Abstract
Multi-detector coronary computed tomography angiography (CTA) is a promising modality for widespread clinical application because of its non-invasive nature and high diagnostic accuracy as found in previous studies using 64 to 320 simultaneous detector rows. It is, however, limited in its ability to detect myocardial ischemia. In this manuscript we describe the design of the CORE320 study (“Combined Coronary Atherosclerosis and Myocardial Perfusion Evaluation Using 320 Detector Row Computed Tomography”). This prospective, multicenter, multinational study is unique in that it is designed to assess the diagnostic performance of combined 320-row CTA and myocardial CT perfusion imaging (CTP) in comparison to the combination of invasive coronary angiography and single photon emission computed tomography myocardial perfusion imaging (SPECT-MPI). The trial is being performed at 16 medical centers located in 8 countries worldwide. Computed tomography has the potential to assess both anatomy and physiology in a single imaging session. The co-primary aims of the CORE320 study is to define the per-patient diagnostic accuracy of the combination of coronary CTA and myocardial CTP to detect physiologically significant coronary artery disease compared to 1) the combination of conventional coronary angiography and SPECT-MPI and 2) conventional coronary angiography alone. If successful, the technology could revolutionize the management of patients with symptomatic CAD.
Keywords: computed tomography, coronary angiography, myocardial perfusion imaging, study design and implementation, multicenter study
Introduction
Coronary artery disease (CAD) is now the leading cause of morbidity and mortality worldwide (1). Conventional coronary angiography (CCA) is the current gold standard for determining the presence or absence of significant coronary luminal stenosis. However, due to the high cost and inherent risks involved with this invasive procedure (such as stroke, myocardial infarction, death, need for emergency surgical intervention; and a major complication rate of 0.1-0.3%) (1), the non-invasive assessment of CAD is desirable in well defined groups of patients with suspected CAD. Advances in multi-detector computed tomography (CT) have made the non-invasive imaging of the coronary arterial lumen and wall feasible (2). Multiple studies have shown that coronary CT angiography (CTA) has a high sensitivity and negative predictive value when compared to CCA in patients with low or intermediate risk for CAD (2-8). CTA supersedes all other non-invasive imaging modalities in its capability to rule out disease; however, CTA does not provide information on the physiologic significance of coronary luminal stenosis (9, 10). Previous studies have shown that the patients who benefit most from invasive therapies (ie. angiogplasty and bypass surgery) are those with proven myocardial ischemia (11, 12). Multiple prior studies have shown that a ≥ 50% coronary luminal stenosis identified by CTA is a poor predictor of reversible ischemia with positive predictive values ranging from 29-58% (13-16). The optimal non-invasive diagnostic test for evaluating CAD would be a test that can measure both coronary luminal stenosis and myocardial ischemia in patients with moderate to severe disease.
Preclinical and single center studies have evaluated the capability of combining coronary CTA and myocardial CT perfusion imaging (CTP) to detect atherosclerosis causing myocardial perfusion abnormalities (17-23). These single center studies have shown that CTP compares well with single photon emission computed tomography myocardial perfusion imaging (SPECT-MPI) and is accurate in detecting obstructive atherosclerosis causing myocardial ischemia. As a next step to the validation of myocardial CTP imaging we describe the study design and the methodological implementation of the CORE320 multicenter, multinational diagnostic study.
Methods
Objectives
Prior to data analysis, the CORE320 steering committee specified the two co-primary objectives of the CORE320 study. They are as follows: test the diagnostic accuracy, on a per-patient basis, of 320-CT for identifying the combination of CTA defined coronary artery stenosis ≥ 50% and a corresponding myocardial perfusion defect on CTP in a patient with suspected or know CAD compared with the following reference standards: 1) the combination of a quantitative coronary angiography (QCA) defined stenosis ≥ 50% and a corresponding myocardial perfusion defect on single photon emission computed tomography (SPECT-MPI) and 2) a stenosis ≥ 50% on QCA alone. The primary diagnostic parameter will be the area under the receiver operating characteristic curve (ROC). The CORE320 steering committee selected two co-primary objectives with the goal of comparing the combination of CTA and CTP to the combination of an anatomic (CCA) and physiologic test (SPECT-MPI) and, based on the limitations for SPECT-MPI in the setting of left main and three vessel disease, to an anatomic reference standard (CCA) alone (24, 25). In addition to the co- primary objectives of this study, eighteen secondary objectives have been outlined for further analyses. These objectives have been divided into analyses based on patient level, vessel-territory, segmental, and clinical outcome measures. The details of each of these secondary objectives are outlined in Table 1.
Table 1. Secondary Objectives.
| Patient-Based Hypotheses |
|
| Vessel-Territory Based Hypotheses |
|
| Segmental-Based Hypothesis | The combination of a ≥ 50% coronary stenosis (or a non-evaluable coronary segment) by quantitative 320 × 0.5 mm CTA with a perfusion defect defined by adenosine CTP, corresponds to the combination of a ≥ 50% coronary stenosis detected by quantitative conventional angiography in the same segment, with a perfusion defect defined by SPECT-MPI in the same territory. |
| Outcome-Based Hypotheses |
|
Abbreviations: CTA: computed tomography angiography; CTP: computed tomography perfusion; CCA: conventional catheter angiography; CT: computed tomography; SPECT-MPI: single photon emission computed tomography myocardial perfusion imaging
Design Overview
The CORE320 study is a multicenter, multinational, diagnostic study designed to compare the accuracy of combined coronary CTA and myocardial CTP against the combination of CCA and SPECT-MPI to detect a patient with atherosclerosis and corresponding myocardial ischemia. The study is registered at www.clinicaltrials.gov, identifier: NCT00934037 and is being conducted at 16 centers, with four centers in the United States, four centers in Japan, two centers in Brazil and Singapore, one center in Germany, Canada, the Netherlands, and Denmark. All centers use the same 320 row detector CT scanner (Aquilion ONE, Toshiba Medical Systems, Nasu, Japan). The study sponsor, Toshiba Medical Systems, is not involved in any stage of the study design, data acquisition, data analysis, or manuscript preparation.
