Abstract
Interleukin-9 (IL-9) has attracted renewed interest owing to the identification of its expression by multiple T helper (TH) cell subsets, including TH2 cells, TH9 cells, TH17 cells and regulatory T (TReg) cells. Here, we provide a broad overview of the conditions that are required for cells to produce IL-9 and describe the cellular targets and nature of the immune responses that are induced by IL-9.
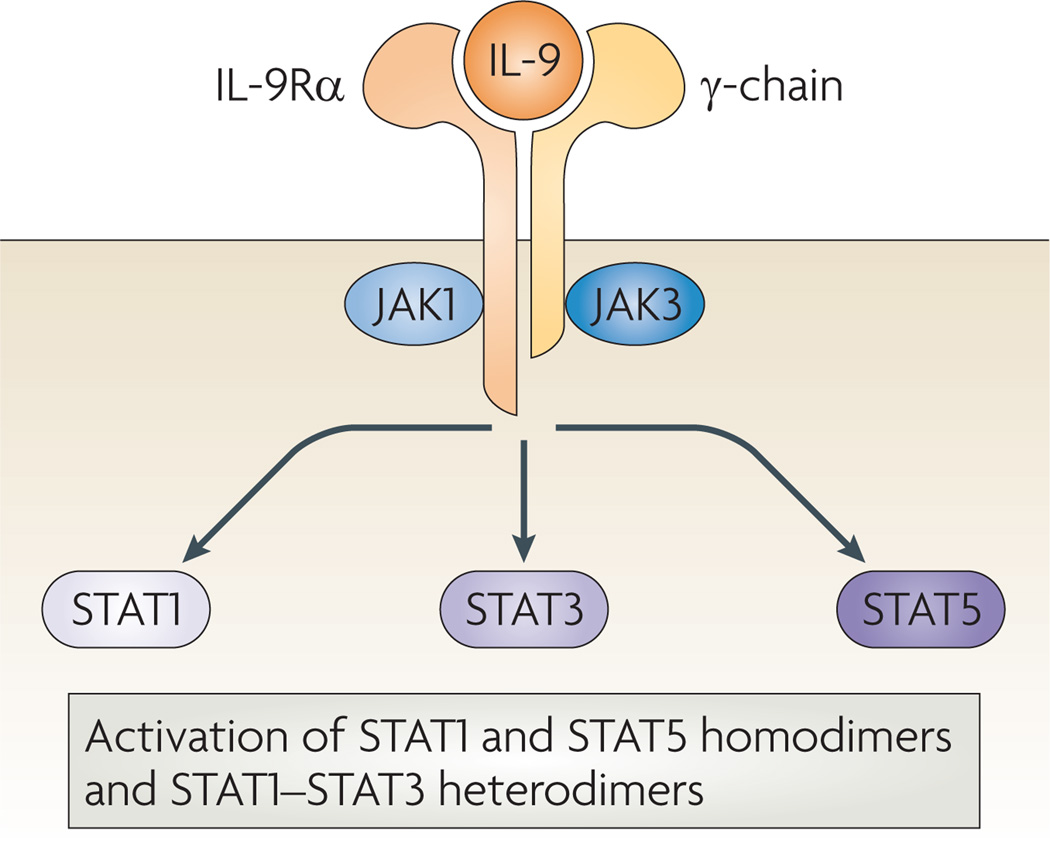
Interleukin-9 (IL-9) was originally identified in mice as a T cell growth factor and is a member of the common γ-chain-receptor cytokine family, with other members including IL-2, IL-4, IL-7, IL-15 and IL-21. The IL9 gene loci of both humans and mice have a similar organization, consisting of five exons, and share a 55% amino acid homology at the protein level. The IL-9 receptor consists of the cytokine-specific IL-9 receptor α-chain (IL-9Rα) and the γ-chain1. IL-9-induced receptor activation promotes the cross-phosphorylation of Janus kinase 1 (JAK1) and JAK3. This cross-phosphorylation leads to the downstream activation of signal transducer and activator of transcription (STAT) complexes, specifically STAT1 homodimers, STAT5 homodimers and STAT1–STAT3 heterodimers2–4 (FIG. 1). Although the contribution of IL-9 to the activation of these pathways in primary cells has not been fully elucidated, it may be of relevance in vivo.
