Abstract
Recent progress in the field of the stem cell research has given new hopes to treat and even cure diverse degenerative disorders and incurable diseases in human. Particularly, the identification of a rare population of adult stem cells in the most tissues/organs in human has emerged as an attractive source of multipotent stem/progenitor cells for cell replacement-based therapies and tissue engineering in regenerative medicine. The tissue-resident adult stem/progenitor cells offer the possibility to stimulate their in vivo differentiation or to use their ex vivo expanded progenies for cell replacement-based therapies with multiple applications in human. Among the human diseases that could be treated by the stem cell-based therapies, there are hematopoietic and immune disorders, multiple degenerative disorders, such as Parkinson’s and Alzeimeher’s diseases, type 1 or 2 diabetes mellitus as well as eye, liver, lung, skin and cardiovascular disorders and aggressive and metastatic cancers. In addition, the genetically-modified adult stem/progenitor cells could also be used as delivery system for expressing the therapeutic molecules in specific damaged areas of different tissues. Recent advances in cancer stem/progenitor cell research also offer the possibility to targeting these undifferentiated and malignant cells that provide critical functions in cancer initiation and progression and disease relapse for treating the patients diagnosed with the advanced and metastatic cancers which remain incurable in the clinics with the current therapies.
Keywords: Adult stem/progenitor cells, Regenerative medicine, Cancer stem/progenitor cells, Targeted therapies, Gene therapy
Introduction
Recent progress in the stem cell research, especially on the tissue-resident adult stem cell biology, has suspired great optimism and given new hopes in offering the possibility to use these undifferentiated cells or their further differentiated progenies for cell replacement in regenerative medicine and cancer therapy in human [1–16]. All of multipotent adult stem/progenitor cells are characterized by an unlimited self-renewal capacity and are able to give rise to the mature cell lineages in tissue from which they originate along the lifespan of an individual [2–9,11–14,16–25]. Moreover, certain adult stem/progenitor cell types, and more particularly, bone marrow (BM)-derived stem/progenitor cells may also be attracted at distant extramedullary peripheral sites after intense injuries and participate to the tissue repair through remodeling and regeneration process of damaged areas [2, 8, 11, 16, 26–30]. These unique features of adult stem/progenitor cells make them a relevant and suitable source of immature cells for the repair of damaged tissues after severe injuries based on the body’s own regenerative potential. The in vivo stimulation of tissue-resident adult stem cells or use of their ex vivo expanded and differentiated progenies is emerging as a promising approach with multiple important clinical applications for cell replacement-based therapies and tissue engineering [1, 2, 4–9, 11–15, 23, 31–35]. Among the human inherited and degenerative disorders that could be treated by stem cell-based therapies, there are age-related functional defects, hematopoietic and immune disorders, type 1 or 2 diabetes mellitus, lung, liver and cardiovascular diseases, neurodegenerative and eye disorders and aggressive and recurrent cancers [8, 9, 11, 13, 15, 16, 18, 20, 28–30, 36–60].
Numerous recent investigations revealed that the accumulation of genetic and/or epigenic alterations occurring in stem/progenitor cells during aging and severe injuries including chronic inflammatory atrophy could trigger their malignant transformation into cancer stem/progenitor cells [11, 16, 23–25, 31, 32, 34, 36, 61–67]. Thus, the targeting of the deregulated signaling pathways in the cancer stem/progenitor cells, which may contribute to their sustained growth, survival, invasion, metastasis and/or treatment resistance during cancer progression, is also of particular therapeutic interest for eradicating these cancer-initiating cells [11, 16, 23–25, 36, 67, 68]. The elimination of cancer stem/progenitor cells should permit to counteract the cancer progression and disease recurrence. Thereby, the targeting of cancer stem/progenitor cells and their local microenvironment should lead to a complete remission of patients diagnosed with advanced and metastatic cancers which remain lethal in the clinics with the current surgical, hormonal, radiation and/or chemotherapeutic treatments. We describe here the anatomic localization, known specific biomarkers and functional characteristics of diverse human tissue-resident adult stem/progenitor cell types. The emphasis is on the recent research on adult stem/progenitor cells and their niches in term of their functions in the tissue regeneration in physiological and pathophysiological conditions. We also summarize and discuss the therapeutic potentials of adult stem/progenitor cells to give rise to particular cell lineages in the well-defined culture conditions ex vivo as well as in animal models in vivo and clinical settings. The provided information should help to develop novel therapeutic strategies that could be translated into clinical applications for treating and curing the patients with diverse degenerative disorders and lethal diseases including the metastatic and recurrent cancers.
Tissue-Resident Adult Stem Cell Types and Their Therapeutic Implications
Diverse poorly-differentiated adult stem/progenitor cell types, which have generally small size relative to the terminally differentiated cells and express specific stemness markers, such as CD133, CD44, nestin and/or ABCG2, have recently been identified in the most mammalian tissues/organs [11, 16, 36]. The adult stem cells have notably been isolated by fluorescence-activated cell sorting (FACS) with the specific antibodies directed against specific stem cell markers as well as by side population (SP) technique based on their ability to efflux Hoechst 33342 dye due to their high expression of ATP-binding cassette (ABC) transporters such as ABCG2 [11, 16, 36, 69–71]. Among the tissues and organs harboring a very small number of specific multipotent and undifferentiated adult stem/progenitor cells, there are BM, vascular walls, adipose tissues, skeletal muscles, heart and brain as well as epithelium of lung, liver, pancreas, digestive tract, skin, limbus, retina, breast, ovaries, prostate and testis [2–9, 11–14, 16–25, 69–71]. Several efforts have permitted to establish the unique features of each tissue-resident adult stem/progenitor cell type and their specialized local microenvironment as well as their critical functions in homeostatic state maintenance and tissue regeneration [1, 5, 7, 11, 15, 16, 72–75]. In general, the adult stem/progenitor cells are localized within a specialized microenvironment designated as niche consisting of the neighboring cells such as fibroblasts, endothelial cells and/or stromal components that tightly regulate their functions through the direct interactions and release of specific soluble factors [1, 5, 7, 11, 15, 16, 24, 25, 72–74]. All of the multipotent or bipotent adult stem/progenitor cell types display a long-term self-renewing capacity and can give rise in appropriate conditions including after intense injury to all of mature and specialized cell types of distinct lineages in the tissues/organs from which they originate or in certain cases to cell lineages at distant sites [1, 2, 4–9, 11–16, 23–25, 31, 32, 34, 61]. Despite certain adult stem/progenitor cells found in BM, skin and gastrointestinal tract usually show a rapid turnover to replenish the cell loss along lifespan, other adult stem/progenitor cell types remain under a quiescent state and rarely divide in normal conditions, and undergo only a sustained proliferation after intense tissue injuries [2, 3, 11, 16, 73]. The expansion of adult stem/progenitor cell pool within niche is accomplished through a symmetric cell division that gives rise to two identical stem cell daughters. The generation of differentiated cell lineages rather generally implicates an asymmetric division of a stem cell that gives rise to one stem cell daughter and one cell termed early transit-amplifying (TA)/intermediate cell [2, 11, 16, 21, 24, 25, 72, 73]. The early TA/intermediate cells, which possess a high proliferative potential and migratory ability, may exit the stem cell niche and give rise to late TA cells. The changes in the local environment of early and late TA/intermediate cells during amplification process and their migration at distant sites from niche may also influence the phenotype of their further and terminally differentiated progenies, and thereby contribute to the populational asymmetry and cellular diversity characterizing each tissue and organ [11, 16, 76]. The tissue regeneration mediated via adult stem/progenitor cells is usually accompanied by environmental changes in the niche and orchestrated by several growth factor and cytokine initiated cascades. Among them, there are epidermal growth factor (EGF)-epidermal growth factor receptor (EGFR), sonic hedgehog (SHH)-patched receptor (PTCH)/Gli, wingless ligand (Wnt)/β-catenin, Notch, bone morphogenic proteins (BMPs), and stromal cell-derived factor-1 (SDF-1)-CXC chemokine receptor 4 (CXCR4) signaling pathways [11, 16, 25]. These soluble factors may be released by tissue-resident activated stem/progenitor cells and stromal cells including the myofibroblasts, endothelial cells and immune cells such as macrophages attracted at the injured areas. BM-derived stem/progenitor cells may also contribute to the tissue repair by the release of diverse factors and/or to transdifferentiate into specific cell types within the host tissue. Hence, the tissue-resident adult stem/progenitor cells are unique as compared with other embryonic, umbilical cord, fetal and placenta-derived stem cell sources, in being enriched in an anatomic location that may be easy to access. Thus, in this way the adult stem cells may be stimulated in vivo in their respective environment by the exogenous application of specific growth factors and cytokines in the damaged areas that restores the endogenous tissue regeneration program.
Certain aberrant molecular pathways and deregulated interactions with the niche components resulting to a dysfunctional behavior of adult stem cells have been identified and associated with the occurrence of particular human degenerative disorders and aggressive cancers [7, 11, 16, 31, 72, 73]. Hence, the supply of new functional adult stem cells or their further differentiated progenies offers great therapeutic potentials for regenerating damaged tissues as well as gene delivery vehicle for treating and even curing diverse degenerative diseases and recurrent cancers. We describe here in more detailed manner, the specific biomarkers and functional properties of tissue-resident adult stem cells and their niches, with a particular emphasis on the adult stem/progenitor cells localized in BM, adipose tissues, muscles, heart, brain, gastrointestinal tract, liver and pancreas as well as their potential therapeutic applications in cell replacement-based therapies for treating diverse degenerative disorders and diseases in human.
BM-Derived Stem/Progenitor Cells
Hematopoietic Stem Cells
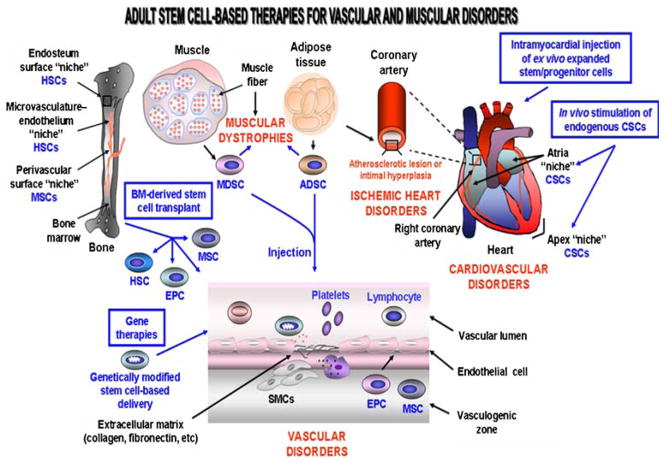
The BM-derived hematopoietic stem cells (HSCs) provide a critical role by continually renewing all of the new mature and differentiated hematopoietic cell lineages in peripheral circulation including leucocytes, erythrocytes and thrombocytes along lifespan of an individual [11, 16, 72]. The most immature and quiescent multipotent HSCs, which are characterized by the expression of specific biomarkers including CD34− or CD34+/CD38−/low/Thy1+/CD90+/C-kit−/lo/Lin−/CD133+/vascular endothelial growth factor receptor 2 (VEGFR2+) are co-localized with the osteoblasts in a specialized niche within a BM region designated as endosteum (Fig. 1) [11, 16, 72, 73, 77]. Moreover, another subpopulation of HSCs, which is found in a BM microvasculature- sinusoidal endothelium niche, appears to represent the stem cells that may rapidly supply new mature blood cell lineages which have a short live into peripheral circulation (Fig. 1) [16, 72, 74, 77].
Fig. 1.
Scheme showing the possible molecular events and cellular changes associated with the development of vascular disorders and the potent cellular and gene therapies for restoring the damaged walls of blood vessels. The recruitment of smooth muscle cells (SMCs) which may participate to the vascular disorder formation by the release of extracellular matrix components such as collagen and fibronectin and immune cells including macrophage in injury area is shown. The stem cell-based therapy using genetically-modified stem/progenitor cells or BM-derived stem/progenitor cell transplant is also illustrated. ADSCs, adipose tissue-derived stem cells; CSCs, cardiac stem cells; EPCs, endothelial progenitor cells; HSCs, hematopoietic stem cells; MDSCs, muscle-derived stem cells; MSCs, mesenchymal stem cells
Mesenchymal Stem Cells and Endothelial Progenitor Cells
The BM stroma as well as the walls of large and small blood vessels in most tissues/organs including brain, spleen, liver, kidney, lung, muscle, thymus and pancreas also contain the multipotent mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs) [11, 16, 29, 30, 78–82]. Much of the work conducted on adult stem/progenitor cells has focused on MSCs found within the BM stroma. More particularly, the MSCs expressing CD49a and CD133 markers are localized in a perivascular niche in BM, and may give rise to the osteoblasts that are co-localized with HSCs, and which may support the hematopoieisis by producing the growth factors and cytokines that promote the expansion and/or differentiation of HSCs (Fig. 1) [11, 16, 83, 84]. The BM-derived or tissue-resident MSCs can generate diverse mesodermal cell lineages involved in osteogenesis, adipogenesis, cartilage and muscle formation including the osteoblasts, osteocytes, adipocytes, chondrocytes, myoblasts and myocytes under appropriate culturing conditions ex vivo and in vivo. Moreover, MSCs may also be induced to differentiate into fibroblasts, neuronal cells, pulmonary cells, pancreatic islet β cells, corneal epithelial cells and cardiomyocytes ex vivo and/or in vivo using specific growth factors and cytokines [11, 16, 21, 85–91]. In the case of EPCs, which are derived like HSCs from the embryonic hemangioblasts, they may be distinguished by the expression of different biomarkers, including CD34+ or CD34−, CD133, vascular endothelial growth factor receptor- 2 (VEGFR-2), also designated as Flk-1 (fetal liver kinase-1), stem cell factor (SCF) receptor KIT and CXCR4 [24]. EPCs may contribute in a significant manner to give rise to mature endothelial cells that form new vascular walls of vessels after intense injury and vascular diseases (Fig. 1) [11, 16, 29, 30, 79, 81, 92]. The critical role of circulating EPCs in endothelial cell maintenance after tissue injury is notably supporting by the observation that their number and function is inversely associated with the progression of atherosclerosis and an enhanced risk of cardiovascular diseases. Hence, all of the above functional properties of BM-resident stem cells made them the promising sources of immature cells for treating numerous degenerative and vascular disorders in human.
