Abstract
Objectives. We identified potential determinants and cause-specific sources of excess infant mortality among Native Hawaiians.
Methods. We compared infant mortality rates among Native Hawaiians and Whites by using data from the 2002 to 2009 Hawai’i State Linked Birth/Infant Death Cohort File. We evaluated the components of excess infant mortality by age and underlying cause of death as well as maternal sociodemographic, behavioral, and chronic condition disparities.
Results. The Native Hawaiian infant mortality rate was more than twice that for Whites (7.9 vs 3.5/1000 live births). Excess Native Hawaiian infant mortality was equally apportioned to neonatal and postneonatal deaths. Preterm-related causes of death accounted for 43.9% of the infant mortality disparity, followed by sudden unexpected infant death (21.6%) and injury (5.6%). In multivariable models, maternal educational inequality accounted for the largest portion of the neonatal mortality disparity (20.9%); younger maternal age (12.2%) and smoking (9.5%) were the only significant contributors to the postneonatal mortality disparity.
Conclusions. Addressing educational inequalities, promoting safe sleep practices, and reducing smoking among Native Hawaiian mothers would help to eliminate excess infant mortality.
As an indigenous population, Native Hawaiians share a similar sociopolitical status with American Indians and Alaska Natives and are recognized in the Native American Programs Act with access to some but not all of the assistance programs and tribal rights accorded to American Indians.1 Although the health status of Native Hawaiians is relatively understudied, people of Native Hawaiian ancestry have been shown to suffer a greater burden of ill health, including higher rates of cardiovascular disease, diabetes, and obesity.2,3 Higher mortality rates are observed across the life span, including the most premature of deaths—among infants.4 Indeed, the infant mortality rate (IMR) is a sentinel population health metric because it reflects the cumulative health experience of women and families as well as society’s ability to care for a most vulnerable and dependent subgroup. In 2002, the latest year with national estimates on Asian and Pacific Islander subgroups,5 the IMR among Native Hawaiians was the second highest (after African Americans) of any racial/ethnic group and 66% higher than for Whites.6 Previous studies have generally alluded to predominantly postneonatal determinants of excess infant death among Native Hawaiians, but all analyses rely on data from the 1980s or earlier.7–11 Advances in the application of analytic techniques to quantify components of disparities12,13 and new strategies to promote infant health (e.g., preconception health14 and safe sleep practices15) support the need for an updated examination to identify avenues for preventive action.
We examined the determinants and cause-specific sources of excess infant mortality among Native Hawaiians relative to Whites in a contemporary cohort of births in Hawai’i, where the majority of Native Hawaiians reside. According to the 2010 Census, 55% of those who report being of Native Hawaiian race, alone or in combination with other races, reside in Hawai’i.16
METHODS
Data were from the Hawai’i State Linked Birth/Infant Death Cohort Files from 2002 to 2009. Deaths in the state are routinely linked to their corresponding birth certificates if the deceased were born in Hawai’i. Because infant deaths can occur up to 1 year after birth, deaths among infants born in one calendar year cohort can occur in the next calendar year. Thus, the 2002 to 2009 cohort file includes deaths in 2002 to 2010. Deaths that occur outside of Hawai’i are not included, and they are generally considered beyond the purview of Hawai’i state health efforts. During the study period (2002–2009), 99.7% (914/917) of infant deaths among those born in Hawai’i were matched. We analyzed data for births and linked infant deaths among those born to resident women of White (n = 33 683 births; n = 119 deaths) or Native Hawaiian (n = 40 917 births; n = 323 deaths) race. We selected Whites as the reference group because they have the lowest IMR of all major racial groups in Hawai’i.17 In the race-coding conventions used in Hawai’i, a designation of White generally refers to single race, but a designation of Native Hawaiian may refer to persons with a combination of races in their makeup.18 The 8 data years from 2002 to 2009 provided the maximum statistical power for multivariable analyses without significant trending in mortality rates.