The study protocol was entirely developed by the CORE320 Steering Committee. The imaging components occur after enrollment and consist of four tests: 1) coronary CTA; 2) adenosine stress myocardial CTP; 3) SPECT-MPI; and 4) CCA. At study baseline, blood serum is being collected and stored for biomarker analyses. Biomarker analyses will focus on four functional categories: (1) lipid metabolism (lipoprotein profile, total cholesterol, triglycerides, high density lipoprotein, low density lipoprotein), (2) inflammatory (C-reactive protein, myeloperoxidase, soluble intercellular adhesion molecule-1), (3) myocardial stress (ProBNP), and (4) glucose metabolism (fructosamine).
All enrolled patients referred for a clinically indicated CCA are required to have a SPECT-MPI using either symptom limited exercise or pharmacologic stress test in the previous 60 days. SPECT-MPI can be a study performed for clinical purposes on a CORE320 validated SPECT camera or can be performed under the research protocol, see Figure 1. Prior to CCA, patients will undergo a combined CTA and adenosine stress CTP. SPECT-MPI, CCA, CTA, and CTP are to all be completed within 60 days of each other. The CT exam was performed at least 24 hours prior to the CCA. The overall design of the CORE320 study is depicted in Figure 1.
Figure 1.
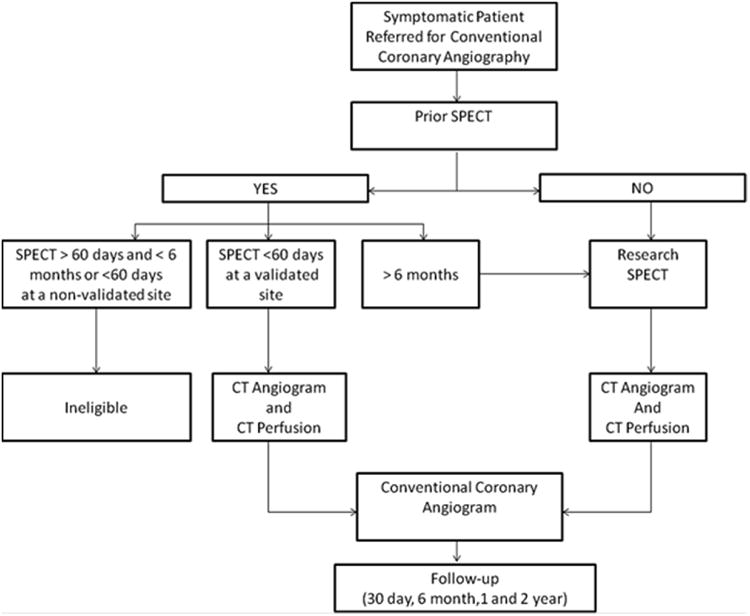
CORE320 study design. Workflow of patient enrollment, data acquisition, and data handling. Reproduced with permission from George, et al. AJR Am J Roentgenol 2011;197:829-37.
Study investigators, referring physicians, and participants are blinded to the CT results; however results of the clinical or research SPECT-MPI are available to the referring physician and the invasive cardiologist. All further clinical decisions are based on the patient's clinical standard of care and the clinically indicated invasive coronary angiogram. Each imaging reading center (CTA, CTP, CCA, and SPECT-MPI) is blinded to all clinical data and analyses from other imaging core laboratories. All enrolled participants provide informed consent approved by both central and local institutional review boards (IRB). Evaluation for the presence of non-cardiac findings on CTA is performed locally at each center by a radiologist according to local institutional regulatory standards. Clinical follow-up is scheduled for 30 days, 6 months, one year, and two years. Clinical status and interval events are reviewed via telephone with the participant, review of medical records, or written communication with the participant. Specific clinical outcomes to be captured include: the occurrence of death, myocardial infarction, stroke, coronary revascularization (including percutaneous coronary intervention or coronary artery bypass surgery), or hospitalizations. Study participant safety will be monitored by an independent data safety and monitoring board and IRB.
Patient Selection/Inclusion/Exclusion Criteria
The CORE320 study was designed to include participants between 45-85 years of age, who were referred for clinically driven CCA for suspected or known CAD and who were willing and able to sign written informed consent. Women of child-bearing potential are required to have a negative pregnancy test within 24 hours of the CT study. Patients will be excluded from participation if they had any of the following:
Atrial fibrillation or uncontrolled tachyarrhythmia
Advanced atrioventricular block (second or third degree heart block)
Evidence of acute coronary syndrome with TIMI risk score greater than 5 or elevated cardiac enzymes in the past 72 hours
Known or suspected moderate or severe aortic stenosis
Evidence of severe symptomatic heart failure (NYHA class III or IV)
Previous coronary artery bypass or other cardiac surgery
Coronary artery intervention within the last 6 months
History of allergic reaction to iodinated contrast media
History of contrast-induced nephropathy
Multiple myeloma
Previous organ transplantation
Elevated serum creatinine (>1.5 mg/dl) or calculated creatinine clearance of <60 ml/min (using the Cockcroft-Gault formula)
Contraindications to vasodilatory stress (SBP < 90, recent use of dipyridamole and dipyridamole containing medications, recent use of methylxanthines like aminoplylline and caffeine)
Unstable acute coronary syndrome or MI
Profound sinus bradycardia (less than 40 beats per minute)
Known or suspected intolerance or contraindication to beta-blockers (including: known allergy to beta-blockers, history of moderate to severe bronchospastic lung disease including moderate to severe asthma, severe pulmonary disease with the use of inhaled bronchodilator over the past year)
Body mass index >40
Presence of intracardiac devices like ICD or metallic implants within the imaging field of view
History of high radiation exposure (≥ 2 nuclear or MDCT studies or ≥ 5.0 rems) in the 18 months prior to consent,
Dual isotope studies, thallium studies, or sestamibi studies that included rest and stress performed in 2 days protocol
History of a clinical SPECT performed within the previous 6 months but more than 60 days of screening or in a non-validated center within 60 days prior to screening
The presence of any other history or condition that the investigator judged to be a significant reason for exclusion
We anticipate a similar demographic breakdown as in the previous study performed by the same group of investigators, CORE-64, in terms of age distribution, ethnic diversity, and gender. The study included 71% Caucasian, 5% African American or African Brazilian, 20% Asian, and 4% Other (American Indian and Pacific Islander) with 28% of participants being Hispanic. The CORE-64 study and the CORE320 study eligibility criteria are similar and a majority of the enrollment centers are the same (5, 26).
