Abstract
Background
Immunosuppressive therapy may potentially alter the natural disease course of scleroderma. There have been reports of using mycophenolate mofetil (MMF) for the treatment of scleroderma skin disease.
Objective
To analyse the experience of using MMF for the treatment of active diffuse cutaneous scleroderma.
Methods
The authors compared the change in mean modified Rodnan skin scores (mRSS) in an MMF cohort at baseline with scores at 3, 6, 9 and 12 months and with those of historical controls from a pooled analysis of three multicentre randomised clinical trials of recombinant human relaxin, d-penicillamine and oral bovine type I collagen.
Results
Improvement in mRSS after treatment with MMF compared with baseline was seen as early as 3 months and continued through the 12-month follow-up. The mRSS of the MMF cohort was not different from that of the historical controls at 6 months (MMF −3.05±7.4 vs relaxin −4.83±6.99, p=0.059), but was significantly lower at 12 months (MMF −7.59±10.1 vs d-penicillamine −2.47±8.6, p<0.001; collagen −3.4±7.12, p=0.002). General and muscle severity scores and quality of life measures also improved compared with baseline. Pulmonary function remained stable.
Conclusions
MMF may benefit skin disease in patients with diffuse scleroderma, but prospective studies are required to determine its role.
INTRODUCTION
Currently, no one drug has been proven to successfully control the scleroderma disease process, but immunosuppressive therapy, particularly when given at the early inflammatory phase of diffuse skin involvement, could potentially alter the natural course of the disease. The main aim of this observational study was to assess the use of mycophenolate mofetil (MMF) for the treatment of active diffuse cutaneous scleroderma.
PATIENTS AND METHODS
Patient selection
Patients treated with MMF were identified through the Johns Hopkins Scleroderma Center database, which prospectively collects data on all patients at first visit and every 6-month visit thereafter. All cases fulfilled the American College of Rheumatology classification criteria for systemic sclerosis (SSc).1 Only patients classified as having diffuse disease who were started on MMF primarily for the treatment of active skin disease were included in the analysis.2 Patients were excluded if no baseline skin score was available before MMF therapy initiation, if they were using MMF as maintenance therapy after successful treatment with another drug, or if they were on MMF for reasons other than scleroderma skin disease (eg, active lung disease).
Data for the comparison historical control group were taken from the pooled analysis of three large multicentre randomised clinical trials of d-penicillamine (d-pen), recombinant human relaxin (Relaxin) and oral bovine type I collagen (Collagen).3 In this pooled analysis, the placebo and active treatment arms were combined as each individual trial showed no treatment response. The overall mean change in skin scores was available at 6 months for the Relaxin group, and at 12 months for the d-pen and Collagen groups, and were compared with those of our MMF cohort at 6 and 12 months, respectively. The primary analysis included all of the patients who met our entry criteria, hereafter referred to as the ‘MMF cohort’. Secondary analyses were done on the following groups: (1) ‘MMF alone’, patients who continued on MMF without the concurrent use of another agent; (2) ‘combination therapy’, patients who were also on another agent in addition to MMF; and (3) ‘flare’ subgroups, patients who initially underwent MMF discontinuation or dose reduction but needed to restart MMF or increase the dose of MMF for the sole reason of increased skin disease as defined by a rise in the mRSS.
Clinical assessment
Clinical assessments at baseline and after 3, 6, 9 and 12 months (±1 month) of MMF therapy were chosen as time points for analysis. Disease onset was defined by the first non-Raynaud’s symptom. Severity of skin was quantified using the modified Rodnan skin score (mRSS).4,5 The Health Assessment Questionnaire Disability Index (HAQ-DI) score and Medsger’s organ-specific severity scores (MSS) were noted at baseline and at 12 months (±3 months).6,7 The percent predicted force vital capacity (FVC) and percent predicted diffusing capacity of carbon monoxide (DLCO) from pulmonary functions tests (PFTs) at baseline and at 12 months (±6 months) were used to monitor lung status. PFTs were performed at various sites and all measurements of FVC and DLCO were standardised according to National Health and Nutrition Examination Survey and Knudson et al, respectively.8,9
Treatment
Our usual practice was to start patients on MMF (Cellcept; Roche Pharmaceuticals, Basel, Switzerland) 500 mg twice daily for 1 to 2 weeks. If there were no significant side effects, the dose was increased to 1000 mg twice daily. Titration to a maximum dose of 1500 mg twice daily was done in patients based on tolerance and response by the treating physicians.