Measures
We examined IMRs and disparities between births to Native Hawaiian and White women by age at death (neonatal, 0–27 days; postneonatal, 28–364 days), underlying cause of death (by International Classification of Diseases,10th Revision code19), and birth weight or gestational age categories. These are conventional techniques for infant mortality investigations to evaluate the summary contribution of various causes of death that occur at different ages; neonatal deaths are largely attributable to preterm birth and congenital anomalies, and postneonatal deaths tend to be more sensitive to infant care practices and access to primary health care (e.g., sudden unexpected infant death [SUID], injury, and infection).20–23
We determined underlying cause of death by a National Center for Health Statistics computer algorithm that evaluates all of the causes listed by the certifying physician.24 We grouped underlying cause-of-death codes by common categories of infant death: preterm related,25 congenital malformations (Q00–Q99), SUID (R95, R99, W75), injury (U01, V01–W74, W76–Y84), and infection (A00–B99, G00, G03, J00–J21, J40–J42). The definition of preterm-related causes of death followed the classification developed by the Centers for Disease and Control and Prevention (gestational age < 37 weeks and underlying cause-of-death code K550, P000, P010, P011, P015, P020, P021, P027, P070–P073, P102, P220–229, P250–279, P280, P281, P360–369, P520–523, or P77).25,26 The SUID category comprised sudden infant death syndrome, unknown cause, and accidental suffocation or strangulation in bed, because of evidence of shifting reporting practices between these codes.27
Analyses
To evaluate the components of the infant mortality gap connected to low birth weight and preterm birth, we conducted a Kitagawa analysis to distinguish the contribution of maternal health (i.e., percentage low birth weight and preterm birth) versus access to risk-appropriate care (i.e., birth weight– and gestational age–specific mortality rates).22 This method, described in detail elsewhere,12,22,28 provides a decomposition of the absolute difference in the overall IMR into the differences in the proportion born at a given category of birth weight or gestational age and differences in the mortality rates among infants born at that same birth weight or gestational age category. Birth weight data were missing in 0.04% of births, but these cases accounted for 4.0% of deaths among Native Hawaiians and only 0.8% of White deaths; we therefore imputed birth weight with the hot-deck technique used by the National Center for Health Statistics.26 Because of concerns regarding the validity of gestational age data derived from the last menstrual period,29 the Hawai’i Department of Health prefers the clinical estimate, which served as the basis of gestational age assessment for our analysis. We based our calculation on the last menstrual period when the clinical estimate was missing (0.2%).
In addition, we performed a multivariable regression analysis to estimate the contribution of racial differences in sociodemographic, behavioral, and medical risk factors to disparities in infant, neonatal, and postneonatal mortality rates. We based our selection of risk factors available on the birth certificate on previous associations with perinatal outcomes; these were maternal age, education, marital status, county of residence, parity, plurality, smoking during pregnancy, and any chronic condition (cardiac, lung, or renal disease; anemia; hypertension; diabetes; and hemoglobinopathy). Although we considered including the timing of prenatal care entry, our results indicated counterintuitive confounded effects, because women who enter care later in pregnancy tend to have an extended or nonpreterm delivery by definition. Missing data for any given variable generally represented less than 1%, cumulating to 1.4% overall; we placed missing values in the reference group (mean imputation) to preserve the full study population. We performed Oaxaca decomposition13,30–32 to assess the contribution of prevalence differences in each factor to infant mortality disparities. This approach generally requires the use of additive or linear models where the mean of the outcome equals the sum of the mean values of the predictors multiplied by their coefficients; thus we used ordinary least squares regression.32 For binary responses, this type of regression is known as a linear probability model and provides unbiased estimators of coefficients but requires robust standard errors to account for heteroskedasticity.33 The overall crude disparity in infant mortality 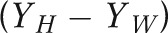 is equal to the unexplained disparity from the adjusted model
is equal to the unexplained disparity from the adjusted model 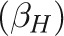 plus the sum of the adjusted effects (β coefficients) multiplied by the prevalence difference for each factor
plus the sum of the adjusted effects (β coefficients) multiplied by the prevalence difference for each factor 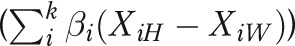 :
:
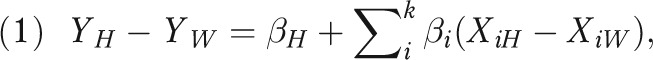 |
where H = Hawaiian and W = White.
