B GAL4 drivers. To circumvent this early lethality, we recombined mef2-GAL4 with the temperature-sensitive GAL80ts repressor (Lee and Luo, 1999) and mated these flies with the appropriate UAS-RNAi line. The resulting progeny were raised at the non-permissive temperature (18°C) throughout embryogenesis to repress leaky GAL4 expression and then shifted to the permissive temperature (29°C) to initiate RNAi induction at the end of the first instar larval stage. As expected (Liu and Geisbrecht, 2011), filleted L3 larvae with decreased levels of if, mys or ILK RNAi, all exhibited muscle attachment defects (Fig. 7B–D). However, the localization of Dim7 was differentially affected in these different genotypes. In control animals (mef2-GAL4, GAL80ts) Dim7 accumulated at the ends of muscles (Fig. 7A′–A″). The subcellular localization of Dim7 was dramatically altered upon loss of αPS2 (Fig. 7B–B″) or βPS (Fig. 7C′–C″) where it was found at high levels in the nuclei rather than being tethered to the MASs (Fig. 7C). Knockdown of ILK had no effect on the maintenance of Dim7 at the cell periphery in both attached and detached muscles in these larvae (Fig. 7D′,D″) or in ilk mutant embryos with detached muscles (supplementary material Fig. S8). These results suggest that integrins or integrin-associated proteins (exclusive of ILK) are responsible for tethering Dim7 to the ends of the muscles.
Fig. 7.
Loss of integrins redistributes Dim7 from the ends of muscles to myofiber nuclei. (A–D‴) Confocal micrographs of the L3 musculature to show the repeated sarcomeric structure of actin filaments (phalloidin; red) and the subcellular localization of Dim7 (green). Severe muscle detachment phenotypes are observed upon knockdown of PSα2 integrin, PSβ integrin or ILK levels (B–D‴). In control mef2-GAL4, GAL80ts larvae, Dim7 is enriched at the MASs (A–A‴). Upon loss of either integrin heterodimer subunit, Dim7 disappears from the muscle ends and is present at high levels in the myofiber nuclei (B–C‴). However, in muscles with decreased ILK, Dim7 is still tethered to the cell periphery, even in detached muscles (D–D‴).
The ability of Dim7 to control ElmoEDE accumulation (Fig. 6) or integrins to mediate Dim7 localization (Fig. 7) at the MASs obviated the need to examine whether integrins modulate ElmoEDE localization. Inclusion of the Gal80ts element had no effect on the accumulation of ElmoEDE to the ends of myofibers (Fig. 8A,A′). The MAS accumulation of ElmoEDE was attenuated in larvae with low levels of either the αPS2 (Fig. 8B,B′) or βPS (Fig. 8C,C′). Consistent with the results obtained for Dim7 localization, loss of ILK did not alter ElmoEDE protein accumulation at the ends of either attached or detached muscles (Fig. 8D,D′). Loss of Dystrophin (Dys) or FAK had no effect on ElmoEDE MAS accumulation, suggesting that ElmoEDE localization is integrin dependent (supplementary material Fig. S8).
Fig. 8.
Integrins are required for the localization of activated Elmo. (A–D′) Muscles in third instar larval were labeled with phalloidin to visualize F-actin (red) along with the activated Elmo fusion protein (green). Compared with localization of ElmoEDE at the MASs (A,A′), this accumulation at the muscles ends is reduced in PSα2 integrin (B,B′) or PSβ integrin (C,C′) knockdown myofibers. Knockdown of ILK levels have no effect on ElmoEDE levels (D,D′). (E) The pixel intensity of ElmoEDE in the indicated genetic backgrounds are measured across muscle 7. Note the levels of ElmoEDE accumulation at the attachment sites (arrowheads in A–D′) upon knockdown of integrins are approximately half of the levels observed in controls.