Figure 1. The IL-9 receptor signalling complex.
Interleukin-9 (IL-9) activates a heterodimeric receptor that consists of the IL-9 receptor α-chain (IL-9Rα) and the γ-chain and promotes the crossphosphorylation of Janus kinase 1 (JAK1) and JAK3. This leads to the activation of signal transducer and activator of transcription 1 (STAT1), STAT3 and STAT5 and the upregulation of IL-9-inducible gene transcription.
Here, we summarize the cellular sources of IL-9, focusing principally on T cells. We also describe the main targets of IL-9 in vivo and discuss the downstream effects of IL-9-induced signalling on leukocyte function and overall immunity.
Cellular sources of IL-9
TH2 cells
IL-9 was first linked to T helper 2 (TH2) cells, which express IL-4, IL-5 and IL-13, following the report that levels of IL-9 expression were high in the TH2-prone BALB/c mouse strain but low in the TH1-prone C57BL/6 mouse strain during infection with Leishmania major. The same study showed that T cells were the main producers of IL-9 in vivo and that the levels of IL-9 expression correlated with the expansion of TH2 cell populations. Futhermore, blockade of IL-4, which is a crucial mediator of TH2 cell differentiation, could suppress IL-9 production5. Owing to the recent characterization of TH9 cells (which express IL-9 but not the key signature cytokines of other TH cell subsets, such as IL-4 (TH2 cells), interferon-γ (IFNγ) (TH1 cells) and IL-17 (TH17 cells)) it is now unclear whether the correlation between IL-9 levels and TH2 cell numbers is due to the direct production of this cytokine by TH2 cells or whether TH2-cell derived IL-4 supports the development of TH9 cells from either naive CD4+ T cells or TH2 cells. Currently, only one group has reported low but detectable co-expression of IL-4 and IL-9 during in vitro differentiation of TH2 cells6. Surprisingly, to date there have been no reports addressing whether these cytokines are co-expressed by effector CD4+ T cell populations that differentiate in vivo during a TH2-type immune response.
TH9 cells
TH9 cells are a recently described subset of TH cells that express IL-9 but not other TH cell lineage-specific cytokines or transcription factors7,8 (FIG. 2). The most definitive work that supports the existence and functional relevance of TH9 cells in vivo has come from the study of mice with a T cell-specific deletion of the transcription factor PU.1. These mice do not develop IL-9-dependent allergic inflammation in the lungs and have only low levels of IL-9 in bronchoalveolar lavage fluid, but still develop normal numbers of TH2 cells. This finding indicates that, at least in this setting, TH9 cells are the main T cell subset expressing IL-9 and that PU.1 is crucial for their development6. Another transcription factor that is associated with TH9 cell development both in vitro and in vivo is interferon-regulatory factor 4 (IRF4)9, which also regulates TH2 and TH17 cell differentiation10–12. Characterization of TH9 cells in vitro has shown that their generation is dependent on transforming growth factor-β (TGFβ) and IL-4 (REF. 13). The addition of IL-25 along with these two cytokines can further enhance IL-9 production by T cells in vitro. TH9 cells from IL-25-deficient mice have decreased IL-9 expression and reduced airway inflammation in a model of asthma14. There have also been conflicting reports that various cytokines, such as IL-1β, IL-6, IL-10, IL-12, IL-21, IFNα and IFNβ, may have an additive effect in promoting TH9 cell generation in the presence of TGFβ and IL-4 in vitro15,16. In addition, culturing human memory T cells in the presence of TGFβ (alone or in combination with IL-1β, IL-4, IL-6, IL-12, IL-21 or IL-23) was shown to promote IL-9 production with or without co-expression of IFNγ and IL-17 (REFS 16,17).
Figure 2. Expression of IL-9 by distinct T cell subsets.