The percentage of the disparity explained by each factor can be calculated as 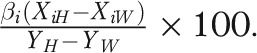 The contribution of racial differences in the effects of factors (interactions) could also be evaluated, but we did not incorporate interactions because we found none to be significant. We conducted descriptive analyses in SAS version 9.2 (SAS Institute, Cary, NC) and the Oaxaca decomposition in Stata SE version 11.1 (StataCorp LP, College Station, TX) with the OAXACA add-on command.34
The contribution of racial differences in the effects of factors (interactions) could also be evaluated, but we did not incorporate interactions because we found none to be significant. We conducted descriptive analyses in SAS version 9.2 (SAS Institute, Cary, NC) and the Oaxaca decomposition in Stata SE version 11.1 (StataCorp LP, College Station, TX) with the OAXACA add-on command.34
RESULTS
The IMR among Native Hawaiians averaged 7.9 deaths per 1000 live births over the study period, more than twice the rate for Whites (3.5/1000; Table 1). With an average annual number of about 5000 births, the excess IMR difference among Native Hawaiians (4.4/1000) translates to about 22 deaths annually that would have been prevented if Native Hawaiians had the same risk of infant death as Whites. Although the relative infant mortality disparity was considerably greater at postneonatal than neonatal ages (relative risk = 4.0 vs 1.8), the absolute disparity was the same because of the greater overall risk of neonatal mortality. Thus, excess infant mortality among Native Hawaiians was equally apportioned to neonatal and postneonatal deaths. We detected no significant differences in infant mortality between those with full or partial Hawaiian ancestry and no significant trends over time for either Whites or Native Hawaiians (data not shown).
TABLE 1—
Native Hawaiian and White Infant Mortality Rates by Age at Death: Hawai’i, 2002–2009
| Deaths |
|||||
| Age at Death | Native Hawaiians,a No. (No./1000 Live Births) | Whites,b No. (No./1000 Live Births) | RR (95% CI) | Rate Difference (95% CI) | Excess Infant Deaths,c % |
| Neonatal (0–27 d) | 206 (5.0) | 95 (2.8) | 1.8 (1.4, 2.3) | 2.2 (1.3, 3.1) | 50.8 |
| Postneonatal (28–364 d) | 117 (2.9) | 24 (0.7) | 4.0 (2.6, 6.2) | 2.1 (1.6, 2.7) | 49.2 |
| Total | 323 (7.9) | 119 (3.5) | 2.2 (1.8, 2.2) | 4.4 (3.3, 5.4) | 100.0 |
Note. CI = confidence interval; RR = rate ratio.
Among 40 917 births.
Among 33 683 births.
Calculated as the age-specific rate difference divided by the total rate difference.
Among the major categories of cause of infant death, Native Hawaiian mortality exceeded that of Whites for preterm related, SUID, and injury (Table 2). Excess preterm-related deaths among Native Hawaiians accounted for 43.9% of the infant mortality disparity. SUID and injury accounted for another 21.6% and 5.6% of the infant mortality gap, respectively. Although too few White infant deaths from infection occurred to report a cause-specific IMR, a significant racial disparity could be detected (data not shown).
TABLE 2—
Cause-Specific Infant Mortality Rates Among Native Hawaiians and Whites: Hawai’i, 2002–2009
| Deaths |
|||||
| Underlying Cause of Deatha | Native Hawaiians, No. (No./100 000 Live Births) | Whites, No. (No./100 000 Live Births) | RR (95% CI) | Rate Difference (95% CI) | Excess Infant Deaths,b % |
| Preterm relatedc | 133 (325.0) | 45 (133.6) | 2.4 (1.7, 3.4) | 191.4 (123.8, 259.1) | 43.9 |
| Congenital anomaliesd | 27 (66.0) | 25 (74.2) | 0.9 (0.5, 1.5) | –8.2 (–46.5, 30.1) | –1.9 |
| Sudden unexpected infant deathe | 58 (141.8) | 16 (47.5) | 3.0 (1.7, 5.2) | 94.2 (51.0, 137.5) | 21.6 |
| Injuryf | 16 (39.1) | 5 (14.8) | 2.6 (1.0, 7.2) | 24.3 (1.1, 47.4) | 5.6 |
| Infectiong | 12 (29.3) | … | … | … | … |
Note. CI = confidence interval; RR = rate ratio. Ellipsis indicates number too small to report (< 5).