Therapeutic Applications of BM-Derived Stem/Progenitor Cells
The autologous or allogeneic transplantation of BM or peripheral blood (PB) can lead to the homing and engraftment of functional HSCs, MSCs and EPCs and/or their differentiated progenies at BM and distant damaged tissues. Thus, this supports the feasibility of this strategy for improving the tissue remodeling and healing processes after severe injury (Fig. 1) [11, 16, 79, 81, 87, 88, 90]. More specifically, HSC transplants, alone or in combination therapy, may be used for the treatment of patients with autoimmune diseases, refractory and severe aplastic anemias, congenital thrombocytopenia, osteoporosis, cardiovascular disorders, chronic inflammatory Bowel disorders (IBD) including Crohn’s disease and ulcerative colitis, diabetes mellitus, leukemias, multiple myelanomas and Hodgkin’s and non-Hodgkin’s lymphomas and aggressive tumors [1, 2, 11, 16, 57–60, 93–97]. It has also been reported that MSCs, EPCs and their progenies can contribute to the regenerative process of several tissues including bone, cartilage, tendon, muscle, adipose, brain, lung, heart, pancreas, kidney and eye [98, 99]. Thus, these immature cells may constitute a cellular source for the treatment of diverse human disorders including osteogenesis imperfecta, atherosclerotic lesions, ischemic cardiovascular and muscular diseases (Fig. 1) [11, 16, 79, 81, 87, 88, 90, 100]. Importantly, adult BM-derived and tissue-resident MSCs are little immunogenic and display immunomodulatory and anti-inflammatory effects in host in vivo [96, 99, 101]. Therefore, these therapeutic properties of MSCs also support their possible clinical applications to prevent the tissue/organ allograft rejection and severe acute graft-versus-host diseases as well as to treat the autoimmune disorders such as inflammatory bowel disease and inflammation of the heart muscle walls associated with autoimmune myocarditis in which immunomodulation and tissue repair are required [96, 99, 102]. Indeed, MSCs can prolong skin allograft survival and reverse severe acute graft-versus-host disease in vivo supporting their use in treating skin diseases as well as in the maxillofacial surgery [99, 103].
In counterpart, the migration and proliferation of vascular smooth-muscle cells (SMCs) derived from BM cells including HSCs and MSCs in vascular injured area leading to an excessive cell accumulation may however contribute to the development of vascular pathologies such as intimal hyperplasia and atherosclerotic lesions (Fig. 1) [104, 105]. The recruited SMCs may mediate their detrimental effects through the synthesis of the extracellular matrix components such as collagen and fibronectin that accumulate on the luminal side of the damaged vessel walls, and thereby induce an occlusive vascular remodeling that may result in an ischemic heart attack or restenosis (Fig. 1) [104]. Therefore, future studies to optimize the BM-derived cell transplantation strategies and establish the specific mechanism( s) of action and physiological effects of HSCs, MSCs and EPCs at long term is essential in order to improve their therapeutic and curative benefits and prevent their detrimental clinical effects in treated patients.
Adipose Tissue-Derived Stem Cells and Their Therapeutic Applications
Adipose tissue is a highly specialized, complex and active metabolic and endocrine structure that contributes to the energy storage under form of fat. In mammals, the adipose-tissues are found in diverse anatomic compartments and designed as subcutaneous adipose tissue, internal organ-surrounding adipose tissue and interstitial adipose tissue [106]. Adipose tissue, like BM, is derived from the embryonic mesenchyme and contains a stroma-vascular fraction. More specifically, adipose tissue is mainly constituted of mature adipocytes, loose connective tissue matrix, nerve tissue and stromal host cells including immature MSC-like cells, fibroblasts, vascular smooth muscle cells, endothelial cells, and immune cells such as the resident hematopoietic progenitor cells and macrophages [107]. Recent studies have permitted to identify a putative adult stem/progenitor cell population termed as processed lipoaspirate (PLA) cells or adipose tissue-derived stem cells (ADSC), within the human adipose compartment [13, 107–111]. The stromal immature cell population, termed processed lipoaspirate (PLA) cells can be easily isolated from human lipoaspirates and, like BM-derived human MSCs express the CD29, CD44, CD71, CD90, CD105/SH2 and SH3 [108]. Importantly, it has also been noticed that the PLA cells could be distinguished from BM-derived stromal human MSCs by its unique expression of antigen CD49d (a4-integrin) and CD106 (VCAM) while they did not express MSC marker, CD106 [108]. Moreover, it has been shown that the ADSCs may be differentiated into functional cells expressing the specific markers of mesodermal (adipocytes, chondrocytes, osteocytes, myocytes, cardiomyocytes and endothelial/vascular cells, endodermal (hepatocytes and endocrine pancreatic cells) or ectodermal (neurons) tissue origin in vitro and/or in vivo under well definite culture containing specific differentiation factors [13, 52–54, 107–111]. Since human adipose tissue containing ADSCs can be easily obtained by surgical resection, tumescent lipoaspiration, or ultrasound-assisted lipoaspiration, it constitutes another promising source enriched in immature cells for cellular therapy for diverse human diseases. Among them, there are the clinical management of diverse bone, cartilage and musculoskeletal disorders, muscular dystrophies, cardiovascular and liver disorders, neuronal diseases and diabetes mellitus as well as the bioengineering of fat and musculoskeletal tissue reconstitution (Fig. 1; Table 1) [13, 52–54, 109, 112, 113].
Table 1.
Tissue-resident adult stem/progenitor cells and their therapeutic applications in disease treatment
| Adult stem cell/progenitor cell source and type | Differentiated cells | Treated degenerative disorders and diseases |
|---|---|---|
| BM and vascular walls | ||
| HSCs | Myleloid and lymphoid cells, platelets | Autoimmune diseases, anemias, thrombocytopenia, leukemias, aggressive solid tumors |
| MSCs | Osteoblasts | Osteoporosis, Osteogenesis imperfecta |
| Chondrocytes | Cartilage disorders, osteoarthritis | |
| Muscular cells | Muscular disorders | |
| HSCs, MSCs | Neural cells | Nervous system disorders |
| Cardiomyocytes | Heart disorders | |
| Insulin-producing β cells | Type 1 or 2 diabetes mellitus | |
| Hepatocytes | Liver disorders | |
| EPCs | Endothelial cells | Vascular disorders |
| Adipose tissue/skeletal muscle | ||
| ADSCs and MDSCs | Muscle cells | Muscular disorders (muscular Duchenne and Becker dystrophies, neuromuscular disorders) |
| Osteoblasts | Osteoporosis, Osteogenesis imperfecta | |
| Chondrocytes | Cartilage disorders, osteoarthritis | |
| Endothelial cells | Vascular disorders | |
| Cardiomyocytes | Heart disorders | |
| Neural cells | Nervous system disorders | |
| ADSCs | Insulin-producing βcells | Type 1 or 2 diabetes mellitus |
| Hepatocytes | Liver disorders | |
| Heart | ||
| CSCs | Cardiomyocytes | Heart disorders |
| Brain | ||
| NSCs | Neurons, Astrocytes | Nervous system disorders |
| Oligodentrocytes | Myelin disorders | |
| Insulin-producing βcells | Type 1 or 2 diabetes mellitus | |
| Eye | ||
| CESCs | Corneal epithelial cells | Corneal disorders |
| Conjunctival SCs | Conjunctival epithelial cells | Conjunctival epithelial injury |
| CE-RSCs | Retinal progenitor cells | Retinal disorders |
| Skin | ||
| KSCs, bESCs and eNCSCs | Skin cells | Skin and hair disorders |
| Gastroinestinal tract | ||
| ISCs and GSCs | Intestinal and stomach cells | Chronic inflammatory bowel diseases, ulcers |
| Pancreas | ||
| PSCs | Insulin-producing βcells | Type 1 or 2 diabetes mellitus |
| Hepatocytes | Liver disorders | |
| Liver | ||
| HOCs | Hepatocytes, cholangiocytes | Hepatitis, acute liver failure, cirrhosis |
| Cardiomyocytes | Heart failures | |
| Lung | ||
| BASCs | Lung cells (Bronchiolar Clara Cells and alveolar cells | Interstitial lung diseases, cystic fibrosis, asthma, chronic bronchitis, emphysema |
BASCs = bronchioalveolar stem cells, bESCs = bulge epithelial stem cells, CE-RSCs = ciliary epithelium-retinal stem cells, CESCs = corneal epithelial stem cells, CSCs = cardiac stem cells, EPCs = endothelial progenitor cells, eNCSCs = epidermal neural crest stem cells, GSCs = gastric stem cells, HOCs = hepatic oval cells, HSCs = hematopoietic stem cells, ISCs = intestinal stem cells, KSCs = keratinocyte stem cells, MDSCs = muscle-derived stem cells, MSCs = mesenchymal stem cells, NSCs = neural stem cells, PSCs = pancreatic stem cells, SCs = stem cells
Muscle-Derived Stem Cells and Their Therapeutic Applications
Adult skeletal muscles contain two distinct stem/progenitor cells, the muscle-derived stem cells (MDSCs) and satellite cell population that may actively participate to myofiber regenerative process and repair of injured or diseased musculoskeletal tissues [20, 114]. Muscle-committed satellite cells expressing the markers such as M-cadherin, myogenic factor 5 (MYF5) and paired box gene 7 (PAX7) transcription factors, and neural cell adhesion molecule-1, are quiescent progenitor cells located at the periphery of skeletal myofibers under homeostatic conditions [20, 115]. The satellite cells endowed with self-renewal ability may be activated and to trigger a migration and differentiation into myogenic cells in vitro and after muscle injury in vivo [20, 115]. In addition, the multipotent MDSCs, which may correspond to the more immature progenitor cells relative to satellite cells, can give rise to satellite cells and more committed progenies such as musculoskeletal, osteogenic, chondrogenic, vascular, cardiac and peripheral nerve (Schwann cells and perineurium) cell lineages in vitro under specific conditions and induce new myofiber formation in animal models in vivo [19, 20, 51, 114, 116, 117]. Muscle stem/progenitor cell-based regenerative therapy and orthopaedic tissue engineering using ex vivo gene therapy, are promising approaches for the treatment of muscle atrophy with aging, muscle wasting (cachexia) and various musculoskeletal and neuromuscular degenerative disorders such as muscular Duchenne and Becker dystrophies and amyotrophic lateral sclerosis as well as the urological degenerative disorders and cardiovascular disorders (Fig. 1; Table 1) [20, 114, 118]. More specifically, Duchenne muscular dystrophy (DMS) is a severe X-linked recessive muscle disease occurring majoritly in boys, which is associated with a defect on the gene encoding protein dystrophin on the sarcolemma of muscle fiber, and that results in a rapidly progressive weakness of the body’s muscles [113, 119]. At present time, no curative treatment for DMS exists and the current therapies principally consist to delay its progression and provide palliative cares that will result to the death of young patients. Importantly, the results from a phase I trial have revealed that the autologous transplantation of CD133+ MDSCs was safe, without systemic secondary effects and improved the symptoms of DMS in treated patients [119]. Recently, MDSC or ADSC injection based-therapies have also emerging as a potent alternative therapeutic option for the remedial treatment of deficient urethral functions such as the repair of the damaged urethral sphincter associated with the stress urinary incontinence [118].
The genetic and/or epigenic alterations and changes in the microenvironment “niche” of adult MDSCs and/or satellite cells or the embryonicmuscle precursorsmay however lead to defective skeletal muscle differentiation and rhabdomyosarcoma development [120–123]. The metastatic forms of embryonal, alveolar and pleomorphic rhabdomyosarcomas have a poor clinical management and prognosis. Thus, among the possible therapeutic approaches to treat the rhabdomyosarcomas, the targeted therapies consisting to the toxic gene product delivery in satellite tumor cells by the carriers such as MSCs may represent a promising strategy [120]. In addition, it has also been observed that the injection of MDSCs or MDSC engineered to overexpress vascular endothelial growth factor (VEGF) into an animal model of acute myocardial infraction induced angiogenesis and improved cardiac function suggesting that the MDSCs could constitute an adjuvant therapy for treating the cardiovascular disorders (Fig. 1) [124].
Cardiac Stem/Progenitor Cells and Their Therapeutic Applications
The heart is a vital muscular organ that by its repeated and rhythmic contractions is responsible for pumping the blood through the circulatory system and delivering the oxygenated blood via the systemic circulation to all parts of the body. The heart muscle is constituted by the cardiac involuntary striated muscle cells also called cardiomyocytes or cardiac myocytes. Importantly, the cell renewal in the adult myocardium may be accomplished along lifespan via the activation of cardiac stem/progenitor cells (CSCs) found within the specialized niches localized at the apex and atria of the heart (Fig. 1) [9, 11, 16–18, 125, 126]. CSCs are able to give rise to three major cell types constituting the myocardium including cardiomyocytes, smooth muscles and vascular endothelial cells in physiological and pathological conditions. With this regard, in infracted myocardium of patients with heart failure or animal models, the regenerative process appears notably to occur through the differentiation of resident small interstitial cells expressing nestin, KIT, Sca-1 and efflux transporters, P-glycoprotein and ABCG2 into cardiomyocytes, endothelial cells, smooth muscle cells, neuronal cells and fibroblasts [127]. Therefore, the in vivo stimulation of these endogenous CSCs or the intravascular, intramyocardial or catheter-based delivery of ex vivo expanded CSCs or their further differentiated progenies may constitute the therapeutic strategies for the cardiac cell replacement-based therapies (Fig. 1; Table 1) [9, 11, 16–18, 128–131]. Particularly, the CSC-based therapies could be used to replace the aged, dysfunctional or lost CSCs by new functional cardiomyocytes and regenerate coronary vessels after cardiac injury. The transplantation of genetically modified cells also offers great promise by permitting to delivery a specific therapeutic gene product such as angiogenic factors or survival agents of endogenous cardiomyocytes in the ischemic or non-ischemic heart diseased areas (Fig. 1). For instance, it has been observed that the transplantation of tumor necrosis factor receptor (TNFR) gene-modified MSCs induced an anti-inflammatory effect and inhibited the apoptotic death of resident cardiomyocytes, and thereby improved the left ventricular function in rat with acute myocardial infraction [132]. Similarly, the transplantation of adenovirus carrying human vascular endothelial growth factor 165 (Ad-hVEGF165) gene-transfected MSCs into rats with ischemic heart disease significantly promoted the host-derived angiogenesis and produced effective myogenesis as compared to MSCs transplant [133]. The transplantation of angiogenin-overexpressing human MSCs obtained after infection with adenovirus containing angiogenin gene (AdAng) also improved the heart perfusion and function in a porcine model of chronic ischemia as compared to MSC (AdNull) [134]. These treatment types, alone or in conjunction with the conventional medical therapies by using pharmaceutical agents such as angiotensin- converting enzyme (ACE) inhibitors, β-adrenergic blockers, and nitroglycerin, should permit to improving the myocardiac regeneration and long-term outcome of patients diagnosed with heart failures resulting from ischemic heart disease, hypertension and myocardial infarction [9, 11, 16–18, 37, 128–131, 135].
