Abstract
Absorption of light converts bilirubin-IXalpha in solution to a mixture of what are probably cis-trans geometric isomers. This reaction is much faster than other photochemical reactions of bilirubin and reaches photoequilibrium before losses due to photooxidation are significant. At room temperature in the dark in the presence of trifluoroacetic acid or iodine or simply on standing, the photoproducts revert to the natural isomer. They also revert under visible light. Their formation and reversion can be followed by chromatography on polyamide and by absorbance difference spectroscopy.
Full text
PDF
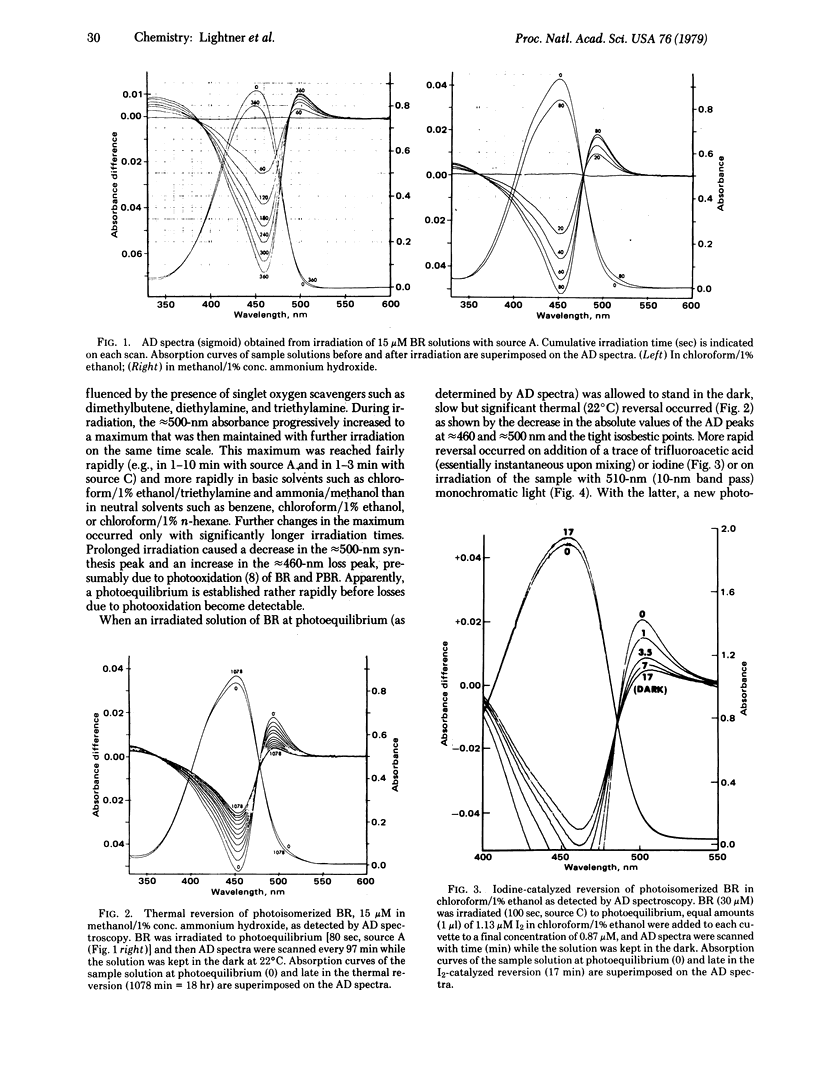
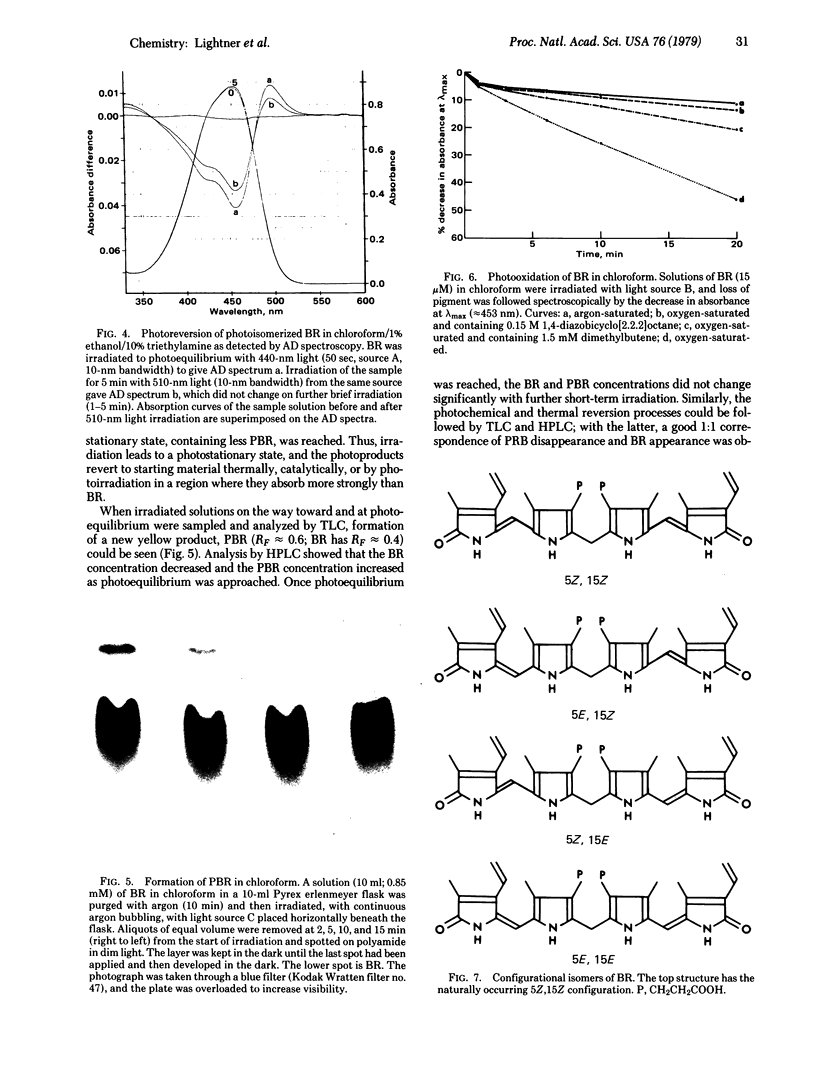

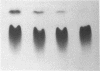
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnett R., Davies J. E., Hursthouse M. B., Sheldrick G. M. The structure of bilirubin. Proc R Soc Lond B Biol Sci. 1978 Jun 23;202(1147):249–268. doi: 10.1098/rspb.1978.0066. [DOI] [PubMed] [Google Scholar]
- Davies R. E., Keohane S. J. Early changes in light-irradiated solutions of bilirubin: a spectrophotometric analysis. Photochem Photobiol. 1973 May;17(5):303–312. doi: 10.1111/j.1751-1097.1973.tb06358.x. [DOI] [PubMed] [Google Scholar]