Different differentiation pathways of effector CD4+ T cell subsets are shown, as well as the transcription factors that are necessary for their differentiation and some of the cytokines that they produce. Interleukin-9 (IL-9) has been reported to be expressed by T helper 2 (TH2), TH9, TH17 and regulatory T (TReg) cell subsets, and all of these subsets, except TH2 cells, require transforming growth factor-β (TGFβ) for IL-9 production. AHR, aryl hydrocarbon receptor; FOXP3, forkhead box P3; GATA3, GATA-binding protein 3; IFNγ, interferon-γ; IRF4, interferon-regulatory factor 4; ROR, retinoic acid receptor-related orphan receptor; TNF, tumour necrosis factor.
TH17 cells
There have also been reports that TH17 cells can produce IL-9. In vivo differentiated TH17 cells, which were identified by their expression of either IL-17F or the TH17-associated transcription factor retinoic acid receptor-related orphan receptor-γt (RORγt), could produce IL-9 after re-stimulation with phorbol 12-myristate 13-acetate and ionomycin18. In addition, some groups also reported co-expression of IL-17 and IL-9 during TH17 cell generation in vitro18,19. However, IL-23, which is important for the expansion of TH17 cells after their initial differentiation, has been shown to inhibit the expression of IL-9 by T cells in mice19; therefore, this finding suggests that the expression of IL-9 by TH17 cells could be transient and may decrease over time.
Human TH17 cells have also been shown to produce IL-9 in vitro. After multiple rounds of differentiation in vitro, some TH17 cells acquire expression of IL-9. In memory T cells, TH17-promoting cytokines, such as IL-1β and IL-21, can also enhance the co-production of IL-9 and IL-17. However, these cultures also contain IL-9-producing T cells that do not co-express IL-17 and are presumably bona fide TH9 cells16.
TReg cells
There have been contradictory reports regarding the expression of IL-9 by TReg cells. Data from mice have shown that co-expression of forkhead box P3 (FOXP3) and IL-9 can occur in TReg cells that are found in tolerant allografts in vivo20, as well as in purified TReg cell populations in vitro18,21. However, other groups did not report co-expression of FOXP3 and IL-9 in T cell cultures that were generated in vitro8,19. Similarly, there have been conflicting data from studies of TReg cells from healthy human donors15–17. As one group showed that retinoic acid can downregulate IL-9 expression by TReg cells22, a possible explanation for the discrepancy between the data is that there may be differences in the levels of retinoic acid present in cultures in vitro and in the local microenvironments in vivo.
For all of the above CD4+ T cell subsets, TGFβ seems to be the unifying factor for promoting IL-9 production; an inability to respond to TGFβ in vivo prevents IL-9 expression by T cells8. The main exception to this observation is that TGFβ does not seem to be necessary for IL-9 production by TH2 cells; however, one possible explanation for this finding could be the presence of endogenous TGFβ in the culture media that is used for TH2 cell differentiation.
Mast cells as a source of IL-9
Although mainly studied as a target of IL-9, mast cells have also been reported to produce this cytokine. Production occurs in an autocrine manner in response to IL-9-induced signals and as a consequence of the crosslinking of IgE molecules on the surface of mast cells. Crosslinking of surface IgE molecules results in mast cell degranulation and leads to the immediate release of numerous pre-formed mediators. Two of these products, histamine and IL-1β, can promote further IL-9 production23,24. As IL-9 is a growth factor for mast cells, it is thought that these pathways promote the survival and expansion of mast cells during an active immune response.
Natural killer T cells
Studies using natural killer T (NKT) cells from naive mice have shown that following stimulation with IL-2, but not IL-15, these cells can produce IL-9. IL-2 stimulation also led to some coexpression of IL-4, IL-5 and IL-13, but not IFNγ, by IL-9-producing NKT cells25. In a mouse model of allergic airway inflammation, deficiency of CD1d-restricted NKT cells correlated with decreased IL-9 expression and reduced mast cell recruitment to the lungs. This finding suggested that NKT cells can promote IL-9 responses in vivo26.