Sample Size
We determined that a sample size (number of evaluable patients) of 400 is needed to detect the difference in area under the receiver operating characteristic curve between the null (H0) and alternative (H1) hypothesis values indicated using a one-sided test with a significance level of 5% with at least 80% power (Table 2) (27, 28). The sample size determination was based on the primary objective, with the patient being the unit of analysis. Secondary analyses examining diagnostic accuracy based on a vessel and segment level analysis will be performed using statistical methods to adjust for the effects of within patient clustering.
Table 2. Sample Size Calculation.
| AUC | H0: 0.80 | H0: 0.80 | H0: 0.80 | H0: 0.85 | H0: 0.85 | H0: 0.90 |
|---|---|---|---|---|---|---|
| Prevalence | H1: 0.85 | H1: 0.90 | H1: 0.95 | H1: 0.90 | H1: 0.95 | H1: 0.95 |
| 0.20 | 900 | 205 | 80 | 700 | 150 | 460 |
| 0.25 | 740 | 168 | 64 | 568 | 120 | 372 |
| 0.30 | 634 | 144 | 57 | 487 | 104 | 314 |
| 0.35 | 560 | 129 | 49 | 426 | 92 | 275 |
| 0.40 | 510 | 115 | 45 | 385 | 83 | 245 |
| 0.45 | 472 | 107 | 41 | 354 | 76 | 223 |
| 0.50 | 446 | 100 | 38 | 330 | 70 | 204 |
| 0.55 | 428 | 97 | -- | 313 | 66 | 193 |
| 0.60 | 419 | 94 | -- | 302 | 64 | 184 |
Abbreviations: AUC: area under receiver operating characteristic curve; H0: null hypothesis; H1: alternative hypothesis
Image Acquisition
The CORE320 CTA and CTP acquisition methods (developed by the CT methods sub-committee) are described in detail elsewhere (29) and the CT acquisition parameters are summarized in Table 3. Patients with heart rates >60 beats per minute receive 75-150 mg of oral metoprolol. Coronary calcium scanning precedes the coronary CTA. The CTA/rest CTP study is then performed using sublingual nitroglycerin, real-time bolus tracking, and a prospective ECG triggered protocol over 1-2 heart beats. Tube current and voltage, contrast dose and exposure window are adjusted according to Table 3. Twenty minutes later stress CTP will be acquired during a 4 to 5 minute adenosine infusion (140 mcg/kg/min) using real-time bolus tracking and a prospective ECG triggered protocol over 1-2 heart beats. Tube current and voltage, contrast dose and exposure window are adjusted according to Table 3.
Table 3. CT Acquisition Parameters.
| CTA | CTP | |||
|---|---|---|---|---|
| Heart Rate ≤65(bpm) | Heart Rate >65(bpm) | Heart Rate ≤65(bpm) | Heart Rate >65(bpm) | |
| Tube current (mA)Body mass index | ||||
| Body mass index | ||||
| Female | ||||
| ≤19.9 | 300 | 300 | 270 | 270 |
| 20.0-24.9 | 370 | 340 | 300 | 300 |
| 25.0-29.9 | 400 | 340 | 350 | 300 |
| 30.0-34.9 | 450 | 450 | 370 | 300 |
| 35.0-39.9 | 460 | 460 | 400 | 300 |
| Male | ||||
| ≤19.9 | 350 | 350 | 350 | 300 |
| 20.0-24.9 | 400 | 400 | 370 | 350 |
| 25.0-29.9 | 450 | 440 | 400 | 350 |
| 30.0-34.9 | 520 | 520 | 450 | 350 |
| 35.0-39.9 | 550 | 550 | 470 | 350 |
| Center of Exposure Window (%) | 75 | 60 | 85 | 85 |
| Exposure Window (%) | 10 | 40 | 20 | 20 |
| Contrast Injection | ||||
| Volume (ml), Rate (ml/sec) | ||||
| Weight | ||||
| <60 kg | 50 (4.0) | 50 (4.0) | 50 (4.0) | 50 (4.0) |
| 60-70 kg | 60 (4.5) | 60 (4.5) | 60 (4.5) | 60 (4.5) |
| 71-100 kg | 60 (5.0) | 60 (5.0) | 60 (5.0) | 60 (5.0) |
| >100 kg | 70 (5.0) | 70 (5.0) | 70 (5.0) | 70 (5.0) |
| Detector Collimation (mm) | 240-320 | 240-320 | 240-320 | 240-320 |
| Detector Width (mm) | 0.5 | 0.5 | 0.5 | 0.5 |
| Tube Voltage (kV) | 120 | 120 | 120 | 120 |
| Gantry rotation time (seconds) | 0.350-0.375 | 0.350-0.375 | 0.350-0.375 | 0.350-0.375 |
Abbreviations: CTA; computed tomography angiography, CTP; computed tomography myocardial perfusion imaging, BPM; beats per minute.
Conventional angiographic methods are identical to those utilized in CORE-64 and are described in detail elsewhere (5, 26). The SPECT-MPI protocols utilized in CORE320 are summarized in Figure 2. An example of an entire CORE320 data set from a run in participant is depicted in Figure 3.
Figure 2.