Discussion
We previously showed that Dim7 localizes to developing muscle–tendon insertion sites and removal of Dim7 has severe consequences in muscle attachment maintenance (Liu and Geisbrecht, 2011). Studies herein extend these observations to elucidate the functional contribution of Dim7–Elmo in regulating Drosophila muscle attachment. Our results show that Dim7 is an upstream adaptor protein that recruits Elmo in response to integrin adhesion and/or signaling. Thus, we propose that the spatial and temporal regulation of Elmo-Mbc activity results in regulation of the Rac-mediated actin cytoskeleton changes at the MASs.
The Dim7–Elmo complex functions in late embryonic myogenesis for stable establishment of MASs
The ‘myospheroid’ phenotype in elmo or msk mutants resembles attachment defects first characterized in mutated genes that encode integrins, ILK and Talin, and is not due to earlier developmental defects in myogenesis. A similar number of cells expressing the muscle differentiation factor DMef2 was present in elmo or msk mutants, indicating that muscle specification was not affected (Geisbrecht et al., 2008; Liu and Geisbrecht, 2011). Mutations in genes essential for muscle migration and targeting also lead to detached muscles. For example, in kon/perd or grip mutants, the early arrest of migrating myotubes resulting from defective migration eventually leads to a linkage failure between the muscle and tendon cells (Estrada et al., 2007; Schnorrer et al., 2007; Swan et al., 2006; Swan et al., 2004). In mutant embryos with reduced levels of Elmo or Dim7, the muscle detachment phenotype did not appear to result from muscle migration defects. First, the spatiotemporal accumulation of Elmo and Dim7 is developmentally regulated. Both proteins are not detected at the leading edges of migrating muscles, but begin to accumulate at MASs after stage 15. Second, we did not observe a failure of muscle ends contacting their corresponding attachment sites in elmo or msk mutants at late stage 15, when muscle migration was almost complete (Liu and Geisbrecht, 2011).
The recruitment of active Elmo protein to the MAS by Dim7
Both membrane localization and Rac-dependent cell spreading of the uninhibited, active version of Elmo is enhanced compared with native WT Elmo in cultured mammalian cells (Patel, et al., 2010). These in vitro results are in agreement with our in vivo analysis, which found ElmoEDE is enriched at larval muscle ends compared with the poor accumulation of full-length Elmo–YFP. This may reflect a potential regulatory mechanism controling the subcellular localization of Elmo from the cytoplasm to the muscle ends upon the release of Elmo autoinhibition. Within different cells or tissues, various proteins may regulate Elmo localization to the cell periphery, or other sites where active Elmo is needed. In cultured mammalian epithelial cells, membrane recruitment of the Elmo–Dock180 complex is dependent on active RhoG for cell spreading (Katoh and Negishi, 2003). Consistent with a functional role for membrane-targeted Elmo, active Elmo promotes cell elongation in HeLa cells when co-expressed with RhoG (Patel et al., 2010).
Our data argues that adaptor proteins may be required in muscle cells for activated Elmo membrane recruitment. Decreased levels of ElmoEDE are observed at the polarized ends of muscle insertion sites when Dim7 levels are decreased. It is still not clear if Dim7 binding is required for the conformational change that results in Elmo activation or if an activated Dim7–Elmo complex already exists within the cell and is recruited as a complex upon integrin activation. Furthermore, we do not observe a complete loss of ElmoEDE protein levels, suggesting that either Dim7 protein levels are not depleted enough or other proteins in addition to Dim7 play a role in Elmo membrane recruitment. Alternatively, post-translational modification(s), such as phosphorylation, could be an additional mechanism for the relief of Elmo autoinhibition. Thus, we conclude that in muscle, Dim7 is an essential adaptor protein for the polarized membrane localization of active Elmo or the active Elmo–Mbc complex downstream of integrin signaling pathway.
