Abstract
The rapid progression from aggressive primary cancers into locally advanced and invasive and/or metastatic diseases remains a big obstacle for an early diagnosis and curative therapeutic intervention for cancer patients. The late-stage leukemias and disseminated and metastatic sarcomas, melanomas, brain tumors and epithelial cancers are the devastating diseases associated with a high rate of recurrence after treatment with the conventional clinical therapies including surgery, ionizing radiation, hormonal therapy and systemic chemotherapy, which generally lead to the death of patients. Therefore, the establishment of the molecular events underlying cancer initiation and progression into locally invasive and metastatic diseases is of major interest in basic cancer research as well as for the development of new effective clinical therapeutic options against the recurrent and lethal cancers. Recent advances have led to the identification of specific oncogenic products that are implicated in the malignant transformation of adult stem/progenitor cells into leukemic or tumorigenic and migrating cancer stem/progenitor cells during cancer progression. Of therapeutic interest, the molecular targeting of deregulated signaling elements in cancer stem/progenitor cells and their local microenvironment represents a new potential strategy for the development of more effective clinical treatments against aggressive cancers. Particularly, the combined use of chemotherapeutic drugs to eradicate cancer-initiating cells with hematopoietic stem cell or genetically-modified stem cell transplant is emerging as potential cancer treatments that hold great promise in the area of clinical cancer research. These targeting and stem cell-based therapies may offer the ultimate hope for treating and even curing the patients diagnosed with locally advanced cancers at high risk of recurrence, metastatic and/or relapsed cancers in the clinics.
Keywords: Adult stem cells, Neoplastic stem cells, Regenerative medicine, Gene therapy
Recent advancements in the early diagnostic and prognostic tests and therapeutic management of cancers by surgery, radiotherapy, hormonal therapy, and/or chemotherapy have led in last few years to a major improvement of clinical treatments and survival outcome for the patients diagnosed with diverse types of localized cancers.1–15 Unfortunately, the progression from organ-confined cancers into the locally invasive and metastatic disease states frequently leads to resistance to the conventional treatments and disease recurrence, which is lethal for the patients.8–19 This is due, in part, to the occurrence of some genetic abnormalities leading to an aberrant expression and/or activation of a complex network of oncogenic signaling elements (MYC, NF-κB, PI3K/Akt, Bcl-2, survivin and/or fusion proteins resulting from chromosomal rearrangements) and down-regulation or inactivation of tumor suppressor gene products [p53, PTEN and/or Rb] in cancer cells (Figure 1).8–18, 20–32 Particularly, the sustained activation of numerous tumorigenic signaling cascades initiated by hormones and/or distinct growth factors, cytokines and their cognate receptors in cancer stem/progenitor cells and/or their early progenies may contribute to their sustained growth, survival, invasion and/or metastatic spread during cancer progression, treatment resistance and disease relapse.9–14, 20–22, 26–38 Hence, most patients who undergo potential curative treatments for locally advanced cancers and/or disseminated disease stages may subsequently relapse due to the persistence of cancer stem/progenitor cells and/or their early progenies in primary neoplasms and/or micrometastases at distant sites.3, 10–15, 20, 22, 25, 26, 29–32, 39–48 Therefore, the development of novel therapeutic strategies to eradicate the total mass of cancer stem/progenitor cells and their further differentiated progenies is of great clinical therapeutic interest.
Figure 1.
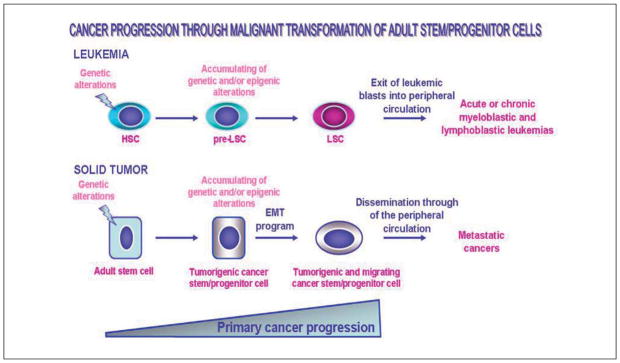
Scheme showing the potential cellular oncogenic events associated with the malignant transformation of tissue-resident adult stem/progenitor cells into cancer stem/progenitor cells during the development of leukemias and solid epithelial tumors. The genetic alterations in bone marrow-resident hematopoietic stem cells (HSC), which may generate the non-malignant intermediate hematopoietic progenitor cells, the precancerous-LSC (pre-LSC) as well as the accumulating genetic and/or epigenic alterations in these pre-LSC immature cells leading to their malignant transformation into LSCs and leukemia development, are illustrated. Moreover, the accumulation of genetic and/or epigenic alterations in the epithelium-resident stem/progenitor cells occurring during primary cancer progression and whose molecular events may result in their malignant transformation into tumorigenic stem/progenitor cells and tumor formation is indicated. The acquisition of a migratory phenotype by tumorigenic stem/progenitor cells during the epithelial-mechenchymal transition (EMT) program which may result in their dissemination through systemic circulation and metastases at distant sites is also shown.
Numerous preclinical and clinical investigations have been undertaken to develop new therapeutic strategies for improving the efficacy of current chemotherapeutic regimens against locally advanced cancers at high risk of relapse as well as metastatic and recurrent cancers. Recent significant advances have been made on the development of novel combination therapies by targeting cancer stem/progenitor cells and their progenies for counteracting cancer progression and disease relapse.3, 9–15, 20–22, 24, 26–32, 35, 36, 43, 45–47, 49–56 In general, it has been observed that the combinations of different classes of anticarcinogenic agents that target distinct oncogenic signaling elements, are generally more effective than individual drugs for eradicating the cancer-initiating cells at the primary neoplasms and distant metastatic sites.10–14, 26, 29–32, 53–57 Unfortunately, the development of diverse resistance mechanisms by cancer stem/progenitor cells and their differentiated progenies to these chemotherapeutic regimens may still lead to the most aggressive cancer forms and disease relapse.3, 4, 6, 9–15, 18, 20, 21, 25, 26, 29–32, 40, 45–48, 58–60 Of particular interest, the treatment consisting of molecular targeting therapy, high-dose chemotherapy, high-dose sequential chemotherapy or high-intensity ionizing radiation combined with a subsequent autologous or allogenic stem cell transplantation or genetically-modified stem cell-based therapy may constitute effective therapeutic strategies against the most of aggressive, metastatic and recurrent cancers.8, 11–14, 26, 29, 32, 61–66 The stem cell transplant may remedy the systemic toxicity including the myeloablative effect often associated with the use of chemotherapeutic drugs at high doses. In addition, the gene therapies using genetically-modified stem cell transplant may constitute a potential approach for specifically delivering the anti-carcinogenic agents including anti-angiogenic factors or toxins in cancer stem/progenitor cells and their early progenies.12–14, 26, 29, 32, 67–74 Among the cancer types that can be treated by these treatment types, there are relapsed/refractory leukemias, multiple myeloma and Hodgkin’s and non-Hodgkin’s lymphomas as well as locally advanced and/or metastatic solid tumors such as sarcomas, melanomas, neuroblastoma, medulloblastoma, lung, kidney, breast, ovarian, prostatic, pancreatic and colorectal cancers.11–14, 26, 29, 32, 58, 65, 66, 75–89 The choice to use these stem cell-based therapeutic strategies for treating patients with aggressive cancers, and thereby counteract the recurrence of the disease, generally depends on factors such as the availability of appropriate transplant donors for the patients, age, overall health and specific oncogenic gene expression profiling of patients. In regard with this, we describe the molecular events that are often associated with cancer initiation and transition to invasive, metastatic and recurrent states. The emphasis is on the genetic and/or epigenic alterations occurring in leukemic or tumorigenic cancer stem/progenitor cells during cancer progression. Of particular interest, we also review recent advances on the development of therapeutic approaches consisting of the molecular targeting of cancer stem/progenitor cells and their local microenvironment, cellular replacement and gene therapies that may be used for treating and even curing the aggressive and recurrent cancers.
New concepts on the implications of cancer stem/progenitor cells in cancer progression and disease relapse
Numerous recent lines of evidence have revealed that the accumulation of genetic and/or epigenetic alterations occurring in the tissue-resident adult stem cells and/or their early progenies may contribute to their malignant transformation into leukemic or tumorigenic and migrating cancer stem/progenitor cells during cancer initiation and progression (Figure 1).10–14, 22, 26, 29–32, 34–37, 41, 44, 45, 47, 90, 91 More specifically, the highly leukemic or tumorigenic cancer stem/progenitor cells expressing the stem cell-like markers, such as CD133, CD44, Oct-3/4, c-KIT and/or ATP-binding cassette (ABC) multidrug transporters, have recently been isolated from leukemias and primary and/or secondary neoplasms from patients with skin, brain, gastrointestinal tract, pancreas, liver, breast, prostate and ovarian cancers as well as established cancer cell lines.10–14, 26, 29–32, 34, 39, 48, 91–113 These multipotent and poorly differentiated cancer stem/progenitor cells also designated as cancer- or tumor-initiating cells were able to give rise to the total cancer cell mass in vitro and to reconstitute the patient’s original leukemia or tumor in animal models in vivo.39, 90–92, 94–103, 105, 114, 115 Importantly, certain experimental lines of evidence have also indicated that the cancer stem/progenitor cells, which express high levels of anti-apoptotic factors, ABC multidrug efflux pumps and enhanced DNA repair mechanisms could be more resistant than their differentiated progenies to radiation, hormonal and/or chemotherapeutic treatments.10–15, 20–22, 25, 26, 29–32, 42–44 Hence, these immature cancer-initiating cells can then provide major functions in cancer progression to the aggressive and metastatic disease states, and resistance to current clinical treatments and disease relapse.10–14, 20, 21, 25, 26, 29–32, 39, 40, 45–48, 59, 60 In this matter, the Authors are reporting the molecular events that are often associated with the early and late stages of cancer progression and treatment resistance by focusing on the critical implications of accumulating genetic abnormalities and the activation of diverse growth factors and cytokines in the malignant transformation of cancer stem/progenitor cells and/or their early progenies.