According to International Classification of Diseases, 10th Revision (ICD-10) categories.19
Calculated as the cause-specific rate difference divided by the total rate difference.
According to Centers for Disease Control and Prevention definition: gestational age < 37 weeks and underlying cause-of-death ICD-10 code of K550, P000, P010, P011, P015, P020, P021, P027, P070–P073, P102, P220–229, P250–279, P280, P281, P360–369, P520–523, or P77.25,26
ICD-10 codes Q00–Q99.
ICD-10 codes R95, R99, and W75.
ICD-10 codes V01–W74 and W76–Y36.
ICD-10 codes A00–B99, G00, G03, and J00–J42.
Consistent with the underlying cause-of-death results, about half of the infant mortality gap could be explained by the greater proportion of low birth weight and preterm birth among Native Hawaiians than Whites (Table 3). In particular, Native Hawaiians were 2.2 times as likely as Whites to be born at the smallest size (< 1000 g) and earliest gestational age (< 28 weeks), when the mortality risk is highest, and this disparity alone accounted for about 45% of the infant mortality gap and the majority of the total low birth weight–preterm birth contribution. In general we found no significant racial differences in the mortality rate within each low birth weight or preterm gestational age category (data not shown).
TABLE 3—
Contribution of Low Birth Weight and Preterm Birth to the Native Hawaiian–White Infant Mortality Gap: Hawai’i, 2002–2009
| Birth Distribution |
Infant Mortality Rate/1000 Live Births |
Excess Infant Deaths Attributable to Distributional Differencesa |
||||||||
| Variable | Native Hawaiians, % | Whites, % | Prevalence RR (95% CI) | Prevalence Rate Difference (95% CI) | Native Hawaiians | Whites | RR (95% CI) | Rate Difference (95% CI) | No./1000 | Total, % |
| Birth weight, g | ||||||||||
| < 1000 | 0.9 | 0.4 | 2.2 (1.8, 2.7) | 0.5 (0.4, 0.6) | 452.7 | 386.4 | 1.2 (0.9, 1.5) | 66.4 (−31.8, 164.5) | 1.9 | 45.4 |
| 1000–1499 | 0.7 | 0.6 | 1.3 (1.1, 1.5) | 0.2 (0.0, 0.3) | 55.9 | 65.7 | 0.9 (0.4, 1.7) | −9.7 (−52.8, 33.4) | 0.1 | 2.2 |
| 1500–1999 | 1.4 | 1.2 | 1.1 (1.0, 1.3) | 0.2 (0.0, 0.4) | 27.4 | 19.1 | 1.4 (0.6, 3.3) | 8.3 (−10.4, 27.0) | 0.0 | 1.0 |
| 2000–2499 | 5.2 | 3.9 | 1.3 (1.2, 1.4) | 1.3 (1.0, 1.6) | 7.9 | 6.8 | 1.2 (0.5, 2.6) | 1.1 (−4.7, 6.9) | 0.1 | 2.3 |
| Total low birth weight | 8.3 | 6.2 | 1.3 (1.3, 1.4) | 2.1 (1.7, 2.5) | 61.5 | 39.1 | 1.6 (1.2, 2.0) | 22.4 (10.8, 34.0) | 2.2 | 50.9 |
| Gestational age, wk | ||||||||||
| < 28 | 0.9 | 0.4 | 2.2 (1.8, 2.6) | 0.5 (0.4, 0.6) | 416.9 | 368.1 | 1.1 (0.9, 1.5) | 48.8 (−44.3, 141.9) | 2.0 | 45.9 |
| 28–31 | 0.9 | 0.7 | 1.2 (1.1, 1.5) | 0.2 (0.1, 0.3) | 31.7 | 48.0 | 0.7 (0.3, 1.4) | −16.3 (−48.2, 15.5) | 0.1 | 1.7 |
| 32–33 | 1.3 | 1.0 | 1.3 (1.1, 1.5) | 0.3 (0.1, 0.4) | 36.0 | 8.9 | 4.1 (1.2, 13.6) | 27.1 (8.3, 45.9) | 0.1 | 1.5 |
| 34–36 | 8.3 | 6.4 | 1.3 (1.2, 1.4) | 1.9 (1.5, 2.3) | 5.9 | 5.1 | 1.2 (0.6, 2.4) | 0.8 (−3.2, 4.8) | 0.1 | 2.5 |
| Total preterm | 11.4 | 8.6 | 1.3 (1.3, 1.4) | 2.9 (2.4, 3.4) | 44.8 | 27.5 | 1.6 (1.3, 2.1) | 17.3 (8.9, 25.7) | 2.2 | 51.6 |
Note. CI = confidence interval; RR = rate ratio.