In addition, NKT cells that have undergone transformation to nasal NKT cell lymphoma cell lines also produce IL-9; in this setting, IL-9 functions as an autocrine growth factor. Histological sections from patients with nasal NKT cell lymphomas contained large numbers of infiltrating IL-9-producing NKT cells, which suggested that IL-9 may promote disease progression27. It is currently unclear whether IL-9 can also have effects on other cell types in the nasal mucosa of these patients. For example, the effects of IL-9 on mast cells might contribute to cancer progression by promoting the production of angiogenic factors that increase the vasculature of the tumour.
Immune cell targets of IL-9
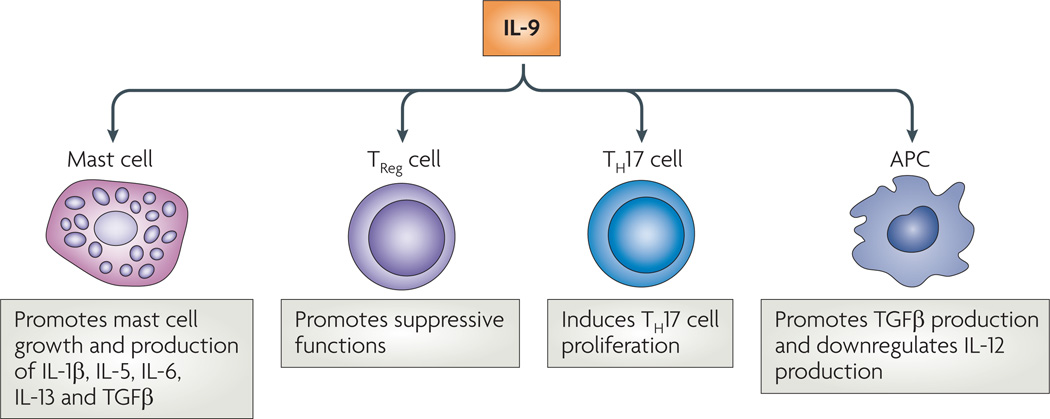
The downstream effects of IL-9 have been primarily associated with mast cells; however, this does not preclude IL-9 from exerting effects on other cell types. Here, we summarize the observed effects of IL-9 on mast cells and other cell types (FIG. 3). However, as there is currently no way to definitively confirm the expression of IL-9Rα on specific, rather than heterogenous, cell populations, it can be difficult to determine the relative importance of each individual cell type.
Figure 3. Targets of IL-9 function.
Interleukin-9 (IL-9) has been shown to have various effects on different cell types. These effects include activating mast cells to secrete several products, including IL-13, which exerts its effects on the epithelial cells of the lung and gut. In addition, IL-9 seems to have a direct effect on regulatory T (TReg) cells, T helper 17 (TH17) cells and antigen-presenting cells (APCs). TGFβ, transforming growth factor-β.
Mast cells
One of the main targets of IL-9 is the mast cell and, as mentioned earlier, initial studies described a role for IL-9 in promoting the expansion of mast cell populations28. Subsequent work in mice that were deficient for both IL-9 and IL-9Rα showed that IL-9 is not required for the generation of mast cell precursors, as the basal numbers of mast cells in these mice were normal. However, mice that were deficient for IL-9 or IL-9Rα showed defective expansion and recruitment of mast cell populations in response to intestinal nematode infection or following the induction of experimental autoimmune encephalomyelitis (EAE)18,29. IL-9 can induce mast cell production of TGFβ, which can have pro-inflammatory downstream effects on neurons in a murine stroke model and on epithelial cells during intestinal inflammation30,31. Following antigen-specific crosslinking of surface IgE molecules on mast cells, IL-9 can enhance mast cell expression of several cytokines, including IL-1β, IL-5, IL-6, IL-9, IL-10 and IL-13 (REF. 24). Of these cytokines, IL-5 and IL-13 are of particular interest, as the previously described direct effects of IL-9 in promoting eosinophilia and mucus production in the lung and the gut instead seem to be indirect effects mediated through the induction of these cytokines by IL-9 (REFS 32–36).