Cellular origin and clinical treatments of aggressive cancers
The precise establishment of the cellular origin and molecular events involved in cancer initiation and transition into locally aggressive, metastatic and recurrent disease stages is of major importance for the development of new effective diagnostic and prognostic methods and therapeutic interventions for cancer patients. Some recent studies revealed that the genetic and/or epigenic alterations leading to an aberrant activation of diverse developmental signaling cascades, which are involved in stringent control of the self-renewal and/or differentiation of tissue-resident adult stem cells, may occur with aging or during intense injuries such as inflammatory atrophies and fibrosis, and thereby promote leukemia or tumor initiation (Figure 1).10–15, 20, 22, 25, 26, 29–32, 42, 116 Furthermore, the accumulation of different genetic and/or epigenic alterations in cancer stem/progenitor cells may also confer to them a more malignant behavior during cancer progression and may be associated with the occurrence of highly aggressive cancer subtypes.10–15, 26, 29–32, 36, 46, 47, 59, 96, 97, 117 More specifically, the activation of diverse tumorigenic cascades initiated through distinct growth factors, cytokines and integrins such as epidermal growth factor (EGF)-EGFR system, hedgehog, Wnt/β-catenin, Notch, stem cell factor (SCF)/KIT, platelet-derived growth factor (PDGF)/PDGFR, stromal cell-derived factor-1 (SDF-1)-CXC chemokine receptor, transforming growth factor-β (TGF-β/TGF-βR) and/or integrin pathways in cancer stem/progenitor cells and their progenies may contribute to their sustained growth, survival, invasion and/or drug resistance.10–15, 26, 29–32, 36, 46, 47, 59, 96, 97, 117, 118 In addition, cancer progression is also accompanied by changes in the specialized microenvironment, niche of cancer stem/progenitors cells and stromal components, including host cells such as activated myofibroblasts, stellate cells and/or immune cells.10–14, 26, 29–32, 119–123 The release of diverse soluble growth factors and cytokines by host stromal cells may notably contribute in a paracrine manner to the acquisition of a more malignant behavior by cancer stem/progenitors cells. In regard with this, we describe the recent observations indicating the critical functions of cancer stem/progenitors cells in cancer progression and disease relapse with a particular emphasis on the cellular origin of leukemias, sarcomas, cutaneous and non-cutaneous melanomas, brain tumors and diverse epithelial cancers as well as novel molecular targeting and stem cell-based therapeutic approaches for the treatment of aggressive and recurrent cancer subtypes.
Cellular origin and clinical treatments of leukemias
Leukemias are the types of hematological malignancies that arise from the genetic abnormalities occurring in the bone morrow (BM)-resident primitive immature hematopoietic stem cells (HSCs) or more committed lymphoid or myeloid precursors. The accumulation of genetic alterations may result in the generation of precancerous- leukemic stem/progenitor cells (pre-LSCs) also designated as precancerous stem cells (p-CSCs) followed by their malignant transformation into leukemic stem/progenitor cells (LSCs), showing abnormal proliferation and differentiation abilities (Figure 1).4, 5, 11–14, 26, 32, 92, 115, 124–127 These molecular events subsequently culminate to the release of unfunctional leukemic blasts from BM into the bloodstream and their dissemination through the body’s circulatory system. Among the leukemia types, there are acute and chronic lymphoid/lymphocytic/lymphoblastic leukemias (ALLs and CLLs) leading to deregulated lymphoid cell lineage and acute and chronic myeloid/myelogenous/myeloblastic leukemias (AMLs or CMLs) that are accompanied by the genetic alterations in he myeloid cell lineage in BM, and which lead to an accumulation of the unfunctional blood cells. In most cases, the development of leukemia is accompanied by specific chromosomal translocations in immature hematopoietic stem/progenitor cells that generate the abnormal fusion proteins as well as enhanced expression and activation of some hematopoietic growth factor and cytokine signaling cascades [hedgehog, Wnt/β-catenin, Notch, KIT and/or FMS-like tyrosine kinase 3 (FLT3) pathways] which may contribute to their malignant transformation.11–14, 26, 32, 35, 43, 51, 127, 128 The accumulation of different genetic alterations in hematopoietic stem/progenitor cells generally results in more aggressive leukemia forms that are less responsive to current chemotherapeutic treatments and associated with a higher rate of cancer recurrence and death.6, 8, 11–14, 17,18, 25, 26, 32, 51, 127
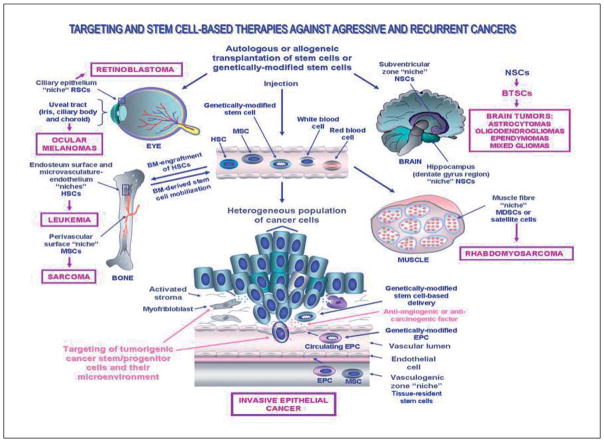
The clinical treatment of leukemias may consist of chemotherapy, radiotherapy, molecular targeting therapy without or with allogeneic stem cell transplantation combined with immunosuppressive therapy (Figure 2).11–14, 26, 32, 58, 76, 84, 85, 116 More specifically, the use of cytoreductive conditioning regimens, such as high-dose chemotherapy or ionizing radiations plus allogeneic stem cell transplantation, is generally performed as therapeutic intervention for treating the more aggressive leukemia forms at the advanced and late stages of disease or relapsed/refractory leukemias. The myeloablative treatment, which usually consists of a combination of chemotherapeutic drugs such as cyclophosphamide with busulfan or total body irradiation, permits eradicates the leukemic cells.13, 66, 76, 129 The transplantation procedure is subsequently carried out with isolated BM- or PB-derived HSCs that have been collected from BM or by aphaeresis after their mobilization into PB by using a granulocyte-stimulating factor (G-CSF), granulocyte colony-stimulating factor (GM-CSF) and/or synthetic chemical compounds like bicyclam derivative, AMD 3100 (Plerixafor) (Figure 2).13, 130, 131 Alternatively, bank-stored umbilical cord cells, including UC blood and placenta cells and fetal tissues, may also be used as a HSC source for an allogeneic transplantation procedure when no appropriate graft donor is available.11, 13, 132–134 In regard with this, UC blood also contains a substantial amount of CD16−/CD56+ natural killer cells that might be expanded in the presence of interleukin, IL-12 or IL-15 and that display a high rate of proliferation and cytotoxic effects against some cancers, and more particularly leukemias.135 In fact, the intravenously infusion of allogeneic stem cell transplant generally leads to the engraftment and homing of immature HSCs to BM. (Figure 2).11, 13, 65, 116 Hence, the new-engrafted HSCs can occupy the empty niche, and thereby this allows for the replacement of the HSCs and their progenies destroyed by the myeloablative treatment such as high-dose chemotherapy or total body irradiation. The BM-engrafted HSCs can repopulate the hematopoietic cells in BM and produce new functional blood cells, and thereby improve the immune response.13, 65 Alternatively, autologous stem cell transplant after purging the leukemic cells or purified HSC transplant may also occasionally be used when no appropriate donors are available or for old patients or patients with co-morbidities.13, 84, 136 Autologous transplantation offers the advantage of reducing the graft rejection and infection and to prevent the risk of the graft-versus-host disease and the immunogenic effect usually associated with allogeneic transplantation. More recently, it has also been reported that other treatment types consisting of reduced-intensity myeloablative conditioning regimens such as intermittent chemotherapy or mini-transplant by using smaller doses of chemotherapeutic drugs could be used in certain cases for the patients at high risk of developing severe secondary effects with the conventional myeloablative treatments.13, 136 Moreover, the graft-versus-host disease, including the inflammatory effect, which is mediated usually by T cells from a donor, may also be prevented by retrieving T cells of the graft prior to the transplantation.137 In addition, the chemoprotection against myelotoxicity induced by cytoreductive treatment may also be counteracted by genetic manipulations in HSCs or other hematopoietic cells, conferring them and their progenies resistance to certain cytotoxic effects of drugs, such as the expression of multidrug resistance-1 (MDR1) gene product, P-glycoprotein.138
Figure 2.
Scheme showing the potential targeting and stem cell-based therapeutic applications in cellular and gene therapies against aggressive and recurrent cancers. The clinical treatment consisting of an injection of an autologous or allogeneic stem cell transplant of bone marrow (BM)-derived stem cells, peripheral blood (PB) HSCs or genetically-modified stem cells into diseased areas or peripheral circulation in the same patient or a host patient diagnosed with locally advanced or metastatic cancer is illustrated. The anatomic localization of the tissue-resident adult stem/progenitor cells and their putative niches in the human adult eye, bone, brain, muscle and epithelium as well as their malignant transformation into cancer stem/progenitor cells are shown. The aggressive cancers including leukemia, sarcomas, brain tumors and invasive epithelial cancer that might benefit the targeting and/or stem cell-based therapies are also indicated. BTSCs, brain tumor stem cells; EPC, endothelial progenitor cell; HSCs, hematopoetic stem cells; MDSCs, muscle-derived stem cells; MSCs, mesenchymal stem cells; NSCs, neural stem cells and RSCs, retinal stem cells.