Statistical analysis
A paired two-tailed Student t test was used to compare the baseline mRSS with those at 3, 6, 9 and 12 months within the MMF cohort. A χ2 test for categorical variables and an unpaired two-tailed Student t test for continuous variables were used to compare the baseline parameters between the MMF cohort and historical controls. The MSS is an ordinal measurement; hence central tendency was reported as a median with non-parametric testing (paired Wilcoxon signed rank test) to determine equality of the distributions from baseline to 12 months. A value of p<0.05 was considered to be statistically significant.
RESULTS
A total of 98 patients were included in the primary analysis (table 1).
Table 1.
Baseline characteristics in the mycophenolate mofetil (MMF), recombinant human relaxin (Relaxin), d-penicillamine (d-pen) and oral bovine type I collagen (Collagen) groups
| Variable | MMF (n=98) | Relaxin (n=231) | d-Pen (n=134) | Collagen (n=168) |
|---|---|---|---|---|
| Age (years) | 48.4±11.1 (1.00) | 47.3±10.3 (0.387) | 43.7±12.4 (0.003) | 50.8±12.2 (0.111) |
| Female, % (p value) | 83 (1.00) | 85.2 (0.663) | 77.6 (0.436) | 79.2 (0.595) |
| Race | ||||
| Caucasian, % (p value) | 76 (1.00) | 74 (0.885) | 67.9 (0.265) | 76.2 (0.900) |
| African-American, % (p value) | 14 (1.00) | 13.3 (0.973) | 19.4 (0.399) | 16.1 (0.831) |
| Other, % (p value) | 8 (1.00) | 12.8 (0.288) | 12.7 (0.377) | 7.7 (0.901) |
| Mean disease duration (months) | 21.9±27.6 (1.00) | 26.4±16.4 (0.068) | 9.5±4.1 (<0.001) | 41.8±31.9 (<0.001) |
| Modified Rodnan skin score (0–51) | 24.4±9.5 (1.00) | 27.3±6.9 (0.001) | 21.0±8.0 (0.006) | 26.1±7.8 (0.079) |
| Health Assessment Questionnaire Disability Index (0–3) | 1.10±0.64 (1.00) | 1.18±0.71 (0.337) | 1.04±0.67 (0.493) | 1.22±0.72 (0.173) |
| Antibody profile | ||||
| Anti-centromere (ACA) (94/98 tested), n (%) | 2 (2) | |||
| Anti-topoisomerase antibody (SCL-70) (94/98 tested), n (%) | 24 (24) | |||
| Antinuclear antibody (ANA) + nucleolar (SCL-70 negative) (73/98 tested), n (%) | 26 (27) |
Values are mean±SD (p value) unless stated otherwise.
The mean disease duration at initiation of MMF was 21.9±27.6 months with a median of 12.5 months (IQR 8–23). The mean disease duration of our MMF cohort was comparable to the Relaxin group (26.4±16.4 months; p=0.068), but it was significantly higher than the d-pen group (9.5±4.1 months; p<0.001) and significantly lower than the Collagen group (41.8±31.9 months; p<0.001). Treatment details are listed in table 2.
Table 2.