Determined by the Kitagawa method, the racial difference in percentage born in a certain birth weight–gestational age category multiplied by the average of race-specific infant mortality rates for that category (excess deaths attributable to birth weight– or gestational age–specific mortality rate differences not shown because of lack of statistical significance).
Native Hawaiian mothers were more likely than White mothers to have several sociodemographic and health risk factors: they were more likely to be adolescent and unmarried, to have lower levels of educational attainment and higher parity, to smoke, and to have a chronic condition (Table 4). In adjusted models, all covariates were significantly related to neonatal, postneonatal, or overall infant mortality, with the exception of chronic conditions. In total, these covariates explained 20.6% of the overall infant mortality disparity and a greater share of excess postneonatal than neonatal deaths (37.5% vs 4.2%). However, educational inequality explained 20.9% of the neonatal mortality gap (11.6% overall), but differences in parity and multiple births favored Native Hawaiians. Younger maternal age (12.2%) and higher smoking rates (9.5%) among Native Hawaiians were the only statistically significant contributors to the postneonatal mortality disparity.
TABLE 4—
Multivariable Decomposition of the Native Hawaiian-White Infant Mortality Disparity: Hawai’i, 2002–2009
| Covariate Distributiona |
Adjusted Linear Parameter Estimates, Deaths/1000 Live Births |
Disparity Explained by Covariate Distributional Differencesb |
||||||
| Maternal Characteristic | Native Hawaiians, % | Whites, % | Total Infant Mortality, b (SE) | Neonatal Mortality, b (SE) | Postneonatal Mortality, b (SE) | Total Infant Mortality, % (SE) | Neonatal Mortality, % (SE) | Postneonatal Mortality, % (SE) |
| Race | ||||||||
| Native Hawaiian | 100.0 | 0.0 | 3.46* (0.65) | 2.12* (0.54) | 1.34* (0.37) | |||
| White (Ref) | 0.0 | 100.0 | ||||||
| Maternal age, y | ||||||||
| < 20 | 15.8 | 3.8 | 2.34* (1.17) | 0.49 (0.97) | 1.85* (0.66) | 3.2 (4.4) | −5.5 (7.2) | 12.2* (5.0) |
| 20–24 | 31.5 | 25.1 | −0.43 (0.73) | −0.70 (0.60) | 0.28 (0.41) | |||
| 25–34 (Ref) | 42.8 | 53.5 | ||||||
| ≥ 35 | 10.0 | 17.6 | 1.48 (0.88) | 1.80* (0.73) | −0.32 (0.34) | |||
| Education | ||||||||
| < High school | 14.7 | 6.7 | 4.28* (1.22) | 2.72* (1.01) | 1.56* (0.69) | 11.6* (4.0) | 20.9* (6.6) | 1.9 (4.4) |
| High school | 52.3 | 34.7 | 1.29 (0.84) | 1.82* (0.70) | −0.53 (0.48) | |||
| Some college | 20.7 | 25.8 | 1.31 (0.87) | 1.50* (0.72) | −0.18 (0.50) | |||
| ≥ College (Ref) | 12.3 | 32.8 | ||||||
| Marital status | ||||||||
| Married (Ref) | 41.8 | 81.8 | 7.0 (6.2) | 4.6 (10.1) | 9.6 (7.0) | |||
| Unmarried, father listed | 50.0 | 14.3 | 0.12 (0.72) | −0.31 (0.59) | 0.43 (0.41) | |||
| Unmarried, father not listed | 8.2 | 3.9 | 6.08* (1.25) | 4.86* (1.03) | 1.21 (0.71) | |||
| County of residence | ||||||||
| Oahu (Ref) | 62.0 | 68.8 | 0.1 (1.5) | 0.2 (2.4) | 0.0 (1.7) | |||
| Hawai'i | 20.0 | 12.5 | −0.02 (0.78) | 0.07 (0.65) | −0.09 (0.44) | |||
| Maui | 12.5 | 13.6 | −1.68 (0.87) | −0.75 (0.72) | −0.93 (0.49) | |||