For instance, CML is a myeloproliferative disease that is characterized in the most cases by the chromosomal translocation t(9;22) (q34;q11) called the Philadelphia (Ph+) chromosome.12, 13, 16, 26, 139 This chromosomal rearrangement generate a constitutively activated BCR-ABL fusion oncoprotein endowed with a tyrosine kinase activity which mediated its transforming effect, at least, in part, by speeding up cell division and inhibiting DNA repair. The treatment of patients with BCR-ABL+ (Ph+) CMLs consisting of molecular targeting of BCR-ABL protein by using a specific inhibitor of its tyrosine kinase activity such as imatinib mesylate, dasatinib or nilotinib may lead to a complete remission in the early chronic phase of the disease.7, 8, 12, 13, 16–18, 26 Unfortunately, the persistence of primitive quiescent CML-LSCs or the occurrence of additional genetic abnormalities during disease progression to an accelerated phase and ultimately to a terminal phase, blast crisis may result in treatment resistant, disease relapse and a high rate of fatalities.8, 12, 13, 16–18, 26, 46, 51, 127, 139–141 At the late stages of disease progression, including blast cryptis, the combination of diverse agents targeting distinct oncogenic cascades or high-dose chemotherapy plus stem cell transplantation may thus represent the more effective therapeutic options.12, 13, 16–18, 26, 139, 142, 143 Similarly, the Ph+ AMLs and FIP1L1-PDGFRA positive chronic eosinophilic leukemia may also be treated by using the agents like imatinib mesylate.7, 16, 18 Moreover, acute promyelocytic leukemia (APL), which is an AML subtype derived from a t(15;17) translocation, may generate a promyelocytic leukemia-retinoic acid receptor α (PML-RARα) fusion protein that induces an inhibition of myeloid differentiation.144 Although the patients diagnosed with APL and other AML subtypes generally respond in the early stages to clinical therapy such as the combination of all-trans-retinoic acid (ATRA) for APL or cytarabine (ara-C) for AMLs plus anthracycline-based chemotherapy, the disease often progresses to the more aggressive leukemia forms that are resistant to these treatment types.4–6, 12, 13, 26 In fact, although a high rate of patients may show a complete remission with these chemotherapeutic treatments, the persistence of immature AML cells at an undetectable level with the available diagnostic methods may lead at a recurrence of leukemia. For patients at high risk of relapse or with refractory-relapsed AMLs, a potentially curative therapeutic treatment option consists then of using ATRA plus other cytotoxic drugs such as arsenic trioxide for APL or high-dose chemotherapy plus allogeneic stem cell transplants to eradicate the residual AML cells, thereby to achieving a potential curative treatment of patients.4–6 Importantly, the data of a recent study have also indicated that BM-resident MSCs isolated from patients with acute myeloid leukemia (AML) may exhibit abnormal biological properties including a limited proliferation capacity and impaired differentiation ability.145 Therefore, these observations underline the importance of providing new functional MSCs endowed with the immunomodulatory properties in BM-derived cell-based transplantation therapies for restoring the normal hematopoiesis in patients with AML.65
Cellular origin and clinical treatments of sarcomas
The sarcomas are the malignant solid tumors derived from connective or supportive tissues surrounding the organs, and which may originate at some parts of the body including in the bone, cartilage, muscles, fat, fibrous tissues, blood vessels, lymph vessels and nerve tissues.64, 146–154, 154–164 Although the causes of the sarcoma development are not precisely known, it has been reported that certain genetic alterations, including specific mutations, inherited disorders, viral infection and/or high level of inflammation, can increase the risk of developing sarcomas. Among the most common forms of sarcoma occurring commonly in adults, there are the gastrointestinal stromal tumors (GISTs), which may arise in the gastrointestinal tract including the wall of the stomach, intestines, rectum or esophagus. GISTs are thought to originate from the interstitial cells of Cajal (ICCs) or a precursor of these cells found in the wall lining of the GI tract constituting a part of the autonomic nervous system, and which act as the pacemaker cells in controlling intestinal motility.148, 150–152 GIST progression may result in the dissemination and metastatic spread of these cancer cells at diverse sites such as the liver, omentum and peritoneal cavity.
Other types of sarcomas, which occur more frequently in children than in adults, also include the bone cancers such as osteosarcoma and Ewing’s sarcoma, retinoblastoma and rhabdomyosarcoma (Figure 1). More specifically, several lines of evidence have revealed that the bone osteoblastic and Ewing’s sarcomas, which are among the most aggressive mesenchymal malignancies in childhood and young adults, may originate from the malignant transformation of primitive stem cell-like MSCs.12, 146, 147, 149, 153–155 The osteoblastic sarcoma development, which is often accompanied by germinal mutations in p53 and Rb tumor suppressor genes, may notably result in the aggressive cancer forms that can metastasize at distant sites including the lungs.146, 147, 153, 165, 166 In the case of Ewing’s sarcoma, which is a member of Ewing’s family tumors (EFTs), the occurrence of a chromosomal translocation resulting in EWS-FLI-1 fusion oncoprotein that acts as an aberrant transcriptional activator, appears to contribute to tumor development.154 Additionally, the ocular retinoblastoma appear to arise from tumorigenic retinal cells with properties like multipotent retinal stem/progenitors (RSCs) in the nerve tissues at the back of the eye (Figure 2).156–159 The ocular retinoblastoma, which are often accompanied by leukocoria (white pupil) and misaligned eyes (strabismus) usually occurs in young children and its causes may be hereditary or nonhereditary (sporadic).160 The retinoblastoma is generally associated with the mutations in the Rb tumor suppressor gene that gives an unfunctional Rb gene product. In fact, the mutant Rb protein remains always under its phosphorylated form and it does not interact with E2F transcription factor. Thereby, cell division occurs and leads to the malignant transformation of cells. The ocular retinoblastoma progression may lead to metastatic spread and the formation of diverse secondary cancers including osteosarcoma and Ewing’s sarcoma and usually has a poor prognosis.167, 168
In general, the treatment of the sarcoma depends on the location of the disease and the aggressiveness and grade of the tumors at the time of diagnosis. The localized and low grade sarcomas are usually treated by surgery, radiation therapy and/or chemotherapy, while high grade sarcomas including metastatic and relapsed sarcomas are treated by radiation, chemotherapy, alone or in combination with stem cell transplantations.61–63, 87, 160, 169 Moreover, the treatment of metastatic GISTs, which frequently harbor the activating mutations in KIT (also known as CD117) and PDGFRA tyrosine kinase receptors, may also include diverse molecular targeting-based therapies including the use of agents such as dual KIT/PDGFA tyrosine kinase inhibitor, imatinib mesylate or sunitinib.148, 150–152, 170, 171
In addition, embryonic, alveolar and pleomorphic forms of rhabdomyosarcoma are rare types of sarcoma occurring principally in muscle tissues in children and younger adults. The rhabdomyosarcoma appear to arise of genetic and/or epigenic alterations in the embryonic muscle precursor or adult muscle-derived stem cells (MDSCs) and/or satellite cells that results in a defective skeletal muscle proliferation and differentiation.32, 161–164 More particularly, different genetic alterations occurring in the p53 and Rb tumor suppressor genes as well as the activation of MYCN and Ras proto-oncogenes, human telomerase reverse transcriptase (hTERT) catalytic subunit and/or different growth factor cascades such as hedgehog may lead to different rhabdomyosarcoma subtypes.161, 164 The treatment of localized rhabdomyosarcomas primarily consists of chemotherapy, radiation therapy and in certain cases, surgery, when the tumor is accessible, alone or in combination. When the tumor is not resectable or has metastasized, the long-term survival of patients is poor and the disease remains incurable with these treatments. Another potential therapeutic approach to manage the metastatic rhabdomyosarcomas may thus consist of the high-dose chemotherapy and/or stem cell-based therapies such as the use of genetically-modified MSCs that can act as a carrier to specifically deliver the toxic gene products in MDSC or satellite cell-derived tumor cells.63, 64, 66 Interestingly, the results from a recent preclinical study have also revealed the possibility of using the pharmacological agents inhibiting the cyclin D-dependent Cdk4 and Cdk6 kinase activity to possibly promote the myogenic differentiation in rhabdomyosarcoma, and thereby inhibit the tumor growth.172
Cellular origin and clinical treatments of cutaneous and non-cutaneous melanomas
Although the localized cutaneous melanomas diagnosed in the early stages are usually curable by surgical resection of malignant tumors, invasive melanomas that have penetrated deeper into the skin and spread to distant sites represent one of the most aggressive form of skin cancer that may cause the death of the patients.3, 173 Cutaneous melanomas appear to arise from the malignant transformation of adult pluripotent epidermal neural crest stem cells (eNCSC) or their progenitors localized in bulge areas of hair follicles into tumorigenic cancer progenitors, and whose cells are the precursors of the pigmented cells, melanocytes that produce the pigment melanin that give the colors to our skin, hair, and eyes.3, 11, 12, 105 Moreover, the change in the stromal environment of tumor cells including the activation of fibroblasts and their conversion into myofibroblasts may also contribute to melanoma progression.174
In general, an enhanced risk for an individual to develop cutaneous melanomas depends on several factors including sun exposure, familial genetic predisposition, such as the genetic alterations in BRAF (v-raf murine sarcoma viral oncogene homolog B1), p53 and/or p16 genes, and the activation of growth factor cascades, such as hedgehog and Notch pathways.3, 173, 175 The potential management of invasive, metastatic and/or relapsed melanomas may consist of metastasectomy, chemotherapy such as dacarbazine (DTIC), alone or in combination with other chemotherapeutic drugs and radiotherapy without or plus stem cell-based transplantation therapies.3, 173 Moreover, certain patients with aggressive and incurable melanomas may also choose to enroll in novel clinical experimental trials with new treatments such as immunotherapy-based melanoma vaccines, chemoimmunotherapies with immunosuppressive agents such as interferon-α, gene therapies and anti-angiogenic therapy (thalidomine, angiostatin and endostatin), which represent the promising research areas on developing novel melanoma treatments.176 In regard with this, it has recently been reported that the melanoma stem cells can be efficiently targeted by using cancer testis antigens (CTA)-directed immunotherapeutic strategies.177 Moreover, the combined treatment with 5-fluorocy-tosine plus engineered NSCs expressing cytosine deaminase, which acts as a pro-drug activating enzyme, resulted in a significant reduction in the tumor border in animal models with established melanoma brain metastasis in vivo.72
In addition, the non-cutaneous malignant melanomas may also originate in different body compartments such as soft tissues and eyes and be associated with specific genetic alterations and changes in the stromal microenvironment.174, 178–183 More specifically, the most common non-cutaneous melanomas, uveal melanomas are rare and aggressive intraocular primary cancers of the ureal tract, which is the pigmented layer of the eye that includes the iris, ciliary body, and choroids (Figure 2). Uveal melanomas are thought to derive from the neuroectodermal-like cells that give rise to the melanocytes in the pigmented layer of the uveal tract of the eyes. The potential curative treatments of uveal melanomas may consist of local tumor resection, radiotherapy, and ultimately to the enucleation procedure. Although the small and localized intraocular melanomas are generally not aggressive and curable by these therapeutic intervention types, the locally advanced and invasive forms, and more particularly the ciliary body or choroidal melanomas, are very serious and devastating diseases that may cause blindness and result in metastatic and lethal disease stages. The poor prognosis of patients with aggressive uveal melanomas underlines the need to further identify their precise cellular origin as well as the molecular mechanisms leading to the metastatic forms in order to offer ultimate hope for tumor control and vision preservation.179 In respect with this, the molecular targeting of diverse oncogenic signaling elements including receptor tyrosine kinases, PI3K/Akt, CXCR4 and angiogenic factors involved in the uveal melanoma progression and metastasis to the liver, offers great promise for developing new combination therapies against the aggressive and metastatic uveal melanomas.178–184
Cellular origin and clinical treatments of brain tumors
The embryonic, pediatric and adult brain tumors include distinct cancer subtypes with different cellular origins which may be associated with the inherited disorders and specific genetic alterations occurring in primitive stem/progenitor cells in the developing brain or adult central nervous system (CNS).12, 22, 185–187 Among the brain tumors, some arise from embryonic neuroectodermal stem cells during embryogenesis (neuroblastoma, pheochromocytoma, ependymoblastoma and pineoblastoma) as well as from neural stem cells (NSCs) and/or more committed neuronal or glial cell lineage precursors (astrocytomas, oligodendrogliomas, ependymomas and mixed gliomas) in post-natal and adult life (Figure 2).12, 22, 37, 47, 58, 94–97, 104, 185–189 Brain tumors are generally constituted by a heterogeneous population of cancer cells containing a mixture of the astrocytes, oligodendrocytes and/or ependymal cell-like tumor cells in different proportions.190, 191 The malignant transformation of NSCs or their early progenies into brain tumor stem cells (BTSCs) may be accompanied by the sustained activation of distinct mitotic cascades such as EGF-EGFR, SHH-PTCH, Wnt/β-catenin or and/or Notch pathways as well as the changes in their local microenvironment, niche.11, 12, 22, 32, 45, 189, 192 For instance, the primary glioblastoma multiformes (GBMs), which are among the most aggressive brain tumor forms, are frequently associated with the overexpression of EGFR.12, 22, 47, 96, 97, 193 In contrast, secondary GBMs are usually less aggressive cancers and usually progress more slowly from low-grade tumors, as compared to primary GBMs.12, 22, 47, 194 Secondary GBMs appear to be often related with the occurrence of p53 tumor suppressor gene mutations. Hence, these two GBM forms have different therapeutic management in the clinical practice.
