Abstract
Objectives. We used data from multiple national health surveys to systematically track the health of the US adult population.
Methods. We estimated trends in quality-adjusted life expectancy (QALE) from 1987 to 2008 by using national mortality data combined with data on symptoms and impairments from the National Medical Expenditure Survey (1987), National Health Interview Survey (1987, 1994–1995, 1996), Medical Expenditure Panel Survey (1992, 1996, 2000–2008), National Nursing Home Survey (1985, 1995, and 1999), and Medicare Current Beneficiary Survey (1992, 1994–2008). We decomposed QALE into changes in life expectancy, impairments, symptoms, and smoking and body mass index.
Results. Years of QALE increased overall and for all demographic groups—men, women, Whites, and Blacks—despite being slowed by increases in obesity and a rising prevalence of some symptoms and impairments. Overall QALE gains were large: 2.4 years at age 25 years and 1.7 years at age 65 years.
Conclusions. Understanding and consistently tracking the drivers of QALE change is central to informed policymaking. Harmonizing data from multiple national surveys is an important step in building this infrastructure.
Despite tremendous expenditures on medical care and public health in the United States, there has not been a systematic effort to measure the overall impact of spending on population health. Without a comprehensive population health measure, we cannot track health changes in conjunction with changes in medical treatment and public health practices, or systematically consider the impact of possible interventions.1–4 Thus, ongoing measurement of the health of the nation has been recommended by multiple commissions and panels.5,6,7
Essential attributes of a national population health measure are that it is detailed, combines mortality and morbidity into a single metric, is consistent over time, and provides information on the entire US population.1–4 It should also extend beyond disease and consider both physical and mental functioning and well-being.8,9 Length of life is tracked consistently in the United States.10 However, the measurement of health-related quality of life (HRQOL) is fragmented. The HRQOL instruments that combine groups of questions on functioning and symptoms into a general health score—for example the EuroQol-5D (EQ-5D),11 Short Form-6D,12 Health Utilities Index,13 Quality of Well-Being Scale14,15—have rarely been included in multiple years of US national surveys. Scores on existing instruments have been imputed in national data in some years,9,16 and broader self-rated health questions have been used over a longer period of time.17 A measure that combines self-care ability with an overall health rating (the Health and Activity Limitation Index) was also proposed for use in health tracking.18 However these measures do not take advantage of the rich data covering multiple dimensions of health, have sometimes trended in different directions across national surveys,19 and do not provide specific and actionable information on the particular symptoms and impairments underlying health change.9
Population coverage has also been incomplete; although some studies of health trends in the elderly have included those in institutions,20–24 most have not. Finally, in tracking health, it is important to examine disparities across demographic groups, which are known to differ in morbidity and mortality rates,25,26 and to take into account the effects of differences in behavioral risk factors, particularly obesity and smoking, which have been found to have the largest effects on mortality.27,28
Our goal was to examine US trends in quality-adjusted life expectancy (QALE) using as rich a set of health indicators as possible in as broad a population as possible and to parse out the major factors driving changes in health over time: changes in mortality, changes in the prevalence of symptoms and impairments, and changes in obesity and smoking. We used data for the community and the institutionalized populations aged 25 years and older from 1987, 1994–1995, and 2000–2008 to track and disaggregate QALE.
METHODS
We defined health as the remaining quality-adjusted years of life a typical person of a given age can expect to live.29 We considered changes in QALE for a typical 25-year-old person and a typical 65-year-old person.
Data
For life expectancy, we used national reports of mortality rates at each age.10,30 Data for HRQOL were from multiple sources: the National Medical Expenditure Survey (1987), the National Health Interview Survey (1987, 1994–1995, 1996), the Medical Expenditure Panel Survey (1992, 1996, 2000–2008), the National Nursing Home Survey (1985, 1995, and 1999) and institutionalized respondents to the Medicare Current Beneficiary Survey (1992, 1994–2008). Data on smoking and body mass index (BMI; defined as weight in kilograms divided by the square of height in meters) were from the National Health and Nutrition Examination Survey (NHANES; 1988–1994, 2000–2008). We weighted all surveys to be representative of the US population (surveys described in the appendix, available as a supplement to the online version of this article at http://www.ajph.org). Because many of the HRQOL indicators were designed for the adult population, we restricted analyses to individuals aged 25 years or older.
We grouped symptoms and impairments into 7 broad domains: problems with primary activity (working, keeping house, school) or social activity; physical activity limitations (self-care, performance of tasks for routine needs, and specific movement difficulties); mental health symptoms; sensory problems (vision and hearing impairment); pain; cognitive impairment and low energy. Table 1 provides a list of comparable symptoms and impairments measured in each survey. The appendix provides details on the samples, the wording of each survey’s symptom and impairment questions, and the disutility weights assigned to them. The appendix also discusses cross-survey differences and adjustments performed to address them.
TABLE 1—
Symptoms and Impairments Comparable Over Time: United States, 1987–2008
| Community Population |
Institutionalized Population |
||||
| Domain | 1987 NMES or NHISa | 1994 and 1995 NHIS | 2000–2008 MEPS | 1985, 1995, 1999 NNHS | 1994–2008 MCBS |
| Primary activity | Major, social | Major, social | Major, social | … | Social |
| Physical activity | Self-care, routine needs, bending, lifting, walking | Self-care, routine needs, bending, lifting, walking, standing, reaching, dexterity | Self-care, routine needs, bending, lifting, walking, standing, reaching, dexterity | Self-care, routine needs, bending, lifting, walking | Self-care, routine needs, bending, lifting, walking, reaching, dexterity |
| Mental | Depressive, anxious | … | Depressive, anxious | … | … |
| Sensory | Vision, hearing | … | Vision, hearing | Vision, hearing | Vision, hearing |
| Pain | … | … | Pain interfered with normal work | … | … |
| Cognitive | … | … | Cognitive impairment | … | … |
| Energy | … | … | Low energy | … | … |
Note. MCBS = Medicare Current Beneficiary Survey; MEPS = Medical Expenditure Panel Survey; NHIS = National Health Interview Survey; NMES = National Medical Expenditure Survey; NNHS = National Nursing Home Survey. Details on the wording of each survey’s symptom and impairment questions, along with a discussion of cross-survey differences and adjustments performed to address them, are provided in the appendix (available as a supplement to the online version of this article at http://www.ajph.org).