T cell subsets
There are some indications that IL-9 can target certain T cell subsets, specifically, TH17 cells and TReg cells. In the case of TH17 cells, IL-9 seems to function as an autocrine growth factor that facilitates the expansion of TH17 cell populations in vitro19. This is also supported by the decreased accumulation of TH17 cells that is seen in IL-9Rα-deficient mice during EAE18; however, because this is a global defect in vivo, the possibility that this is an indirect effect of IL-9 deficiency cannot be entirely dismissed. By contrast, IL-9 does not seem to facilitate the survival or expansion of TReg cells. Instead, standard suppression assays show that TReg cells from IL-9Rα-deficient mice are less able to inhibit the proliferation of effector CD4+ T cells in vitro19. This suggests that IL-9 can enhance the regulatory function of TReg cells through an unknown mechanism.
Antigen-presenting cells
Although a careful analysis of the specific antigen-presenting cell (APC) subsets that express the IL-9 receptor has not yet been carried out, there are indications that professional APCs are also targets of IL-9. During lipopolysaccharide-induced activation of a heterogenous population of macrophages and monocytes, IL-9 can promote the expression of TGFβ; this results in a decrease in the oxidative burst of these cells, as well as in decreased expression of tumour necrosis factor (TNF)37,38. In addition, the culture of peripheral blood mononuclear cells (PBMCs), which contain both T cells and monocytes, with IL-9 leads to decreased production of IL-12 and IFNγ in response to treatment with extracts from Mycobacterium tuberculosis. Under these conditions, it was also shown that the addition of IL-9-specific blocking antibodies to the cultures upregulates PBMC production of IL-12 (REF. 39). Overall, these data suggest that IL-9 might limit the capacity of APCs to induce TH1-type immune responses.
IL-9 and immunity
IL-9 can function as both a positive and negative regulator of immune responses (TABLE 1). In general, it seems that IL-9 has detrimental roles during allergy and autoimmunity. However, during parasitic infections, IL-9 can help to clear the pathogen, and during skin transplantation, IL-9 can promote the maintenance of a tolerant environment.
Table 1.
Sources and targets of IL-9 during immune responses
| Immune model | Sources of IL-9 | Targets of IL-9 | Effects of IL-9 | Refs |
|---|---|---|---|---|
| Allergy | ||||
| Allergic airway inflammation | NKT cells and TH9 cells | Mast cells | Promotes allergic inflammation | 6,9,26,33,36,40 |
| Oral antigen-induced anaphylaxis | TH2 cells and TH9 cells | Mast cells | Promotes allergic inflammation | 41 |
| Autoimmunity | ||||
| EAE | TH17 cells and TH9 cells | Mast cells and TH17 cells | Promotes EAE | 18, 42–44 |
| TH17 cells and TH9 cells | TReg cells | Inhibits EAE | 19 | |
| Type 1 diabetes | TH17 cells | Unknown | Unknown | 16 |
| Parasitic infection | ||||
| Lung infection with Schistosoma mansoni | TH2 cells and TH9 cells | Mast cells | No effect on granuloma formation | 29 |
| Intestinal infection with Trichuris muris | TH2 cells and TH9 cells | Mast cells | Promotes parasite expulsion | 45–47 |
| Transplantation | ||||
| Skin allograft transplantation | TReg cells | Mast cells and TReg cells | Promotes allograft tolerance | 20,48 |
EAE, experimental autoimmune encephalomyelitis; NKT, natural killer T; TH, T helper; TReg, regulatory T.
Allergy
During some allergic responses in the lung, which are traditionally thought of as being TH2 cell-mediated, IL-9 contributes to disease by promoting mast cell expansion and production of IL-13, which in turn promotes the release of mucus that contributes to airway hyperresponsiveness33,35,40. The initial influx of mast cells in this model is likely to be driven by IL-9 that is derived from NKT cells26. In addition, TH9 cells also seem to contribute to disease in this model, as mice with PU.1-deficient T cells and a global IL-25 deficiency are protected from allergic airway inflammation6,14. Similarly, during food allergy, IL-9 production (presumably by TH2 cells and/or TH9 cells) also contributes to mastocytosis and, indirectly, to increased intestinal permeability41.