The therapeutic treatment of the brain tumors in the clinics largely vary with the cancer subtype, its anatomic localization and grade at the time of diagnosis and may include surgery, radiotherapy and chemotherapy and stem cell transplant, delivered alone or in combination.12, 58, 66, 77, 80, 86, 88 The targeted therapy with new drug classes that are able to penetrate the blood-brain barrier such as temozolomide or nitrosourea agents, such as carmustine (also called BCNU) and lomustine (CCNU), may also be used for treating patients with aggressive and recurrent brain tumors. In regard with this, certain recent experimental studies have also revealed the potential benefit of targeting the developmental signaling cascades such as hedgehog, EGFR, Wnt/β-catenin, Notch pathways or CDK1 and CDK2 checkpoint kinases by using the specific inhibitor, alone or in combination therapy to eradicate BTSCs, thereby improving the current therapies.12, 26, 30–32, 49, 59, 195 For instance, it has been observed that a selective inhibitor of the smoothened (SMO) hedgehog signaling element, cyclopamine alone inhibited the self-renewal capacity of CD133+ glioma cancer stem cell cultures established from human GBM tumor samples in vitro.195 Moreover, the use of cyclopamine at a lower concentration in combination with the current therapeutic drug, temozolomide also induced an additive or synergistic anti-proliferative and apoptotic effect on the gliomasphere cells.195 Importantly, a long-term cyclopamine treatment of gliomasphere cells expressing a high expression of the stemness genes (PTCH1 receptor, GLI1 transcription factor, Nanog, Oct-4, SRY-box containing gene 2 “SOX2”, BMI1 polycomb ring finger oncogene and proliferating cell nuclear antigen “PCNA”) also eradicated all of these BTSCs in culture, and induced the regression of glioma tumors established from the gliomasphere cells in nude mice in vivo, without systemic toxicity.195 Interestingly, it has been observed that the treatment of the mice bearing orthotopic U87 glioma cell xenografts with anti-VEGF monoclonal antibody, bevacizumab markedly reduced the microvasculature density and tumor growth of vessel-associated self-renewing CD133/nestin expressing BTSCs.196 Additionally, the genetically-modified migrating NSCs or other stem cell types, which are able to migrate through the CNS and reach the extracranial neoplastic sites also offer great promise for specifically delivering the cytotoxic drugs to the brain tumor site.64, 67–69, 71, 72 As a matter of fact, it has been observed that the transplantation of fetal-derived NSCs engineered to express IL-12 or tumor necrosis factor-α (TNF-α) related apoptosis inducing ligand (TRAIL), resulted in their specific recruitment within intracranial glioma, and the release of therapeutic gene product concomitant with an inhibition of tumor growth.69, 73, 74
Cellular origin and clinical treatments of epithelial cancers
The malignant solid tumors derived from multipotent adult stem/progenitor cells in epithelium represents the most common cancer group and includes the skin, head and neck, thyroid, lung, cervical, renal, liver, gastrointestinal, colon, bladder, pancreatic, breast, ovarian and prostatic cancers.10, 14, 26, 29–32, 34, 36, 39, 42, 90, 91, 98–103, 106, 110–113, 117, 197–204 The epithelial cancers generally result from the accumulation of distinct genetic and/or epigenic alterations occurring in adult stem/progenitor cells resident within the basal compartment near the epithelial basement membrane concomitant with the changes in their local microenvironment that lead to in their transformation into tumorigenic cancer stem/progenitor cells with abnormal proliferation and differentiation abilities (Figure 1).10–15, 26, 29–33 Moreover, the tumorigenic cancer stem/progenitor cells may also acquire a migratory phenotype during the epithelial-mesenchymal transition (EMT) program whose molecular event may lead to their invasion in the stromal compartment and metastatic spread at distant tissues/organs in the body.10–14, 26, 29–32
Although the localized cancers may be successfully treated by tumor resection, radiotherapy, hormonal therapy and/or chemotherapy, the invasive and metastatic forms are the aggressive diseases associated with the development of resistance to current therapeutic options with a high rate of recurrence and death of cancer patients.10–14, 26, 29–32 The combined use of adult stem cell- or genetically-modified stem cell transplant or the mobilization of HSCs, alone or in combination therapies with high-dose chemotherapy or ionizing radiation, may thus constitute potential therapeutic strategies for treating and curing the aggressive, metastatic and recurrent cancers such as kidney, lung, pancreatic, breast, ovarian and prostatic cancers.11, 13, 58, 65, 66, 75, 76, 82–86, 88, 89, 205 However, the timing of the injection of HSCs during disease progression as well as the number of grafted cells are among the major factors influencing the success rate and survival of patients.
Of therapeutic interest, the recent identification of diverse deregulated growth factor cascades (EGF-EGFR. hedgehog, Wnt/β-catenin and/or Notch), oncogenic signaling elements (telomerase, Scr, Bcl-2, NF-kB, PI3K/AKT and/or COX-2), ABC multidrug efflux pumps and DNA repair mechanisms that provide a critical function for the sustained growth, survival, invasion, metastases and/or treatment resistance of cancer stem/progenitor cells offers the possibility of targeting these signaling elements (Figure 2).11–13, 20, 26, 30, 31, 33, 45, 206 This should allow us to eradicate the cancer-initiating cells, thereby improving the current clinical therapies and preventing the disease relapse. The targeting of the local microenvironment of cancer stem/progenitor cells, including the host cells such as myofibroblasts and immune cells that support their malignant transformation as well as the use of anti-angiogenic agents may also constitute an adjuvant treatment for counteracting the cancer progression to metastatic and lethal disease states (Figure 2).12, 13, 26, 30, 31
In addition, the use of specific delivery techniques for the administration of anti-carcinogenic drugs in tumor may also constitute promising approaches for targeting cancer stem/progenitor cells, and their early progenies which express specific biomarkers and oncogenic elements. Among the available strategies, there is the targeted delivery of therapeutic agents into tumors by using the conjugation/fusion of drugs to tumor-specific antibodies, encapsulation of chemotherapeutic drugs in liposomes, or other carriers such as nanoparticules, as well as the use of genetically-engineered stem/progenitor cells as vehicles.207–210 More specifically, gene therapies by using genetically-modified stem cells as carriers for the delivery of anti-angiogenic or cytotoxic agents at specific tumoral sites represent, promising strategies for treating numerous aggressive and metastatic cancers (Figure 2).11, 67, 69, 71
Conclusions and perspectives
Recent advances on tissue-resident adult stem/progenitor cell biology and their malignant counterpart, cancer stem/progenitor cells, has significantly improved our understanding of the molecular events that may contribute to cancer initiation and progression to aggressive and metastatic disease stages, resistance to current clinical treatments and cancer recurrence. Further investigations are however necessary to further elucidate the causes leading to leukemia and solid tumor development. Specifically, it will be important to establish the oncogenic gene expression patterns specific to each cancer stem/progenitor cell type in order to identify better diagnostic biomarkers and prognostic indicators. These additional studies should allow us to develop new methods for preventing disease relapse or detecting residual disease after treatment. The development of new combination therapies by molecular targeting the cancer-initiating cells and their local microenvironment and stem cell-based therapies is also of great clinical therapeutic interest for developing new effective treatments for curing the aggressive, metastatic, recurrent and lethal cancers.
Acknowledgments
The Authors of this manuscript are supported by the grants from the US Department of Defense (PC04502, OC04110) and the National Institutes of Health (CA78590, CA111294). We thank Ms. Kristi L. Berger for editing the manuscript.