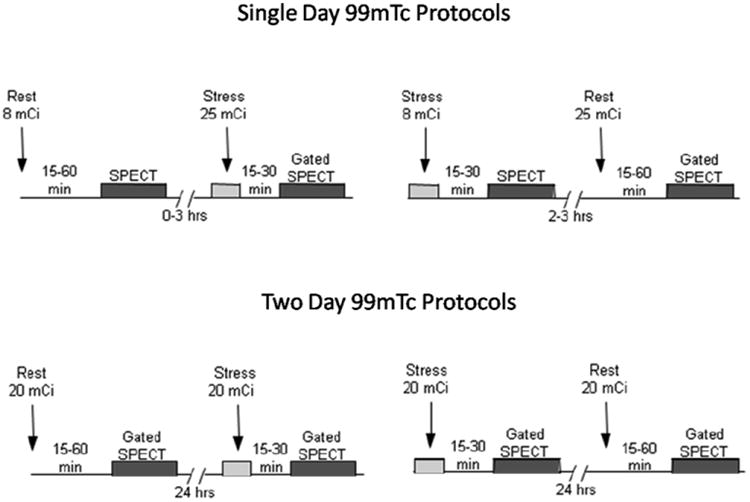
Depiction of one day and two day SPECT myocardial imaging protocols utilized in the CORE320 study. The rest/stress or stress/rest gated 99mTc SPECT imaging protocols are used with vasodilators (adenosine, dipyridamole, or regadenoson) or symptom limited exercise.
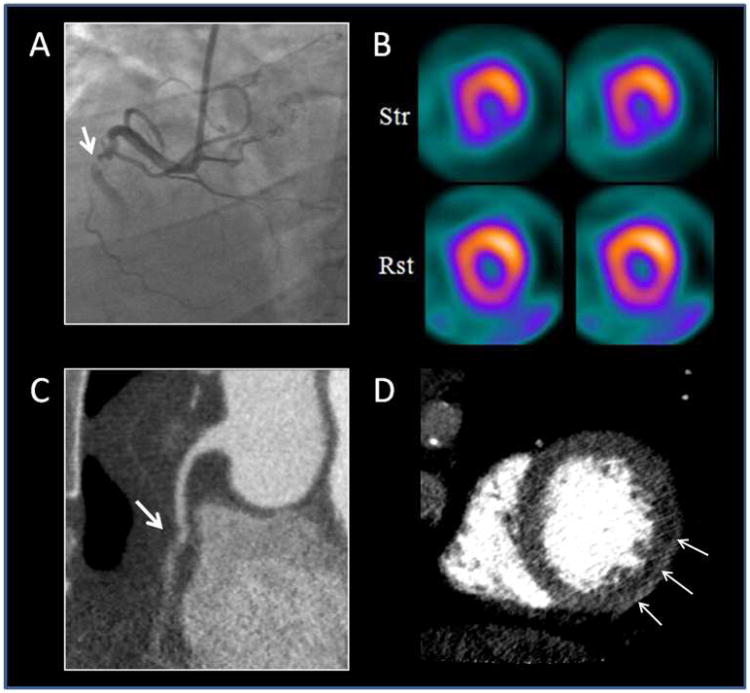
Figure 3.
Depicts a CORE320 participant's entire imaging dataset. Panel A depicts a right coronary artery with a 100% occlusion using conventional coronary angiography. Panel B depicts an inferior and inferolateral wall, partially reversible perfusion defect on a SPECT scan. Panel C depicts a 100% occlusion in the right coronary artery on a CT coronary angiography. Panel D depicts an inferolateral wall perfusion defect on the CT myocardial perfusion image.
Reading Center Framework -Data handling, analysis
Clinical data is being obtained at baseline and entered into an electronic data collection, data management, and data extraction system (DATATRAK International, Mayfield Heights, OH) using an Internet/Hybrid database design at each of the field centers. SPECT-MPI images in DICOM format are electronically transmitted via AGMednet (Boston, MA) Image Transfer System from the field centers directly to the SPECT-MPI Reading Center (M. Dicarli; Brigham and Women's Hospital) and the Coordinating Center's DICOM repository (Johns Hopkins Hospital). DICOM image data from the clinically driven index CCA and potential intervention are transmitted via WebPax (Heart Imaging Technologies Durham, NC) and deposited directly into the Coordinating Center's DICOM repository for storage and data analyses in the CCA Core Laboratory (J. Miller; Johns Hopkins Hospital). The CT raw image data is transferred on Blu-ray Disc™ to the CT reading center for centralized image reconstruction and analysis (J. Lima, R. George, and A. Zadeh; Johns Hopkins Hospital).
The CCA and CTA studies are interpreted and quantified by two experienced readers and final results are obtained by consensus according to previously published methods (5, 26, 29). SPECT-MPI and CTP studies are independently interpreted by two experienced readers and differences are resolved by consensus. SPECT-MPI and CTP will use a 13 segment myocardial model as previously described (29). Each reading center enters secondary data (results from quantitative and qualitative image interpretation) into a web-based OpenClinica relational database (Akaza Research, Waltham, MA) which has been developed and is managed by the coordinating center. This relational database is designed to recognize and trigger studies needing consensus and/or adjudication within each reading center or between two reading centers (reference standard - CCA/SPECT and test case – CTA/CTP). Consensus processes occur within the framework of each core-laboratory while the adjudication processes are designed to assign perfusion territories to specific vessels using previously established models (30, 31). The adjudication processes can only begin after completion and locking of all qualitative and quantitative procedural measurements for any given study and are performed according to pre-established rules between the reference standard reading centers (CCA and SPECT-MPI) and between the two arms of the CT reading center (CTA and CTP). The CT adjudication committee consists of three members (AZ, RG, and JL), with expertise in CT. Adjudication between the SPECT-MPI and the CCA reading centers will involves three members (MDC, JM, and JB) from the SPECT-MPI and the CCA reading centers with expertise in both imaging modalities.
Statistical Methods
The primary analysis will calculate the diagnostic accuracy on a per-patient level of the combination of quantitative CTA and visual CTP measurements compared to the reference standards: 1) the combination of quantitative CCA and visual SPECT-MPI measurements and 2) quantitative CCA alone. In the first primary analysis, a positive patient will be defined as having at least one vessel with a ≥ 50% diameter stenosis defined by quantitative CCA and a corresponding territorial perfusion defect by SPECT-MPI. In the second primary analysis, a positive patient will be defined as having at least one vessel with a ≥ 50% diameter stenosis defined by quantitative CCA.