In addition, several lines of evidence have also revealed that the functional and contractile cardiomyocytes and/or vascular endothelial cells could be derived from other stem/progenitor cell sources including embryonic stem cells (ESCs), umbilical cord blood (UCB)-derived stem cells (CD133+ cells, HSCs or MSCs), amniotic epithelial stem cells (AECs), BM-derived stem cells (CD133+ cells, HSCs, MSCs or EPCs), ADSCs, MDSCs, pancreatic stem cells (PSCs) and adult testicular stem cells or their progenies in vitro and/or in vivo under specific differentiation conditions [9, 11, 13, 16, 18, 37, 38, 87, 88, 110, 125, 130, 136–139]. Interestingly, it has been observed that the co-culture of adult rat liver stem cells or human PSCs in a cardiomyocyte microenvironment consisting to the rat neonatal cardiac cells or human myocardial biopsies, respectively promoted their differentiation into autonomously contracting cardiomyocyte- like cells in vitro [140]. Hence, these stem/progenitor cell types or their further differentiated progenies could be used for improving the myocardial and vascular regeneration and cardiac function. In support with this, the results from some investigations have revealed the potential benefit to use these stem cell types or their further differentiated progenies with the cardiomyogenic properties to repair the damaged myocardium in animal injury models in vivo [13, 16, 17, 37, 38, 87, 88, 110, 128–130, 138, 139]. For instance, it has been observed that the transplantation of ex vivo differentiated cardiomyocytes derived from human ESCs resulted in a stable cardiomyocyte engraftment and improvement of myocardial performance in rat with extensive myocardial infraction [141]. The data from small clinical trials have also revealed that the transplantation of human BM-derived stem cells, mobilized PB cells or purified CD133+ BM-derived stem cells into patients with advanced ischemic heart diseases generally improved the vascularization process and/or myocardial function [37, 131, 136, 142, 143]. More specifically, the BM-derived cells, ADSCs and MDSCs may contribute to the repair of the injured cardiovascular system via multiple molecular mechanisms. Among them, there are the transdifferentiation of these adult stem cells into new cardiomyocytes, smooth muscle cells and/or endothelial cells as well as their release of diverse paracrine factors such as hepatocyte growth factor (HGF), insulin-like growth factor (IGF-I) and VEGF that may in turn stimulates the angiogenesis and endogenous CSCs and inhibit their apoptotic/necrotic cell death (Fig. 1) [92, 124, 144–146]. More specifically, BM-derived MSCs recruited to the infracted heart in animal model in vivo seem principally to mediate their therapeutic cardioprotective effects through the releasing of paracrine growth factors and cytokines including HGF that promotes the repair of cardiac lesion by inducing re-vascularization of diseased vessels and stimulating resident CSCs that contribute to repair of damaged cardiac lesion [102, 144, 147].
Unfortunately, in certain cases, MSCs may also differentiate into aortic smooth muscle cells and contribute to intimal hyperplasia development after coronary vascular injurywhose pathological effect may be attenuated by co-culture with late-outgrowth endothelial cells (Fig. 1) [105]. Moreover, it has also been observed that MSCs may display cytogenic instability and may differentiate toward progenies endowed with unwanted phenotype in vivo such as osteocytes and adipocytes that are undesirable for their therapeutic application in the cardiac repair [148]. All of these above detrimental effects of MSCs must be considered before their use to treat cardiovascular diseases in clinical setting.
There are great therapeutic potential and clinical interests of using these diverse stem/progenitor cell types for curing cardiovascular diseases in humans, and more particularly for treating late-stage heart failure patients that have little hope of survival without an opportunity of heart transplantation due to a massive loss of functional cardiomyocyte mass. In counterbalance, additional work appears however necessary for establish more precisely the specific biomarkers and anatomic localization, niche of endogeneous CSCs within heart and the intrinsic and extrinsic factors that regulate their self-renewal and differentiation ability. Moreover, the functional properties of transplanted stem/progenitor cells and the progenies, their cytogenetic stability at long-term, and the molecular mechanisms at the basis of observed effects in the animal models in vivo and clinical setting also require additional studies. Particularly, it will be important to ascertain the therapeutic effects on cardiac function associated with each stem/progenitor cell type found in BM-derived transplants. An optimization of cell delivery methods and number of cell injected as well as the establishment of possible interactions between the cardiac cell-replacement therapies and conventional pharmacotherapies currently used to treat the ischemic and non-ischemic heart diseases in the clinics and their specific therapeutic potential after long-term treatment also merit further investigations. These future works are necessary for minimizing the potential clinical risks associated with cell replacement therapy before their possible applications as effective cellular or gene therapies of cardiovascular diseases in the safe conditions in humans.
Neural Stem/Progenitor Cells and Their Therapeutic Applications
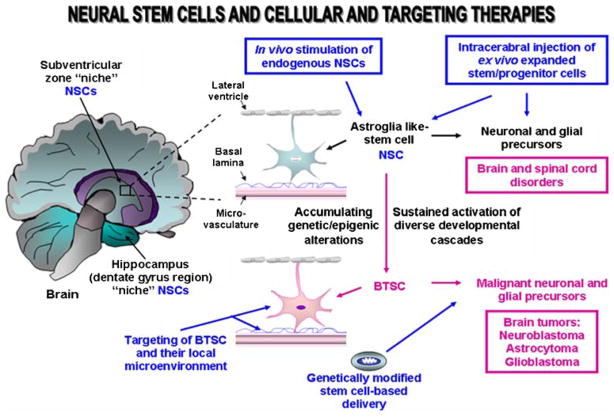
Although the mammalian adult central and peripheral nervous systems have been considered during long time as non-renewal tissues, accumulating body of evidence over the past few years to reverse this dogma by showing that the neurogenesis may occur in adult life through self-renewal and multipotent adult neural stem/progenitor cells present in central and peripheral nervous tissues. More specifically, neural stem cells (NSCs) found in the adult human brain are localized within two specific neurogenic regions designated as the lateral subventricular zone of lateral ventricle in the forebrain and dentate gyrus in hippocampus (Fig. 2) [5, 11, 15, 16, 149]. Multipotent CD133+/nestin NSCs with an astroglia-like cell phenotype are endowed with a self-renewal potential and capable to give rise to the progenitors that can proliferate and migrate at distant damaged areas of brain where they can generate further differentiated and functional progenies [5, 11, 15, 16, 149, 150]. More particularly, NSCs found in the subraventricular zone, can give rise to three principal neural cell lineages, including mature neurons and glial cells, astrocytes and oligodentrocytes while them localized in the subgranular cell layer of hippocampus may generate the granule cell projection neurons [5, 11, 15, 16, 149]. Hence, NSCs and their progenitors can participate to regenerate and repair the injured tissues after neurological damages and trauma in human. NSCs may notably give rise to diverse neural and glial cell lineages in appropriate conditions ex vivo and in vivo [11, 16, 39, 149, 151, 152]. Numerous developmental signaling cascades [EGF-EGFR, SHH-PTCH/GLI, Wnt/β-catenin, Notch, basic fibroblast growth factor (bFGF), nerve growth factor (NGF), neuregulins, BMPs, platelet-derived growth factors (PDGFs), ciliary neutrophic factor, VEGF, thyroid hormone T3, dopamine, TGF-β, integrins, Ephrins/Ephs, leukemia inhibitory factor (LIF) and/or RNA-binding proteins, Musashi (Msi-1 and Msi-2)] may contribute to the stringent regulation of the proliferation and cell fate decision of NSCs and astroglial progenitor cells in developing and adult CNS [5, 16]. In regard with this, the sustained activation of these mitotic cascades including EGF-EGFR and SHH-PTCH pathways in NSCs may also result in their malignant transformation and brain tumor formation [16, 24, 25, 31, 34, 36, 67]. The local microenvironment of NSCs also may influence their behavior. The changes in the niche components including neighboring endothelial cells co-localized with NSCs in the subraventricular zone may assume a critical function during regeneration process as well as during the progression of several neuropathologic diseases including the brain cancers which may arise from the alterations occurring in NSCs and their microenvironments [5, 11, 15, 16, 149]. More recently, the adult stem/progenitor cells derived from neural crest-derived stem cells have also been identified in peripheral nervous system within a germinal center designated carotid body (CB) [153]. Multipotent CB-resident adult stem cells, which represent the glia-like sustentacular cells expressing the glial markers can give rise to the dopaminergic glomus cells that produce the glial cell line-derived neurotrophic factor [153].
Fig. 2.
Scheme showing the anatomic localization of the neural stem/progenitor cells and their putative niches in human adult brain as well as their malignant transformation into brain tumor stem/progenitor cells. The disorders that could be treated by stem cell-based and targeting therapies are indicated. BTSCs, brain tumor stem cells; NSCs, neural stem/progenitor cells
The identification of NSCs has important therapeutic repercussions by offering the possibilities to stimulate them in vivo or replace these immature cells by new one for treating diverse CNS degenerative disorders including Parkinson’s, Alzheihmer’s, Lou Gehrig’s and Huntington’s diseases, temporal lobe epilepsy, stroke, multiple sclerosis and amyotrophic lateral sclerosis (Table 1) [11, 15, 16, 39–42, 149, 154–159]. Several lines of evidence revealed that the ex vivo expanded NSCs or their progenies may be transplanted in damaged areas of brain where they can proliferate, survive, migrate, and differentiate into functional neural and glial cell in vivo [11, 16, 157–160]. For instance, it has been reported that the transplantation of adult neural precursor cells (aNPCs) from the brain of adult transgenic mice into the spinal cords of adult Shiverer (shi/shi) mice with congenitally dysmyelinated adult CNS axons, give rise to the cells expressing the oligodendrocyte markers and resulted in formation of nodes of Ranvier and improved axonal conduction [157]. Importantly, it has also been reported that the transplanted dopaminergic neurons derived from mouse ESCs survived for more than 32 weeks and displayed the functional properties into an animal model of Parkinson’s disease [159]. In regard with this, the intrastriatal transplantation of CB-stem/progenitor cells or their progenies also offers great promise as antiparkinsonian therapy [153]. Additionally, ESCs, fetal stem/progenitor cells, UC-derived stem cells, AECs, BMSCs including MSCs, ADSCs, and pluripotent epidermal neural crest stem cells (eNCSCs) found in bulge areas within the hair follicle of the skin may also be induced to differentiate or transdifferentiate into functional neurons (tubulin-β and Tuj1), astrocytes (glial fibrillary acidic protein, GFAP) or oligodendrocytes (O4) in vitro and/or in vivo [11, 13, 16, 39, 161–163]. These observations support the feasibility to use these immature cells or their further differentiated progenies for treating diverse incurable neurodegenerative diseases. Nevertheless, before the clinical applications of NSCs, CB-resident stem/progenitor cells and other stem/progenitor cell sources for neurorestoration therapies, future investigations are required in order to more precisely establish the extrinsic and intrinsic factors that control their behavior within the niche in vivo as well as their therapeutic advantages at long term after treatment initiation.
Ocular Stem Cells
Corneal and Conjunctival Epithelial Stem Cells
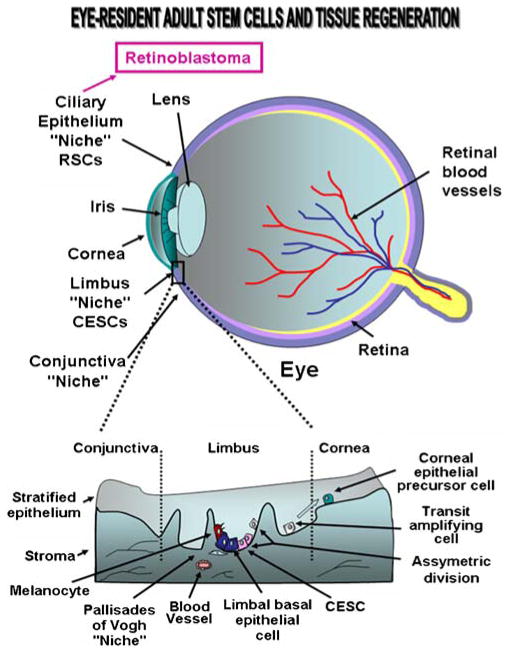
The human ocular surface is covered by the corneal, limbal, and conjunctival epitheliums. These stratified and squamous epitheliums, which consist from a basement membrane cover up by the epithelial cell layers, are connected via a connective tissue, the stroma (Fig. 3). Functionally, all of these three epithelial regions of the ocular surface may protect against fluid loss and pathogen entrance while the connective tissue and stromal cells may act for providing the oxygen and nutrients. More particularly, the cornea is avascular under the normal physiological conditions and constitutes the transparent front part of the eye that provides critical functions in permitting at the light to reach the retina and protecting against insults from external environment. Several lines of evidence have revealed that the renewal of the mature corneal epithelial cells may be accomplished via the activation of a small population of corneal epithelial stem cells (CESCs) found within a distant compartment know as the limbus situated at the junctional zone between the cornea and conjunctiva [4, 11, 16, 69, 164, 165]. CESCs also designated as limbal stem cells, appear notably to be concentrated within the basal cell layer of limbal epithelial crypts in a definite structure termed as Palisades of Vogh (Fig. 3). The limbal CESCs, which express several markers [p63, ABCG2, α9- and β1-integrins, EGFR, cytokeratin 19 (K19), α-enolase, CD71, CCAAT enhancer binding protein δ (C/EBPδ), Bmi-1 polycomb ring finger oncogene and Msi-1] but do not form gap junctions, possess the ability to reconstitute an intact and functional corneal epithelium in vivo [4, 69–71, 164–166]. In fact, despite CESCs are generally maintained under a mitotically quiescent state in homeostatic conditions prevalent in their specialized local microenvironment “niche”, the changes in their local microenvironment, and more particularly after corneal epithelium injury, may result in their activation [71, 167, 168]. The extrinsic signals provided by activated limbal stromal cells including melanocytes, myofibroblasts and endothelial cells as well as resident MSCs and BM-derived cells attracted at limbus may contribute to the stimulation of CESCs and generation of further differentiated progenies. For instance, after ocular injury, the limbal CESCs are activated by a complex network of growth factors such as EGF, FGF, TGF and PDGF and inflammatory cytokines and can produce by asymmetric division the stem cell daughters that remain undifferentiated and replenish the limbal CESC pool and the early TA/intermediate cells (Fig. 3) [169]. Early TA/intermediate cells in turn may proliferate and give rise to late TA/intermediate cells that migrate to the corneal surface epithelium where they undergo a differentiation into corneal epithelial cell precursors (Fig. 3). The corneal epithelial cell precursors display a rate of proliferation inferior to that of CESCs in vitro and correspond to a basal cell population that are involved in the replenishment of the corneal epithelium cells, which have a limited lifespan of less than 1 year in homeostatic conditions [4]. Hence, a transient renewal of the corneal epithelial cell precursors located in basal cell layer derived from limbal CESCs, may contribute to replace unfunctional or lost corneal epithelial cells after wounding, and thereby repair the damaged areas of corneal surface. On the other hand, the limbal CESCs in the adult cornea are also able to transdifferentiate into neurons and glia-like cells ex vitro in the presence of BMP and under heterotopic transplantation.
Fig. 3.