References
- 1.Brawley OW, Kramer BS. Cancer screening in theory and in practice. J Clin Oncol. 2005;23:293–300. doi: 10.1200/JCO.2005.06.107. [DOI] [PubMed] [Google Scholar]
- 2.van der Merwe DE, Oikonomopoulou K, Marshall J, Diamandis EP. Mass spectrometry: uncovering the cancer proteome for diagnostics. Adv Cancer Res. 2006;96:23–50. doi: 10.1016/S0065-230X(06)96002-3. [DOI] [PubMed] [Google Scholar]
- 3.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–7. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 4.Tallman MS. Treatment of relapsed or refractory acute promyelocytic leukemia. Best Pract Res Clin Haematol. 2007;20:57–65. doi: 10.1016/j.beha.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Zhou GB, Zhang J, Wang ZY, Chen SJ, Chen Z. Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: a paradigm of synergistic molecular targeting therapy. Philos Trans R Soc Lond B Biol Sci. 2007;362:959–71. doi: 10.1098/rstb.2007.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng X, Seshire A, Ruster B, Bug G, Beissert T, Puccetti E, et al. Arsenic but not all-trans retinoic acid overcomes the aberrant stem cell capacity of PML/RARalpha-positive leukemic stem cells. Haematologica. 2007;92:323–31. doi: 10.3324/haematol.10541. [DOI] [PubMed] [Google Scholar]
- 7.Jovanovic JV, Score J, Waghorn K, Cilloni D, Gottardi E, Metzgeroth G, et al. Low-dose imatinib mesylate leads to rapid induction of major molecular responses and achievement of complete molecular remission in FIP1L1-PDGFRA positive chronic eosinophilic leukemia. Blood. 2007;109:4635–40. doi: 10.1182/blood-2006-10-050054. [DOI] [PubMed] [Google Scholar]
- 8.Goldman J, Gordon M. Why do chronic myelogenous leukemia stem cells survive allogeneic stem cell transplantation or imatinib: does it really matter? Leuk Lymphoma. 2006;47:1–7. doi: 10.1080/10428190500407996. [DOI] [PubMed] [Google Scholar]
- 9.Mimeault M, Brand RE, Sasson AA, Batra SK. Recent advances on the molecular mechanisms involved in pancreatic cancer progression and therapies. Pancreas. 2005;31:301–16. doi: 10.1097/01.mpa.0000175893.04660.1b. [DOI] [PubMed] [Google Scholar]
- 10.Mimeault M, Batra SK. Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis. 2006;27:1–22. doi: 10.1093/carcin/bgi229. [DOI] [PubMed] [Google Scholar]
- 11.Mimeault M, Batra SK. Recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells. 2006;24:2319–45. doi: 10.1634/stemcells.2006-0066. [DOI] [PubMed] [Google Scholar]
- 12.Mimeault M, Hauke R, Mehta PP, Batra SK. Recent advances on cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Mol Cell Med. 2007;11:981–1011. doi: 10.1111/j.1582-4934.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mimeault M, Hauke R, Batra SK. Stem cells — A revolution in therapeutics—Recent advances on the stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther. 2007;82:252–64. doi: 10.1038/sj.clpt.6100301. [DOI] [PubMed] [Google Scholar]
- 14.Mimeault M, Batra SK. Stem cell applications in disease research: Recent advances on stem cell and cancer stem cell biology and their therapeutic implications. In: Faraday Allen V, Dyer Jonathon T., editors. Progress in stem cell applications. New York: Nova Publisher; 2008. In press. [Google Scholar]
- 15.Sato M, Shames DS, Gazdar AF, Minna JD. A translational view of the molecular pathogenesis of lung cancer. J Thorac Oncol. 2007;2:327–43. doi: 10.1097/01.JTO.0000263718.69320.4c. [DOI] [PubMed] [Google Scholar]
- 16.Copland M, Jorgensen HG, Holyoake TL. Evolving molecular therapy for chronic myeloid leukaemia—are we on target? Hematology. 2005;10:349–59. doi: 10.1080/10245330500234195. [DOI] [PubMed] [Google Scholar]
- 17.Copland M, Hamilton A, Elrick LJ, Baird JW, Allan EK, Jordanides N, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–9. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 18.Mauro MJ. Defining and managing imatinib resistance. Hematology Am Soc Hematol Educ Program. 2006:219–25. doi: 10.1182/asheducation-2006.1.219. [DOI] [PubMed] [Google Scholar]
- 19.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 20.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 21.Milas L, Raju U, Liao Z, Ajani J. Targeting molecular determinants of tumor chemo-radioresistance. Semin Oncol. 2005;32:S78–S81. doi: 10.1053/j.seminoncol.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 22.Nicolis SK. Cancer stem cells and “stemness” genes in neuro-oncology. Neurobiol Dis. 2007;25:217–29. doi: 10.1016/j.nbd.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–86. doi: 10.1093/carcin/bgm052. [DOI] [PubMed] [Google Scholar]
- 24.Fuster JJ, Sanz-Gonzalez SM, Moll UM, Andres V. Classic and novel roles of p53: prospects for anticancer therapy. Trends Mol Med. 2007;13:192–9. doi: 10.1016/j.molmed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 25.de Jonge-Peeters SD, Kuipers F, de Vries EG, Vellenga E. ABC transporter expression in hematopoietic stem cells and the role in AML drug resistance. Crit Rev Oncol Hematol. 2007;62:214–26. doi: 10.1016/j.critrevonc.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Mimeault M, Hauke R, Batra SK. Recent advances on the molecular mechanisms involved in drug-resistance of cancer cells and novel targeting therapies. Clin Pharmacol Ther. 2007;29 doi: 10.1038/sj.clpt.6100296. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mimeault M, Pommery N, Henichart JP. New advances on prostate carcinogenesis and therapies: involvement of EGF-EGFR transduction system. Growth Factors. 2003;21:1–14. doi: 10.1080/0897719031000094921. [DOI] [PubMed] [Google Scholar]
- 28.Mimeault M, Bonenfant D, Batra SK. New advances on the functions of epidermal growth factor receptor and ceramides in skin cell differentiation, disorders and cancers. Skin Pharmacol Physiol. 2004;17:153–66. doi: 10.1159/000078818. [DOI] [PubMed] [Google Scholar]
- 29.Mimeault M, Mehta PP, Hauke R, Batra SK. Functions of normal and malignant prostatic stem/progenitor cells in tissue regeneration and cancer progression and novel targeting therapies against advanced prostate cancers. Endocr Rev. 2008:21. doi: 10.1210/er.2007-0040. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mimeault M, Batra SK. Interplay of distinct growth factors during epithelial-mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann Oncol. 2007;18:1605–19. doi: 10.1093/annonc/mdm070. [DOI] [PubMed] [Google Scholar]
- 31.Mimeault M, Batra SK. Functions of tumorigenic and migrating cancer progenitor cells in cancer progression and metastasis and their therapeutic implications. Cancer Metastasis Rev. 2007;26:203–14. doi: 10.1007/s10555-007-9052-4. [DOI] [PubMed] [Google Scholar]
- 32.Mimeault M, Batra SK. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008:21. doi: 10.1007/s12015-008-9008-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen BY, Liu JY, Chang HH, Chang CP, Lo WY, Kuo WH, et al. Hedgehog is involved in prostate basal cell hyperplasia formation and its progressing towards tumorigenesis. Biochem Biophys Res Commun. 2007;357:1084–9. doi: 10.1016/j.bbrc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 34.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–82. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 35.Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol. 2004;51:1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–9. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 37.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–36. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 38.Massard C, Deutsch E, Soria JC. Tumour stem cell-targeted treatment: elimination or differentiation. Ann Oncol. 2006;17:1620–4. doi: 10.1093/annonc/mdl074. [DOI] [PubMed] [Google Scholar]
- 39.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 40.Kurbel S. Selective reduction of estrogen receptor (ER) positive breast cancer occurrence by estrogen receptor modulators supports etiological distinction between ER positive and ER negative breast cancers. Med Hypotheses. 2005;64:1182–7. doi: 10.1016/j.mehy.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 41.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–7. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 42.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Brenner MK, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101:14228–33. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizo A, Vellenga E, de Haan G, Schuringa JJ. Signaling pathways in self-renewing hematopoietic and leukemic stem cells: do all stem cells need a niche? Hum Mol Genet. 2006;2:R210–R219. doi: 10.1093/hmg/ddl175. [DOI] [PubMed] [Google Scholar]
- 44.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–31. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 46.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tso CL, Shintaku P, Chen J, Liu Q, Liu J, Chen Z, et al. Primary glioblastomas express mesenchymal stem-like properties. Mol Cancer Res. 2006;4:607–19. doi: 10.1158/1541-7786.MCR-06-0005. [DOI] [PubMed] [Google Scholar]
- 48.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–33. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 49.Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–52. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 50.Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7:86–95. doi: 10.1186/bcr1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sengupta A, Banerjee D, Chandra S, Banerji SK, Ghosh R, Roy R, et al. Deregulation and cross talk among Sonic hedgehog, Wnt, Hox and Notch signaling in chronic myeloid leukemia progression. Leukemia. 2007;21:949–55. doi: 10.1038/sj.leu.2404657. [DOI] [PubMed] [Google Scholar]
- 52.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2006;431:707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 53.Mimeault M, Moore E, Moniaux N, Hénichart JP, Depreux P, Lin MF, et al. Cytotoxic effects induced by a combination of cyclopamine and gefitinib, the selective hedgehog and epidermal growth factor receptor signaling inhibitors, in prostate cancer cells. Int J Cancer. 2006;118:1022–31. doi: 10.1002/ijc.21440. [DOI] [PubMed] [Google Scholar]