| Kauai | 5.5 | 5.1 | −2.92* (1.27) | −2.28* (1.05) | −0.64 (0.72) | |||
| Parity | ||||||||
| First child | 35.6 | 46.5 | 1.60* (0.65) | 2.06* (0.54) | −0.46 (0.37) | −6.3* (2.3) | −16.4* (3.8) | 4.2 (2.7) |
| Second or third child (Ref) | 47.2 | 45.4 | ||||||
| ≥ fourth child | 17.3 | 8.1 | −1.06 (0.91) | −1.49* (0.76) | 0.43 (0.52) | |||
| Plurality | ||||||||
| Singleton (Ref) | 97.3 | 96.4 | −3.5* (0.8) | −6.4* (1.5) | −0.4 (0.5) | |||
| Multiple | 2.7 | 3.6 | 16.28* (1.63) | 15.34* (1.35) | 0.94 (0.93) | |||
| Smoking | ||||||||
| No (Ref) | 88.9 | 96.7 | 5.0* (2.5) | 0.6 (3.5) | 9.5* (3.6) | |||
| Yes | 11.1 | 3.3 | 2.82* (1.10) | 0.18 (0.91) | 2.64* (0.62) | |||
| Chronic condition | ||||||||
| No (Ref) | 78.3 | 90.7 | 3.4 (2.6) | 6.2 (4.3) | 0.4 (2.9) | |||
| Yes | 21.8 | 9.3 | 1.18 (0.78) | 1.11 (0.65) | 0.07 (0.44) | |||
| Total explained | 20.6* (8.3) | 4.2 (13.4) | 37.5* (9.6) | |||||
All covariates differed significantly by race as determined by the χ2 test; P < .01.
Obtained from Oaxaca decomposition derived from adjusted coefficients multiplied by covariate distributional differences (summed over multiple category variables); positive numbers indicate a Native Hawaiian disadvantage that helps to explain disparity; negative numbers indicate a Native Hawaiian advantage that would serve to increase disparity if the covariate distribution were equalized to that of the White population; total percentage explained is the sum over all covariates, which equals the difference between the unadjusted and adjusted racial disparity divided by the unadjusted racial disparity.
*P < .05.
DISCUSSION
In our updated examination of infant mortality among Native Hawaiians, we documented a large disparity between Native Hawaiians and Whites, with infants born to Native Hawaiian mothers more than twice as likely to die as those born to White mothers in Hawai’i. This disparity translates to about 22 excess Native Hawaiian infant deaths per year in Hawai’i that would not occur if infants born to Native Hawaiian mothers had the same mortality risk as those born to White mothers. Although the relative disparity was greater for postneonatal than neonatal deaths, excess Native Hawaiian infant mortality was equally apportioned to neonatal and postneonatal deaths because of the greater overall risk of neonatal death. Although the authors did not expressly evaluate this, previous analyses showed an equal absolute disparity in both neonatal and postneonatal mortality between Native Hawaiians and Whites.6,9,10
Consistent with the results for neonatal and postneonatal mortality, nearly half of all excess deaths were attributable to preterm-related causes (43.9%), followed by SUID (21.6%) and injury (5.6%). Previous studies also documented higher Native Hawaiian death rates from similar causes.10,11 The excess neonatal, preterm-related deaths among Native Hawaiians appeared to result from an excess of the most extremely preterm or low–birth weight births rather than differences in the gestational age– or birth weight–specific mortality risks. This suggests a role for disparities in maternal or women’s health rather than access differences to risk-appropriate neonatal care.