Treatment details
| Mean follow-up period post-MMF initiation (months) (mean±SD) | 39.0±23.9 |
| Mean treatment length (months) (mean±SD) | 19.2±14.0 |
| Maximum dose | |
| 1 g | 2 (2) |
| 2 g | 41 (42) |
| 3 g | 52 (53) |
| Other | 3 (3): 0.5 g (1); 1.5 g (1); 2.5 g (1) |
| Side effects | |
| Infection | 11 (11) |
| Anaemia | 2 (2) |
| Nausea/vomiting | 6 (6) |
| Abdominal pain | 3 (3) |
| Diarrhoea | 6 (6) |
| Increased reflux symptoms | 4 (4) |
| Pseudo-obstruction | 2 (2) |
| Headache | 1 (1) |
| Medications prior to MMF | 41 (42) |
| Corticosteroids | 30 (31) |
| Azathioprine | 4 (4) |
| Cyclophosphamide | 9 (9) |
| d-Penicillamine | 11 (11) |
| IVIG | 0 |
| Methotrexate | 23 (23) |
| Medications used in conjunction with MMF | 28 (29) |
| Corticosteroids | 21 (21) |
| Azathioprine | 0 |
| Cyclophosphamide | 0 |
| d-Penicillamine | 0 |
| IVIG | 11 (11) |
| Methotrexate | 17 (17) |
All values are n (%) unless stated otherwise.
IVIG, intravenous immunoglobulins; MMF, mycophenolate mofetil.
Comparisons with baseline
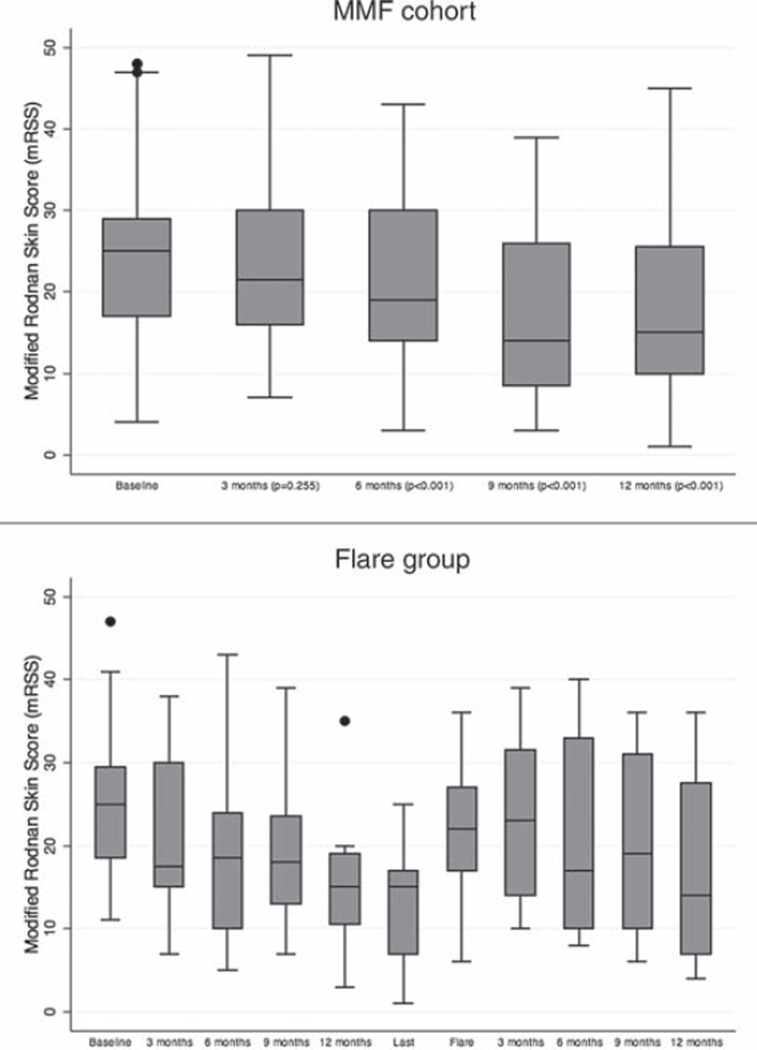
The mean mRSS of the MMF cohort decreased progressively over time from baseline (24.4±9.5). At 3, 6, 9 and 12 months the mean mRSS were 23.4±10.1, 21.4±10.6, 17.5±10.3 and 17.5±10.4, respectively. Statistical significance was reached at 6, 9 and 12 months when compared with baseline skin scores (all p<0.001), but not at 3 months (p=0.255) (figure 1A). After 12 months, there was a statistically significant improvement in the general and muscle severity scores (p=0.013 and p=0.003, respectively), but no differences were seen in the pulmonary (p=0.490), renal (p=0.317), cardiac (p=0.655), joint (p=0.103) and peripheral vascular scores (p=0.061). There was a worsening of the gastrointestinal (GI) severity score (p=0.025). HAQ-DI score improved significantly after 12 months compared with baseline (1.1±0.6 vs 0.94±0.7; p<0.001). Comparison of PFTs at baseline (FVC 79.4±17.6%; DLCO 77.4±21.5%) with those at 12 months (FVC 80.7±17.7%, DLCO 79.2±23.8%) revealed no difference in the mean FVC and DLCO (p=0.264 and 0.336, respectively).