Schematic structure of human eye showing the stem/progenitor cell localization in conjunctival, limbal and corneal epitheliums and retina as well as the disorders that could be treated by stem cell-based therapies. The corneal epithelial stem cells (CESCs) located in basal layer of the limbal epithelium and the migration of early and late transit-amplifying (TA) cells from limbus to the corneal basal cell layer during corneal epithelium regeneration is also illustrated. RSCs, retinal stem cells
Retinal Stem Cells
The retina is a multi-layered sensory tissue constituted from neural cells that lines the back of the eye. During mammalian eye development, the neural crest-derived optic vesicle under the influence of the mesenchyme gives rise to the neural and non-neural tissues including the neural retina, the pigmented epithelium, the iris, the ciliary epithelium of the ciliary body and the optic stalk [170]. Importantly, the retina may also continue to grow after birth and new cells may be generated during lifespan after injury due to the presence of retinal stem cell (RSCs) resident in a region adjacent to the retina, the ciliary epithelium (CE) located in the pigmented ciliary bodies of the adult mammalian eye (Fig. 3) [11, 16, 170–173]. These multipotent RSCs located in the pigmented ciliary bodies, also designated CE stem cells, express several stem cell markers, including telomerase, neural markers such as nestin, and retinal progenitor markers such as Pax 6 [170–172]. The stringent regulation of the proliferation versus differentiation of RSCs in CE is accomplished through the activation of distinct mitogenic and differentiation signaling cascades such as FGF2, EGF, hedgehog, KIT, and Notch pathways, which are also known to be important regulators of neurogenesis [11, 170, 171]. The activated RSCs in CE can give rise to neural spheres containing the cell types that in turn can differentiate in vitro into distinct adult retinal progenitor populations, including retinal ganglion cells, as well as rod photoreceptors, bipolar cells, and Müller glia, which are derived from early and late stages of retinal histogenesis, respectively [171, 172]. In spite of this advance, future works are required to establish the functional properties of RSCs and the niche components as well as their possible implication in the replenishment of the functional retinal cell types in normal and pathological conditions in vivo.
Other Ocular Stem Cell Types
In addition to CESCs and RSCs, another putative stem cell population, which appears to be sequestered in a niche localized at the region between the corneal endothelium and the trabecular meshwork, may also give rise to both the mature cells that constitute the corneal endothelium and trabeculae [174]. Moreover, the conjunctival epithelial stem cells enriched in the bulbar and forniceal conjunctiva, may contribute to the conjunctival epithelium regeneration [175]. Importantly, multipotent MSC-like cells expressing octamer-binding protein/Nanog/Rex/CD29/CD44/CD166/CD13/SH2/SH3 have also been isolated from the stroma in human eye conjunctiva biopsies. These immature cells can differentiate toward the osteogenic, adipogenic, chondrogenic and neurogenic lineages in vitro [176]. Hence, all these above adult stem/progenitor cell types found in different ocular compartments in adult eye may contribute to the maintenance of the eye homeostasis and visual acuity along lifespan. Future research on the regulatory mechanisms of self-renewal and fate decision of ocular stem cells, and more particularly on the specific signals from their local microenvironment should permit to establish more precisely the influence of the stromal cells and/or extracellular components on their behavior. These additional investigations should lead to more successfully surgical engraftment procedures of the ocular stem cells. In regard with this, we reviewed here the recent advances in the development of novel ocular stem cell-based transplantation strategies for treating the diseased eyes.
Eye Diseases and Stem Cell-Based Treatments
The development of novel stem cell-based transplantation strategies has improved considerably the efficacy of treatments for corneal and retinal diseases, and thereby lead to the new surgical methods to preserve or restore the vision [177, 178]. The current surgical approaches in ophthalmic practice to manage severe ocular surface disease consist to the epithelial tissue transplantation with or without the use of bio-engineering support into the diseased eye. Among these procedures which aim at replacing unfunctional or loss conjunctival and/or limbal stem cells, there are limbal autograft, conjunctival autograft, conjunctival limbal autograft, living related conjunctival allograft, keratolimbal allograft, with the biological supports such as amniotic membrane. Moreover, the in vivo stimulation of immature limbal CESCs, conjunctival stem cells and RSCs in CE in human adult eye, which possess the capacity to give rise to the epithelial and neural cell lineages, also offers the possibility to stimulate these adult stem cell types for the repair of damaged corneal and conjunctival epithelia and retina after severe injuries.
The occurrence of limbal and cunjunctival epithelium damages may cause a partial or complete loss of limbal CESCs and/or conjunctival stem cells, and thereby this deficiency in turn may lead to an interruption of supply of the essential corneal epithelial cell precursors arising from the TA/intermediate cells and result in a partially or completely defective cornea regeneration process (Fig. 3). In the case of a major corneal epithelium degeneration that affects whole ocular surface due to a partial or total limbal stem/progenitor cell deficiency, the cornea does not maintain its natural functions, and thereby the corneal wound healing may result in the persistence of recurrent epithelial defects. Numerous growth factors and inflammatory cytokines released by corneal epithelial cells, keratocytes, endothelial cells and recruited immune cells may act in cooperation to promote the corneal wound healing [169]. Moreover, in certain pathologies, the corneal remodeling may be accompanied by a neovascularization of corneal stroma, ulceration, chronic inflammation, opacification, and scarring associated with the invasion of the corneal surface by the conjunctival epithelial cells “conjunctivalization”, and ultimately may lead to blindness and a partial or complete loss of vision [169, 179]. The available treatments for severely injured ocular surface may consist to stimulate the residual limbal CESCs in patient’s limbus in vivo by using appropriate growth factors or alternatively to perform an autologous or allogenic graft transplant of the new exogenous and functional limbal CESCs from patient’s or host donor’s limbus onto damaged ocular surface of diseased eye. The limbal CESCs explants may be cultured and expanded ex vivo on appropriate extracellular carrier/matrix consisting to human amniotic membrane, anterior lens capsule, human AECs used as feeder layers or fibrin based substrates which can help maintain and support their expansion and survival in acting as the microenvironmental components within the niche [11, 16, 180]. A new effective strategy of treating limbal CESCs deficiency-derived eye disorders in clinic is to transplant a bio-engineered graft by expanding limbal and/or cunjunctival epithelial stem cells ex vivo on amniotic membrane [4, 11, 16, 180]. The amniotic membrane covered by host epithelium constitutes an ideal extracellular matrix for eye surgery because it displays the biological and physical properties that are very similar to the ocular surface tissue of eye. Particularly, the amniotic membrane does not contain blood vessels, has a similar thickness and is relatively transparent like the cornea surface. Hence, the amniotic membrane exhibits important clinical beneficial effects since it may promote the ocular surface healing by supporting epithelial cell attachment, growth and differentiation of CESCs into functional corneal epithelial cells and providing anti-scarring, anti-angiogenic, and anti-inflammatory properties [4, 11, 16, 180]. The amniotic membrane transplant, especially when performed with limbal and/or conjunctival stem cell autograft, is now being used extensively in ophthalmic practice. The surgically transplantation of resultant bioengineered composite as autologous or allogenic graft tissues onto the damaged corneal surface of eye may represent an effective strategy for ocular surface reconstruction. This therapeutic treatment may be used for treat the patients with partial or total limbal and/or conjunctival stem cell deficiency, persistent epithelial defects due to chemical or thermal burns of the ocular surface, bullous keratopathy, glaucoma, oculoplastic and refractive surgery and primary pterygium [11, 16, 181, 182]. Additionally, the results from recent investigations in animal models have also revealed the possibility to use BM-derived human mesenchymal cell transplants, which can engraft at injured cornea and act in synergy with HSCs, to promote cornea wound healing after alkaline or chemical burn [167, 183]. Moreover, it has been reported that the differentiation of human ESCs into corneal epithelial-like cells may be performed in vitro using limbal fibroblasts’ conditioned medium which may provide the collagen IV acting as CESC’s niche component [184]. Similarly, the ESC-derived neural progenitors which express the regulatory factors that are implicated in retinal differentiation also may differentiate along photoreceptor lineage in response to epigenetic cues suggesting their potential application for treating certain retinal diseases [185].
The ocular stem cell-based therapies might then offer an alternative to patients with ocular lesions and a unique therapeutic chance for restoring the vision of patients with severe or complete corneal-limbal epithelial and retinal defects. Future research on the identification of specific biomarkers and potent growth factors, cytokines and immunoregulatory molecules that may influence the self-renewal capacity and differentiating rate of limbal and retinal stem/progenitor cells in normal and pathological conditions is however necessary for the optimization of their in vivo or ex vivo expansion and use in the optimal and safe conditions as therapeutic treatment to care the diseased eye and recovery of the functional vision. This should improve the ex-vivo expansion techniques of ocular stem cells, reduce the risk of graft rejection which represents yet one of the major cause of stem cell-based treatment failure, and thereby result in a high success rate of ocular surface transplantation in clinical opthamologic practice.
Gastrointestinal Stem/Progenitor Cells and Their Therapeutic Applications
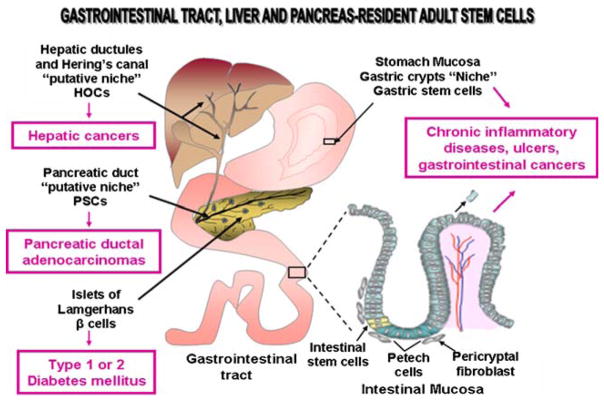
The mucosal surface of the gastrointestinal tract is lined by a simple columnar epithelium that is organized under form of invaginations, designated as the crypt-villus structures, which are surrounded by the connective tissue (Fig. 4). More specifically, the intestinal epithelium acts as a physical barrier which protects against the aggressions from external environment including the microbial infection. The gastrointestinal epithelium is a complex tissue with high rate of cell turnover where the cells that reach the villus tip are shed from intestinal and gastric epithelium and constantly replaced by new specialized epithelial cells. In regard with this, several accumulating lines of evidence indicated that the regeneration of intestinal epithelial cells in villi is provided through the precursor cells arising from a multipotent intestinal stem cells located at the base of each crypt of Lieberkühn (Fig. 4). More specifically, the small intestinal epithelial stem cells expressing markers such as Msi-1, CD24, KIT and leucine-rich-repeat-containing G-protein-coupled receptor 5 (Lgr5) appear to reside up at the crypt base within a protective niche containing the surrounding mesenchymal cells [11, 16, 186–188]. These stem cells can give rise to all cell types within each crypt including the Paneth cells that migrate down at the base of crypt as well as the highly proliferative precursor cells that migrate up at the bottom the crypt where they differentiate into absorptive enterocytes, mucus-secreting globlet cells and peptide hormone-secreting enteroendocrine cells that form villus epithelium (Fig. 4) [3, 11, 186, 188]. Similarly, the stem/progenitor cells in the colon, which are localized at the crypt base (due to the absence of Paneth cells), may also give rise to the proliferative progenitors that differentiate toward all lineages during epithelium regeneration [186–188]. Moreover, the millions of glands constituting the stomach also appear to arise from gastric stem/progenitor cells residing in a niche localized in the isthmus region within each gastric gland [2, 189]. The gastric stem/progenitor cells can give rise to the progenitors that may undergo a bipolar migration and differentiate into all the different types of specialized cell lineages including parietal, zymogenic and pit cells that form the functional gastric glands lining the basis of the stomach. Each gastric gland is also constituted of mesenchymal cells and extracellular matrix components that regulate the functions of gastric stem/progenitor cells through mesenchymal-epithelial interactions [2, 31, 189, 190].
Fig. 4.
Scheme showing the anatomic localization of the adult stem/progenitor cells and their putative niches in epithelium lining the gastrointestinal tract, hepatic ductules and Hering’s canal and pancreatic duct epithelium. The disorders that could be treated by stem cell-based therapies are indicated. HOCs, hepatic oval cells; PSCs, pancreatic stem/progenitor cells
The self-renewal of gastrointestinal stem/progenitor cells and differentiation of their progenies localized along crypt-villi axis is tightly regulated through the interplay of different developmental growth factor signaling including EGF, FGFs, IGF, hedgehog, Wnt/β-catenin, Notch, BNP and/or TGF-β signaling cascades [2, 31, 186, 188, 189]. Moreover, the BM-derived and gastrointestinal tract-resident mesenchymal progenitor cells may also contribute to the gastrointestinal tissue regeneration by providing new endothelial cells and pericryptal myofibroblasts that may improve the vasculogenesis, and thereby promote the epithelial repair after injury [188, 191]. The genetic and/or epigenic alterations in the gastrointestinal stem/progenitor cells or changes in their local microenvironment “niche” including the host stromal cells, resident and BM-derived myofibroblasts and immune cells, may lead to different disorders including chronic inflammatory bowel diseases such as Crohn’s autoimmune disease, intestinal and gastric ulcers and cancers [2, 3, 31, 47, 97, 189, 190, 192, 193]. Therefore, the gastrointestinal stem/progenitor cell- or BM-derived stem cell-based therapies may constitute a promising approach for improving the repair of the damaged areas of intestinal and gastric epithelium after severe injury and in gastrointestinal diseases as well as to develop new gastrointestinal tissue engineering strategies. The molecular targeting of gastrointestinal cancer stem/progenitor cells also may represent a potential therapy for treating the aggressive cancer forms as described below in more detailed manner.
Hepatic Oval Cells and Their Therapeutic Applications
Mammalian adult liver constitutes the largest glandular organ of the body and provides multiple important physiological functions related to metabolism, glycogen storage and detoxication of harmful substances from the blood. Therefore, the liver possesses unique and high capacity of tissue repair for triggering a rapid regenerative response after hepatic tissue injury, and thereby providing a constant renewal of mature liver cell mass [194–196]. In pathophysiological conditions, mature hepatocytes in fetal and adult livers can undergo several cell division cycles and are responsible for continuous hepatic cell replacement during liver injury [194, 195]. In addition, a small population of postnatal hepatic stem/progenitor cells, also designated as hepatic oval cells (HOCs), may also be activated following chronic or extensive damages of the liver, and more particularly in an environment inhibiting the hepatocyte replication [8, 11, 16, 194–197]. The HOCs appear to reside in the smallest units of the intrahepatic bile ducts within the periportal region of adult liver, termed the canals of Hering and terminal bile ductules and/or periductular region, from where they can migrate into the liver parenchyma (Fig. 4) [8, 11, 16, 195, 197]. The HOCs with an oval nuclei and limited cytoplasm as well as their early progenies may express several phenotypic markers of hepatic cells [α-fetoprotein (AFP) and albumin], biliary cells [γ-glutamyl-transferase, CK7 and 19, OV-6 (an anticytokeratin 19 antibody), OC2 (anti-myeloperoxidase)] as well as HSCs [CD34, Thy-1, Flt3-receptor and KIT]. More recently, the analyses of the cell surface markers in adult rat progenitor cells of injury rat liver have also revealed that they expressed CD133, claudin-7, cadherin 22, mucin-1, ros-1 and Gabrp [198]. The HOCs display a bipotent ability and can give rise to a heterogeneous population of TA/intermediate cells that in turn may mature into both parenchymal cells, hepatocytes and epithelial cells of the biliary duct, cholangiocytes, and thereby contribute to liver repair [8, 195, 199, 200]. In particular, the activation of a complex signaling network involving numerous growth factor and cytokine pathways [Wnt/β-catenin, HGF/tyrosine kinase receptors for HGF (c-met), EGF/TGF-α/EGFR, tumor necrosis factor (TNF), VEGF, SCF and SDF-1] appears to play a critical role in the regulation of the proliferation, survival and differentiation of HOCs into mature hepatocytes and cholangiocytes during hepatic tissue regeneration [8, 11, 16, 31, 195, 196].