- 54.Mimeault M, Johansson SL, Venkatraman G, Moore E, Henichart JP, Depreux P, et al. Combined targeting of epidermal growth factor receptor and hedgehog signaling by gefitinib and cyclopamine cooperatively improves the cytotoxic effects of docetaxel on metastatic prostate cancer cells. Mol Cancer Ther. 2007;6:967–78. doi: 10.1158/1535-7163.MCT-06-0648. [DOI] [PubMed] [Google Scholar]
- 55.Mimeault M, Venkatraman G, Johansson SL, Moore E, Henichart JP, Depreux P, et al. Novel combination therapy against metastatic and androgen-independent prostate cancer by using gefitinib, tamoxifen and etoposide. Int J Cancer. 2007;120:160–9. doi: 10.1002/ijc.22268. [DOI] [PubMed] [Google Scholar]
- 56.Mimeault M, Mehta PP, Hauke R, et al. Improvement of cytotoxic effects of mitoxantrone on hormone-refractory metastatic prostate cancer cells by co-targeting epidermal growth factor receptor and hedgehog signaling cascades. Growth Factors. doi: 10.1080/08977190801930935. In press. [DOI] [PubMed] [Google Scholar]
- 57.Dancey JE, Chen HX. Strategies for optimizing combinations of molecularly targeted anticancer agents. Nat Rev Drug Discov. 2006;5:649–59. doi: 10.1038/nrd2089. [DOI] [PubMed] [Google Scholar]
- 58.Ceschel S, Casotto V, Valsecchi MG, Tamaro P, Jankovic M, Hanau G, et al. Survival after relapse in children with solid tumors: a follow-up study from the Italian off-therapy registry. Pediatr Blood Cancer. 2006;47:560–6. doi: 10.1002/pbc.20726. [DOI] [PubMed] [Google Scholar]
- 59.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 60.Chen MS, Woodward WA, Behbod F, Peddibhotla S, Alfaro MP, Buchholz TA, et al. Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci. 2007;120:468–77. doi: 10.1242/jcs.03348. [DOI] [PubMed] [Google Scholar]
- 61.Burke MJ, Walterhouse DO, Jacobsohn DA, Duerst RE, Kletzel M. Tandem high-dose chemotherapy with autologous peripheral hematopoietic progenitor cell rescue as consolidation therapy for patients with high-risk Ewing family tumors. Pediatr Blood Cancer. 2007;49:196–8. doi: 10.1002/pbc.21182. [DOI] [PubMed] [Google Scholar]
- 62.Schlemmer M, Wendtner CM, Falk M, Abdel-Rahman S, Licht T, Baumert J, et al. Efficacy of consolidation high-dose chemotherapy with ifosfamide, carboplatin and etoposide (HD-ICE) followed by autologous peripheral blood stem cell rescue in chemosensitive patients with metastatic soft tissue sarcomas. Oncology. 2006;71:32–9. doi: 10.1159/000100447. [DOI] [PubMed] [Google Scholar]
- 63.Lang P, Pfeiffer M, Muller I, Schumm M, Ebinger M, Koscielniak E, et al. Haploidentical stem cell transplantation in patients with pediatric solid tumors: preliminary results of a pilot study and analysis of graft versus tumor effects. Klin Padiatr. 2006;218:321–6. doi: 10.1055/s-2006-942256. [DOI] [PubMed] [Google Scholar]
- 64.Okada T, Ozawa K. Vector-producing tumor-tracking multipotent mesenchymal stromal cells for suicide cancer gene therapy. Front Biosci. 2008;13:1887–91. doi: 10.2741/2808. [DOI] [PubMed] [Google Scholar]
- 65.Ringden O. Immunotherapy by allogeneic stem cell transplantation. Adv Cancer Res. 97C:25–60. doi: 10.1016/S0065-230X(06)97002-X. [DOI] [PubMed] [Google Scholar]
- 66.Avramova B, Jordanova M, Michailov G, Konstantinov D, Christosova I, Bobev D. Myeloablative chemotherapy with autologous peripheral blood stem cell transplantation in patients with poor-prognosis solid tumors - Bulgarian experience. J BUON. 2006;11:433–8. [PubMed] [Google Scholar]
- 67.Yu JJ, Sun X, Yuan X, Lee JW, Snyder EY, Yu JS. Immunomodulatory neural stem cells for brain tumour therapy. Expert Opin Biol Ther. 2006;6:1255–62. doi: 10.1517/14712598.6.12.1255. [DOI] [PubMed] [Google Scholar]
- 68.Fodde R. Stem cells and metastatic cancer: fatal attraction? PLoS Med. 2006;3:e482. doi: 10.1371/journal.pmed.0030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mapara KY, Stevenson CB, Thompson RC, Ehtesham M. Stem cells as vehicles for the treatment of brain cancer. Neurosurg Clin N Am. 2007;18:71–80. doi: 10.1016/j.nec.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt NO, Przylecki W, Yang W, Ziu M, Teng Y, Kim SU, et al. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005;7:623–9. doi: 10.1593/neo.04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci. 2006;7:75–84. doi: 10.1038/nrn1829. [DOI] [PubMed] [Google Scholar]
- 72.Aboody KS, Bush RA, Garcia E, Metz MZ, Najbauer J, Justus KA, et al. Development of a tumor-selective approach to treat metastatic cancer. PLoS ONE. 2006;1:e23. doi: 10.1371/journal.pone.0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL, Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657–63. [PubMed] [Google Scholar]
- 74.Ehtesham M, Kabos P, Gutierrez MA, Chung NH, Griffith TS, Black KL, et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62:7170–4. [PubMed] [Google Scholar]
- 75.Bregni M, Bernardi M, Ciceri F, Peccatori J. Allogeneic stem cell transplantation for the treatment of advanced solid tumors. Springer Semin Immunopathol. 2004;26:95–108. doi: 10.1007/s00281-004-0164-4. [DOI] [PubMed] [Google Scholar]
- 76.Small TN, Young JW, Castro-Malaspina H, Prockop S, Wilton A, Heller G, et al. Intravenous busulfan and melphalan, tacrolimus, and short-course methotrexate followed by unmodified HLA-matched related or unrelated hematopoietic stem cell transplantation for the treatment of advanced hematologic malignancies. Biol Blood Marrow Transplant. 2007;13:235–44. doi: 10.1016/j.bbmt.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Perez-Martinez A, Lassaletta A, Gonzalez-Vicent M, Sevilla J, Diaz MA, Madero L. High-dose chemotherapy with autologous stem cell rescue for children with high risk and recurrent medulloblastoma and supratentorial primitive neuroectodermal tumors. J Neurooncol. 2005;71:33–8. doi: 10.1007/s11060-004-4527-4. [DOI] [PubMed] [Google Scholar]
- 78.Corradini P, Tarella C, Zallio F, Dodero A, Zanni M, Valagussa P, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia. 2006;20:1533–8. doi: 10.1038/sj.leu.2404306. [DOI] [PubMed] [Google Scholar]
- 79.Oyan B, Koc Y, Ozdemir E, Kars A, Turker A, Tekuzman G, et al. High dose sequential chemotherapy and autologous stem cell transplantation in patients with relapsed/refractory lymphoma. Leuk Lymphoma. 2006;47:1545–52. doi: 10.1080/10428190600570958. [DOI] [PubMed] [Google Scholar]
- 80.von Allmen D, Grupp S, Diller L, Marcus K, Ecklund K, Meyer J, et al. Aggressive surgical therapy and radiotherapy for patients with high-risk neuroblastoma treated with rapid sequence tandem transplant. J Pediatr Surg. 2005;40:936–41. doi: 10.1016/j.jpedsurg.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 81.George RE, Li S, Medeiros-Nancarrow C, Neuberg D, Marcus K, Shamberger RC, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006;24:2891–96. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 82.Dillman RO, Barth NM, VanderMolen LA, Allen K, Beutel LD, Chico S. High-dose chemotherapy and autologous stem cell rescue for metastatic breast cancer: superior survival for tandem compared with single transplants. Am J Clin Oncol. 2005;28:281–8. doi: 10.1097/01.coc.0000156917.43490.65. [DOI] [PubMed] [Google Scholar]
- 83.Carella AM, Beltrami G, Corsetti MT, Nati S, Musto P, Scalzulli P, et al. Reduced intensity conditioning for allograft after cytoreductive autograft in metastatic breast cancer. Lancet. 2005;366:318–20. doi: 10.1016/S0140-6736(05)66989-9. [DOI] [PubMed] [Google Scholar]
- 84.Barfield RC, Hale GA, Burnette K, Behm FG, Knapp K, Eldridge P, et al. Autologous transplantation of CD133 selected hematopoietic progenitor cells for treatment of relapsed acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;48:349–353. doi: 10.1002/pbc.20687. [DOI] [PubMed] [Google Scholar]
- 85.Sora F, Piccirillo N, Chiusolo P, Laurenti L, Marra R, Bartolozzi F, et al. Mitoxantrone, carboplatin, cytosine arabinoside, and methyl-prednisolone followed by autologous peripheral blood stem cell transplantation: a salvage regimen for patients with refractory or recurrent non-Hodgkin lymphoma. Cancer. 2006;106:859–66. doi: 10.1002/cncr.21634. [DOI] [PubMed] [Google Scholar]
- 86.Iwasaki Y, Nagata K, Nakanishi M, Natuhara A, Kubota Y, Ueda M, et al. Double-cycle, high-dose ifosfamide, carboplatin, and etoposide followed by peripheral blood stem-cell transplantation for small cell lung cancer. Chest. 2005;128:2268–73. doi: 10.1378/chest.128.4.2268. [DOI] [PubMed] [Google Scholar]
- 87.Matsubara H, Makimoto A, Higa T, Kawamoto H, Sakiyama S, Hosono A, et al. A multidisciplinary treatment strategy that includes high-dose chemotherapy for metastatic retinoblastoma without CNS involvement. Bone Marrow Transplant. 2005;35:763–6. doi: 10.1038/sj.bmt.1704882. [DOI] [PubMed] [Google Scholar]
- 88.Hale GA. Autologous hematopoietic stem cell transplantation for pediatric solid tumors. Expert Rev Anticancer Ther. 2005;5:835–46. doi: 10.1586/14737140.5.5.835. [DOI] [PubMed] [Google Scholar]
- 89.Frickhofen N, Berdel WE, Opri F, Haas R, Schneeweiss A, Sandherr M, et al. Phase I/II trial of multicycle high-dose chemotherapy with peripheral blood stem cell support for treatment of advanced ovarian cancer. Bone Marrow Transplant. 2006;38:493–9. doi: 10.1038/sj.bmt.1705472. [DOI] [PubMed] [Google Scholar]
- 90.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–9. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 91.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. 1. 2007. pp. 313–23. [DOI] [PubMed] [Google Scholar]