Autoimmunity
In addition to having a pathogenic role in allergic responses in the lung and gut, IL-9 may contribute to the development of autoimmune disease. During EAE, both TH17 cells and TH9 cells have the capacity to produce IL-9. In addition, antibody-mediated blockade of IL-9, or IL-9Rα deficiency, attenuates disease progression18,42. This seems to be partly due to the direct effects of IL-9 on mast cells, as there is a decreased accumulation of these cells in the periphery18. However, in contrast to these findings, another study has reported that IL-9Rα-deficient mice develop more severe EAE19. Although these discrepancies cannot be readily explained, there is mounting evidence to suggest that IL-9 that is produced during EAE and other autoimmune diseases has pro-inflammatory effects; for example, the adoptive transfer of antigen-specific TH9 cells promotes EAE disease43, and the decrease in EAE severity that is seen following the induction of oral tolerance is associated with reduced levels of IL-9 (REF. 44). In addition, patients with diabetes were shown to have increased levels of IL-9+ TH17 cells in the blood (REF. 16).
Parasitic infections
In the context of certain nematode infections, such as Trichuris muris infection, IL-9 can promote the isolation and/or expulsion of parasites from the gastrointestinal tract. In these circumstances, it is thought that TH2 cells and/or TH9 cells are the main source of IL-9 and that the main targets of IL-9 are mucosal mast cells. IL-9 can stimulate the release of mast cell products that contribute to eosinophilia, mucus production, increased intestinal permeability and muscle contraction45–47. The net effect of these immune effector mechanisms is the physical expulsion of the parasite. By contrast, during lung parasitic infections, such as those caused by Schistosoma mansoni, IL-9-deficient mice do not show any functional defects in the formation of granulomas to isolate parasitic eggs, although decreased mucus production and mast cell accumulation are observed29.
Transplant tolerance
During the transplantation of skin allografts, TReg cells, rather than TH9 cells, seem to be one of the major sources of IL-9, which is possibly involved in the recruitment of mast cells that mediate tolerance without degranulation20,48. IL-9 may also promote the ability of TReg cells that are present at the graft site to suppress ongoing immune effector responses against the skin allograft. Currently, it is unknown whether TReg cell-derived IL-9 has a central role in promoting tolerance at other transplantation sites.
Concluding remarks
Interest in IL-9 has been renewed recently, partly fuelled by reports that IL-9 can be expressed by several distinct T cell subsets, particularly following exposure to TGFβ. However, as much of this work has been based on in vitro studies, a re-examination of the main cell sources of IL-9 during different types of immune response in vivo is needed. This is particularly true of TH2-type immune responses, as data from models of allergic lung inflammation suggest that TH9 cells, rather than TH2 cells, are the main source of IL-9 in this setting6,14. In addition, as TH17 and TReg cells have been shown to be capable of producing IL-9 during autoimmunity and transplantation, the contribution of this cytokine to these processes also warrants further investigation18–20.
Acknowledgements
This work is supported by the Medical Research Council (MRC) Centre for Transplantation, King’s College London, UK. E.C.N. is supported by a postdoctoral fellowship from the National Multiple Sclerosis Society, USA.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Contributor Information
Randolph J. Noelle, Department of Microbiology and Immunology, Dartmouth Medical School; and The Norris Cotton Cancer Center, Lebanon, New Hampshire 03756, USA Medical Research Council (MRC) Centre for Transplantation, King’s College London, King’s Health Partners, Guy’s Hospital, London SE1 9RT, UK.