Integrins function upstream of the Dim7–Elmo complex
What is the relationship between the integrin adhesome and the Dim7–Elmo complex? We propose two possibilities, which are not mutually exclusive. One is that the Dim7–Elmo–Mbc complex assembles at MASs through integrin-mediated ‘outside-in’ signaling. Upon ligand binding to ECM molecules, integrin activation results in Dim7–Elmo–Mbc complex localization for the spatiotemporal regulation of Rac activity to maintain dynamic actin filament adhesion at the MASs. We predict that localization of activated Elmo to the MASs is a prerequisite regulatory mechanism for actin cytoskeleton remodeling through Rac to maintain stable attachments. This hypothesis is supported by three lines of evidence: (1) muscle attachment defects upon loss of Dim7 or Elmo are only observed after the establishment of the integrin adhesion complex and onset of muscle contraction; (2) muscle detachment in msk mutants can be rescued by expressing low levels of activated Rac; and (3) the enrichment of Dim7 and ElmoEDE proteins at the ends of muscle fibers is greatly reduced in integrin-deficient larvae.
The other possibility is that accumulation of the Dim7–Elmo complex to the ends of muscles regulates ‘inside-out’ signaling to dynamically regulate integrin affinity for strong ligand binding and stable muscle attachments. Previously, we reported that Dim7 acts upstream of the Vein–Egfr signaling pathway in muscle to tendon cell signaling (Liu and Geisbrecht, 2011). Combined with previous reports that muscle-specific Vein secretion is dependent on the adhesive role of βPS integrin (Martin-Bermudo, 2000; Yarnitzky et al., 1997), the Dim7–Elmo complex may be internally required for integrins to regulate Vein secretion. A decrease in Vein–Egfr signaling and loss of tendon cell terminal fate results in a reduction in ECM secretion and weakened integrin attachment to the ECM. This is consistent with the observation that msk or elmo mutants phenocopy embryos with reduced or excessive amounts of the αPSβPS integrin complex, where pointed muscle ends result in smaller muscle attachments. Future studies analyzing Dim7–Elmo–Mbc complex localization and function in the background of integrin deletion constructs which separate the ‘inside-out’ and ‘outside-in’ signaling pathways will be essential to uncover more detailed molecular mechanisms.
What is the relationship between the Dim7–Elmo–Mbc→Rac signaling pathway and the integrin-mediated adhesome complex assembly (including Talin and the IPP complex)? We propose that actin filaments within the muscle cell are anchored to the muscle cell membrane through the IPP complex, whereas regulation of MAS–actin remodeling is controlled by the Dim7–Elmo–Mbc→Rac pathway. Our data suggests that these two complexes assemble independently at the muscle ends. In msk mutant embryos, both ILK and Talin properly accumulate at the MASs, suggesting that Dim7 is not responsible for their localization (Liu and Geisbrecht, 2011). Similarly, we can still detect both MAS-enriched Dim7 and active Elmo at two ends of the muscles in ILK-deficient larvae, even in fully detached muscles. In a vertebrate cell culture model, Elmo2 was found to physically interact with ILK for the establishment for cell polarity (Ho and Dagnino, 2012; Ho et al., 2009). Thus, it is possible that our approaches have not fully knocked down Ilk levels or that the Dim7–Elmo recruitment by Ilk is redundant with another attachment site protein. Alternatively, an upstream scaffold protein may function to recruit both the IPP and Dim7–Elmo complexes to the MASs. It is probable that these two complexes are temporally regulated in embryogenesis, where the actin remodeling complex is not needed until initial muscle–tendon initiation has been established.
Materials and Methods
Fly genetics
Fly stocks were raised on standard cornmeal medium at 25°C unless indicated otherwise. The y1,w1118 strain was used as the wild-type control. The following fly stocks and/or alleles were used in this study: msk4/TM3-lacZ [a gift from Lizabeth Perkins (Baker et al., 2002)]; mef2-GAL4 (Liu and Geisbrecht, 2011); sns-GAL4 (Kocherlakota et al., 2008); mef2-GAL4, Gal80ts (Liu and Geisbrecht, 2012); UAS-elmo (Geisbrecht et al., 2008); UAS-mbc (Balagopalan et al., 2006); mef2-GAL4, rac1J11, rac2− (Geisbrecht et al., 2008); mbcD11.2/TM3-lacZ (Erickson et al., 1997); mbcD11.2, twist-GAL4/TM3-lacZ (Biersmith et al., 2011); mef2-GAL4, msk4 (Liu and Geisbrecht, 2011). The following stocks were obtained from the Bloomington Stock Center: y1w; msk5P{neoFRT}80B/TM3, P{ftz-lacZ.ry+}TM3, Sb1 (BL-23879); sr-GAL4/TM6 (BL-26663); UAS-msk (BL-23944); UAS-mskRNAi (BL-27572); UAS-Zasp RNAi (BL-31561); UAS-mys RNA (BL-33642); UAS-if RNAi (BL-27544); UAS-ILK RNAi (BL-35374).