Figure 1.
(A) Course of the modified Rodnan skin thickness score (mRSS) for the mycophenolate mofetil (MMF) cohort. (B) Course of the mRSS for the ‘flare’ group. Data are shown as box plots. Each box represents the IQR, indicating the first (25th percentile) and third (75th percentile) quartiles. Lines inside the boxes represent the median. Whiskers represent 1.5 times the first and third quartile. Circles indicate outliers.
A secondary analysis was performed on 45 patients: the ‘MMF alone’ group. The mean mRSS at baseline was 23.9±9.7 and decreased significantly to 21.8±9.2 at 3 months (p=0.028), 19.8±10.1 at 6 months (p<0.001), 14.6±8.5 at 9 months (p<0.001) and 13.9±8.2 at 12 months (p<0.001).
Twenty-eight of the 98 patients on MMF were also on concurrent therapeutic agents (‘combination therapy’ group). Seventeen were on a regimen of methotrexate plus MMF. Eleven patients were on a regimen of MMF plus intravenous immunoglobulins. The mean baseline mRSS was 24±9.8 and those at 3, 6, 9 and 12 months were 22.4±8.9, 22.6±10.5, 19.4±11.2 and 16.4±9.2, respectively. The skin scores were not significantly different from baseline at 3, 6 and 9 months (p=0.169, p=0.632, p=0.460, respectively); however, the mRSS at 12 months was significantly lower than at baseline (p=0.044). The 6-month mean change in mRSS of the ‘combination group’ was also significantly less than that of the ‘MMF alone’ group (−0.83±7.6 vs −4.64±6.2; p=0.047); however, at 12 months, the mean change in mRSS between both groups was not significantly different (−7.73±13.5 vs −10.14±8.6; p=0.496).
Interestingly, a subgroup of 20 patients, the ‘flare’ group, flared after MMF was discontinued or tapered to a lower dose. The mean duration of treatment before MMF discontinuation/taper was 17.9±7.9 months. The mean time to re-initiation/escalation of MMF dose after discontinuation/taper was 7.5±12.5 months (median 3.5 months; IQR 2–6.5). The mean baseline mRSS was 25.3±9.5 and decreased to 21.9±10 at 3 months, 18.8±11.2 at 6 months, 19.3±9.4 at 9 months, and 13.5±6.2 at 12 months (figure 1B).
Comparison with Relaxin trial at 6 months
The mean baseline mRSS of the MMF cohort (24.4±9.5) was significantly lower than that of the Relaxin group (27.3±6.9; p=0.001). There was no significant difference comparing the 6-month change in mean mRSS between the Relaxin population (−4.83±6.99) and the MMF cohort (−3.05±7.4; p=0.059). Similarly, no significant differences in 6-month changes in skin scores were detected when comparing the Relaxin group (−4.83±6.99) with the ‘MMF alone’ (−4.6±6.3, p=0.877) or the ‘flare’ groups (−5.9±6.6; p=0.569). However, when comparing the 6-month mean change in mRSS between the ‘combination therapy’ group (−0.83±7.6) and the Relaxin group, the Relaxin group actually showed a greater decrease in mRSS that met statistical significance (p=0.016).