Numerous studies have revealed the beneficial effect to transplant ex vivo expanded fetal or adult HOCs or to activate the pool of endogenous HOCs for inducing the liver regeneration after acute liver failure and chronic liver diseases including in the late-stage cirrhoses that are associated with an important hepatocyte necrosis and loss [195, 196]. For instance, it has been observed that the transplantation of wild-type (dipeptidyl peptidase IV [DPPIV(+)]) rat fetal liver stem/progenitor cells may lead to their liver incorporation into DPPIV(−) mutant F344 rats and differentiation into mature hepatocytes and bile duct cells [201]. The transplanted progenitor cells, which repopulated about 23.5% of the total hepatic cell mass and exhibited a greater proliferative activity and a lower rate of apoptotic death relative to host hepatocytes, shown a longterm liver replacement at 6 months after cell transplantation [201]. In regard with this, it has also been noticed that the Thy-1− stem/progenitor cells expressing α-fetoprotein, albumin, CK19, E-cadherin and CXCR4 isolated from embryonic day 14 rat fetal liver substantially repopulated normal adult rat liver and totally repopulated retrorsine-treated rat liver while the Thy-1+/CD45+/CXCR4+ subpopulation was only incorporated after severe hepatic injury [202]. This suggests then that fetal Thy-1− liver progenitor cells may represent a most immature progenitor cell population than Thy-1+ counterpart with distinct regenerative properties [202]. In support with this, a recent study has revealed that Thy-1 expression was induced during transit-amplification process of the oval cell population while Thy-1 mRNA was undetectable in the α-fetoprotein-expressing oval cells during liver regeneration after tissue injury in animal models and patients with liver failure [203]. More recently, it has also been reported that a population of human liver stem cells (HLSCs) expressing nestin, vimentin, albumin, α-fetoprotein and several mesenchymal stem cell markers such as CD29, CD73, CD44, and CD90 could also contribute to the regeneration of the liver parenchyma in severe-combined immunodeficient mice in vivo [204].
In addition, the hepatocyte-like cells can also be derived from ESCs, UCB, BM and pancreatic stem/progenitor cells under specific culture conditions in vitro and/or in vivo, and thereby these cells could then constitute the alternative extrahepatic sources for liver regeneration in pathological conditions [8, 26, 151, 205]. As a matter of fact, the transplantation of BM-derived cells or enriched HSCs in the animal models in vivo has been observed to restore the HOCs and their progenitors and improve the liver regeneration after injury [11, 16, 26, 28]. However, despite the beneficial effects associated with the BM-derived cells, numerous studies have also indicated that the transdifferentiation of BM-derived cells into hepatocytes did not occur or only occur at a low frequency under certain physiological and pathological conditions [8, 11, 205, 206]. Thus, other molecular mechanisms including the vascular regeneration mediated by BM-derived cells may also be responsible at least in part for the improvement of liver repair after specific damages. In addition, among the most important risk factors associated with the liver cancer development there are chronic viral and alcoholic hepatitis (also known as chronic liver inflammation) and chronic liver diseases leading to cirrhosis. In certain cases of the chronic liver diseases as well as the hepatocellular carcinoma (HCC), bile duct cancers such as intrahepatic cholangiocarcinomas and hepatoblastoma, the dysplastic foci of activated hepatic progenitor cells may be detected in diseased area suggesting that them could constitute a potent cellular target for treatment of these hyperproliferative liver disease and cancer types [11, 16, 197, 207–209].
Additional works appear to be essential for establishing more precisely the factors that control the incorporation, homing, expansion, migration and differentiation of HOCs and extrahepatic stem/progenitor cells in the liver under specific normal and pathological conditions in vivo as well as their specific functions in liver carcinogenesis. The differences between human and rat liver anatomy also underline the necessity to consider the species differences before to extrapolate the data obtained from animal models into human applications. Since hepatic progenitor cells can be easily expanded, genetically manipulated and potentially used in transplantation for a long-term replacement of hepatic cells, they constitute a promising strategy that could lead to development of new cell-based therapies for chronic liver diseases in human.
Pancreatic Stem/Progenitor Cells and Their Therapeutic Applications
The pancreas is a glandular organ constituted by an exocrine compartment, comprising the ductal epithelium and acinar cells that produce digestive enzymes, and the endocrine islets of Langerhans containing four different types of cells: insulin-producing β cells, glucagon-releasing α cells, somatostatin-producing δ cells and pancreatic polypeptide-containing cells (Fig. 4) [11, 210]. The pancreatic β cells, which represent the major type of endocrine cells, are co-localized near at a vascular basement membrane and produce the insulin that is released into bloodstream, and which in turn controls the level of blood glucose in the peripheral circulation [211]. Recent lines of evidence have revealed that the human and rodent mature insulin-producing islet β cells could arise from adult pancreatic stem/progenitor cells (PSCs) expressing ductal epithelial cell markers, cytokeratin 19 (CK19high), neural (nestin) and endocrine nuclear pancreatic and duodenal homeobox factor-1 (PDX-1) markers and/or more committed nestin+/PDX-1+/CD19low islet precursors localized in the ductal regions and/or within islet compartment (Fig. 4) [10–12, 16, 210, 212–215]. Therefore, these poorly differentiated adult putative PSCs or their early progenies within the adult pancreas could represent a potential source of β cells for cell replacement or gene therapy for treating the type 1 or 2 diabetes mellitus. More particularly, the in vivo stimulation of putative PSCs or transplantation of ex vivo expanded pancreatic β cells in the host diseased recipient may constitute a therapeutic strategy for restoring the β cell mass and treating the type 1 or 2 diabetes mellitus [10, 11, 13, 16, 48–50, 216, 217]. In addition, the use of functional pancreatic insulin-producing β cell-like progenitors derived from other stem cell types [embryonic, fetal and UCB stem/progenitor cells, human amniotic epithelial cells (hAECs), placental-derived multipotent stem cells (PDMSCs), and adult stem cells including HSCs, MSCs, HOCs, NSCs, hAECs, ADSCs] also may represent an alternative therapeutic strategy for the treatment of type 1 or 2 diabetes mellitus (Table 1) [10, 11, 13, 16, 50, 82, 91, 137, 151, 216, 218–221].
Additional in vivo studies appear however to be necessary for establishing the beneficial effects to use these stem cell types and their further differentiated progenies for treating patients suffering from type 1 or 2 diabetes mellitus or other human pancreatic diseases in the clinics. Particularly, it will be essential to establish the effects of insulin-producing progenitors on the restoration and normalization of blood sugar levels after long-term treatment. On the other hand, the targeting of the malignant counterparts of PSCs including pancreatic cancer stem/progenitor cells and their local microenviroment involved in the development of autoimmune or chronic pancreatitis and ductal pancreatic adenocarcinomas also offer great promises for the development of new therapeutic approaches for treating these pancreatic disorders [24, 65, 222, 223].
Cancer Stem/Progenitor Cells and Novel Targeting Therapy
Recent progress in stem cell field has also revealed the critical implications of leukemic and tumorigenic cancer stem/progenitor cells in the initiation and progression of the most the aggressive and recurrent cancers and treatment resistance [11, 16, 24, 25, 32, 36, 62–65, 67, 224]. Furthermore, further differentiated tumor cells and activated stromal cells including myofibroblasts as well as BM-derived cells including immune cells, macrophages, HSCs and EPCs attracted at the tumoral sites, also may actively collaborate during each step of the tumor formation at primary and secondary sites [16, 24, 25, 36, 67, 225–228]. Therefore, the targeting of the cancer stem/progenitor cells and their local microenviroment involved in the cancer development offers great promises for the development of new therapeutic approaches for treating the aggressive, metastatic and recurrent cancers in the clinics [16, 24, 25, 36, 65, 67]. More specifically, the molecular targeting of the oncogenic cascades such as EGFR, hedgehog and/or Wnt/β-catenin, oncogenic signaling elements [telomerase, Scr, Bcl-2, nuclear factor-kappa beta (NF-kB), phosphoinositide- 3 kinase (PI3K)/AKT, cyclooxygenase-2 (COX-2)] and ABC multidrug efflux pumps that assume a critical function in regulating the stem cell self-renewal, differentiation, survival and/or drug resistance of cancer stem/progenitor cells, in certain leukemia subtypes, multiple myeloma and numerous solid cancers is of particular therapeutic interest for eradicating the cancer-initiating cells [11, 16, 24, 25, 31, 35, 36, 67, 68, 229]. The targeting of the microenvironment of cancer stem/progenitor cells including the host cells such as myofibroblasts that support their malignant transformation as well as the use of anti-angiogenic agents also may constitute an adjuvant treatment for counteracting the cancer progression to metastatic and lethal disease states [16, 24, 25, 36, 67]. For instance, it has been observed that the treatment of the mice bearing orthotopic U87 glioma cell xenografts with anti-VEGF monoclonal antibody, bevacizumab markedly reduced the microvasculature density and tumor growth and this anti-angiogenic effect was also accompanied by a decrease of the number of vessel-associated self-renewing CD133+/nestin+ brain tumor stem cells (BTSCs) (Fig. 2) [230]. Moreover, the gene therapies by using genetically-modified stem cells as carriers for the delivery of anti-angiogenic or cytotoxic agents at specific tumoral sites may also represent other therapeutic strategies for treating numerous aggressive and metastatic cancers (Fig. 2) [11, 100, 231–233]. For instance, it has been observed that genetically-modified migrating NSCs, which are able to migrate through the CNS and reach the extracranial neoplastic sites, may be transplanted in the animal models in vivo and specifically attracted to tumoral sites due to the release of chemotactic signals such as VEGF and SDF-1 [231, 233, 234]. Importantly, the combined treatment with 5-fluorocytosine plus engineered NSCs expressing cytosine deaminase, which acts as a pro-drug activating enzyme, resulted in significant cytotoxic effects on melanoma cells as well as a reduction in tumor border in animal models with established melanoma brain metastasis in vivo [234].
Conclusion and Future Research
Recent progress revealed the major implications of adult stem/progenitor cells in tissue homeostasis and regeneration after severe injuries as well as their malignant counterparts in diverse human pathologies including aggressive cancers. A better understanding of specific features of each tissue-resident adult stem/progenitor cell is however essential for the successful formulation of therapeutic approaches to treat particular degenerative disorders and diseases by cell replacement therapy. Further research is required to establish the gene expression patterns of normal and malignant adult stem/progenitor cells versus their differentiated progenies in order to identify their specific biomarkers. Furthermore, the identification of the intrinsic and extrinsic factors that govern the decision between the self-renewal versus differentiation of tissue-resident adult stem cells as well as the influence of the extracelllular signals from their local microenvironment “niche” on their behavior in vivo is also of immense interest for the design of new therapeutic strategies. The characterization of the biological properties of ex vivo expanded adult stem/progenitor cells and their further differentiated progenies in diverse animal models in vivo after long-term treatment also merits future investigations. These additional studies on the adult stem/progenitor cells and their progenies should ultimately lead to the development of new effective therapies for treating and even curing numerous degenerative disorders which remain incurable with the current therapeutic treatments in the clinics.
Acknowledgments
The authors on this manuscript are supported by the grants from the U.S. Department of Defense (PC04502, OC04110) and the National Institutes of Health (CA78590, CA111294). We thank Ms. Kristi L. Berger for editing the manuscript.
Abbreviations
- ABC
ATP-binding cassette
- ADSCs
adipose tissue-derived stem cells
- ATP
adenosine triphosphate
- BM
bone marrow
- BMP
bone morphogenic protein
- CB
carotid body
- CESCs
corneal epithelial stem cells
- CE
ciliary epithelium
- CNS
central nervous system
- CSCs
cardiac stem/progenitor cells
- CXCR4
CXC chemokine receptor-4
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EPCs
endothelial progenitor cells
- ESCs
embryonic stem cells
- FGF
fibroblast growth factor
- hAECs
human amniotic epithelial cells
- HGF
hepatocyte growth factor
- HOV
hepatic oval cells
- HSCs
hematopoietic stem cells
- IGF
insulin-like growth factor
- MDSCs
muscle-derived stem cells
- MSCs
mesenchymal stem cells
- NCSCs
neural crest stem cells
- NSCs
neural stem cells
- Oct-3/4
octamer-binding protein
- PTCH
patched receptor
- SDF-1
stromal cell-derived factor-1
- TA
transit-amplifying
- UC
umbilical cord
- SHH
sonic hedgehog ligand
- UCB
umbilical cord blood
- PSCs
pancreatic stem cells
- RSCs
retinal stem cells
- SMCs
smooth-muscle cells
- VEGF
vascular endothelial growth factor
- Wnt
Wingless ligand
Contributor Information
Murielle Mimeault, Email: mmimeault@unmc.edu, Department of Biochemistry and Molecular Biology, University of Nebraska Medical Center, Omaha, NE 68198-5870, USA, Eppley Institute for Cancer and Allied Diseases, University of Nebraska Medical Center, Omaha, NE 68198-5870, USA.