NHIS 1987, 1994–1995, and 1996 used for primary activity limitation, self-care (activities of daily living), and routine needs (instrumental activities of daily living).
Health Related Quality of Life and Quality Adjusted Life Expectancy Calculation
We calculated HRQOL scores based on 2 considerations: the prevalence of particular symptoms and impairments in the population (measured at several points in time) and the impact of each impairment and symptom on HRQOL (calculated at a single point in time). To estimate the impact of symptoms and impairments on HRQOL, we used a previously published method31 to relate each symptom and impairment to a 100-point rating of overall health (the visual analog scale from the EQ-5D11) measured in the 2002 Medical Expenditure Panel Survey. We then calculated disutility weights for each impairment and symptom independent of the others. Weights not only are based on the coefficients for the main effects but also reflect the effects of interactions between all pairs of symptoms and impairments. To do this, we calculated mean predicted scores from the regressions, first assuming that everyone reported the item (worst case) and then assuming no one reported the item (best case). The difference between these mean predicted scores yields a weight that captures the broadest possible impact of having the symptom or impairment in light of the other symptoms and impairments that people have.
To calculate HRQOL scores, we applied these weights to the predicted rates of symptoms and impairments reported by 10-year age group in each year. We also added a decrement equal to the intercept of the regression to all HRQOL scores to represent the average utility loss not accounted for by our set of symptoms and impairments. Previous analyses31 supported holding constant the impact of each impairment and symptom over time, with changes in the prevalence of symptoms and impairments driving changes in population HRQOL.
To adjust for the fact that more symptoms and impairments were available in the 2000s than in earlier years, we calculated 2 additional HRQOL scores for each 10-year age group in 2000: one using only the symptoms and impairments available in the 1987 National Medical Expenditure Survey, and one using only those in the 1994–1995 National Health Interview Survey. For each 10-year age group, we calculated the difference in 2000 between HRQOL scores using the smaller number of symptoms and impairments versus the larger set. We used this difference to adjust the 1987 and 1994–1995 scores downward by 10-year age group to account for the fact that the scores in those years were based on fewer symptoms and impairments.
To calculate QALE, mean HRQOL for each 10-year age group was used to adjust remaining life expectancy at each age. We used a delta method to estimate standard errors in QALE trends, taking into account the error associated with each of the QALE components.32
Disaggregation of Quality-Adjusted Life Expectancy Trends
We disaggregated overall QALE change into 3 contributing factors: changes in mortality not attributable to obesity and smoking trends, changes in the prevalence of each symptom and impairment not attributable to changes in obesity and smoking, and changes in obesity and smoking prevalence.
To estimate the effects of obesity and smoking, we divided the population into 12 cells on the basis of BMI categories (normal weight, overweight, obese, and morbidly obese) and smoking categories (never, former, and current smoker). We estimated the distribution of the population into these categories using NHANES data, smoothing obesity and smoking rates across ages using regression analysis. The first and second phases of NHANES III data (1988–1991 and 1992–1994) were used to estimate rates for 1987 and 1994–1995, respectively, and continuous NHANES data were used for 2000 onward. More details are given in the online appendix.
We calculated the relative risks of mortality for each smoking–BMI category by using combined NHANES I, II, and III mortality follow-up data, analogous to the methodology in Stewart et al.27 The effect of obesity and smoking changes on life expectancy was the difference between the actual improvement in life expectancy over time and the improvement forecast when holding constant obesity and smoking rates at baseline levels.
To calculate the contribution of life expectancy changes to QALE trends net of the impact of obesity and smoking, we simulated the increase in QALE that would have occurred if neither obesity or smoking nor impairments or symptoms had changed from their baseline rates.
To calculate the impact of obesity and smoking changes on QALE via HRQOL, we first calculated predicted rates of symptoms and impairments in each 10-year age group and calendar year for each BMI–smoking category. We then simulated QALE changes holding BMI and smoking constant at their base rates. The difference between the simulated and actual QALE, holding constant life expectancy, was the impact of changes in obesity and smoking on QALE change via their effects on HRQOL. Finally, the effect of symptom and impairment change net of obesity and smoking was the simulated QALE change holding constant both life expectancy and smoking and BMI rates.
RESULTS
Table 2 shows rates of reported symptoms and impairments in 1987, 1994–1995, 2000, and 2008 (age-normed to the 2000 population distribution), as well as tests of trends in each impairment and symptom from the first time it was measured. A similar portion of the symptoms and impairments improved significantly versus worsening significantly in their prevalence across both non-elderly and elderly adults. There were improvements among both age groups in ability to meet routine needs, in depressive and anxious moods, and in vision. However, walking and self-care problems worsened significantly for the nonelderly. Though anxiety declined dramatically in both age groups from 1987 to 2000, this decline has not continued among young and middle-aged adults. Increasing among both age groups since 1994 and 1995 were difficulty with bending, lifting, standing, and reaching. Cognitive impairment worsened significantly among the elderly.