Immunostaining and microscopy
Embryos were collected on agar-apple juice plates and aged at 25°C. Rescue experiments were conducted at 29°C unless otherwise specified. For immunostaining, embryos or dissected third instar larva were fixed with 4% formaldehyde and stained as described previously (Geisbrecht et al., 2008; LaBeau-DiMenna et al., 2012). Primary antibodies used were: anti-MHC (mouse 1∶500; Susan Abmayr, The Stowers Institute for Medical Research, Kansas City, MO); anti-Elmo (1∶3000 for western and 1∶400 for immunochemistry) (Geisbrecht et al., 2008); anti-Msk (rabbit 1∶3000 for western and 1∶400 for immunochemistry) (Lorenzen et al., 2001); anti-TM (rat 1∶50; Babraham Institute, Cambridge, UK); βPS-integrin [mouse 1∶50; Developmental Studies Hybridoma Bank (DSHB)]; anti-Tig (mouse 1∶1000) (Fogerty et al., 1994); anti-FAK[pY397] (rabbit 1∶1000; Invitrogen); anti-dpERK/MAPK (rabbit 1∶100; Cell Signaling).
Secondary antibody used for tyramide enhancement was goat anti-rabbit-HRP (1∶200, Jackson Laboratories). Secondary antibodies for fluorescent immunostaining were Alexa Fluor 488 or Alexa Fluor 546 (1∶400; Molecular Probes). Phalloidin 594 (or 647) was used for F-actin labeling (Molecular Probes). Tyramide enhancement was used to improve signal intenisty for anti-Msk and anti-dp-ERK-MAPK (Cell Signaling). Fluorescence images were collected on an Olympus Fluoview300 and processed using Adobe Photoshop Elements 2.0.
In situ proximity ligation assay
Duolink™ in situ proximity ligation assays (PLA) were performed to detect in vivo interactions between Elmo and Dim7 in embryonic myogenesis until larval stages. PLA provides a fluorescent signal when the target proteins are localized within 40 nm. The freshly collected WT embryos and dissected third instar larva are fixed, permeabilized and incubated with anti-Elmo and anti-Dim7 primary antibodies followed by secondary antibodies conjugated to complementary PLA probes. Duolink hybridization, ligation, amplification and detection media were administered according to the manufacturer's instructions (Olink Biosciences). Fluorescence duo-link signals were collected on the Olympus Fluoview300 and processed using Adobe Photoshop Elements 2.0. For control samples, no primary antibodies, but only secondary antibodies were added, followed by standard amplification, and detection methods.
Molecular biology and site-directed mutagenesis
All Elmo deletion constructs were generated using standard PCR-based techniques. For all FLAG-tagged constructs, sequences encoding the FLAG epitope were added to the 3′ end of the appropriate Elmo sequences, with a stop codon engineered in the oligonucleotide after the tag. The ORF of the full length elmo cDNA (Geisbrecht et al., 2008) was cloned in the proper reading frame into the Gateway entry vector (Drosophila GATEWAY™ cloning system, Invitrogen). The amino acid changes in the EID region of FL Elmo (L194E/I196D/L197E) were introduced via standard site-directed mutagenesis methods (QuikChange II Site-Directed Mutagenesis Kit, Agilent Technologies). ElmoFL-YFP and Elmo EDE-YFP fusions were generated using the pTWV gateway destination vectors (pUAST-based vector, EYFP fusions at the C-terminal end) with the Drosophila Gateway Cloning System according to the instruction manual.