Surinder K. Batra, Email: sbatra@unmc.edu, Department of Biochemistry and Molecular Biology, University of Nebraska Medical Center, Omaha, NE 68198-5870, USA, Department of Pathology and Microbiology, University of Nebraska Medical Center, Omaha, NE 68198-5870, USA, Eppley Institute for Cancer and Allied Diseases, University of Nebraska Medical Center, Omaha, NE 68198-5870, USA
References
- 1.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. American Journal of Pathology. 2006;169(2):338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brittan M, Wright NA. Gastrointestinal stem cells. Journal of Pathology. 2002;197(4):492–509. doi: 10.1002/path.1155. [DOI] [PubMed] [Google Scholar]
- 3.Potten CS, Ellis JR. Adult small intestinal stem cells: identification, location, characteristics, and clinical applications. Ernst Schering Research Foundation Workshop. 2006;60:81–98. doi: 10.1007/3-540-31437-7_7. [DOI] [PubMed] [Google Scholar]
- 4.Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Experimental Eye Research. 2004;78(3):433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Watts C, McConkey H, Anderson L, Caldwell M. Anatomical perspectives on adult neural stem cells. Journal of Anatomy. 2005;207(3):197–208. doi: 10.1111/j.1469-7580.2005.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Xie T. Stem cell niche: Structure and function. Annual Review of Cell and Developmental Biology. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 8.Guettier C. Which stem cells for adult liver? Annales de Pathologie. 2005;25(1):33–44. doi: 10.1016/s0242-6498(05)80097-5. [DOI] [PubMed] [Google Scholar]
- 9.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiological Reviews. 2005;85(4):1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 10.Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nature Biotechnology. 2005;23(7):857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 11.Mimeault M, Batra SK. Recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells. 2006;24:2319–2345. doi: 10.1634/stemcells.2006-0066. [DOI] [PubMed] [Google Scholar]
- 12.Koblas T, Zacharovova K, Berkova Z, et al. Isolation and characterization of human CXCR4-positive pancreatic cells. Folia Biologica (Praha) 2007;53(1):13–22. [PubMed] [Google Scholar]
- 13.Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25(4):818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 14.Herrera MB, Bruno S, Buttiglieri S, et al. Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 2006;24(12):2840–2850. doi: 10.1634/stemcells.2006-0114. [DOI] [PubMed] [Google Scholar]
- 15.Lim DA, Huang YC, Alvarez-Buylla A. The adult neural stem cell niche: lessons for future neural cell replacement strategies. Neurosurgery Clinics of North America. 2007;18(1):81–92. doi: 10.1016/j.nec.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Mimeault M, Hauke R, Batra SK. Stem cells – A revolution in therapeutics–Recent advances on the stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clinical Pharmacology and Therapeutics. 2007;82:252–264. doi: 10.1038/sj.clpt.6100301. [DOI] [PubMed] [Google Scholar]
- 17.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 18.van Vliet P, Sluijter JP, Doevendans PA, Goumans MJ. Isolation and expansion of resident cardiac progenitor cells. Expert Reviews in Cardiovascular Therapy. 2007;5(1):33–43. doi: 10.1586/14779072.5.1.33. [DOI] [PubMed] [Google Scholar]
- 19.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends in Cell Biology. 2005;15(12):666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Peault B, Rudnicki M, Torrente Y, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Molecular Therapy. 2007;15(5):867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths MJ, Bonnet D, Janes SM. Stem cells of the alveolar epithelium. Lancet. 2005;366(9481):249–260. doi: 10.1016/S0140-6736(05)66916-4. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Driskell RR, Engelhardt JF. Airway glandular development and stem cells. Current Topics in Developmental Biology. 2004;64:33–56. doi: 10.1016/S0070-2153(04)64003-8. [DOI] [PubMed] [Google Scholar]
- 23.Mimeault M, Batra SK. Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis. 2006;27:1–22. doi: 10.1093/carcin/bgi229. [DOI] [PubMed] [Google Scholar]
- 24.Mimeault M, Hauke R, Mehta PP, Batra SK. Recent advances on cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. Journal of Molecular and Cellular Medicine. 2007;11:981–1011. doi: 10.1111/j.1582-4934.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mimeault M, Batra SK. Interplay of distinct growth factors during epithelial-mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Annals of Oncology. 2007;18:1605–1619. doi: 10.1093/annonc/mdm070. [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Pan X, Chen G, et al. Hematopoietic stem cells mobilized by granulocyte colony-stimulating factor partly contribute to liver graft regeneration after partial orthotopic liver transplantation. Liver Transplantationantation. 2006;12(7):1129–1137. doi: 10.1002/lt.20822. [DOI] [PubMed] [Google Scholar]
- 27.Herzog EL, Krause DS. Engraftment of marrow-derived epithelial cells: the role of fusion. Proceedings of the American Thoracic Society. 2006;3(8):691–695. doi: 10.1513/pats.200605-109SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh SH, Witek RP, Bae SH, et al. Bone marrow-derived hepatic oval cells differentiate into hepatocytes in 2- acetylaminofluorene/partial hepatectomy-induced liver regeneration. Gastroenterology. 2007;132(3):1077–1087. doi: 10.1053/j.gastro.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34−/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circulation research. 2006;98(3):E20–E25. doi: 10.1161/01.RES.0000205765.28940.93. [DOI] [PubMed] [Google Scholar]
- 30.Schatteman GC, Dunnwald M, Jiao C. Biology of bone marrow-derived endothelial cell precursors. American Journal of Physiology. Heart and Circulatory Physiology. 2007;292(1):H1–H18. doi: 10.1152/ajpheart.00662.2006. [DOI] [PubMed] [Google Scholar]
- 31.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432(7015):324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 32.Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Research. 2005;65(20):9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 33.Berns A. Stem cells for lung cancer? Cell. 2005;121(6):811–813. doi: 10.1016/j.cell.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Galderisi U, Cipollaro M, Giordano A. Stem cells and brain cancer. Cell Death and Differentiation. 2006;13:5–11. doi: 10.1038/sj.cdd.4401757. [DOI] [PubMed] [Google Scholar]
- 35.Woodward WA, Chen MS, Behbod F, Rosen JM. On mammary stem cells. Journal of Cell Science. 2005;118(Pt 16):3585–3594. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]
- 36.Mimeault M, Hauke R, Batra SK. Recent advances on the molecular mechanisms involved in drug-resistance of cancer cells and novel targeting therapies. Clinical Pharmacology and Therapeutics. 2008 doi: 10.1038/sj.clpt.6100296. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMullen NM, Pasumarthi KB. Donor cell transplantation for myocardial disease: does it complement current pharmacological therapies? Canadian Journal of Physiology and Pharmacology. 2007;85(1):1–15. doi: 10.1139/Y06-105. [DOI] [PubMed] [Google Scholar]
- 38.Behfar A, Perez-Terzic C, Faustino RS, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. Journal of Experimental Medicine. 2007;204(2):405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garbuzova-Davis S, Willing AE, Saporta S, et al. Progress in Brain Research. 2006;157:207–222. doi: 10.1016/S0079-6123(06)57014-1. [DOI] [PubMed] [Google Scholar]
- 40.Chang YC, Shyu WC, Lin SZ, Li H. Regenerative therapy for stroke. Cell Transplant. 2007;16(2):171–181. [PubMed] [Google Scholar]
- 41.Geraerts M, Krylyshkina O, Debyser Z, Baekelandt V. Concise review: therapeutic strategies for Parkinson disease based on the modulation of adult neurogenesis. Stem Cells. 2007;25(2):263–270. doi: 10.1634/stemcells.2006-0364. [DOI] [PubMed] [Google Scholar]
- 42.Trzaska KA, Rameshwar P. Current advances in the treatment of Parkinson’s disease with stem cells. Current Neurovascular Research. 2007;4(2):99–109. doi: 10.2174/156720207780637199. [DOI] [PubMed] [Google Scholar]
- 43.Gharaee-Kermani M, Gyetko MR, Hu B, Phan SH. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy. Pharmaceutical Research. 2007;24(5):819–841. doi: 10.1007/s11095-006-9216-x. [DOI] [PubMed] [Google Scholar]
- 44.Andrade CF, Wong AP, Waddell TK, Keshavjee S, Liu M. Cell-based tissue engineering for lung regeneration. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2007;292(2):L510–L518. doi: 10.1152/ajplung.00175.2006. [DOI] [PubMed] [Google Scholar]
- 45.Sharma AD, Cantz T, Manns MP, Ott M. The role of stem cells in physiology, pathophysiology, and therapy of the liver. Stem Cell Review. 2006;2(1):51–58. doi: 10.1007/s12015-006-0009-8. [DOI] [PubMed] [Google Scholar]
- 46.Fiegel HC, Lange C, Kneser U, et al. Fetal and adult liver stem cells for liver regeneration and tissue engineering. Journal of Cellular and Molecular Medicine. 2006;10(3):577–587. doi: 10.1111/j.1582-4934.2006.tb00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox JG, Wang TCJ. Inflammation, atrophy, and gastric cancer. Clinical Investigator. 2007;117(1):60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fellous TG, Guppy NJ, Brittan M, Alison MR. Cellular pathways to beta-cell replacement. Diabetes/Metabolism Research and Reviews. 2007;23(2):87–99. doi: 10.1002/dmrr.692. [DOI] [PubMed] [Google Scholar]
- 49.Santana A, Ensenat-Waser R, Arribas MI, Reig JA, Roche E. Insulin-producing cells derived from stem cells: recent progress and future directions. Journal of Cellular and Molecular Medicine. 2006;10(4):866–883. doi: 10.1111/j.1582-4934.2006.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 50.Lu P, Liu F, Yan L, et al. Stem cells therapy for type 1 diabetes. Diabetes Research and Clinical Practice. 2007;78:1–7. doi: 10.1016/j.diabres.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Kuroda R, Usas A, Kubo S, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis and Rheumatism. 2006;54(2):433–442. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 52.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circulation Research. 2007;100(9):1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dragoo JL, Carlson G, McCormick F, et al. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Engineering. 2007;13:1615–1621. doi: 10.1089/ten.2006.0249. [DOI] [PubMed] [Google Scholar]
- 54.Helder MN, Knippenberg M, Klein-Nulend J, Wuisman PI. Stem cells from adipose tissue allow challenging new concepts for regenerative medicine. Tissue Engineering. 2007;13:1799–1808. doi: 10.1089/ten.2006.0165. [DOI] [PubMed] [Google Scholar]
- 55.Limb GA, Daniels JT, Cambrey AD, et al. Current prospects for adult stem cell-based therapies in ocular repair and regeneration. Current Eye Research. 2006;31(5):381–390. doi: 10.1080/02713680600681210. [DOI] [PubMed] [Google Scholar]
- 56.MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 57.Rossi DJ, Bryder D, Weissman IL. Hematopoietic stem cell aging: Mechanism and consequence. Experimental Gerontology. 2007;42(5):385–390. doi: 10.1016/j.exger.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barfield RC, Hale GA, Burnette K, et al. Autologous transplantation of CD133 selected hematopoietic progenitor cells for treatment of relapsed acute lymphoblastic leukemia. Pediatric Blood Cancer. 2007;48(3):349–353. doi: 10.1002/pbc.20687. [DOI] [PubMed] [Google Scholar]
- 59.Sora F, Piccirillo N, Chiusolo P, et al. Mitoxantrone, carboplatin, cytosine arabinoside, and methylprednisolone followed by autologous peripheral blood stem cell transplantation: a salvage regimen for patients with refractory or recurrent non- Hodgkin lymphoma. Cancer. 2006;106(4):859–866. doi: 10.1002/cncr.21634. [DOI] [PubMed] [Google Scholar]
- 60.Small TN, Young JW, Castro-Malaspina H, et al. Intravenous busulfan and melphalan, tacrolimus, and short-course methotrexate followed by unmodified HLA-matched related or unrelated hematopoietic stem cell transplantation for the treatment of advanced hematologic malignancies. Biology of Blood and Marrow Transplantation. 2007;13(2):235–244. doi: 10.1016/j.bbmt.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 61.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nature Immunology. 2004;5(7):738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 62.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 63.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23(43):7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 64.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Research. 2005;65(8):3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 65.Hermann PC, Huber SL, Herrler T. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cells. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nature Reviews Cancer. 2006;6(6):425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 67.Mimeault M, Batra SK. Functions of tumorigenic and migrating cancer progenitor cells in cancer progression and metastasis and their therapeutic implications. Cancer Metastasis Reviews. 2007;26(1):203–214. doi: 10.1007/s10555-007-9052-4. [DOI] [PubMed] [Google Scholar]
- 68.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nature Reviews Cancer. 2005;5(4):275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 69.de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23(1):63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. Journal of Cell Science. 2005;118(Pt 8):1715–1724. doi: 10.1242/jcs.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Umemoto T, Yamato M, Nishida K, Yang J, Tano Y, Okano T. Limbal epithelial side-population cells have stem cell-like properties, including quiescent state. Stem Cells. 2006;24(1):86–94. doi: 10.1634/stemcells.2005-0064. [DOI] [PubMed] [Google Scholar]
- 72.Wilson A, Trumpp A. Bone-marrow haematopoietic- stem-cell niches. Nature Reviews. Immunology. 2006;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 73.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311(5769):1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 74.Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Annals of the New York Academy of Sciences. 2007;1106:41–53. doi: 10.1196/annals.1392.005. [DOI] [PubMed] [Google Scholar]
- 75.Murphy MJ, Wilson A, Trumpp A. More than just proliferation: Myc function in stem cells. Trends in Cell Biology. 2005;15(3):128–137. doi: 10.1016/j.tcb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 76.Urbanek K, Cesselli D, Rota M, et al. Stem cell niches in the adult mouse heart. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(24):9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 78.Delorme B, Chateauvieux S, Charbord P. The concept of mesenchymal stem cells. Regeneración médica. 2006;1(4):497–509. doi: 10.2217/17460751.1.4.497. [DOI] [PubMed] [Google Scholar]
- 79.Muller P, Beltrami AP, Cesselli D, Pfeiffer P, Kazakov A, Bohm M. Myocardial regeneration by endogenous adult progenitor cells. Journal of Molecular and Cellular Cardiology. 2005;39(2):377–387. doi: 10.1016/j.yjmcc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 80.da Silva ML, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of Cell Science. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 81.Zengin E, Chalajour F, Gehling UM, et al. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133(8):1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- 82.Seeberger KL, Dufour JM, Shapiro AM, Lakey JR, Rajotte RV, Korbutt GS. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Laboratory Investigation. 2006;86(2):141–153. doi: 10.1038/labinvest.3700377. [DOI] [PubMed] [Google Scholar]
- 83.Bernardo ME, Emons JA, Karperien M, et al. Human mesenchymal stem cells derived from bone marrow display a better chondrogenic differentiation compared with other sources. Connective Tissue Research. 2007;48(3):132–140. doi: 10.1080/03008200701228464. [DOI] [PubMed] [Google Scholar]