TABLE 2—
Rates of Symptoms and Impairments and Behavioral Risk Factors Over Time: United States, 1987–2008
| 1987 (NMES/NHIS), % | 1994–1995 (NHIS), % | 2000 (MEPS), % | 2008 (MEPS), % | Percentage Point Change | Pa | |
| Age 25–64 y | ||||||
| Primary activity | ||||||
| Major activity | 6.6 | 7.3 | 5.7 | 6.3 | −0.3 | <.001 |
| Social activity | … | 3.4 | 3.5 | 0.1 | .387 | |
| Physical activity | ||||||
| Self-care | 0.6 | 0.4 | 0.7 | 0.7 | 0.1 | .084 |
| Routine needs | 1.7 | 1.9 | 1.6 | 1.7 | 0 | .003 |
| Walkingb | 4.9 | 5.1 | 5.4 | 6.2 | 1.3 | .001 |
| Bendingc | … | 5.1 | 5.3 | 6.2 | 1.1 | .001 |
| Lifting | … | 4.0 | 3.8 | 4.0 | 0 | .056 |
| Standing | … | 4.5 | 5.0 | 5.3 | 0.8 | .009 |
| Reaching | … | 2.2 | 3.3 | 3.8 | 1.6 | <.001 |
| Dexterity | … | 2.2 | 2.2 | 2.3 | 0.1 | .785 |
| Mental health | ||||||
| Depressed mood | 29.8 | … | 29.2 | 25.0 | −4.8 | <.001 |
| Anxiety | 34.3 | … | 27.3 | 39.6 | 5.2 | <.001 |
| Sensory | ||||||
| Vision | 9.7 | … | 4.2 | 4.8 | −4.9 | <.001 |
| Hearing | 5.8 | … | 3.6 | 4.5 | −1.3 | <.001 |
| Pain | … | … | 41.5 | 42.1 | 0.6 | .691 |
| Cognitive | … | … | 2.4 | 3.1 | 0.7 | .238 |
| Low energy | … | … | 54.9 | 43.9 | −11.0 | .501 |
| Behavioral risk factors | ||||||
| Current smoker | 32.7 | 29.0 | 26.9 | 26.0 | −6.7 | <.001 |
| Obese (BMI ≥ 30 kg/m2) | 23.5 | 26.7 | 32.6 | 36.4 | 12.9 | <.001 |
| Age ≥ 65 y | ||||||
| Primary activity | ||||||
| Major activity | 28.3 | 28.1 | 22.6 | 22.5 | −5.8 | .237 |
| Social activity | … | … | 11.3 | 11.1 | −0.1 | .938 |
| Physical activity | ||||||
| Self-care | 9.7 | 10.9 | 10.1 | 8.6 | −1.1 | .233 |
| Routine needs | 15.8 | 17.0 | 14.6 | 13.6 | −2.2 | .001 |
| Walkingb | 33.1 | 30.1 | 29.1 | 29.3 | −3.8 | .606 |
| Bendingc | … | 22.2 | 26.3 | 26.6 | 4.4 | .001 |
| Lifting | … | 19.8 | 22.1 | 21.0 | 1.2 | .002 |
| Standing | … | 22.8 | 26.5 | 25.6 | 2.8 | .002 |
| Reaching | … | 10.7 | 19.7 | 18.0 | 7.2 | <.001 |
| Dexterity | … | 9.0 | 13.1 | 11.6 | 2.6 | .961 |
| Mental health | ||||||
| Depressed mood | 34.8 | … | 30.7 | 23.5 | −6.7 | <.001 |
| Anxiety | 36.6 | … | 25.6 | 28.2 | −12.7 | <.001 |
| Sensory | ||||||
| Vision | 28.3 | … | 11.8 | 10.8 | −17.5 | <.001 |
| Hearing | 21.7 | … | 20.5 | 20.1 | −1.6 | .563 |
| Pain | … | … | 67.1 | 64.7 | −2.4 | .042 |
| Cognitive | … | … | 7.5 | 9.4 | 1.9 | .032 |
| Low energy | … | … | 69.6 | 54.2 | −15.4 | .007 |
| Behavioral risk factors | ||||||
| Current smoker | 12.8 | 10.4 | 10.1 | 8.2 | −4.6 | <.001 |
| Obese (BMI ≥ 30 kg/m2) | 20.2 | 24.3 | 30.1 | 32.7 | 12.5 | <.001 |
Note. BMI = body mass index (defined as weight in kilograms divided by the square of height in meters); MEPS = Medical Expenditure Panel Survey; NHIS = National Health Interview Survey; NMES = National Medical Expenditure Survey. Rates of all impairments, symptoms, and behavioral risk factors are shown adjusted to the 2000 population distribution by 10-year age groups. These are predicted rates from regression models using 10-year age categories, gender, race (Black or White), gender × race, and smoking and BMI categories. Analyses in MEPS (2000–2008) were in pooled data with dummy variables for survey year, interacted with age. Excluded were those of “other” race (not White or Black), those with a BMI less than 18.5, and those with incomplete data on smoking and BMI. In NHIS, where a large portion of the samples (51% in 1987, 74% in 1994–1995) were missing smoking status because of only 1 adult per household being asked about it in a supplement (in all households in 1987 and half of households in 1994–1995), we included a dummy variable for missing on smoking in the regressions to avoid losing data on these individuals. P values are for a variable with levels representing years from the time when the symptom or impairment was first measured. We estimated risk factor rates from National Health and Nutrition Examination Survey data.
We tested significance of changes in the noninstitutionalized population by using logistic regressions, controlling for 10-year age group, gender, race, gender × race, and BMI–smoking category.
Walking rates excluding difficulty walking a mile. Rates including walking a mile for age 25–64 years were 6.0% in 2000 and 6.8% in 2008 (P = .001). For age ≥ 65 years, rates including 1 mile were 30.9% and 31.5% (P = .641).
Bending and lifting were asked as part of a combined question in 1987, and rates of these limitations combined were 13% for the nonelderly and 47% for age ≥ 65 years. Decline for bending and lifting combined from 1987 to 2008, was significant (P <.001) for both ages 25–64 years and ≥ 65 years.