Elizabeth C. Nowak, Department of Microbiology and Immunology, Dartmouth Medical School; and The Norris Cotton Cancer Center, Lebanon, New Hampshire 03756, USA
References
- 1.Renauld JC, et al. Expression cloning of the murine and human interleukin 9 receptor cDNAs. Proc. Natl Acad. Sci. USA. 1992;89:5690–5694. doi: 10.1073/pnas.89.12.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer JH, Liu KD, You Y, Lai SY, Goldsmith MA. Heteromerization of the γ chain with the interleukin-9 receptor α subunit leads to STAT activation and prevention of apoptosis. J. Biol. Chem. 1998;273:9255–9260. doi: 10.1074/jbc.273.15.9255. [DOI] [PubMed] [Google Scholar]
- 3.Demoulin JB, et al. A single tyrosine of the interleukin-9 (IL-9) receptor is required for STAT activation, antiapoptotic activity, and growth regulation by IL-9. Mol. Cell Biol. 1996;16:4710–4716. doi: 10.1128/mcb.16.9.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demoulin JB, Van Roost E, Stevens M, Groner B, Renauld JC. Distinct roles for STAT1, STAT3, and STAT5 in differentiation gene induction and apoptosis inhibition by interleukin-9. J. Biol. Chem. 1999;274:25855–25861. doi: 10.1074/jbc.274.36.25855. [DOI] [PubMed] [Google Scholar]
- 5.Gessner A, Blum H, Rollinghoff M. Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology. 1993;189:419–435. doi: 10.1016/S0171-2985(11)80414-6. [DOI] [PubMed] [Google Scholar]
- 6.Chang HC, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nature Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dardalhon V, et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nature Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veldhoen M, et al. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 9.Staudt V, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010 Jul 29; doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Brustle A, et al. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nature Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 11.Lohoff M, et al. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proc. Natl Acad. Sci. USA. 2002;99:11808–11812. doi: 10.1073/pnas.182425099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rengarajan J, et al. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J. Exp. Med. 2002;195:1003–1012. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt E, et al. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-β and IL-4, and is inhibited by IFN-γ. J. Immunol. 1994;153:3989–3996. [PubMed] [Google Scholar]
- 14.Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. Regulation of IL-9 expression by IL-25 signaling. Nature Immunol. 2010;11:250–256. doi: 10.1038/ni.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong MT, et al. Regulation of human Th9 differentiation by type I interferons and IL-21. Immunol. Cell Biol. 2010;88:624–631. doi: 10.1038/icb.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beriou G, et al. TGF-β Induces IL-9 production from human Th17 cells. J. Immunol. 2010;185:46–54. doi: 10.4049/jimmunol.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putheti P, Awasthi A, Popoola J, Gao W, Strom TB. Human CD4 memory T cells can become CD4+ IL-9+ T cells. PLoS ONE. 2010;5:e8706. doi: 10.1371/journal.pone.0008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowak EC, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J. Exp. Med. 2009;206:1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elyaman W, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc. Natl Acad. Sci. USA. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu LF, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nature Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 22.Hill JA, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+ CD44hi cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stassen M, et al. Murine bone marrow-derived mast cells as potent producers of IL-9: costimulatory function of IL-10 and kit ligand in the presence of IL-1. J. Immunol. 2000;164:5549–5555. doi: 10.4049/jimmunol.164.11.5549. [DOI] [PubMed] [Google Scholar]
- 24.Wiener Z, Falus A, Toth S. IL-9 increases the expression of several cytokines in activated mast cells, while the IL-9-induced IL-9 production is inhibited in mast cells of histamine-free transgenic mice. Cytokine. 2004;26:122–130. doi: 10.1016/j.cyto.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Lauwerys BR, Garot N, Renauld JC, Houssiau FA. Cytokine production and killer activity of NK/T-NK cells derived with IL-2, IL-15, or the combination of IL-12 and IL-18. J. Immunol. 2000;165:1847–1853. doi: 10.4049/jimmunol.165.4.1847. [DOI] [PubMed] [Google Scholar]