Secondary analyses will be performed at the patient and vascular territory level according to the secondary objectives outlined in Table 1. Moreover, we will also perform a patient based analysis to determine the diagnostic capability of the combination of CTA and CTP imaging to predict subsequent coronary revascularization.
In order to assess the diagnostic accuracy of CTA and CTP compared CCA plus SPECT-MPI we will consider the result from the combination of CTA and CTP as continuous measures. Both measures will be available at the patient and vascular territory level. The analysis will be based on the area under the receiver operating characteristic (ROC) curve (AUC). Construction of the ROC curve using the two CT measures is based on the result that the curve is based on the risk score has the same ROC curve as the optimal curve based on the likelihood ratio (32). The risk score will be estimated by logistic regression analysis with CTA and CTP as predictor variables. The resulting risk score (the linear predictor from the logistic regression) will be used to construct the ROC curve. The AUC and its standard error will be estimated nonparametrically using previously described methods (32).
Conduct of the Study
Study Organization
The Steering Committee is responsible for the overall design, conduct, and supervision of the study. The Steering Committee consists of a representative from each participating site, a member of each of the reading centers (CTA, CTP, SPECT-MPI, CCA), and a member from the data coordinating center. The Steering Committee functions independently from the sponsor of the study.
Safety Monitoring
All field centers had the study protocol and informed consent form approved by their local IRBs, reviewed by a centralized IRB, and an independent Data and Safety Monitoring Board (DSMB). Where required at a national level, national agency approvals for protocol and radiation exposure were obtained (Germany, Canada, and Brazil). For any individual patient, consideration is given immediately for immediate study withdrawal, if serious adverse events occur (i.e. adverse reaction to intravenous contrast or adenosine or change in clinical course). Notifications of events occurring within 30 days of study enrollment are transmitted within 24 hours of occurrence electronically to the local study coordinator and Principal Investigator (PI) and the central coordinator and overall study PI (J. Lima). The coordinating center reports pertinent safety data and study progress to the DSMB every 100 patients enrolled. The DSMB chairman (Warren Laskey, MD, University of New Mexico) and its members are experts in the fields of radiology, cardiology, and nephrology and are completely independent of the study management and investigators. The DSMB initially reviewed all study protocols and determined an ongoing process of evaluation of study progress and data safety. The DSMB has recommended protocol modifications, and monitors study progression continuously.
Quality Assurance and Training
This study was designed to implement quality assurance using a three phase training period for all participating study physicians, coordinators, and technologists. Phase one training included an onsite presentation of the study design, rationale, and CT acquisition protocol; a teleconference describing the SPECT-MPI camera qualification process and outlining of the conventional angiography data transfer methodology and an online training module for the instructions on entering data into the DATATRAK clinical database. Phase two training required each site to perform three rest only CTAs and transmit all DICOM data to the corresponding reading centers. Phase three training was performed under IRB approval as a run in period. Each field center was required to enroll one patient into the study and perform all study procedures (combined CTA and CTP, SPECT-MPI, and CCA) according to the protocol specifications. Prior to commencing enrollment at a field center, each site was required to complete phase three training without any protocol deviations.
CT Site Accreditation
Sites and their CT technologists were required to complete phase 1-3 training. This included phase 1didactic training, phase 2 practical training with the completion of three rest CTA examinations according to protocol, and completion of a written test with 100% accuracy. Phase 3 training required the site to enroll at least one participant in the run in phase of the study with no protocol deviations. Upon successful completion of phases 1-3, a site received CT accreditation.
SPECT-MPI and CCA Site Accreditation
All SPECT cameras used in the study were required to undergo accreditation for quality assurance prior to commencement and throughout the enrollment period. The SPECT qualification process was multifocal involving evaluation of both camera physics and image quality. To account for variability in imaging equipment and image acquisition techniques, the nuclear core laboratory evaluated images for quality control following guidelines of the American Society of Nuclear Cardiology (33).
The physics qualification process included completion of a qualification form depicting camera specifications, camera maintenance, and local quality control procedures. In addition, a phantom study was performed on each camera and images were transmitted to the nuclear core laboratory for evaluation. A nuclear core lab physicist reviewed the qualification data and phantom images. In addition, three clinical test cases (one case with normal myocardial perfusion and two cases with abnormal myocardial perfusion) were transmitted to the Core Lab for evaluation. All submitted images were reviewed by the Core Lab Director for final approval.
For CCA accreditation, sites were required to submit three clinically indicated conventional angiography qualifying films to the angiographic core laboratory for review of image quality, protocol compliance, and data handling methodology.
Radiation Exposure
Using the dose-length product on the scanner and converted to effective dose using a factor of 0.014, the standard methodology outlined in the European Guidelines on Quality Criteria for Computed Tomography (34), the total mean radiation dose for CACS, CTA, and CTP is estimated to be 9-13 mSv with the total CT radiation dose capped at 25.5 mSv by the central IRB (29). The total CT dose is dependent upon a participant's gender, heart rate, and body mass index. Beta-blockers are being used to manage heart rate to reduce the radiation dose. The average total dose for the CT studies during the run-in period was 11.61 ± 2.63 mSv, well below the protocol cap of 25.5 mSv. In participants that undergo a research SPECT-MPI study the estimated effective radiation dose is 13 mSv. 99mTc-labeled radioisotopes are injected with 8 mCi at rest followed by a second injection of 25 mCi at stress. The estimated radiation dose for all research procedures included in this study is 22.0 – 38.5 mSv.
Presentations and Publications Committee
A Presentations and Publications Committee (P&P) has been instituted to oversee the dissemination of study results. The P&P Committee consists of selected Steering Committee members and is chaired by a radiologist (M. Clouse) and co-chaired by a cardiologist (J. Brinker). The P&P Committee is responsible for the review and approval of the final study presentation and manuscript. The committee will insure data integrity and promote timely publication/presentation of the primary endpoint. The committee will also approve secondary analyses from the study database, and review and approve all presentations and manuscripts that describe results from data collected in the CORE320 Study.