Taking account of the changes in all of the symptoms and impairments and their associated disutility weights, HRQOL was essentially unchanged among the nonelderly and increased among the elderly, particularly the oldest old. Men had the highest HRQOL, with similar levels among Blacks and Whites. Black women had the lowest HRQOL. (Plots of HRQOL trends are in the appendix, available as a supplement to the online version of this article at http://www.ajph.org)
Quality Adjusted Life Expectancy Trends
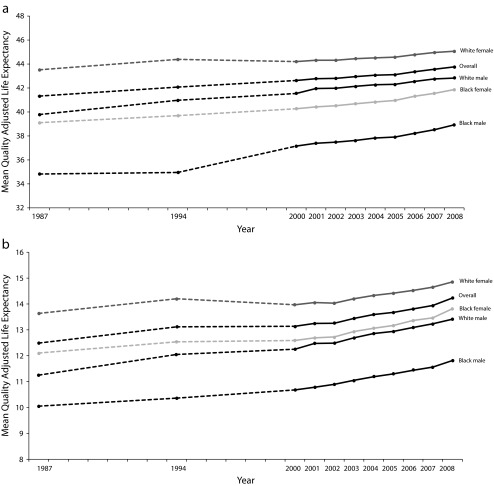
Trends in overall population health (QALE) in 1987, 1994–1995, and 2000–2008 are shown overall and by gender and race in Figure 1 for a typical 25-year-old person (Figure 1a) and a typical 65-year-old person (Figure 1b). Overall, mean QALE rose by 2.4 years, or 6% (P < .005) at age 25 years and 1.7 years (14%; P = .007) at age 65 years. White women had the highest and Black men the lowest QALE levels. White men had higher levels than Black women at age 25 years but not at age 65 years. Although life expectancy of Black women exceeded that of White men,10 adjusting for HRQOL reversed this relationship at age 25 years, with higher QALE among White men.
FIGURE 1—
Trends in quality-adjusted life expectancy at (a) age 25 years and (b) age 65 years: United States, 1987–2008.
Note. Dashed lines indicate that data was not available for intervening years. The 95% confidence intervals for overall change are smaller than the width of the line. All trends exclude those of “other” race (not White or Black).
Source. National mortality data combined with data on symptoms and impairments from the National Medical Expenditure Survey (1987), National Health Interview Survey (1987, 1994–1995, 1996), Medical Expenditure Panel Survey (1992, 1996, 2000–2008), National Nursing Home Survey (1985, 1995, and 1999), and Medicare Current Beneficiary Survey (1992, 1994–2008).
Improvement in QALE occurred among all groups, with a narrowing of gender and race gaps over time, and a greater rate of increase among the elderly. From 1987 to 2008, QALE at age 25 years increased by 1.6 years among White women, 3.0 years among White men, 2.8 years among Black women, and 4.1 years among Black men. At age 65 years, QALE increased by 1.2 years among White women, 2.2 years among White men, 1.7 years among Black women, and 1.8 years among Black men.
Disaggregation of Quality-Adjusted Life Expectancy Trends
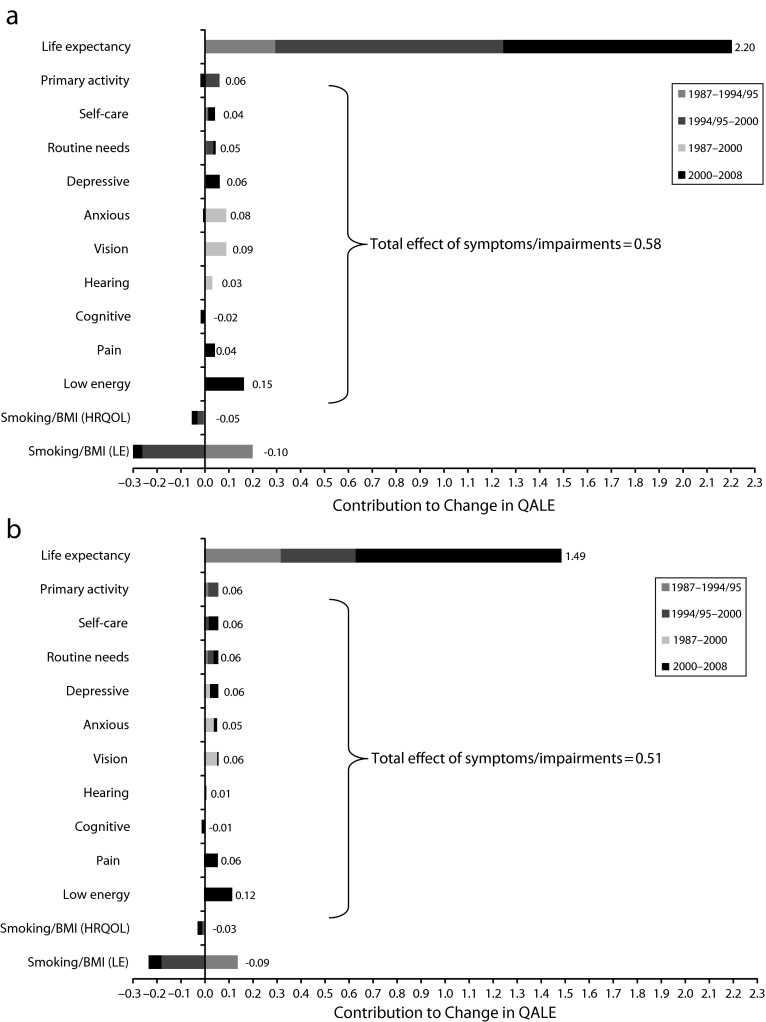
The factors underlying increased overall QALE are summarized in Figure 2. Reduced mortality net of obesity and smoking was the major driver of QALE gains among both 25- and 65-year-old persons. Life expectancy improvements alone accounted for 84% (2.2 years) of the QALE increase at age 25 years and 79% (1.5 years) at age 65 years. The next largest drivers of QALE gains were reductions in the prevalence of symptoms and impairments, particularly low energy (accounting for 6% of the QALE increase at age 25 years and at age 65 years), vision problems (4% at age 25 years and 3% at age 65 years), anxiety (3% at both ages), depressive mood (2% at age 25 years and 3% at age 65 years), and primary activity limitation, self-care, routine needs and pain (each accounting for 1%–2% at age 25 years and 3% at age 65 years). Improvements in primary activity, anxiety, and vision increased QALE before 2000, whereas improvements in energy, depression, pain, and self-care increased QALE more recently. Overall, declines in symptoms and impairments accounted for 27% (0.58 years) of the QALE increase at age 25 years, and 22% (0.51 years) at age 65 years.