- 26.Jones TG, et al. Antigen-induced increases in pulmonary mast cell progenitor numbers depend on IL-9 and CD1d-restricted NKT cells. J. Immunol. 2009;183:5251–5260. doi: 10.4049/jimmunol.0901471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagato T, et al. Expression of interleukin-9 in nasal natural killer/T-cell lymphoma cell lines and patients. Clin. Cancer Res. 2005;11:8250–8257. doi: 10.1158/1078-0432.CCR-05-1426. [DOI] [PubMed] [Google Scholar]
- 28.Renauld JC, Goethals A, Houssiau F, Van Roost E, Van Snick J. Cloning and expression of a cDNA for the human homolog of mouse T cell and mast cell growth factor P40. Cytokine. 1990;2:9–12. doi: 10.1016/1043-4666(90)90037-t. [DOI] [PubMed] [Google Scholar]
- 29.Townsend JM, et al. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–583. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 30.Mesples B, Fontaine RH, Lelievre V, Launay JM, Gressens P. Neuronal TGF-β1 mediates IL-9/mast cell interaction and exacerbates excitotoxicity in newborn mice. Neurobiol. Dis. 2005;18:193–205. doi: 10.1016/j.nbd.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Miller HR, Wright SH, Knight PA, Thornton EM. A novel function for transforming growth factor-β1: upregulation of the expression and the IgE-independent extracellular release of a mucosal mast cell granule-specific β-chymase, mouse mast cell protease-1. Blood. 1999;93:3473–3486. [PubMed] [Google Scholar]
- 32.Steenwinckel V, et al. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J. Immunol. 2009;182:4737–4743. doi: 10.4049/jimmunol.0801941. [DOI] [PubMed] [Google Scholar]
- 33.Steenwinckel V, et al. IL-13 mediates in vivo IL-9 activities on lung epithelial cells but not on hematopoietic cells. J. Immunol. 2007;178:3244–3251. doi: 10.4049/jimmunol.178.5.3244. [DOI] [PubMed] [Google Scholar]
- 34.Temann UA, Ray P, Flavell RA. Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology. J. Clin. Invest. 2002;109:29–39. doi: 10.1172/JCI13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whittaker L, et al. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. Am. J. Respir. Cell. Mol. Biol. 2002;27:593–602. doi: 10.1165/rcmb.4838. [DOI] [PubMed] [Google Scholar]
- 36.Sitkauskiene B, et al. Airway allergen exposure stimulates bone marrow eosinophilia partly via IL-9. Respir. Res. 2005;6:33. doi: 10.1186/1465-9921-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilette C, et al. IL-9 inhibits oxidative burst and TNF-α release in lipopolysaccharide-stimulated human monocytes through TGF-β. J. Immunol. 2002;168:4103–4111. doi: 10.4049/jimmunol.168.8.4103. [DOI] [PubMed] [Google Scholar]
- 38.Pilette C, et al. Oxidative burst in lipopolysaccharideactivated human alveolar macrophages is inhibited by interleukin-9. Eur. Respir. J. 2002;20:1198–1205. doi: 10.1183/09031936.02.00005402. [DOI] [PubMed] [Google Scholar]
- 39.Wu B, et al. IL-9 is associated with an impaired Th1 immune response in patients with tuberculosis. Clin. Immunol. 2008;126:202–210. doi: 10.1016/j.clim.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Cheng G, et al. Anti-interleukin-9 antibody treatment inhibits airway inflammation and hyperreactivity in mouse asthma model. Am. J. Respir. Crit. Care Med. 2002;166:409–416. doi: 10.1164/rccm.2105079. [DOI] [PubMed] [Google Scholar]
- 41.Osterfeld H, et al. Differential roles for the IL-9/IL-9 receptor α-chain pathway in systemic and oral antigen-induced anaphylaxis. J. Allergy Clin. Immunol. 2010;125:469–476. e462. doi: 10.1016/j.jaci.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, et al. Neutralization of IL-9 amerliorates experimental autoimmune encephalomyelitis by decreasing the effector T cell population. J. Immunol . 2010 Aug 30; doi: 10.4049/jimmunol.1000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peron JP, et al. Oral tolerance reduces Th17 cells as well as the overall inflammation in the central nervous system of EAE mice. J. Neuroimmunol. 2010 Jun;25 doi: 10.1016/j.jneuroim.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Khan WI, et al. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect. Immun. 2003;71:2430–2438. doi: 10.1128/IAI.71.5.2430-2438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forbes EE, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J. Exp. Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richard M, Grencis RK, Humphreys NE, Renauld JC, Van Snick J. Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proc. Natl Acad. Sci. USA. 2000;97:767–772. doi: 10.1073/pnas.97.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Vries VC, et al. Mast cell degranulation breaks peripheral tolerance. Am. J. Transplant. 2009;9:2270–2280. doi: 10.1111/j.1600-6143.2009.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]