Discussion
The combination of coronary CTA and myocardial CTP imaging in one test has the potential to obviate some of the risks associated with invasive assessment of atherosclerosis, the capability to detect coronary atherosclerosis and associated myocardial ischemia, reduce costs associated with multiple screening tests, and potentially decrease patients' cumulative radiation exposure. Importantly, also, is the possibility to gain insight into the interplay between ischemia secondary to microvascular disease and that caused by epicardial obstructive atherosclerosis which is not well understood and has become of paramount importance given the rising prevalence of diabetes and hypertension, the two most important etiologies of microvascular disease, and two of the main determinants of epicardial atherosclerosis. Animal models combining both problems are difficult to generate and generally provide an incomplete picture of the human paradigm which is complex, chronic, and difficult to quantify. The proposed method of assessing coronary large and small vessel disease at once is promising in the elucidation of these conditions in the real world of emergency rooms serving diverse populations with high prevalence of both diabetes and hypertension.
Technologically, the development of a 320-detector row scanner has enabled volumetric imaging of the entire heart at a single time point within one cardiac cycle. This new technology has reduced imaging artifacts inherent in subvolume imaging over multiple cardiac cycles (35). Initial studies have reported consistently good image quality and contrast opacification (36), significantly lower radiation exposure as compared to helical acquisition approaches (8)and diagnostic accuracy results similar to 64-detector row studies (8). This study has the potential to demonstrate higher CTA diagnostic accuracy than reported in 64-detector row multi-center and meta-analysis studies due to volumetric imaging, tight heart rate control, and a standardized imaging protocol at a significantly lower radiation dose than 64-detector row studies. Finally, on a broader scale, the study has the potential to provide a safer and more expeditious evaluation of patients with suspected ischemic CAD. Furthermore, the application of study results have the potential to prevent unnecessary revascularization, reduce radiation exposure, and decrease overall health care expenses.
Recruitment Challenges and Methodological Considerations
Enrollment for the study began with a training run-in period in August 2009 and active enrollment began in November 2009. With 16 centers in 8 countries recruiting for a study as complex as CORE320, several challenges have become obvious and deserve discussion. Such enrollment barriers include: eligibility criteria, limited timeframe between experimental procedures and the clinically indicated CCA (particularly in US sites), international shortage of technetium, cumulative radiation exposure, blinding of CTA results, qualification of clinical SPECT scanners, and clinical availability of SPECT studies have all constituted significant challenges for worldwide patient recruitment in CORE320. In order to overcome these recruitment barriers we have activated two back-up field centers and all field centers have expanded SPECT feeder sites to increase patient screening pools. Several changes to the protocol were carried-out following the run-in phase to stream-line procedures and accommodate for the multinational clinical realities and regulatory aspects of the study. Specifically these have included adjusting site specific beta-blocker type and dose, adenosine concentration, and total radiation limits. Enrollment barriers that were beyond the control of the study coordination such as the worldwide technetium shortage have been partly compensated for by the activation of two additional back-up sites in Denmark and Japan.
The study methods are limited in that some participants will receive a research SPECT-MPI study using a standardized protocol and other participants will undergo a clinically indicated SPECT-MPI protocol in a pre-approved nuclear laboratory. Moreover, the inherent limitations of the perfusion gold standard include limited spatial resolution by SPECT-MPI and limited sensitivity in left main and three vessel disease are well recognized but were offset by the realization that SPECT-MPI is the most utilized clinical technology to assess myocardial perfusion clinically and therefore should be included as part of the reference standard of clinical care, in combination with conventional coronary angiography.
Summary
CORE320 is a prospective multinational multicenter clinical study of CT for the combined noninvasive detection of obstructive CAD with corresponding myocardial ischemia. The primary outcome measure is the comparison of the combination of coronary artery stenosis ≥ 50% and a corresponding myocardium perfusion defect identified by CT in a patient with suspected or known CAD, against the same parameters identified by CCA and SPECT-MPI. Image data are being analyzed by four blinded independent reading centers and compliance with study protocol. Safety is being monitored closely by an independent DSMB, a central IRB, and the study Coordinating Center. Enrollment of the required sample size was completed in July 2011 and the study is expected to be completed by mid 2013. We anticipate that the efficacy of this combined approach, if documented against the reference standard methods, could significantly improve the management of patients with suspected CAD in this country and abroad.
List of Abbreviations
- CAD
coronary artery disease
- CCA
conventional coronary angiography
- CT
multi-detector computed tomography
- CTA
coronary computed tomography angiography
- CTP
myocardial computed tomography perfusion imaging
- SPECT-MPI
single photon emission computed tomography myocardial perfusion imaging
- IRB
institutional review board
- ROC
the receiver operating characteristic curve
- ECG
electrocardiogram
- DSMB
data and safety monitoring board
- DLP
dose length product
- P&P
presentations and publications committee
Conflict of Interest/Financial Disclosures
Andrea L. Vavere - None
Gregory G. Simon - None
Richard T. George
Research Support: Toshiba Medical Systems
Consulting: ICON Medical Imaging
Carlos E. Rochitte - None
Andrew E. Arai- None
Julie M. Miller
Institution has grants from Toshiba Medical Systems and Doris Duke Charitable Foundation. Institution receives support for travel to study meetings and educational purposes from Toshiba Medical Systems
Individual and institution receive honoraria from Toshiba Medical Systems
Marcello Di Carli
Significant research grants: Toshiba Medical Systems and Lantheus Medical Imaging
Armin A. Zadeh
A Steering Committee member of the CORE320 study
Marc Dewey
Significant: Research Grants: European Regional Development Fund, German Heart Foundation/German Foundation of Heart Research, Joint program from the German Science Foundation (DFG) and the German Federal Ministry of Education and Research (BMBF) for meta-analyses, GE Healthcare (Amersham), Bracco, Guerbet, and Toshiba Medical Systems.