FIGURE 2—
Factors accounting for changes in quality-adjusted life expectancy from 1987 to 2008 at (a) age 25 years (total improvement = 2.4 y) and (b) age 65 years (total improvement = 1.7 y): United States, 1987–2008.
Note. BMI = body mass index (defined as weight in kilograms divided by the square of height in meters); HRQOL = health-related quality of life; LE = life expectancy; QALE = quality-adjusted life expectancy. Each bar shows the contribution of that factor to improved population health, holding constant the other contributors to population health. For example, changes in life expectancy held constant the health-related quality of life and share of people in different weight and smoking categories. Obesity or smoking compared the gap between QALE change when allowing behavioral risk factors to change versus holding them constant. Symptoms and impairments explaining less than 0.02 years of QALE change at age 25 years are not shown in either figure: social activity, walking, lifting, bending, standing, reaching, and dexterity.
Source. National mortality data combined with data on symptoms and impairments from the National Medical Expenditure Survey (1987), National Health Interview Survey (1987, 1994–1995, 1996), Medical Expenditure Panel Survey (1992, 1996, 2000–2008), National Nursing Home Survey (1985, 1995, and 1999), and Medicare Current Beneficiary Survey (1992, 1994–2008).
During this time period, smoking rates declined while obesity rates climbed (Table 2), with some leveling off in recent years; the net effect was to attenuate the gains in QALE by 0.16 years at age 25 years and 0.12 years at age 65 years (accounting for –6% and –7% of the QALE increase, respectively). The effect of these behavioral risk factors on life expectancy was about 3 times their effect on HRQOL at age 25 years and twice their effect on HRQOL at age 65 years.
DISCUSSION
Population health, as measured by QALE in the US community and institutionalized populations, has increased markedly over the past 2 decades. A typical 25-year-old person will live an additional 2.4 quality-adjusted years today compared with a 25-year-old person in 1987, and a 65-year-old person will live an additional 1.7 quality-adjusted years. For both ages, the major factor driving this change was declining mortality, though HRQOL improvements contributed as well. The most important improvements were in energy, depressive mood, self care, pain, and routine needs. Reductions in anxiety and vision problems were important prior to 2000 but have not continued among the nonelderly.
Changes in obesity and smoking rates have also affected QALE. The 1.6-fold increase in obesity since 1987, though counterbalanced by declines in smoking, attenuated the increases in QALE by more than a tenth of a year. The effect was most pronounced from the mid-1990s to 2000, when obesity showed the greatest increase. Still, this was more than offset by other health trends.
Disparities in QALE by gender and race were large, with White women having the highest and Black men the lowest QALE at both age 25 years and 65 years. Consistent with previous findings that women live longer but report worse health than men,26 both White and Black women had higher life expectancy than men, but lower HRQOL. White and Black men had similar HRQOL levels, whereas the HRQOL of Black women remained lowest across all groups. Adjusting for quality drove the QALE of Black women below that of White men at age 25 years.
Increases in QALE occurred for all groups, and was greater at older ages.33 Gaps by gender and race narrowed over time as has previously been reported.34–36 Black male QALE at age 25 years rose dramatically from the mid- to late 1990s, reflecting the end of a mortality spike begun in the 1980s, driven by HIV infection and homicide.37–39 White women showed the smallest QALE increase at both age 25 years and 65 years, with the literature indicating that their average was held back by increases in mortality among White women who did not complete high school.34,40,41 The continued disparities and the fact that the narrowing of the gap was partially attributable to worsening health among Whites is disconcerting. Combined with the fact that US life expectancy lags behind that of some other countries, this underlies the importance of examining and addressing the factors underlying health disparities.42,43
This is the first study to assess detailed US QALE trends spanning 3 decades and to disaggregate them into the underlying symptoms and impairments. A recent study16 of the noninstitutionalized US adult population, mapping EQ-5D scores from Behavioral Risk Factor Surveillance System data on self-rated health and healthy days, found a QALE increase of 1.1 years at age 18 years from 1993 to 2008, with worsening HRQOL over time17 offset by increases in longevity. By contrast, we found essentially no change in HRQOL over this time period among the nonelderly and some improvement among the elderly when we used a more detailed set of symptoms and impairments. One difference underlying these findings may be that self-reports of general health have declined in some surveys,16,17,19 while many other measures of symptoms and impairments have remained constant or improved, particularly among the elderly.
Several studies have examined changes in particular symptoms and impairments over parts of this time period, and our findings are largely consistent with these. Similar to studies of disability in activities of daily living and instrumental activities of daily living, we found declines in difficulty with self-care and routine needs among the elderly,20,21 but not in self-care among those younger than 65 years.44–46 Also, consistent with studies finding declines in hearing problems47 and some increases in mobility problems since the mid-1990s, we found reductions in hearing problems and increases in reported problems with walking (among the nonelderly), and with bending, standing, lifting, and reaching over this period.44,48 This is congruent with increases in disability that have been found among the working-age population, partially explained by rising obesity and chronic disease.46 The decline in depressive and anxiety symptoms is concurrent with the increase in pharmaceutical treatment of these mental health conditions since the advent of serotonin-reuptake inhibitors in the late 1980s49; however, there is debate about the efficacy level of these medications.50 Furthermore, anxiety declines have not continued among the nonelderly since 2001. Cognitive impairment has increased among the elderly during the 2000s. Increases in energy levels during the 2000s are encouraging, reflecting an element of vitality not captured in other symptom or impairment questions.