- 92.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–43. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 93.Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–6. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003;100:15178–83. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–01. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 96.Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 23:9392–400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 97.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 98.Al-Hajj M, Wicha MS, ito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 100.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 101.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 102.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 103.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–35. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 105.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–37. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 106.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–19. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 107.Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 108.Hadnagy A, Gaboury L, Beaulieu R, Balicki D. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312:3701–10. doi: 10.1016/j.yexcr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 109.Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20:102–7. doi: 10.1038/modpathol.3800720. [DOI] [PubMed] [Google Scholar]
- 110.Immervoll H, Hoem D, Sakariassen PO, Steffensen OJ, Molven A. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 2008;8:48. doi: 10.1186/1471-2407-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10:R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–14. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 113.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, et al. Significance of CD90(+) cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–66. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 114.Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4:1038–45. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 115.Wulf GG, Wang RY, Kuehnle I, Weidner D, Marini F, Brenner MK, et al. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–73. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- 116.Rossi DJ, Bryder D, Weissman IL. Hematopoietic stem cell aging: Mechanism and consequence. Exp Gerontol. 2007;42:385–90. doi: 10.1016/j.exger.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, et al. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–13. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 118.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 119.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–35. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 120.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 121.Ganss R. Tumor stroma fosters neovascularization by recruitment of progenitor cells into the tumor bed. J Cell Mol Med. 2006;10:857–65. doi: 10.1111/j.1582-4934.2006.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 122.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bruno S, Bussolati B, Grange C, Collino F, Graziano ME, Ferrando U, et al. CD133+ renal progenitor cells contribute to tumor angiogenesis. Am J Pathol. 2006;169:2223–35. doi: 10.2353/ajpath.2006.060498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 125.Chen L, Shen R, Ye Y, Pu XA, Liu X, Duan W, et al. Precancerous stem cells have the potential for both benign and malignant differentiation. PLoS ONE. 2007;2:e293. doi: 10.1371/journal.pone.0000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cobaleda C, Gutierrez-Cianca N, Perez-Losada J, Flores T, García-Sanz R, González M, et al. A primitive hematopoietic cell is the target for the leukemic transformation in human philadelphia-positive acute lymphoblastic leukemia. Blood. 2000;95:1007–13. [PubMed] [Google Scholar]
- 127.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–67. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 128.Frankfurt O, Tallman MS. Growth factors in leukemia. J Natl Compr Canc Netw. 2007;5:203–15. doi: 10.6004/jnccn.2007.0020. [DOI] [PubMed] [Google Scholar]
- 129.Matthews RH, Emami M, Connaghan DG, Holland HK, Morris LE. Home administration of high-dose oral busulfan in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39:397–400. doi: 10.1038/sj.bmt.1705610. [DOI] [PubMed] [Google Scholar]
- 130.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 131.De Clercq E. Potential clinical applications of the CXCR4 antagonist bicyclam AMD3100. Mini Rev Med Chem. 2005;5:805–24. doi: 10.2174/1389557054867075. [DOI] [PubMed] [Google Scholar]
- 132.Bonanno G, Perillo A, Rutella S, De Ritis DG, Mariotti A, Marone M, et al. Clinical isolation and functional characterization of cord blood CD133+ hematopoietic progenitor cells. Transfusion. 2004;44:1087–97. doi: 10.1111/j.1537-2995.2004.03252.x. [DOI] [PubMed] [Google Scholar]
- 133.Rollini P, Kaiser S, Faes-van’t HE, Kapp U, Leyvraz S. Long-term expansion of transplantable human fetal liver hematopoietic stem cells. Blood. 2004;103:1166–70. doi: 10.1182/blood-2003-06-1815. [DOI] [PubMed] [Google Scholar]
- 134.Brunstein CG, Setubal DC, Wagner JE. Expanding the role of umbilical cord blood transplantation. Br J Haematol. 2007;137:20–35. doi: 10.1111/j.1365-2141.2007.06521.x. [DOI] [PubMed] [Google Scholar]
- 135.Cohen Y, Nagler A. Umbilical cord blood transplantation—how, when and for whom? Blood Rev. 2004;18:167–79. doi: 10.1016/S0268-960X(03)00064-X. [DOI] [PubMed] [Google Scholar]
- 136.Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19:2304–12. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 137.Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. J Exp Med. 2006;203:73–85. doi: 10.1084/jem.20051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hesdorffer C, Ayello J, Ward M, Kaubisch A, Vahdat L, Balmaceda C, et al. Phase I trial of retroviral-mediated transfer of the human MDR1 gene as marrow chemoprotection in patients undergoing high-dose chemotherapy and autologous stem-cell transplantation. J Clin Oncol. 1998;16:165–72. doi: 10.1200/JCO.1998.16.1.165. [DOI] [PubMed] [Google Scholar]
- 139.Hu Y, Swerdlow S, Duffy TM, Weinmann R, Lee FY, Li S. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc Natl Acad Sci U S A. 2006;103:16870–5. doi: 10.1073/pnas.0606509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Burger H, Nooter K. Pharmacokinetic resistance to imatinib mesylate: role of the ABC drug pumps ABCG2 (BCRP) and ABCB1 (MDR1) in the oral bioavailability of imatinib. Cell Cycle. 2004;3:1502–5. doi: 10.4161/cc.3.12.1331. [DOI] [PubMed] [Google Scholar]
- 141.Jiang X, Zhao Y, Smith C, Gasparetto M, Turhan A, Eaves A, et al. Chronic myeloid leukemia stem cells possess multiple unique features of resistance to BCR-ABL targeted therapies. Leukemia. 2007;21:926–35. doi: 10.1038/sj.leu.2404609. [DOI] [PubMed] [Google Scholar]
- 142.Angstreich GR, Matsui W, Huff CA, Vala MS, Barber J, Hawkins AL, et al. Effects of imatinib and interferon on primitive chronic myeloid leukaemia progenitors. Br J Haematol. 2005;130:373–81. doi: 10.1111/j.1365-2141.2005.05606.x. [DOI] [PubMed] [Google Scholar]
- 143.Holtz M, Forman SJ, Bhatia R. Growth factor stimulation reduces residual quiescent chronic myelogenous leukemia progenitors remaining after imatinib treatment. Cancer Res. 2007;67:1113–20. doi: 10.1158/0008-5472.CAN-06-2014. [DOI] [PubMed] [Google Scholar]
- 144.Lowenberg B, Griffin JD, Tallman MS. Acute myeloid leukemia and acute promyelocytic leukemia. Hematology Am Soc Hematol Educ Program. 2003:82–101. [PubMed] [Google Scholar]
- 145.Zhao ZG, Liang Y, Li K, Li WM, Li QB, Chen ZC, et al. Phenotypic and functional comparison of mesenchymal stem cells derived from the bone marrow of normal adults and patients with hematologic malignant diseases. Stem Cells Dev. 2007;16:637–48. doi: 10.1089/scd.2007.0008. [DOI] [PubMed] [Google Scholar]
- 146.Helman LJ, Meltzer P. chanisms of sarcoma development. Nat Rev Cancer. 2003;3:685–94. doi: 10.1038/nrc1168. [DOI] [PubMed] [Google Scholar]
- 147.Mackall CL, Meltzer PS, Helman LJ. Focus on sarcomas. Cancer Cell. 2002;2:175–8. doi: 10.1016/s1535-6108(02)00132-0. [DOI] [PubMed] [Google Scholar]
- 148.de Silva CM, Reid R. Gastrointestinal stromal tumors (GIST): C-kit mutations, CD117 expression, differential diagnosis and targeted cancer therapy with Imatinib. Pathol Oncol Res. 2003;9:13–9. doi: 10.1007/BF03033708. [DOI] [PubMed] [Google Scholar]
- 149.Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, et al. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005;7:967–76. doi: 10.1593/neo.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ogasawara N, Tsukamoto T, Inada K, Mizoshita T, Ban N, Yamao K, et al. Frequent c-Kit gene mutations not only in gastrointestinal stromal tumors but also in interstitial cells of Cajal in surrounding normal mucosa. Cancer Lett. 2005;230:199–210. doi: 10.1016/j.canlet.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 151.Min KW, Leabu M. Interstitial cells of Cajal (ICC) and gastrointestinal stromal tumor (GIST): facts, speculations, and myths. J Cell Mol Med. 2006;10:995–13. doi: 10.1111/j.1582-4934.2006.tb00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Antonescu CR. Gastrointestinal stromal tumor (GIST) pathogenesis, familial GIST, and animal models. Semin Diagn Pathol. 2006;23:63–9. doi: 10.1053/j.semdp.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 153.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–9. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 154.Riggi N, Cironi L, Provero P, Suvà ML, Kaloulis K, Garcia-Echeverria C, et al. Development of Ewing’s sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res. 2005;65:11459–68. doi: 10.1158/0008-5472.CAN-05-1696. [DOI] [PubMed] [Google Scholar]
- 155.Tirode F, Laud-Duval K, Prieur A, Delorme B, Charbord P, Delattre O. Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11:421–29. doi: 10.1016/j.ccr.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 156.Kyritsis AP, Tsokos M, Triche TJ, Chader GJ. Retinoblastoma—origin from a primitive neuroectodermal cell? Nature. 1984;307:471–3. doi: 10.1038/307471a0. [DOI] [PubMed] [Google Scholar]
- 157.Dyer MA, Bremner R. The search for the retinoblastoma cell of origin. Nat Rev Cancer. 2005;5:91–101. doi: 10.1038/nrc1545. [DOI] [PubMed] [Google Scholar]
- 158.Zhong X, Li Y, Peng F, Huang B, Lin J, Zhang W, et al. Identification of tumorigenic retinal stem-like cells in human solid retinoblastomas. Int J Cancer. 2007;121:2125–31. doi: 10.1002/ijc.22880. [DOI] [PubMed] [Google Scholar]