Modest: Speakers Bureau: Toshiba Medical Systems, Guerbet, Cardiac MR Academy Berlin, and Bayer-Schering. Consultant: Guerbet.
Institutional master research agreements with Siemens Medical Solutions, Philips Medical Systems, and Toshiba Medical Systems. The terms of these arrangements are managed by the legal department of Charité – Universitätsmedizin Berlin
Hiroyuki Niinuma
Significant research grant from Toshiba Medical Systems
Roger Laham- None
Frank J. Rybicki
Research agreements: Toshiba Medical Systems and Bracco Diagnostics
Joanne D. Schuijf - None
Narinder Paul
Research Support from Toshiba Medical Systems
John Hoe
Research grant- Toshiba Medical systems
Speakers Bureau-Toshiba Medical Systems, Bayer Schering Pharma, Infinitt Systems
Sachio Kuribyashi
Research grant – GE Healthcare, Japan
Hajime Sakuma
Departmental Research Grant: Toshiba Corporation
Cesar Nomura - None
Tan Swee Yaw- None
Klaus F. Kofoed- None
Kunihiro Yoshioka- None
Melvin E. Clouse - None
Jeffrey Brinker - None
Christopher Cox - None
Joao AC Lima
Principal Investigator of Grants from Toshiba Medical Systems and Bracco Diagnosticsthat, in part, support the Core320 study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2011 update: A report from the american heart association. Circulation. 2011 Feb 1;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raff GL, Gallagher MJ, O'Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005 Aug 2;46(3):552–557. doi: 10.1016/j.jacc.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 3.Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, Decramer I, Van Hoe LR, Wijns W, Hunink MG. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: Meta-analysis. Radiology. 2007 Aug;244(2):419–428. doi: 10.1148/radiol.2442061218. [DOI] [PubMed] [Google Scholar]
- 4.Hamon M, Morello R, Riddell JW, Hamon M. Coronary arteries: Diagnostic performance of 16- versus 64-section spiral CT compared with invasive coronary angiography--meta-analysis. Radiology. 2007 Dec;245(3):720–731. doi: 10.1148/radiol.2453061899. [DOI] [PubMed] [Google Scholar]
- 5.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008 Nov 27;359(22):2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 6.Meijboom WB, Weustink AC, Pugliese F, van Mieghem CA, Mollet NR, van Pelt N, Cademartiri F, Nieman K, Vourvouri E, Regar E, Krestin GP, de Feyter PJ. Comparison of diagnostic accuracy of 64-slice computed tomography coronary angiography in women versus men with angina pectoris. Am J Cardiol. 2007 Nov 15;100(10):1532–1537. doi: 10.1016/j.amjcard.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 7.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter ACCURACY (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial. J Am Coll Cardiol. 2008 Nov 18;52(21):1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Dewey M, Zimmermann E, Deissenrieder F, Laule M, Dubel HP, Schlattmann P, Knebel F, Rutsch W, Hamm B. Noninvasive coronary angiography by 320-row computed tomography with lower radiation exposure and maintained diagnostic accuracy: Comparison of results with cardiac catheterization in a head-to-head pilot investigation. Circulation. 2009 Sep 8;120(10):867–875. doi: 10.1161/CIRCULATIONAHA.109.859280. [DOI] [PubMed] [Google Scholar]
- 9.Schuetz GM, Zacharopoulou NM, Schlattmann P, Dewey M. Meta-analysis: Noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann Intern Med. 2010 Feb 2;152(3):167–177. doi: 10.7326/0003-4819-152-3-201002020-00008. [DOI] [PubMed] [Google Scholar]
- 10.Morton G, Plein S, Nagel E. Noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann Intern Med. 2010 Jun 15;152(12):827–8. doi: 10.7326/0003-4819-152-12-201006150-00022. author reply 828-9. [DOI] [PubMed] [Google Scholar]
- 11.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003 Jun 17;107(23):2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 12.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF. FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009 Jan 15;360(3):213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 13.Hacker M, Jakobs T, Matthiesen F, Vollmar C, Nikolaou K, Becker C, Knez A, Pfluger T, Reiser M, Hahn K, Tiling R. Comparison of spiral multidetector CT angiography and myocardial perfusion imaging in the noninvasive detection of functionally relevant coronary artery lesions: First clinical experiences. J Nucl Med. 2005 Aug;46(8):1294–1300. [PubMed] [Google Scholar]
- 14.Rispler S, Keidar Z, Ghersin E, Roguin A, Soil A, Dragu R, Litmanovich D, Frenkel A, Aronson D, Engel A, Beyar R, Israel O. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2007 Mar 13;49(10):1059–1067. doi: 10.1016/j.jacc.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 15.Schuijf JD, Wijns W, Jukema JW, Atsma DE, de Roos A, Lamb HJ, Stokkel MP, Dibbets-Schneider P, Decramer I, De Bondt P, van der Wall EE, Vanhoenacker PK, Bax JJ. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol. 2006 Dec 19;48(12):2508–2514. doi: 10.1016/j.jacc.2006.05.080. [DOI] [PubMed] [Google Scholar]
- 16.Gaemperli O, Schepis T, Valenta I, Koepfli P, Husmann L, Scheffel H, Leschka S, Eberli FR, Luscher TF, Alkadhi H, Kaufmann PA. Functionally relevant coronary artery disease: Comparison of 64-section CT angiography with myocardial perfusion SPECT. Radiology. 2008 Aug;248(2):414–423. doi: 10.1148/radiol.2482071307. [DOI] [PubMed] [Google Scholar]
- 17.George RT, Ichihara T, Lima JA, Lardo AC. A method for reconstructing the arterial input function during helical CT: Implications for myocardial perfusion distribution imaging. Radiology. 2010 May;255(2):396–404. doi: 10.1148/radiol.10081121. [DOI] [PubMed] [Google Scholar]