Past studies have conflicted regarding whether morbidity has been compressed into the period just before death.48,51–54 Our results are consistent with the findings of compression of morbidity from the late 1980s through the early 2000s,51–54 and some reversal of this (expansion of morbidity) because of dips in HRQOL in the mid-2000s.48 However, it is disconcerting that trends in several symptoms and impairments flattened or increased over the 2000s, particularly among the nonelderly. Although these increases did not have large effects in holding back QALE improvement, they did have negative effects, only a portion of which were explained by rising obesity.
The most important question raised by these results is why health has improved so much. In terms of health conditions, advances in cardiovascular disease are known to have made the largest contribution to reduced mortality.55 Improvements in health behaviors, medical procedures, and pharmaceuticals have dramatically reduced mortality and disability associated with cardiovascular disease,56 though the magnitude has been debated. In addition to health behaviors and advances in medical care, other factors that can affect health include education and socioeconomic status,34,57 disease management, environmental change, and changes in the nature of work. Future analyses will help to sort out some of these differing explanations. An important strength of our approach is that it enables detailed tracking of changes in symptoms and impairments among those with different diseases over time,58 in conjunction with their costs. It also facilitates prediction of the possible impact of different policy interventions. Our approach can be adapted for use in different data sets; it does not require any specific set of symptoms and impairments. There is a high correlation between HRQOL measured by using a smaller versus a larger set of symptoms and impairments. However, our use of the broad set of questions in the Medical Expenditure Panel Survey data enabled us to derive maximal information about the factors underlying health change.
Limitations
This analysis excluded children; additional work is needed to add this population group. Also, we compared QALE only across demographic groups for whom life expectancy has been separately reported over the time period considered, and sample size did not permit disaggregating QALE change separately for each race, gender, and age group.
Although we considered the set of symptoms and impairments and 2 behavioral risk factors consistently tracked by national surveys over time, other factors have an impact on health as well. Also, although we accounted for the changing distribution of obesity and smoking over time, we did not account for factors such as level of physical activity, smoking frequency, or the duration a person has been obese or smoked.59,60 This could be important because younger cohorts with higher levels of obesity than today’s older cohorts may be sicker than middle-aged and older people are today.61,62 However, offsetting this is improved management of cardiovascular risk factors among all BMI groups.63 As is typical in QALE estimates, we used a period life table method that necessarily assumes that today’s young will change as they age to look like those who are currently middle-aged and elderly, and we extended the same assumption to impairment and symptom profiles over time. Future work could forecast alternate assumptions about the distribution of mortality and morbidity by age group, and examine the effects of other sociodemographic and risk factors.
Although we performed careful adjustments for question differences between surveys and over time (as detailed in the appendix), these may have had some effect on our measurement of change. Finally, the symptoms and impairments we used were self-reported; for impairments that can be physically measured such as sensory and physical abilities, performance is preferred and can be used to adjust self-reported measures.47
Conclusions
We found that QALE has increased over the past 2 decades, driven by improvements in life expectancy and reductions in the prevalence of several symptoms and impairments, most notably low energy, depressive mood, pain, self-care, routine needs, and, prior to 2000, anxiety and vision problems. It is encouraging that this health improvement occurred among Blacks and Whites of both genders, despite being moderated by increases in obesity and in the prevalence of some symptoms and impairments. However, continued disparities in health by race and gender, and the more recent increase in some problems, particularly among the nonelderly, are troubling and merit further attention. Harmonizing data from multiple national surveys provides the groundwork for the evaluation of population health outcomes by disease in conjunction with preventive care, medical treatment, and spending over time. Linking symptoms and impairments to diseases over time will provide a deeper understanding of the underlying causes of health change.
Acknowledgments
This research was supported by the National Institute on Aging (research grants P01 AG31098, P30 AG12810, and P01 AG005842). We appreciate the support of the Harvard University PHSI, and the Lasker Foundation.
Kaushik Ghosh, Marcelo Coca, and Jean Roth provided some advice and assistance with data analysis. Trivellore Raghunathan and Seth Richards-Shubik provided advice on the calculation of confidence intervals for quality-adjusted life expectancy estimates.
Human Participant Protection
Institutional review board approval was received for the larger projects of which this was a part; researchers had no interaction with study participants and did not have access to any individually identifiable private information.
References
- 1.Kindig DA, Asada Y, Booske B. A population health framework for setting national and state health goals. JAMA. 2008;299(17):2081–2083. doi: 10.1001/jama.299.17.2081. [DOI] [PubMed] [Google Scholar]
- 2.Gold M, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 3.Kaltenthaler E, Maheswaran R, Beverley C. Population based health indexes: a systematic review. Health Policy. 2004;68(2):245–255. doi: 10.1016/j.healthpol.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Murray CJL, Salomon JA, Mathers CD. A critical examination of summary measures of population health. Bull World Health Organ. 2000;78(8):981–994. [PMC free article] [PubMed] [Google Scholar]
- 5.Abraham KG, Mackie C. Beyond the Market: Designing Nonmarket Accounts for the United States. Washington, DC: National Academies Press; 2005. Health; pp. 117–140. [Google Scholar]
- 6.Panel to Advance a Research Program on the Design of National Health Accounts, National Research Council. Accounting for Health and Health Care: Approaches to Measuring the Sources and Costs of Their Improvement. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 7.Boskin MJ. Final Report From the Advisory Commission to Study the Consumer Price Index. Washington, DC: US Senate Finance Committee; 1996. Toward a more accurate measure of the cost of living. [Google Scholar]
- 8.Friedman DJ, Starfield B. Models of population health: their value for US public health practice, policy, and research. Am J Public Health. 2003;93(3):366–369. doi: 10.2105/ajph.93.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson P, Kendall EA, Anderson JP, Kaplan RM. Using composite health status measures to assess the nation’s health. Med Care. 1989;27(3, suppl):S66–S76. doi: 10.1097/00005650-198903001-00006. [DOI] [PubMed] [Google Scholar]
- 10. United States life tables. Hyattsville, MD: National Center for Health Statistics, Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/nchs/products/life_tables.htm. Accessed November 29, 2012.