Immunoblotting
For western blots with whole larva, five third instar larvae from each genotype were directly boiled in 300 µl 2× SDS running buffer [100 mM Tris-HCl, pH 6.8, 200 mM dithiothreitol, 4% SDS (electrophoresis grade), 0.2% Bromophenol Blue, 20% glycerol] for 10 minutes. 10 µl from each genotype was subjected to western blotting and probed with anti-YFP (MBL International) or anti-Elmo antibody. Anti-α tubulin was used as the protein loading control.
Supplementary Material
Acknowledgments
We thank our colleagues for the kind gifts of antibodies and fly stocks as indicated in Materials and Methods. We also thank the Bloomington Stock Center at Indiana University and the Vienna Drosophila RNAi Center (VDRC) for fly stocks and the Iowa Developmental Studies Hybridoma Bank and Babraham Institute for antibodies. We are grateful to Zongheng Wang for critical reading of the manuscript.
Footnotes
Author contributions
N.O. contributed to the execution of experimental data. Z.L. and E.R.G. contributed to the conception, design, execution and interpretation of the data being published, and preparing the article.
Funding
This work was supported by the National Institutes of Health [grant number R01 AR060788 to E.R.G.]; and the University of Missouri, Kansas City Graduate Women's Fellowship (GAF) to Z.L. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.132241/-/DC1
References
- Bahri S. M., Choy J. M., Manser E., Lim L., Yang X. (2009). The Drosophila homologue of Arf-GAP GIT1, dGIT, is required for proper muscle morphogenesis and guidance during embryogenesis. Dev. Biol. 325, 15–23 10.1016/j.ydbio.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Baker S. E., Lorenzen J. A., Miller S. W., Bunch T. A., Jannuzi A. L., Ginsberg M. H., Perkins L. A., Brower D. L. (2002). Genetic interaction between integrins and moleskin, a gene encoding a Drosophila homolog of importin-7. Genetics 162, 285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagopalan L., Chen M. H., Geisbrecht E. R., Abmayr S. M. (2006). The CDM superfamily protein MBC directs myoblast fusion through a mechanism that requires phosphatidylinositol 3,4,5-triphosphate binding but is independent of direct interaction with DCrk. Mol. Cell. Biol. 26, 9442–9455 10.1128/MCB.00016-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A., Poukkula M., Cliffe A., Mathieu J., Luque C. M., Fulga T. A., Rørth P. (2007). Two distinct modes of guidance signalling during collective migration of border cells. Nature 448, 362–365 10.1038/nature05965 [DOI] [PubMed] [Google Scholar]
- Biersmith B., Liu Z. C., Bauman K., Geisbrecht E. R. (2011). The DOCK protein sponge binds to ELMO and functions in Drosophila embryonic CNS development. PLoS ONE 6, e16120 10.1371/journal.pone.0016120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozyczko D., Decker C., Muschler J., Horwitz A. F. (1989). Integrin on developing and adult skeletal muscle. Exp. Cell Res. 183, 72–91 10.1016/0014-4827(89)90419-9 [DOI] [PubMed] [Google Scholar]
- Brown N. H. (2000). Cell-cell adhesion via the ECM: integrin genetics in fly and worm. Matrix Biol. 19, 191–201 [DOI] [PubMed] [Google Scholar]
- Bunch T. A., Graner M. W., Fessler L. I., Fessler J. H., Schneider K. D., Kerschen A., Choy L. P., Burgess B. W., Brower D. L. (1998). The PS2 integrin ligand tiggrin is required for proper muscle function in Drosophila. Development 125, 1679–1689 [DOI] [PubMed] [Google Scholar]
- Carmignac V., Durbeej M. (2012). Cell-matrix interactions in muscle disease. J. Pathol. 226, 200–218 10.1002/path.3020 [DOI] [PubMed] [Google Scholar]