- 84.Gindraux F, Selmani Z, Obert L, et al. Human and rodent bone marrow mesenchymal stem cells that express primitive stem cell markers can be directly enriched by using the CD49a molecule. Cell & Tissue Research. 2007;327(3):471–483. doi: 10.1007/s00441-006-0292-3. [DOI] [PubMed] [Google Scholar]
- 85.Wolbank S, Peterbauer A, Fahrner M, et al. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: A comparison with human mesenchymal stem cells from adipose tissue. Tissue Engineering. 2007;13:1173–1183. doi: 10.1089/ten.2006.0313. [DOI] [PubMed] [Google Scholar]
- 86.Chang YJ, Shih DT, Tseng CP, Hsieh TB, Lee DC, Hwang SM. Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells. 2006;24:679–685. doi: 10.1634/stemcells.2004-0308. [DOI] [PubMed] [Google Scholar]
- 87.Hattan N, Kawaguchi H, Ando K, et al. Purified cardiomyocytes from bone marrow mesenchymal stem cells produce stable intracardiac grafts in mice. Cardiovascular Research. 2005;65(2):334–344. doi: 10.1016/j.cardiores.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 88.Kajstura J, Rota M, Whang B, et al. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circulation Research. 2005;96(1):127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 89.Ma Y, Xu Y, Xiao Z, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells. 2006;24:315–321. doi: 10.1634/stemcells.2005-0046. [DOI] [PubMed] [Google Scholar]
- 90.Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochemica et Cytobiologica. 2006;44(4):215–230. [PubMed] [Google Scholar]
- 91.Sun Y, Chen L, Hou XG, et al. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chinese Medical Journal (English) 2007;120(9):771–776. [PubMed] [Google Scholar]
- 92.Balbarini A, Barsotti MC, Di SR, Leone A, Santoni T. Circulating endothelial progenitor cells characterization, function and relationship with cardiovascular risk factors. Current Pharmaceutical Design. 2007;13(16):1699–1713. doi: 10.2174/138161207780831329. [DOI] [PubMed] [Google Scholar]
- 93.Alison MR, Brittan M, Lovell MJ, Wright NA. Markers of adult tissue-based stem cells. Handbook of Experimental Pharmacology. 2006;174:185–227. [PubMed] [Google Scholar]
- 94.Ceschel S, Casotto V, Valsecchi MG, et al. Survival after relapse in children with solid tumors: a follow-up study from the Italian off-therapy registry. Pediatric Blood Cancer. 2006;47(5):560–566. doi: 10.1002/pbc.20726. [DOI] [PubMed] [Google Scholar]
- 95.Ju Z, Jiang H, Jaworski M, et al. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Natural Medicines. 2007;13:742–747. doi: 10.1038/nm1578. [DOI] [PubMed] [Google Scholar]
- 96.Ringden O. Immunotherapy by allogeneic stem cell transplantation. Advances in Cancer Research. 2007;97C:25–60. doi: 10.1016/S0065-230X(06)97002-X. [DOI] [PubMed] [Google Scholar]
- 97.Khalil PN, Weiler V, Nelson PJ, et al. Non-myeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007;132(3):944–954. doi: 10.1053/j.gastro.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 98.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Experimental Hematology. 2005;33(11):1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 99.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. Journal of Internal Medicine. 2007;262(5):509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 100.Iwaguro H, Asahara T. Endothelial progenitor cell culture and gene transfer. Methods in Molecular Medicine. 2005;112:239–247. doi: 10.1385/1-59259-879-x:239. [DOI] [PubMed] [Google Scholar]
- 101.Hoogduijn MJ, Crop MJ, Peeters AM, et al. Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells and Development. 2007;16(4):597–604. doi: 10.1089/scd.2006.0110. [DOI] [PubMed] [Google Scholar]
- 102.Okada H, Suzuki J, Futamatsu H, Maejima Y, Hirao K, Isobe M. Attenuation of autoimmune myocarditis in rats by mesenchymal stem cell transplantation through enhanced expression of hepatocyte growth factor. International Heart Journal. 2007;48(5):649–661. doi: 10.1536/ihj.48.649. [DOI] [PubMed] [Google Scholar]
- 103.Shanti RM, Li WJ, Nesti LJ, Wang X, Tuan RS. Adult mesenchymal stem cells: biological properties, characteristics, and applications in maxillofacial surgery. Journal of Oral and Maxillofacial Surgery. 2007;65(8):1640–1647. doi: 10.1016/j.joms.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 104.Sata M, Saiura A, Kunisato A, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Natural Medicines. 2002;8(4):403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 105.Wang CH, Cherng WJ, Yang NI, et al. Late-outgrowth endothelial cells attenuate ntimal hyperplasia contributed by mesenchymal stem cells after vascular injury. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;28(1):54–60. doi: 10.1161/ATVBAHA.107.147256. [DOI] [PubMed] [Google Scholar]
- 106.Shen W, Wang Z, Punyanita M, Lei J, Sinav A, Kral JG, et al. Adipose tissue quantification by imaging methods: a proposed classification. Obesity Research. 2003;11(1):5–16. doi: 10.1038/oby.2003.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prunet-Marcassus B, Cousin B, Caton D, Andre M, Penicaud L, Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Experimental Cell Research. 2006;312(6):727–736. doi: 10.1016/j.yexcr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 108.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin Y, Liu L, Li Z, et al. Pluripotency potential of human adipose-derived stem cells marked with exogenous green fluorescent protein. Molecular and Cellular Biochemistry. 2006;291(1–2):1–10. doi: 10.1007/s11010-006-9188-5. [DOI] [PubMed] [Google Scholar]
- 110.Valina C, Pinkernell K, Song YH, et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. European Heart Journal. 2007;28(21):2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 111.Dhar S, Yoon ES, Kachgal S, Evans GR. Longterm maintenance of neuronally differentiated human adipose tissue-derived stem cells. Tissue Engineering. 2007;13(11):2625–2632. doi: 10.1089/ten.2007.0017. [DOI] [PubMed] [Google Scholar]
- 112.Niemela SM, Miettinen S, Konttinen Y, et al. Fat tissue: views on reconstruction and exploitation. Journal of Craniofacial Surgery. 2007;18(2):325–335. doi: 10.1097/scs.0b013e3180333b6a. [DOI] [PubMed] [Google Scholar]
- 113.Liu Y, Yan X, Sun Z, et al. Adipose-derived mesenchymal stem cells differentiate into skeletal muscle satellite cells and ameliorate muscular dystrophy in MDX mice. Stem Cells Development. 2007;16(5):695–706. doi: 10.1089/scd.2006.0118. [DOI] [PubMed] [Google Scholar]
- 114.Usas A, Huard J. Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials. 2007;28(36):5401–5406. doi: 10.1016/j.biomaterials.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nature Cell Biology. 2006;8(7):677–687. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- 116.Rouger K, Fornasari B, Armengol VL, et al. Progenitor cell isolation from muscle-derived cells based on adhesion properties. Journal of Histochemistry and Cytochemistry. 2007;55(6):607–618. doi: 10.1369/jhc.6A6954.2007. [DOI] [PubMed] [Google Scholar]
- 117.Tamaki T, Okada Y, Uchiyama Y, et al. Synchronized reconstitution of muscle fibers, peripheral nerves and blood vessels by murine skeletal muscle-derived CD34(−)/45 (−) cells. Histochemistry and Cell Biology. 2007;128(4):349–360. doi: 10.1007/s00418-007-0331-5. [DOI] [PubMed] [Google Scholar]
- 118.Furuta A, Jankowski RJ, Pruchnic R, Yoshimura N, Chancellor MB. The potential of muscle-derived stem cells for stress urinary incontinence. Expert Opinion on Biological Therapy. 2007;7(10):1483–1486. doi: 10.1517/14712598.7.10.1483. [DOI] [PubMed] [Google Scholar]
- 119.Torrente Y, Belicchi M, Marchesi C, et al. Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplant. 2007;16(6):563–577. doi: 10.3727/000000007783465064. [DOI] [PubMed] [Google Scholar]
- 120.Okada T, Ozawa K. Vector-producing tumor-tracking multipotent mesenchymal stromal cells for suicide cancer gene therapy. Frontiers in Bioscience. 2008;13:1887–1891. doi: 10.2741/2808. [DOI] [PubMed] [Google Scholar]
- 121.Pollett JB, Corsi KA, Weiss KR, et al. Malignant transformation of multipotent muscle-derived cells by concurrent differentiation signals. Stem Cells. 2007;25(9):2302–2311. doi: 10.1634/stemcells.2006-0773. [DOI] [PubMed] [Google Scholar]
- 122.Langenau DM, Keefe MD, Storer NY, et al. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes & Development. 2007;21(11):1382–1395. doi: 10.1101/gad.1545007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mercer SE, Ewton DZ, Shah S, Naqvi A, Friedman E. Mirk/Dyrk1b mediates cell survival in rhabdomyosarcomas. Cancer Research. 2006;66(10):5143–5150. doi: 10.1158/0008-5472.CAN-05-1539. [DOI] [PubMed] [Google Scholar]
- 124.Payne TR, Oshima H, Okada M, et al. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. Journal of the American College of Cardiology. 2007;50(17):1677–1684. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 125.Anton R, Kuhl M, Pandur P. A molecular signature for the “master” heart cell. Bioessays. 2007;29(5):422–426. doi: 10.1002/bies.20562. [DOI] [PubMed] [Google Scholar]
- 126.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(35):14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scobioala S, Klocke R, Kuhlmann M, et al. Up-regulation of nestin in the infarcted myocardium potentially indicates differentiation of resident cardiac stem cells into various lineages including cardiomyocytes. The FASEB Journal. 2008 doi: 10.1096/fj.07-8252com. in press. [DOI] [PubMed] [Google Scholar]
- 128.Dawn B, Stein AB, Urbanek K, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(10):3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Urbanek K, Rota M, Cascapera S, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circulation Research. 2005;97(7):663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 130.Christoforou N, Gearhart JD. Stem cells and their potential in cell-based cardiac therapies. Progress in Cardiovascular Diseases. 2007;49(6):396–413. doi: 10.1016/j.pcad.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 131.Sohn RL, Jain M, Liao R. Adult stem cells and heart regeneration. Expert Reviews in Cardiovascular Therapy. 2007;5(3):507–517. doi: 10.1586/14779072.5.3.507. [DOI] [PubMed] [Google Scholar]
- 132.Bao C, Guo J, Lin G, Hu M, Hu Z. TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. Scandinavian Cardiovascular Journal. 2008:1–7. doi: 10.1080/14017430701543556. in press. [DOI] [PubMed] [Google Scholar]
- 133.Gao F, He T, Wang H, et al. A promising strategy for the treatment of ischemic heart disease: Mesenchymal stem cell mediated vascular endothelial growth factor gene transfer in rats. Canadian Journal of Cardiology. 2007;23(11):891–898. doi: 10.1016/s0828-282x(07)70845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang SD, Lu FL, Xu XY, et al. Transplantation of angiogenin-overexpressing mesenchymal stem cells synergistically augments cardiac function in a porcine model of chronic ischemia. Journal of Thoracic and Cardiovascular Surgery. 2006;132(6):1329–1338. doi: 10.1016/j.jtcvs.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 135.Haider HK, Elmadbouh I, Jean-Baptiste M, Ashraf M. Non-viral vector gene modification of stem cells for myocardial repair. Molecular Medicine. 2008;14(1–2):79–86. doi: 10.2119/2007-00092.Haider. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 137.Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multi-lineage differentiation potential. Biology of Reproduction. 2007;77:577–588. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- 138.Bonanno G, Mariotti A, Procoli A, et al. Human cord blood CD133+ cells immunoselected by a clinical-grade apparatus differentiate in vitro into endothelial- and cardiomyocyte-like cells. Transfusion. 2007;47(2):280–289. doi: 10.1111/j.1537-2995.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 139.Yamada Y, Yokoyama S, Wang XD, Fukuda N, Takakura N. Cardiac stem cells in brown adipose tissue express CD133 and induce bone marrow nonhematopoietic cells to differentiate into cardiomyocytes. Stem Cells. 2007;25(5):1326–1333. doi: 10.1634/stemcells.2006-0588. [DOI] [PubMed] [Google Scholar]
- 140.Guldner NW, Kajahn J, Klinger M, Sievers HH, Kruse C. Autonomously contracting human cardiomyocytes generated from adult pancreatic stem cells and enhanced in co-cultures with myocardial biopsies. International Journal of Artificial Organs. 2006;29(12):1158–1166. doi: 10.1177/039139880602901209. [DOI] [PubMed] [Google Scholar]
- 141.Caspi O, Huber I, Kehat I, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. Journal of the American College of Cardiology. 2007;50(19):1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 142.Bartunek J, Vanderheyden M, Vandekerckhove B, et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation. 2005;112(9 Suppl):I178–I183. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 143.Ahmadi H, Baharvand H, Ashtiani SK, et al. Safety analysis and improved cardiac function following local autologous transplantation of CD133(+) enriched bone marrow cells after myocardial infarction. Current Neurovascular Research. 2007;4(3):153–160. doi: 10.2174/156720207781387141. [DOI] [PubMed] [Google Scholar]
- 144.Brada LM, Rosa K, et al. Systemic delivery of adult stem cells improves cardiac function in spontaneously hypertensive rats. Clinical and Experimental Pharmacology and Physiology. 2008;35(2):113–119. doi: 10.1111/j.1440-1681.2007.04820.x. [DOI] [PubMed] [Google Scholar]
- 145.Napoli C, Maione C, Schiano C, Fiorito C, Ignarro LJ. Bone marrow cell-mediated cardiovascular repair: potential of combined therapies. Trends Molecular Medicine. 2007;13(7):278–286. doi: 10.1016/j.molmed.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 146.Sadat S, Gehmert S, Song YH, et al. Cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochemical and Biophysical Research Communications. 2007;363(3):674–679. doi: 10.1016/j.bbrc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 147.Ohnishi S, Ohgushi H, Kitamura S, Nagaya N. Mesenchymal stem cells for the treatment of heart failure. International Journal of Hematology. 2007;86(1):17–21. doi: 10.1532/IJH97.07041. [DOI] [PubMed] [Google Scholar]
- 148.Atsma DE, Fibbe WE, Rabelink TJ, Atsma DE, Fibbe WE, Rabelink TJ. Opportunities and challenges for mesenchymal stem cell-mediated heart repair. Current Opinion in Lipidology. 2007;18(6):645–649. doi: 10.1097/MOL.0b013e3282f0dd1f. [DOI] [PubMed] [Google Scholar]
- 149.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Natural Medicines. 2004;10:S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 150.Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nature Biotechnology. 2002;20(11):1103–1110. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- 151.Trounson A. The production and directed differentiation of human embryonic stem cells. Endocrine Reviews. 2006;27(2):208–219. doi: 10.1210/er.2005-0016. [DOI] [PubMed] [Google Scholar]
- 152.Walton NM, Sutter BM, Chen HX, et al. Derivation and large-scale expansion of multipotent astroglial neural progenitors from adult human brain. Development. 2006;133(18):3671–3681. doi: 10.1242/dev.02541. [DOI] [PubMed] [Google Scholar]
- 153.Pardal R, Ortega-Saenz P, Duran R, Lopez-Barneo J. Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell. 2007;131(2):364–377. doi: 10.1016/j.cell.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 154.Lovell MA, Geiger H, Van Zant GE, Lynn BC, Markesbery WR. Isolation of neural precursor cells from Alzheimer’s disease and aged control postmortem brain. Neurobiology of Aging. 2006;27(7):909–917. doi: 10.1016/j.neurobiolaging.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 155.Ramaswamy S, Shannon KM, Kordower JH. Huntington’s disease: pathological mechanisms and therapeutic strategies. Cell Transplant. 2007;16(3):301–312. doi: 10.3727/000000007783464687. [DOI] [PubMed] [Google Scholar]