- 11.Brooks R, Rabin RE, de Charro F. The Measurement and Valuation of Health Status Using EQ-5D: A European Perspective. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2003. [Google Scholar]
- 12.Brazier JE, Roberts J. Estimating a preference-based index from the SF-12. Med Care. 2004;42(9):851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 13.Horsman J, Furlong W, Feeny D et al. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. doi: 10.1186/1477-7525-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan RM, Bush JW, Berry CC. Health status: types of validity and the index of well-being. Health Serv Res. 1976;11(4):478–507. [PMC free article] [PubMed] [Google Scholar]
- 15.Andresen EM, Rothenberg BM, Kaplan RM. Performance of a self-administered mailed version of the Quality of Well-Being (QWB-SA) questionnaire among older adults. Med Care. 1998;36(9):1349–1360. doi: 10.1097/00005650-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Jia H, Zack MM, Thompson WW. State quality-adjusted life expectancy for U.S. adults from 1993 to 2008. Qual Life Res. 2011;20(6):853–863. doi: 10.1007/s11136-010-9826-y. [DOI] [PubMed] [Google Scholar]
- 17.Zack MM, Moriarty DG, Stroup DF, Ford ES, Mokdad AH. Worsening trends in adult health-related quality of life and self-rated health—United States, 1993–2001. Public Health Rep. 2004;119(5):493–505. doi: 10.1016/j.phr.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson P. Evaluation of a population-based measure of quality of life: the Health and Activity Limitation Index (HALex) Qual Life Res. 1998;7(2):101–114. doi: 10.1023/a:1008897107977. [DOI] [PubMed] [Google Scholar]
- 19.Salomon JA, Nordhagen S, Oza S, Murray CJL. Are Americans feeling less healthy? The puzzle of trends in self-rated health. Am J Epidemiol. 2009;170(3):343–351. doi: 10.1093/aje/kwp144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman VA, Martin LG, Schoeni RF. Recent trends in disability and functioning among older adults in the United States: a systematic review. JAMA. 2002;288(24):3137–3146. doi: 10.1001/jama.288.24.3137. [DOI] [PubMed] [Google Scholar]
- 21.Manton KG. Recent declines in chronic disability in the elderly US population: risk factors and future dynamics. Annu Rev Public Health. 2008;29:91–113. doi: 10.1146/annurev.publhealth.29.020907.090812. [DOI] [PubMed] [Google Scholar]
- 22.Cai L, Lubitz J. Was there compression of disability for older Americans from 1992 to 2003? Demography. 2007;44(3):479–495. doi: 10.1353/dem.2007.0022. [DOI] [PubMed] [Google Scholar]
- 23.Manton KG, Gu X, Lowrimore GR. Cohort changes in active life expectancy in the US elderly population: experience from the 1982–2004 National Long-Term Care Survey. J Gerontol B Psychol Sci Soc Sci. 2008;63(5):S269–S281. doi: 10.1093/geronb/63.5.s269. [DOI] [PubMed] [Google Scholar]
- 24.Crimmins EM, Hayward MD, Hagedorn A, Saito Y, Brouard N. Change in disability-free life expectancy for Americans 70 years old and older. Demography. 2009;46(3):627–646. doi: 10.1353/dem.0.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institute of Medicine. How Far Have We Come in Reducing Health Disparities?: Progress Since 2000: Workshop Summary. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 26.Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res. 2008;20(2):91–102. doi: 10.1007/bf03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on US life expectancy. N Engl J Med. 2009;361(23):2252–2260. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000 [Correction: JAMA. 2005;293(3):293–294] JAMA. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 29.Muennig PA, Gold MR. Using the years-of-healthy-life measure to calculate QALYs. Am J Prev Med. 2001;20(1):35–39. doi: 10.1016/s0749-3797(00)00261-0. [DOI] [PubMed] [Google Scholar]
- 30. Social Security Administration Annual Statistical Supplements. 2003 to 2011, period life table, 4.C6. Available at: http://www.ssa.gov/policy/docs/statcomps/index.html. Accessed November 30, 2012.
- 31.Stewart ST, Woodward RM, Rosen AB, Cutler DM. The impact of symptoms and impairments on overall health in US national health data. Med Care. 2008;46(9):954–962. doi: 10.1097/MLR.0b013e318179199f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kish L, Frankel MR. Inference from complex samples. J Royal Stat Soc. 1974; Series B, 36:1–37.
- 33.Eggleston KN, Fuchs VR. The new demographic transition: most gains in life expectancy now realized late in life. J Econ Perspect. 2012;26(3):137–156. doi: 10.1257/jep.26.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meara ER, Richards S, Cutler DM. The gap gets bigger: changes in mortality and life expectancy, by education, 1981–2000. Health Aff (Millwood) 2008;27(2):350–360. doi: 10.1377/hlthaff.27.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harper S, Lynch J, Burris S, Davey Smith G. Trends in the Black–White life expectancy gap in the United States, 1983–2003. JAMA. 2007;297(11):1224–1232. doi: 10.1001/jama.297.11.1224. [DOI] [PubMed] [Google Scholar]
- 36.Harper S, Rushani D, Kaufman JS. Trends in the Black–White life expectancy gap, 2003–2008. JAMA. 2012;307(21):2257–2259. doi: 10.1001/jama.2012.5059. [DOI] [PubMed] [Google Scholar]
- 37.Kochanek KD, Maurer JD, Rosenberg HM. Causes of death contributing to changes in life expectancy: United States, 1984–89. National Center for Health Statistics. Available at: http://www.cdc.gov/nchs/data/series/sr_20/sr20_023.pdf. Accessed November 30, 2012. [PubMed] [Google Scholar]
- 38.Geronimus AT, Bound J, Colen CG. Excess Black mortality in the United States and in selected Black and White high-poverty areas, 1980–2000. Am J Public Health. 2011;101(4):720–729. doi: 10.2105/AJPH.2010.195537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fryer R, Heaton P, Levitt S, Murphy K. Measuring crack cocaine and its impact. National Bureau of Economic Research working paper 11318. Available at: http://onlinelibrary.wiley.com/doi/10.1111/j.1465-7295.2012.00506.x/full. Accessed August 15, 2013.