- Chanana B., Graf R., Koledachkina T., Pflanz R., Vorbrüggen G. (2007). AlphaPS2 integrin-mediated muscle attachment in Drosophila requires the ECM protein Thrombospondin. Mech. Dev. 124, 463–475 10.1016/j.mod.2007.03.005 [DOI] [PubMed] [Google Scholar]
- Clark K. A., McGrail M., Beckerle M. C. (2003). Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development 130, 2611–2621 10.1242/dev.00492 [DOI] [PubMed] [Google Scholar]
- Côté J. F., Vuori K. (2007). GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 17, 383–393 10.1016/j.tcb.2007.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek P., Somogyi K., Jékely G., Beccari S., Rørth P. (2001). Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107, 17–26 10.1016/S0092-8674(01)00502-5 [DOI] [PubMed] [Google Scholar]
- Erickson M. R., Galletta B. J., Abmayr S. M. (1997). Drosophila myoblast city encodes a conserved protein that is essential for myoblast fusion, dorsal closure, and cytoskeletal organization. J. Cell Biol. 138, 589–603 10.1083/jcb.138.3.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada B., Gisselbrecht S. S., Michelson A. M. (2007). The transmembrane protein Perdido interacts with Grip and integrins to mediate myotube projection and attachment in the Drosophila embryo. Development 134, 4469–4478 10.1242/dev.014027 [DOI] [PubMed] [Google Scholar]
- Fogerty F. J., Fessler L. I., Bunch T. A., Yaron Y., Parker C. G., Nelson R. E., Brower D. L., Gullberg D., Fessler J. H. (1994). Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila alpha PS2 beta PS integrins. Development 120, 1747–1758 [DOI] [PubMed] [Google Scholar]
- Geisbrecht E. R., Haralalka S., Swanson S. K., Florens L., Washburn M. P., Abmayr S. M. (2008). Drosophila ELMO/CED-12 interacts with Myoblast city to direct myoblast fusion and ommatidial organization. Dev. Biol. 314, 137–149 10.1016/j.ydbio.2007.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsohn E., Volk T. (2010). A screen for tendon-specific genes uncovers new and old components involved in muscle-tendon interaction. Fly (Austin) 4, 149–153 10.4161/fly.4.2.11231 [DOI] [PubMed] [Google Scholar]
- Grabbe C., Zervas C. G., Hunter T., Brown N. H., Palmer R. H. (2004). Focal adhesion kinase is not required for integrin function or viability in Drosophila. Development 131, 5795–5805 10.1242/dev.01462 [DOI] [PubMed] [Google Scholar]
- Graner M. W., Bunch T. A., Baumgartner S., Kerschen A., Brower D. L. (1998). Splice variants of the Drosophila PS2 integrins differentially interact with RGD-containing fragments of the extracellular proteins tiggrin, ten-m, and D-laminin 2. J. Biol. Chem. 273, 18235–18241 10.1074/jbc.273.29.18235 [DOI] [PubMed] [Google Scholar]
- Hakeda-Suzuki S., Ng J., Tzu J., Dietzl G., Sun Y., Harms M., Nardine T., Luo L., Dickson B. J. (2002). Rac function and regulation during Drosophila development. Nature 416, 438–442 10.1038/416438a [DOI] [PubMed] [Google Scholar]
- Ho E., Dagnino L. (2012). Epidermal growth factor induction of front-rear polarity and migration in keratinocytes is mediated by integrin-linked kinase and ELMO2. Mol. Biol. Cell 23, 492–502 10.1091/mbc.E11-07-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho E., Irvine T., Vilk G. J., Lajoie G., Ravichandran K. S., D'Souza S. J., Dagnino L. (2009). Integrin-linked kinase interactions with ELMO2 modulate cell polarity. Mol. Biol. Cell 20, 3033–3043 10.1091/mbc.E09-01-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. Y., Wu Y. C. (2010). Engulfment of apoptotic cells in C. elegans is mediated by integrin alpha/SRC signaling. Curr. Biol. 20, 477–486 [DOI] [PubMed] [Google Scholar]