Comparison with the d-pen and Collagen trials at 12 months
The mean baseline mRSS of the MMF cohort was not significantly different from that of the Collagen group (26.1±7.8; p=0.079), but was significantly higher than that of the d-pen group (21.0±8; p=0.006).
A statistically significant greater decrease in mRSS was seen at 12 months in the MMF cohort (−7.59±10.1) over both control groups (d-pen −2.47±8.6, p<0.001; Collagen −3.4±7.12, p=0.002).
Significantly greater decreases in the mean mRSS at 12 months were also seen in the ‘MMF alone’ (−10.1±8.6; d-pen p<0.001; Collagen p<0.001), the ‘combination therapy’ (−7.7±13.5; d-pen p=0.045; Collagen p=0.050) and the ‘flare’ groups (−11.8±7.5; d-pen p=0.008; Collagen p<0.001) at 12 months over the control groups.
DISCUSSION
In our MMF cohort, an improvement in skin scores compared with baseline was detected as early as 3 months and was statistically significantly better than baseline at 6,9 and 12 months. This suggests that long-term treatment may be required before the benefits of MMF can be fully appreciated. It is also possible that a disease-modifying effect of MMF may occur earlier, but the benefit that is measureable by skin softening can only detected after several months. The use of MMF also resulted in improved general and muscle severity scores and subjective measures of quality of life at 12 months. These patients were treated for active skin disease and did not necessarily have active lung disease, but pulmonary function, as indicated in FVC and DLCO, remained stable from baseline. The most commonly reported symptom during the treatment period was gastrointestinal upset. When comparing our cohort with other available studies, we found no difference at 6 months compared with Relaxin, but we did find a significant difference at 12 months from d-pen and Collagen studies. Among our three controls only the Relaxin cohort had a similar duration of disease at the time of treatment as our cohort. However, in the analysis of the three studies we used as controls by Amjadi et al, disease duration did not influence the change in skin score.3 This suggests that MMF may have some benefit over controls after long-term therapy.
We observed that the ‘MMF alone’ group had greater benefit than the ‘combination’ group. This may be explained by the ‘combination’ group consisting of patients thought to have more aggressive disease, with the ‘MMF alone’ group representing mild disease or even ‘responders.’ In the ‘flare’ group, the decrease or discontinuation of MMF resulted in increased skin activity. The pattern of initial improvement followed by worsening and subsequent improvement occurring with reinstitution of MMF gives some evidence that the drug was beneficial in this subset. Several other case series suggest that MMF is useful as a treatment option for SSc10–13 In addition, other studies in the literature have focused on the lung disease with suggestion of some benefit.14–18
Our study is limited by its retrospective design. A larger, prospective placebo-controlled trial is necessary to elucidate the efficacy of MMF for the treatment of active scleroderma skin disease. Since the study relied on chart review, mild adverse effects may not have been recorded and may be underestimated. Being a large tertiary care centre, our patient population may not accurately be representative of SSc in the general population. As is the case with any trial of skin disease in scleroderma, it is important to account for the natural history of changes in skin score. The lack of an internal control was addressed using the pooled published data for three large multicentre randomised controlled trials.3 Since the patients were from three separate multicentre trials, the patients may differ from our MMF cohort (table 1). The differences between the MMF cohort and the historical controls may not allow the results of this study to be directly comparable to those of the controls. It is possible that changes noted may not be different from the natural history of the disease and only further placebo controlled trials will confirm this.
CONCLUSION
From these data, we cannot determine if MMF therapy provides some benefits over the natural course of disease, but if this is the case then long-term therapy is necessary. Prospective studies are required to define the role of MMF in SSc skin disease.
Acknowledgements
The authors would like to thank the Scleroderma Research Foundation and Nancy and Joachim Bechtle for their support of the Johns Hopkins Scleroderma Center.
Funding AAS receives funding from the American College of Rheumatology Research and Education Foundation’s Clinical Investigator Fellowship Award and from the NIH grant P30 AR058885. FB is supported by the NIH K23AR055667 grant.
Footnotes
Competing interests None.