- Davies R. E., Keohane S. J. Some aspects of the photochemistry of bilirubin. Boll Chim Farm. 1970 Oct;109(10):589–598. [PubMed] [Google Scholar]
- Kawai S. [Discussion on decomposition of chloroform]. Yakugaku Zasshi. 1966 Dec;86(12):1125–1132. doi: 10.1248/yakushi1947.86.12_1125. [DOI] [PubMed] [Google Scholar]
- Lightner D. A., Crandall D. C., Gertler S., Quistad G. B. On the formation of biliverdin during photooxygenation of bilirubin in vitro. FEBS Lett. 1973 Mar 15;30(3):309–312. doi: 10.1016/0014-5793(73)80676-3. [DOI] [PubMed] [Google Scholar]
- McDonagh A. F., Assisi F. Commercial bilirubin: A trinity of isomers. FEBS Lett. 1971 Nov 1;18(2):315–317. doi: 10.1016/0014-5793(71)80475-1. [DOI] [PubMed] [Google Scholar]
- McDonagh A. F., Assisi F. The ready isomerization of bilirubin IX- in aqueous solution. Biochem J. 1972 Sep;129(3):797–800. doi: 10.1042/bj1290797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh A. F. The role of singlet oxygen in bilirubin photo-oxidation. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1306–1311. doi: 10.1016/s0006-291x(71)80228-0. [DOI] [PubMed] [Google Scholar]
- Pedersen A. O., Schonheyder F., Brodersen R. Photooxidation of human serum albumin and its complex with bilirubin. Eur J Biochem. 1977 Jan;72(2):213–221. doi: 10.1111/j.1432-1033.1977.tb11242.x. [DOI] [PubMed] [Google Scholar]