- 156.Shetty AK, Hattiangady B. Concise review: prospects of stem cell therapy for temporal lobe epilepsy. Stem Cells. 2007;25(10):2396–2407. doi: 10.1634/stemcells.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Eftekharpour E, Karimi-Abdolrezaee S, Wang J, El BH, Morshead C, Fehlings MG. Myelination of congenitally dysmyelinated spinal cord axons by adult neural precursor cells results in formation of nodes of Ranvier and improved axonal conduction. Journal of Neuroscience. 2007;27(13):3416–3428. doi: 10.1523/JNEUROSCI.0273-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Smidt MP, Burbach JP. How to make a mesodiencephalic dopaminergic neuron. Nature Reviews. Neuroscience. 2007;8(1):21–32. doi: 10.1038/nrn2039. [DOI] [PubMed] [Google Scholar]
- 159.Rodriguez-Gomez JA, Lu JQ, Velasco I, et al. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells. 2007;25(4):918–928. doi: 10.1634/stemcells.2006-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Roy NS, Cleren C, Singh SK, Yang L, Beal MF. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Natural Medicines. 2006;12(11):1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 161.Wu ZY, Hui GZ, Lu Y, Wu X, Guo LH. Transplantation of human amniotic epithelial cells improves hindlimb function in rats with spinal cord injury. Chinese Medical Journal (English) 2006;119(24):2101–2107. [PubMed] [Google Scholar]
- 162.Lee MW, Moon YJ, Yang MS, et al. Neural differentiation of novel multipotent progenitor cells from cryopreserved human umbilical cord blood. Biochemical and Biophysical Research Communications. 2007;358(2):637–643. doi: 10.1016/j.bbrc.2007.04.181. [DOI] [PubMed] [Google Scholar]
- 163.Ning H, Lin G, Lue TF, Lin CS. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 2006;74(9–10):510–518. doi: 10.1111/j.1432-0436.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 164.Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22(3):355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. British Journal of Ophthalmology. 2005;89(5):529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schlotzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Experimental Eye Research. 2005;81(3):247–264. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 167.Ye J, Yao K, Kim JC. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: engraftment and involvement in wound healing. Eye. 2006;20(4):482–490. doi: 10.1038/sj.eye.6701913. [DOI] [PubMed] [Google Scholar]
- 168.Li W, Hayashida Y, Chen YT, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Research. 2007;17(1):26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Progress in Retinal and Eye Research. 2000;19(1):113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 170.Moshiri A, Close J, Reh TA. Retinal stem cells and regeneration. International Journal of Developmental Biology. 2004;48(8–9):1003–1014. doi: 10.1387/ijdb.041870am. [DOI] [PubMed] [Google Scholar]
- 171.Das AV, James J, Rahnenfuhrer J, et al. Retinal properties and potential of the adult mammalian ciliary epithelium stem cells. Vision Research. 2005;45(13):1653–1666. doi: 10.1016/j.visres.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 172.Ahmad I, Das AV, James J, Bhattacharya S, Zhao X. Neural stem cells in the mammalian eye: types and regulation. Seminars in Cell & Developmental Biology. 2004;15(1):53–62. doi: 10.1016/j.semcdb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 173.Xu H, Sta Iglesia DD, Kielczewski JL, et al. Xu Characteristics of progenitor cells derived from adult ciliary body in mouse, rat, and human eyes. Investigative Ophthalmology & Visual Science. 2007;48(4):1674–1682. doi: 10.1167/iovs.06-1034. [DOI] [PubMed] [Google Scholar]
- 174.Whikehart DR, Parikh CH, Vaughn AV, et al. Evidence suggesting the existence of stem cells for the human corneal endothelium. Molecular Vision. 2005;11:816–824. [PubMed] [Google Scholar]
- 175.Pellegrini G, Dellambra E, Paterna P, et al. Telomerase activity is sufficient to bypass replicative senescence in human limbal and conjunctival but not corneal keratinocytes. European Journal of Cell Biology. 2004;83(11–12):691–700. doi: 10.1078/0171-9335-00424. [DOI] [PubMed] [Google Scholar]
- 176.Nadri S, Soleimani M, Kiani J, Atashi A, Izadpanah R. Multipotent mesenchymal stem cells from adult human eye conjunctiva stromal cells. Differentiation. 2008 doi: 10.1111/j.1432-0436.2007.00216.x. in press. [DOI] [PubMed] [Google Scholar]
- 177.Abe T, Yoshida M, Yoshioka Y, et al. Iris pigment epithelial cell transplantation for degenerative retinal diseases. Progress in Retinal and Eye Research. 2007;26(3):302–321. doi: 10.1016/j.preteyeres.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 178.Das AV, Edakkot S, Thoreson WB, James J, Bhattacharya S, Ahmad I. Membrane properties of retinal stem cells/progenitors. Progress in Retinal and Eye Research. 2005;24(6):663–681. doi: 10.1016/j.preteyeres.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 179.Ye J, Song YS, Kang SH, Yao K, Kim JC. Involvement of bone marrow-derived stem and progenitor cells in the pathogenesis of pterygium. Eye. 2004;18(8):839–843. doi: 10.1038/sj.eye.6701346. [DOI] [PubMed] [Google Scholar]
- 180.Chen YT, Li W, Hayashida Y, et al. Human amniotic epithelial cells as novel feeder layers for promoting ex vivo expansion of limbal epithelial progenitor cells. Stem Cells. 2007;25:1995–2005. doi: 10.1634/stemcells.2006-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ye J, Kook KH, Yao K. Temporary amniotic membrane patch for the treatment of primary pterygium: mechanisms of reducing the recurrence rate. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2006;244(5):583–588. doi: 10.1007/s00417-005-0123-3. [DOI] [PubMed] [Google Scholar]
- 182.Nakamura T, Inatomi T, Sotozono C, Koizumi N, Kinoshita S. Successful primary culture and autologous transplantation of corneal limbal epithelial cells from minimal biopsy for unilateral severe ocular surface disease. Acta Ophthalmologica Scandinavica. 2004 Aug;82(4):468–471. doi: 10.1111/j.1395-3907.2004.00285.x. [DOI] [PubMed] [Google Scholar]
- 183.Ma Y, Xu Y, Xiao Z, Yang W, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells. 2006;24(2):315–321. doi: 10.1634/stemcells.2005-0046. [DOI] [PubMed] [Google Scholar]
- 184.Ahmad S, Stewart R, Yung S, et al. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007;25(5):1145–1155. doi: 10.1634/stemcells.2006-0516. [DOI] [PubMed] [Google Scholar]
- 185.Zhao X, Liu J. Differentiation of embryonic stem cells into retinal neurons. Biochemical and Biophysical Research Communications. 2002;297(2):177–184. doi: 10.1016/s0006-291x(02)02126-5. [DOI] [PubMed] [Google Scholar]
- 186.Burke ZD, Thowfeequ S, Peran M, Tosh D. Stem cells in the adult pancreas and liver. Biochemical Journal. 2007;404(2):169–178. doi: 10.1042/BJ20070167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 188.Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Review. 2006;2(3):203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 189.Modlin IM, Kidd M, Lye KD, Wright NA. Gastric stem cells: an update. Keio Journal of Medicine. 2003;52(2):134–137. doi: 10.2302/kjm.52.134. [DOI] [PubMed] [Google Scholar]
- 190.Katoh Y, Katoh M. Hedgehog signaling pathway and gastrointestinal stem cell signaling network (review) International Journal of Molecular Medicine. 2006;18(6):1019–1023. [PubMed] [Google Scholar]
- 191.Aicher A, Heeschen C. Nonbone marrow-derived endothelial progenitor cells: what is their exact location? Circulation Research. 2007;101(9):e102. doi: 10.1161/CIRCRESAHA.107.162438. [DOI] [PubMed] [Google Scholar]
- 192.Schier S, Wright NA. Stem cell relationships and the origin of gastrointestinal cancer. Oncology. 2005;69:9–13. doi: 10.1159/000086625. [DOI] [PubMed] [Google Scholar]
- 193.Andoh A, Bamba S, Brittan M, Fujiyama Y, Wright NA. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacology & Therapeutics. 2007;114(1):94–106. doi: 10.1016/j.pharmthera.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 194.Alison MR, Vig P, Russo F, et al. Hepatic stem cells: from inside and outside the liver? Cell Proliferation. 2004;37(1):1–21. doi: 10.1111/j.1365-2184.2004.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43(2 Suppl 1):S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 196.Cantz T, Manns MP, Ott M. Stem cells in liver regeneration and therapy. Cell & Tissue Research. 2008;331(1):271–282. doi: 10.1007/s00441-007-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25(27):3818–3822. doi: 10.1038/sj.onc.1209558. [DOI] [PubMed] [Google Scholar]
- 198.Yovchev MI, Grozdanov PN, Joseph B, Gupta S, Dabeva MD. Novel hepatic progenitor cell surface markers in the adult rat liver. Hepatology. 2007;45(1):139–149. doi: 10.1002/hep.21448. [DOI] [PubMed] [Google Scholar]
- 199.Chen YK, Zhao XX, Li JG, Lang S, Wang YM. Ductular proliferation in liver tissues with severe chronic hepatitis B: an immunohistochemical study. World Journal of Gastroenterology. 2006;12(9):1443–1446. doi: 10.3748/wjg.v12.i9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Koenig S, Probst I, Becker H, Krause P. Zonal hierarchy of differentiation markers and nestin expression during oval cell mediated rat liver regeneration. Histochemistry and Cell Biology. 2006;126(6):723–734. doi: 10.1007/s00418-006-0204-3. [DOI] [PubMed] [Google Scholar]
- 201.Oertel M, Menthena A, Chen YQ, Shafritz DA. Properties of cryopreserved fetal liver stem/progenitor cells that exhibit long-term repopulation of the normal rat liver. Stem Cells. 2006;24(10):2244–2251. doi: 10.1634/stemcells.2006-0141. [DOI] [PubMed] [Google Scholar]
- 202.Oertel M, Menthena A, Chen YQ, Shafritz DA. Comparison of hepatic properties and transplantation of Thy-1 (+) and Thy-1(−) cells isolated from embryonic day 14 rat fetal liver. Hepatology. 2007;46(4):1236–1245. doi: 10.1002/hep.21775. [DOI] [PubMed] [Google Scholar]
- 203.Dezso K, Jelnes P, Laszlo V, et al. Thy-1 is expressed in hepatic myofibroblasts and not oval cells in stem cell-mediated liver regeneration. American Journal of Pathology. 2007;171(5):1529–1537. doi: 10.2353/ajpath.2007.070273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Herrera MB, Bruno S, Buttiglieri S, et al. Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 2006;24(12):2840–2850. doi: 10.1634/stemcells.2006-0114. [DOI] [PubMed] [Google Scholar]
- 205.Dorrell C, Grompe M. Liver repair by intra- and extrahepatic progenitors. Stem Cell Review. 2005;1(1):61–64. doi: 10.1385/SCR:1:1:061. [DOI] [PubMed] [Google Scholar]
- 206.Rountree CB, Wang X, Ge S, et al. Bone marrow fails to differentiate into liver epithelium during murine development and regeneration. Hepatology. 2007;45(5):1250–1260. doi: 10.1002/hep.21600. [DOI] [PubMed] [Google Scholar]
- 207.Libbrecht L, Desmet V, Van DB, Roskams T. The immunohistochemical phenotype of dysplastic foci in human liver: correlation with putative progenitor cells. Journal of Hepatology. 2000;33(1):76–84. doi: 10.1016/s0168-8278(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 208.Chen QR, Xiang J, Liao B, et al. Evolutive characters of oval cells in experimental hepatocarcinogenesis. AiZheng. 2007;26(7):719–723. [PubMed] [Google Scholar]
- 209.Alison MR. Liver cancer: a disease of stem cells? Panminerva Medicaica. 2006;48(3):165–174. [PubMed] [Google Scholar]
- 210.Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiological Reviews. 2005;85(4):1255–1270. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- 211.Nikolova G, Jabs N, Konstantinova I, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Developmental Cell. 2006;10(3):397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 212.Seaberg RM, Smukler SR, Kieffer TJ, et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nature Biotechnology. 2004;22(9):1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 213.Wang GS, Rosenberg L, Scott FW. Tubular complexes as a source for islet neogenesis in the pancreas of diabetes-prone BB rats. Laboratory Investigation. 2005;85(5):675–688. doi: 10.1038/labinvest.3700259. [DOI] [PubMed] [Google Scholar]
- 214.D’Alessandro JS, Lu K, Fung BP, Colman A, Clarke DL. Rapid and efficient in vitro generation of pancreatic islet progenitor cells from nonendocrine epithelial cells in the adult human pancreas. Stem Cells Development. 2007;16(1):75–89. doi: 10.1089/scd.2006.0073. [DOI] [PubMed] [Google Scholar]
- 215.Lin HT, Chiou SH, Kao CL, et al. Characterization of pancreatic stem cells derived from adult human pancreas ducts by fluorescence activated cell sorting. World Journal of Gastroenterol. 2006;12(28):4529–4535. doi: 10.3748/wjg.v12.i28.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gangaram-Panday ST, Faas MM, de Vos P. Towards stem-cell therapy in the endocrine pancreas. Trends Molecular Medicine. 2007;13(4):164–173. doi: 10.1016/j.molmed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 217.Lock LT, Tzanakakis ES. Stem/Progenitor cell sources of insulin-producing cells for the treatment of diabetes. Tissue Engineering. 2007;13(7):1399–1412. doi: 10.1089/ten.2007.0047. [DOI] [PubMed] [Google Scholar]
- 218.Yao S, Chen S, Clark J, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shim JH, Kim SE, Woo DH, et al. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50(6):1228–1238. doi: 10.1007/s00125-007-0634-z. [DOI] [PubMed] [Google Scholar]
- 220.Kim S, Shin JS, Kim HJ, Fisher RC, Lee MJ, Kim CW. Streptozotocin-induced diabetes can be reversed by hepatic oval cell activation through hepatic transdifferentiation and pancreatic islet regeneration. Laboratory Investigation. 2007;87(7):702–712. doi: 10.1038/labinvest.3700561. [DOI] [PubMed] [Google Scholar]
- 221.Urban VS, Kiss J, Kovacs J, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26(1):244–253. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 222.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. Journal of Clinical Investigation. 2007;117(1):50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kleger A, Busch T, Liebau S, et al. The bioactive lipid sphingosylphosphorylcholine induces differentiation of mouse embryonic stem cells and human promyelocytic leukaemia cells. Cell Signal. 2007;19(2):367–377. doi: 10.1016/j.cellsig.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 224.Ghods AJ, Irvin D, Liu G, et al. Spheres isolated from 9L gliosarcoma rat cell line possess chemoresistant and aggressive cancer stem-like cells. Stem Cells. 2007;25(7):1645–1653. doi: 10.1634/stemcells.2006-0624. [DOI] [PubMed] [Google Scholar]
- 225.Peters BA, Diaz LA, Polyak K, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Natural Medicines. 2005;11(3):261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 226.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ganss R. Tumor stroma fosters neovascularization by recruitment of progenitor cells into the tumor bed. Journal of Cellular and Molecular Medicine. 2006;10(4):857–865. doi: 10.1111/j.1582-4934.2006.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 228.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature Reviews Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 229.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Research. 2007;67(5):2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 231.Yu JJ, Sun X, Yuan X, Lee JW, Snyder EY, Yu JS. Immunomodulatory neural stem cells for brain tumour therapy. Expert Opinion on Biological Therapy. 2006;6(12):1255–1262. doi: 10.1517/14712598.6.12.1255. [DOI] [PubMed] [Google Scholar]
- 232.Mapara KY, Stevenson CB, Thompson RC, Ehtesham M. Stem cells as vehicles for the treatment of brain cancer. Neurosurgery Clinics of North America. 2007;18(1):71–80. doi: 10.1016/j.nec.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 233.Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nature Reviews. Neuroscience. 2006;7(1):75–84. doi: 10.1038/nrn1829. [DOI] [PubMed] [Google Scholar]
- 234.Aboody KS, Bush RA, Garcia E, et al. Development of a tumor-selective approach to treat metastatic cancer. PLoS ONE. 2006;1:e23. doi: 10.1371/journal.pone.0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]