Ethics approval This study was conducted with the approval of The John Hopkins University School of Medicine.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 2.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 3.Amjadi S, Maranian P, Furst DE, et al. Course of the modified Rodnan skin thickness score in systemic sclerosis clinical trials: analysis of three large multicenter, double-blind, randomized controlled trials. Arthritis Rheum. 2009;60:2490–2498. doi: 10.1002/art.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pope JE, Baron M, Bellamy N, et al. Variability of skin scores and clinical measurements in scleroderma. J Rheumatol. 1995;22:1271–1276. [PubMed] [Google Scholar]
- 5.Clements P, Lachenbruch P, Siebold J, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–1285. [PubMed] [Google Scholar]
- 6.Poole JL, Steen VD. The use of the Health Assessment Questionnaire (HAQ) to determine physical disability in systemic sclerosis. Arthritis Care Res. 1991;4:27–31. doi: 10.1002/art.1790040106. [DOI] [PubMed] [Google Scholar]
- 7.Medsger TA, Jr, Silman AJ, Steen VD, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999;26:2159–2167. [PubMed] [Google Scholar]
- 8.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 9.Knudson RJ, Kaltenborn WT, Knudson DE, et al. The single-breath carbon monoxide diffusing capacity. Reference equations derived from a healthy nonsmoking population and effects of hematocrit. Am Rev Respir Dis. 1987;135:805–811. doi: 10.1164/arrd.1987.135.4.805. [DOI] [PubMed] [Google Scholar]
- 10.Derk CT, Grace E, Shenin M, et al. A prospective open-label study of mycophenolate mofetil for the treatment of diffuse systemic sclerosis. Rheumatology (Oxford) 2009;48:1595–1599. doi: 10.1093/rheumatology/kep295. [DOI] [PubMed] [Google Scholar]
- 11.Stratton RJ, Wilson H, Black CM. Pilot study of anti-thymocyte globulin plus mycophenolate mofetil in recent-onset diffuse scleroderma. Rheumatology (Oxford) 2001;40:84–88. doi: 10.1093/rheumatology/40.1.84. [DOI] [PubMed] [Google Scholar]
- 12.Vanthuyne M, Blockmans D, Westhovens R, et al. A pilot study of mycophenolate mofetil combined to intravenous methylprednisolone pulses and oral low-dose glucocorticoids in severe early systemic sclerosis. Clin Exp Rheumatol. 2007;25:287–292. [PubMed] [Google Scholar]
- 13.Herrick AL, Lunt M, Whidby N, et al. Observational study of treatment outcome in early diffuse cutaneous systemic sclerosis. J Rheumatol. 2010;37:116–124. doi: 10.3899/jrheum.090668. [DOI] [PubMed] [Google Scholar]
- 14.Nihtyanova SI, Brough GM, Black CM, et al. Mycophenolate mofetil in diffuse cutaneous systemic sclerosis–a retrospective analysis. Rheumatology (Oxford) 2007;46:442–445. doi: 10.1093/rheumatology/kel244. [DOI] [PubMed] [Google Scholar]
- 15.Zamora AC, Wolters PJ, Collard HR, et al. Use of mycophenolate mofetil to treat scleroderma-associated interstitial lung disease. Respir Med. 2008;102:150–155. doi: 10.1016/j.rmed.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Gerbino AJ, Goss CH, Molitor JA. Effect of mycophenolate mofetil on pulmonary function in scleroderma-associated interstitial lung disease. Chest. 2008;133:455–460. doi: 10.1378/chest.06-2861. [DOI] [PubMed] [Google Scholar]
- 17.Swigris JJ, Olson AL, Fischer A, et al. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissue disease-related interstitial lung disease. Chest. 2006;130:30–36. doi: 10.1378/chest.130.1.30. [DOI] [PubMed] [Google Scholar]
- 18.Liossis SN, Bounas A, Andonopoulos AP. Mycophenolate mofetil as first-line treatment improves clinically evident early scleroderma lung disease. Rheumatology (Oxford) 2006;45:1005–1008. doi: 10.1093/rheumatology/kei211. [DOI] [PubMed] [Google Scholar]