- Ishimaru S., Ueda R., Hinohara Y., Ohtani M., Hanafusa H. (2004). PVR plays a critical role via JNK activation in thorax closure during Drosophila metamorphosis. EMBO J. 23, 3984–3994 10.1038/sj.emboj.7600417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H., Negishi M. (2003). RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature 424, 461–464 10.1038/nature01817 [DOI] [PubMed] [Google Scholar]
- Kinchen J. M., Ravichandran K. S. (2007). Journey to the grave: signaling events regulating removal of apoptotic cells. J. Cell Sci. 120, 2143–2149 10.1242/jcs.03463 [DOI] [PubMed] [Google Scholar]
- Kinchen J. M., Cabello J., Klingele D., Wong K., Feichtinger R., Schnabel H., Schnabel R., Hengartner M. O. (2005). Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature 434, 93–99 10.1038/nature03263 [DOI] [PubMed] [Google Scholar]
- Kocherlakota K. S., Wu J. M., McDermott J., Abmayr S. M. (2008). Analysis of the cell adhesion molecule sticks-and-stones reveals multiple redundant functional domains, protein-interaction motifs and phosphorylated tyrosines that direct myoblast fusion in Drosophila melanogaster. Genetics 178, 1371–1383 10.1534/genetics.107.083808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBeau-DiMenna E. M., Clark K. A., Bauman K. D., Parker D. S., Cripps R. M., Geisbrecht E. R. (2012). Thin, a Trim32 ortholog, is essential for myofibril stability and is required for the integrity of the costamere in Drosophila. Proc. Natl. Proc. Natl. Acad. Sci. USA. 109, 17983–17988 10.1073/pnas.1208408109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Luo L. (1999). Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 10.1016/S0896-6273(00)80701-1 [DOI] [PubMed] [Google Scholar]
- Legate K. R., Montañez E., Kudlacek O., Fässler R. (2006). ILK, PINCH and parvin: the tIPP of integrin signalling. Nat. Rev. Mol. Cell Biol. 7, 20–31 10.1038/nrm1789 [DOI] [PubMed] [Google Scholar]
- Liu Z. C., Geisbrecht E. R. (2011). Moleskin is essential for the formation of the myotendinous junction in Drosophila. Dev. Biol. 359, 176–189 10.1016/j.ydbio.2011.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. C., Geisbrecht E. R. (2012). “Importin” signaling roles for import proteins: the function of Drosophila importin-7 (DIM-7) in muscle-tendon signaling. Cell Adh. Migr. 6, 4–12 10.4161/cam.19774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löer B., Bauer R., Bornheim R., Grell J., Kremmer E., Kolanus W., Hoch M. (2008). The NHL-domain protein Wech is crucial for the integrin-cytoskeleton link. Nat. Cell Biol. 10, 422–428 10.1038/ncb1704 [DOI] [PubMed] [Google Scholar]
- Lorenzen J. A., Baker S. E., Denhez F., Melnick M. B., Brower D. L., Perkins L. A. (2001). Nuclear import of activated D-ERK by DIM-7, an importin family member encoded by the gene moleskin. Development 128, 1403–1414 [DOI] [PubMed] [Google Scholar]
- Luo L., Liao Y. J., Jan L. Y., Jan Y. N. (1994). Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8, 1787–1802 10.1101/gad.8.15.1787 [DOI] [PubMed] [Google Scholar]
- Martin-Bermudo M. D. (2000). Integrins modulate the Egfr signaling pathway to regulate tendon cell differentiation in the Drosophila embryo. Development 127, 2607–2615 [DOI] [PubMed] [Google Scholar]
- Miyamoto Y., Yamauchi J. (2010). Cellular signaling of Dock family proteins in neural function. Cell. Signal. 22, 175–182 10.1016/j.cellsig.2009.09.036 [DOI] [PubMed] [Google Scholar]
- Nolan K. M., Barrett K., Lu Y., Hu K. Q., Vincent S., Settleman J. (1998). Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 12, 3337–3342 10.1101/gad.12.21.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D., Ravichandran K. S. (2010). Emerging roles of brain-specific angiogenesis inhibitor 1. Adv. Exp. Med. Biol. 706, 167–178 10.1007/978-1-4419-7913-1_15 [DOI] [PubMed] [Google Scholar]