- 40.Montez JK, Hummer RA, Hayward MD, Woo H, Rogers RG. Trends in the educational gradient of US adult mortality from 1986 to 2006 by race, gender, and age group. Res Aging. 2011;33(2):145–171. doi: 10.1177/0164027510392388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olshansky SJ, Antonucci T, Berkman L et al. Differences in life expectancy due to race and educational differences are widening, and many may not catch up. Health Aff (Millwood) 2012;31(8):1803–1813. doi: 10.1377/hlthaff.2011.0746. [DOI] [PubMed] [Google Scholar]
- 42.Kulkarni SC, Levin-Rector A, Ezzati M, Murray CJ. Falling behind: life expectancy in US counties from 2000 to 2007 in an international context. Popul Health Metr. 2011;9(1):16. doi: 10.1186/1478-7954-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaupel JW, Zhang Z, van Raalte AA. Life expectancy and disparity: an international comparison of life table data. BMJ Open. 2011;1(1):e000128. doi: 10.1136/bmjopen-2011-000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seeman TE, Merkin SS, Crimmins EM, Karlamangla AS. Disability trends among older Americans: National Health and Nutrition Examination Surveys, 1988–1994 and 1999–2004. Am J Public Health. 2010;100(1):100–107. doi: 10.2105/AJPH.2008.157388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seeman I. Data on disability. Am J Public Health. 2010;100(8):1367. doi: 10.2105/AJPH.2010.193276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhattacharya J, Choudhry K, Lakdawalla D. Chronic disease and severe disability among working-age populations. Med Care. 2008;46(1):92–100. doi: 10.1097/MLR.0b013e3181484335. [DOI] [PubMed] [Google Scholar]
- 47.Ikeda N, Murray CJL, Salomon JA. Tracking population health based on self-reported impairments: trends in the prevalence of hearing loss in US adults, 1976–2006. Am J Epidemiol. 2009;170(1):80–87. doi: 10.1093/aje/kwp097. [DOI] [PubMed] [Google Scholar]
- 48.Crimmins EM, Beltrán-Sánchez H. Mortality and morbidity trends: is there compression of morbidity? J Gerontol B Psychol Sci Soc Sci. 2011;66(1):75–86. doi: 10.1093/geronb/gbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kessler RC, Demler O, Frank RG et al. US prevalence and treatment of mental disorders: 1990–2003. N Engl J Med. 2005;352(24):2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vöhringer PA, Ghaemi SN. Solving the antidepressant efficacy question? Effect sizes in major depressive disorder. Clin Ther. 2011;33(12):B49–B61. doi: 10.1016/j.clinthera.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai L, Lubitz J. Was there compression of disability for older Americans from 1992 to 2003? Demography. 2007;44(3):479–495. doi: 10.1353/dem.2007.0022. [DOI] [PubMed] [Google Scholar]
- 52.Manton KG, Gu X, Lowrimore GR. Cohort changes in active life expectancy in the US elderly population: experience from the 1982–2004 National Long-Term Care Survey. J Gerontol B Psychol Sci Soc Sci. 2008;63(5):S269–S281. doi: 10.1093/geronb/63.5.s269. [DOI] [PubMed] [Google Scholar]
- 53.Crimmins EM, Hayward MD, Hagedorn A, Saito Y, Brouard N. Change in disability-free life expectancy for Americans 70 years old and older. Demography. 2009;46(3):627–646. doi: 10.1353/dem.0.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fries JF, Bruce B, Chakravarty E. Compression of morbidity 1980–2011: a focused review of paradigms and progress. J Aging Res. 2011;2011:261702. doi: 10.4061/2011/261702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cutler DM, Rosen AB, Vijan S. The value of medical spending in the United States, 1960–2000. N Engl J Med. 2006;355:920–927. doi: 10.1056/NEJMsa054744. [DOI] [PubMed] [Google Scholar]
- 56.Cutler DM, Landrum MB, Stewart K. Intensive medical care and cardiovascular disease disability reductions. In: Cutler DM, Wise DA, editors. Health at Older Ages: The Causes and Consequences of Declining Disability Among the Elderly. Cambridge, MA: National Bureau of Economic Research Inc; 2009. pp. 191–222. [Google Scholar]
- 57.Cutler DM, Lleras-Muney A. Understanding differences in health behaviors by education. J Health Econ. 2010;29(1):1–28. doi: 10.1016/j.jhealeco.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart ST, Woodward RM, Cutler DM. A Proposed Method for Monitoring US Population Health: Linking Symptoms, Impairments, Chronic Conditions, and Health Ratings. National Bureau of Economic Research Working Paper 11358. Cambridge, MA: National Bureau of Economic Research; 2005. [Google Scholar]
- 59.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Preston SH, Mehta NK, Stokes A. Modeling obesity histories in cohort analyses of health and mortality. Epidemiology. 2013;24(1):158–166. doi: 10.1097/EDE.0b013e3182770217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reither EN, Olshansky SJ, Yang Y. New forecasting methodology indicates more disease and earlier mortality ahead for today’s younger Americans. Health Aff (Millwood) 2011;30(8):1562–1568. doi: 10.1377/hlthaff.2011.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeitler P, Hirst K, Pyle L et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gregg EW, Cheng YJ, Cadwell BL et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293(15):1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]