- 18.George RT, Jerosch-Herold M, Silva C, Kitagawa K, Bluemke DA, Lima JA, Lardo AC. Quantification of myocardial perfusion using dynamic 64-detector computed tomography. Invest Radiol. 2007 Dec;42(12):815–822. doi: 10.1097/RLI.0b013e318124a884. [DOI] [PubMed] [Google Scholar]
- 19.George RT, Silva C, Cordeiro MA, DiPaula A, Thompson DR, McCarthy WF, Ichihara T, Lima JA, Lardo AC. Multidetector computed tomography myocardial perfusion imaging during adenosine stress. J Am Coll Cardiol. 2006 Jul 4;48(1):153–160. doi: 10.1016/j.jacc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Blankstein R, Jerosch-Herold M. Stress myocardial perfusion imaging by computed tomography a dynamic road is ahead. JACC Cardiovasc Imaging. 2010 Aug;3(8):821–823. doi: 10.1016/j.jcmg.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Blankstein R, Shturman LD, Rogers IS, Rocha-Filho JA, Okada DR, Sarwar A, Soni AV, Bezerra H, Ghoshhajra BB, Petranovic M, Loureiro R, Feuchtner G, Gewirtz H, Hoffmann U, Mamuya WS, Brady TJ, Cury RC. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J Am Coll Cardiol. 2009 Sep 15;54(12):1072–1084. doi: 10.1016/j.jacc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Cury RC, Magalhaes TA, Borges AC, Shiozaki AA, Lemos PA, Junior JS, Meneghetti JC, Cury RC, Rochitte CE. Dipyridamole stress and rest myocardial perfusion by 64-detector row computed tomography in patients with suspected coronary artery disease. Am J Cardiol. 2010 Aug 1;106(3):310–315. doi: 10.1016/j.amjcard.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 23.George RT, Arbab-Zadeh A, Miller JM, Kitagawa K, Chang HJ, Bluemke DA, Becker L, Yousuf O, Texter J, Lardo AC, Lima JA. Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: A pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging. 2009 May;2(3):174–182. doi: 10.1161/CIRCIMAGING.108.813766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berman DS, Kang X, Slomka PJ, Gerlach J, de Yang L, Hayes SW, Friedman JD, Thomson LE, Germano G. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol. 2007 Jul;14(4):521–528. doi: 10.1016/j.nuclcard.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Mahajan N, Polavaram L, Vankayala H, Ference B, Wang Y, Ager J, Kovach J, Afonso L. Diagnostic accuracy of myocardial perfusion imaging and stress echocardiography for the diagnosis of left main and triple vessel coronary artery disease: A comparative meta-analysis. Heart. 2010 Jun;96(12):956–966. doi: 10.1136/hrt.2009.182295. [DOI] [PubMed] [Google Scholar]
- 26.Miller JM, Dewey M, Vavere AL, Rochitte CE, Niinuma H, Arbab-Zadeh A, Paul N, Hoe J, de Roos A, Yoshioka K, Lemos PA, Bush DE, Lardo AC, Texter J, Brinker J, Cox C, Clouse ME, Lima JA. Coronary CT angiography using 64 detector rows: Methods and design of the multi-centre trial CORE-64. Eur Radiol. 2009 Apr;19(4):816–828. doi: 10.1007/s00330-008-1203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obuchowski NA. Sample size calculations in studies of test accuracy. Stat Methods Med Res. 1998 Dec;7(4):371–392. doi: 10.1177/096228029800700405. [DOI] [PubMed] [Google Scholar]
- 28.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982 Apr;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 29.George RT, Arbab-Zadeh A, Cerci RJ, Vavere AL, Kitagawa K, Dewey M, Rochitte CE, Arai AE, Paul N, Rybicki FJ, Lardo AC, Clouse ME, Lima JA. Diagnostic performance of combined noninvasive coronary angiography and myocardial perfusion imaging using 320-MDCT: The CT angiography and perfusion methods of the CORE320 multicenter multinational diagnostic study. AJR Am J Roentgenol. 2011 Oct;197(4):829–837. doi: 10.2214/AJR.10.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George RT, Arbab-Zadeh A, Miller JM, Kitagawa K, Chang H, Bluemke DA, Becker L, Yousuf O, Texter J, Lardo AC, Lima JAC. Adenosine stress 64 and 256 row detector computed tomography angiography and perfusion imaging: A pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging. 2009 Mar 31; doi: 10.1161/CIRCIMAGING.108.813766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the american heart association. Circulation. 2002 Jan 29;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 32.Pepe MS. The statistical evaluation of medical tests for classification and prediction. Oxford: Oxford University Press; 2003. [Google Scholar]
- 33.Mehta R, Ward RP, Chandra S, Agarwal R, Williams KA. American College of Cardiology Foundation, American Society of Nuclear Cardiology. Evaluation of the american college of cardiology Foundation/American society of nuclear cardiology appropriateness criteria for SPECT myocardial perfusion imaging. J Nucl Cardiol. 2008 May-Jun;15(3):337–344. doi: 10.1016/j.nuclcard.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Shrimpton PC, Hillier MC, Lewis MA, Dunn M. National survey of doses from CT in the UK: 2003. Br J Radiol. 2006 Dec;79(948):968–980. doi: 10.1259/bjr/93277434. [DOI] [PubMed] [Google Scholar]
- 35.Steigner ML, Otero HJ, Cai T, Mitsouras D, Nallamshetty L, Whitmore AG, Ersoy H, Levit NA, Di Carli MF, Rybicki FJ. Narrowing the phase window width in prospectively ECG-gated single heart beat 320-detector row coronary CT angiography. Int J Cardiovasc Imaging. 2009 Jan;25(1):85–90. doi: 10.1007/s10554-008-9347-8. [DOI] [PubMed] [Google Scholar]
- 36.Rybicki FJ, Otero HJ, Steigner ML, Vorobiof G, Nallamshetty L, Mitsouras D, Ersoy H, Mather RT, Judy PF, Cai T, Coyner K, Schultz K, Whitmore AG, Di Carli MF. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008 Jun;24(5):535–546. doi: 10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]