- 159.Seigel GM, Hackam AS, Ganguly A, Mandell LM, Gonzalez-Fernandez F. Human embryonic and neuronal stem cell markers in retinoblastoma. Mol Vis. 2007;13:823–32. [PMC free article] [PubMed] [Google Scholar]
- 160.Chintagumpala M, Chevez-Barrios P, Paysse EA, Plon SE, Hurwitz R. Retinoblastoma: review of current management. Oncologist. 2007;12:1237–46. doi: 10.1634/theoncologist.12-10-1237. [DOI] [PubMed] [Google Scholar]
- 161.Koleva M, Kappler R, Vogler M, Herwig A, Fulda S, Hahn H. Pleiotropic effects of sonic hedgehog on muscle satellite cells. Cell Mol Life Sci. 2005;62:1863–70. doi: 10.1007/s00018-005-5072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pollett JB, Corsi KA, Weiss KR, Cooper GM, Barry DA, Gharaibeh B, et al. Malignant transformation of multipotent muscle-derived cells by concurrent differentiation signals. Stem Cells. 2007;25:2302–11. doi: 10.1634/stemcells.2006-0773. [DOI] [PubMed] [Google Scholar]
- 163.Langenau DM, Keefe MD, Storer NY, Guyon JR, Kutok JL, Le X, et al. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev. 2007;21:1382–95. doi: 10.1101/gad.1545007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Linardic CM, Downie DL, Qualman S, Bentley RC, Counter CM. Genetic modeling of human rhabdomyosarcoma. Cancer Res. 2005;65:4490–5. doi: 10.1158/0008-5472.CAN-04-3194. [DOI] [PubMed] [Google Scholar]
- 165.Tataria M, Quarto N, Longaker MT, Sylvester KG. Absence of the p53 tumor suppressor gene promotes osteogenesis in mesenchymal stem cells. J Pediatr Surg. 2006;41:624–32. doi: 10.1016/j.jpedsurg.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 166.Thomas D, Kansara M. Epigenetic modifications in osteogenic differentiation and transformation. J Cell Biochem. 2006;98:757–69. doi: 10.1002/jcb.20850. [DOI] [PubMed] [Google Scholar]
- 167.Aerts I, Pacquement H, Doz F, Mosseri V, Desjardins L, Sastre X, et al. Outcome of second malignancies after retinoblastoma: a retrospective analysis of 25 patients treated at the Institut Curie. Eur J Cancer. 2004;40:1522–9. doi: 10.1016/j.ejca.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 168.Mittal R, Al AS, Sahar O, Behbehani AM. Ewing’s sarcoma as second malignant neoplasm after retinoblastoma: a case report. Med Princ Pract. 2008;17:84–5. doi: 10.1159/000109597. [DOI] [PubMed] [Google Scholar]
- 169.Dadia S, Grimer R. Characteristics, diagnosis and treatment of bone and soft tissue sarcomas. Br J Hosp Med (Lond) 2007;68:589–593. doi: 10.12968/hmed.2007.68.11.27680. [DOI] [PubMed] [Google Scholar]
- 170.Sleijfer S, Wiemer E, Verweij J. Drug Insight: gastrointestinal stromal tumors (GIST)—the solid tumor model for cancer-specific treatment. Nat Clin Pract Oncol. 2008;5:102–11. doi: 10.1038/ncponc1037. [DOI] [PubMed] [Google Scholar]
- 171.Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–32. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 172.Saab R, Bills JL, Miceli AP, Anderson CM, Khoury JD, Fry DW, et al. Pharmacologic inhibition of cyclin-dependent kinase 4/6 activity arrests proliferation in myoblasts and rhabdomyosarcoma-derived cells. Mol Cancer Ther. 2006;5:1299–308. doi: 10.1158/1535-7163.MCT-05-0383. [DOI] [PubMed] [Google Scholar]
- 173.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 174.Ruiter D, Bogenrieder T, Elder D, Herlyn M. Melanoma-stroma interactions: structural and functional aspects. Lancet Oncol. 2002;3:35–43. doi: 10.1016/s1470-2045(01)00620-9. [DOI] [PubMed] [Google Scholar]
- 175.Pinnix CC, Herlyn M. The many faces of Notch signaling in skin-derived cells. Pigment Cell Res. 2007;20:458–65. doi: 10.1111/j.1600-0749.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- 176.den Brok MH, Nierkens S, Figdor CG, Ruers TJ, Adema GJ. Dendritic cells: tools and targets for antitumor vaccination. Expert Rev Vaccines. 2005;4:699–710. doi: 10.1586/14760584.4.5.699. [DOI] [PubMed] [Google Scholar]
- 177.Sigalotti L, Covre A, Zabierowski S, Himes B, Colizzi F, Natali PG, et al. Cancer testis antigens in human melanoma stem cells: Expression, distribution, and methylation status. J Cell Physiol. 2008;215:287–91. doi: 10.1002/jcp.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li H, Alizadeh H, Niederkorn JY. Differential expression of chemokine receptors on uveal melanoma cells and their metastases. Invest Ophthalmol Vis Sci. 2008;49:636–43. doi: 10.1167/iovs.07-1035. [DOI] [PubMed] [Google Scholar]
- 179.Albini A, Fassina G, Nicolo M, Dell’Eva R, Vené R, Cammarota R, et al. Inhibition of a vascular ocular tumor growth by IL-12 gene transfer. Clin Exp Metastasis. 2007;24:485–93. doi: 10.1007/s10585-007-9085-7. [DOI] [PubMed] [Google Scholar]
- 180.Triozzi PL, Eng C, Singh AD. Targeted therapy for uveal melanoma. Cancer Treat Rev. 2008 doi: 10.1016/j.ctrv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 181.Marshall JC, Nantel A, Blanco P, Ash J, Cruess SR, Burnier MN., Jr Transcriptional profiling of human uveal melanoma from cell lines to intraocular tumors to metastasis. Clin Exp Metastasis. 2007;24:353–62. doi: 10.1007/s10585-007-9072-z. [DOI] [PubMed] [Google Scholar]
- 182.Mallikarjuna K, Pushparaj V, Biswas J, Krishnakumar S. Expression of epidermal growth factor receptor, ezrin, hepatocyte growth factor, and c-Met in uveal melanoma: an immunohistochemical study. Curr Eye Res. 2007;32:281–90. doi: 10.1080/02713680601161220. [DOI] [PubMed] [Google Scholar]
- 183.Marshall JC, Fernandes BF, Di Cesare S, et al. The use of a cyclooxy-genase-2 inhibitor (Nepafenac) in an ocular and metastatic animal model of uveal melanoma. Carcinogenesis. 28:2053–8. doi: 10.1093/carcin/bgm091. [DOI] [PubMed] [Google Scholar]
- 184.Di Cesare S, Marshall JC, Fernandes BF, Logan P, Antecka E, Filho VB, et al. In vitro characterization and inhibition of the CXCR4/CXCL12 chemokine axis in human uveal melanoma cell lines. Cancer Cell Int. 2007;7:17. doi: 10.1186/1475-2867-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mannelli M, Simi L, Gagliano MS, Opocher G, Ercolino T, Becherini L, et al. Genetics and biology of pheochromocytoma. Exp Clin Endocrinol Diabetes. 2007;115:160–5. doi: 10.1055/s-2007-970407. [DOI] [PubMed] [Google Scholar]
- 186.Nakagawara A, Ohira M. Comprehensive genomics linking between neural development and cancer: neuroblastoma as a model. Cancer Lett. 2004;204:213–24. doi: 10.1016/S0304-3835(03)00457-9. [DOI] [PubMed] [Google Scholar]
- 187.Ross RA, Spengler BA. Human neuroblastoma stem cells. Semin Cancer Biol. 2007;17:241–7. doi: 10.1016/j.semcancer.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 188.Sanai N, varez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–22. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 189.Eberhart CG. In search of the medulloblast: neural stem cells and embryonal brain tumors. Neurosurg Clin N Am. 2007;18:59. doi: 10.1016/j.nec.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 190.Baehring JM. An update on oligodendroglial neoplasms. Curr Opin Neurol. 2005;18:639–44. doi: 10.1097/01.wco.0000189877.31637.74. [DOI] [PubMed] [Google Scholar]
- 191.Alexander A, Murtha A, Abdulkarim B, Mehta V, Wheatley M, Murray B, et al. Prognostic significance of serial magnetic resonance spectroscopies over the course of radiation therapy for patients with malignant glioma. Clin Invest Med. 2006;29:301–11. [PubMed] [Google Scholar]
- 192.Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–31. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 193.Lubensky IA, Vortmeyer AO, Kim S, Lonser RR, Park DM, Ikejiri B, et al. Identification of tumor precursor cells in the brains of primates with radiation-induced de novo glioblastoma multiforme. Cell Cycle. 2006;5:452–6. doi: 10.4161/cc.5.4.2482. [DOI] [PubMed] [Google Scholar]
- 194.Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8:119–30. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 197.Seigel GM, Campbell LM, Narayan M, Gonzalez-Fernandez F. Cancer stem cell characteristics in retinoblastoma. Mol Vis. 2005;11:729–37. [PubMed] [Google Scholar]
- 198.Alison MR. Liver cancer: a disease of stem cells? Panminerva Med. 2006;48:165–74. [PubMed] [Google Scholar]
- 199.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian inhibiting substance responsiveness. Proc Natl Acad Sci USA. 2006;103:11154–9. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–51. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 201.Haraguchi N, Inoue H, Tanaka F, Mimori K, Utsunomiya T, Sasaki A, et al. Cancer stem cells in human gastrointestinal cancers. Hum Cell. 2006;19:24–9. doi: 10.1111/j.1749-0774.2005.00004.x. [DOI] [PubMed] [Google Scholar]
- 202.Grichnik JM, Burch JA, Schulteis RD, Shan S, Liu J, Darrow TL, et al. Melanoma, a tumor based on a mutant stem cell? J Invest Dermatol. 2006;126:142–53. doi: 10.1038/sj.jid.5700017. [DOI] [PubMed] [Google Scholar]
- 203.Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007;67:4807–15. doi: 10.1158/0008-5472.CAN-06-4608. [DOI] [PubMed] [Google Scholar]
- 204.Maitland NJ, Bryce SD, Stower MJ, Collins AT. Prostate cancer stem cells: a target for new therapies. Ernst Schering Found Symp Proc. 2006;5:155–79. doi: 10.1007/2789_2007_050. [DOI] [PubMed] [Google Scholar]
- 205.Perillo A, Bonanno G, Pierelli L, Rutella S, Scambia G, Mancuso S. Stem cells in gynecology and obstetrics. Panminerva Med. 2004;46:49–59. [PubMed] [Google Scholar]
- 206.Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–96. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5:147–59. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 208.Nobili S, Landini I, Giglioni B, Mini E. Pharmacological strategies for overcoming multidrug resistance. Curr Drug Targets. 2006;7:861–79. doi: 10.2174/138945006777709593. [DOI] [PubMed] [Google Scholar]
- 209.Schatzlein AG. Delivering cancer stem cell therapies - a role for nanomedicines? Eur J Cancer. 2006;42:1309–15. doi: 10.1016/j.ejca.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 210.Thorne SH. Strategies to achieve systemic delivery of therapeutic cells and microbes to tumors. Expert Opin Biol Ther. 2007;7:41–51. doi: 10.1517/14712598.7.1.41. [DOI] [PubMed] [Google Scholar]