- Patel M., Margaron Y., Fradet N., Yang Q., Wilkes B., Bouvier M., Hofmann K., Cote J. F. (2010). An evolutionarily conserved autoinhibitory molecular switch in ELMO proteins regulates Rac signaling. Curr. Biol. 20, 2021–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M., Pelletier A., Côté J. F. (2011). Opening up on ELMO regulation: New insights into the control of Rac signaling by the DOCK180/ELMO complex. Small GTPases 2, 268–275 10.4161/sgtp.2.5.17716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schejter E. D., Baylies M. K. (2010). Born to run: creating the muscle fiber. Curr. Opin. Cell Biol. 22, 566–574 10.1016/j.ceb.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorrer F., Kalchhauser I., Dickson B. J. (2007). The transmembrane protein Kon-tiki couples to Dgrip to mediate myotube targeting in Drosophila. Dev. Cell 12, 751–766 10.1016/j.devcel.2007.02.017 [DOI] [PubMed] [Google Scholar]
- Schweitzer R., Zelzer E., Volk T. (2010). Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development 137, 2807–2817 10.1242/dev.047498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebé-Pedrós A., Roger A. J., Lang F. B., King N., Ruiz-Trillo I. (2010). Ancient origin of the integrin-mediated adhesion and signaling machinery. Proc. Natl. Acad. Sci. USA 107, 10142–10147 10.1073/pnas.1002257107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Wayburn B., Bunch T., Volk T. (2007). Thrombospondin-mediated adhesion is essential for the formation of the myotendinous junction in Drosophila. Development 134, 1269–1278 10.1242/dev.000406 [DOI] [PubMed] [Google Scholar]
- Swan L. E., Wichmann C., Prange U., Schmid A., Schmidt M., Schwarz T., Ponimaskin E., Madeo F., Vorbrüggen G., Sigrist S. J. (2004). A glutamate receptor-interacting protein homolog organizes muscle guidance in Drosophila. Genes Dev. 18, 223–237 10.1101/gad.287604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan L. E., Schmidt M., Schwarz T., Ponimaskin E., Prange U., Boeckers T., Thomas U., Sigrist S. J. (2006). Complex interaction of Drosophila GRIP PDZ domains and Echinoid during muscle morphogenesis. EMBO J. 25, 3640–3651 10.1038/sj.emboj.7601216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakaloglou K., Zervas C. (2012). Parvin-ILK: An intimate relationship. BioArchitecture 2, 91–94 10.4161/bioa.20700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T. (1999). Singling out Drosophila tendon cells: a dialogue between two distinct cell types. Trends Genet. 15, 448–453 10.1016/S0168-9525(99)01862-4 [DOI] [PubMed] [Google Scholar]
- Wang X., Wu Y. C., Fadok V. A., Lee M. C., Gengyo-Ando K., Cheng L. C., Ledwich D., Hsu P. K., Chen J. Y., Chou B. K. et al. (2003). Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12. Science 302, 1563–1566 10.1126/science.1087641 [DOI] [PubMed] [Google Scholar]
- Yan J., Mihaylov V., Xu X., Brzostowski J. A., Li H., Liu L., Veenstra T. D., Parent C. A., Jin T. (2012). A Gβγ effector, ElmoE, transduces GPCR signaling to the actin network during chemotaxis. Dev. Cell 22, 92–103 10.1016/j.devcel.2011.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitzky T., Min L., Volk T. (1997). The Drosophila neuregulin homolog Vein mediates inductive interactions between myotubes and their epidermal attachment cells. Genes Dev. 11, 2691–2700 10.1101/gad.11.20.2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervas C. G., Gregory S. L., Brown N. H. (2001). Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol. 152, 1007–1018 10.1083/jcb.152.5.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.