In our examination of the contribution of compositional differences in sociodemographic and medical risk factors, educational disparities accounted for the largest component of excess neonatal mortality (20.9%) and was likely underestimated because of possible mediators in the multivariable model (e.g., smoking, chronic conditions). Only a third of Native Hawaiian mothers but more than half of White mothers had attained postsecondary education. Greater educational attainment confers health advantages through knowledge and awareness of positive health behaviors as well as the economic resources to access health-promoting goods and services.35 Efforts to reduce educational inequalities would address a fundamental social determinant of health and help to reduce a variety of health disparities, including those in neonatal mortality, possibly via improvements in women’s health status. Improving educational quality and hope for future career potential may also help to reduce adolescent childbearing,36 a factor associated with excess postneonatal mortality among Native Hawaiians. Hawai’i is among the 21 states and the District of Columbia to receive Race to the Top funding—President Barack Obama’s key educational reform initiative—with with a goal of reducing Native Hawaiian educational achievement gaps).37 In addition, the Affordable Care Act has funded models of maternal, infant, and early childhood home visiting that have shown an impact on child development and school readiness as well as on maternal education and vocational training.38 This funding has expanded Hawai’i home visiting services from a single model (Healthy Families America) operating in only 2 locations to a multi-model network serving at-risk communities in the entire state (A. H. H., personal communication with Home Visiting Unit, Hawai’i State Department of Health, August 21, 2013).
Disparities in smoking also contributed significantly to excess postneonatal mortality. Smoke exposure both before and after delivery is a risk factor for SUID and increases susceptibility to respiratory infection.39 Anonymous reporting in the Pregnancy Risk Assessment and Monitoring System reveals greater disparities in postpartum smoking, during a key exposure period for SUID and infection (22.0% among Native Hawaiians vs 8.0% among Whites in 2009).40 The State of Hawai’i has implemented aggressive population-based tobacco control policies, including a public smoking ban in 2006 and large increases in cigarette taxes.41 Even since 2009, the last year of our study, per-pack taxes have risen 60 cents to $3.20, and only 3 other states have a higher tobacco tax (New York, Rhode Island, and Connecticut).41 This combination of state policies,42 along with the Affordable Care Act's requirement that Medicaid cover comprehensive smoking cessation treatments (both counseling and medication) for pregnant women,43 may help to reduce maternal smoking.
Other preventive opportunities suggested by our results include safe sleep campaigns to reduce excess SUID and improved messaging and health care access to reduce deaths from injury and infection. Analyses of the Hawai’i Pregnancy Risk Assessment and Monitoring System have confirmed that Native Hawaiian mothers are more likely to report using nonsupine infant sleep positions (side or stomach), which increase SUID risk.44 Soft bedding and bed sharing are additional risks that can be addressed with education on positioning through health care provider training, media campaigns, and the provision of bedside bassinets, which can support breastfeeding while minimizing the risks of bedsharing.45–47 In response to findings from the Hawai’i Child Death Review team, a culturally appropriate video on safe sleep practices was developed for use in the Special Supplemental Nutrition Program for Women, Infants, and Children and community health clinics. Additional avenues for SUID reduction are establishment of a safe sleep committee and laws requiring the provision of safe sleep counseling and education by all health care providers to expectant mothers and to families in postpartum units and during pediatric visits.48
Several programs in Hawai’i could disseminate these messages and provide resources or referrals for preventing SUID, injury, and parental smoking. The Hawai’i home visiting models implemented through Affordable Care Act funding38 provide screening, services, and counseling to reduce environmental risks for child maltreatment, such as establishing a medical home, providing connections to community resources, and promoting positive parenting practices. The Native Hawaiian Health Care Systems were created by the Native Hawaiian Health Care Act of 198849 to provide essential educational, enabling, and health care services to Native Hawaiians in the State of Hawai’i. They operate on 5 of the Hawaiian islands and are an important service point for Native Hawaiian families that could be used to deliver messages and resources to promote smoking cessation and prevent SUID and other injury deaths.50
Limitations
The advantage of capturing nearly all births and deaths in Hawai’i vital records was tempered by limitations in the measurement of certain risk factors on the birth certificate. For example, medical risk factors such as hypertension and diabetes are underreported on birth certificates,51,52 and others (e.g., maternal obesity) are not collected on the birth certificate currently used in Hawai’i. Thus, the impact of chronic conditions on infant mortality was likely underestimated and consequently their contribution to disparities as well. Other analyses have shown a greater burden of chronic conditions and obesity (not measured here) among Native Hawaiians,2,3,53–56 as well as a connection to infant mortality.57 Linkages between vital records and discharge data would help to reduce this knowledge gap but will require the state vital statistics department to complete linkages with personally identifiable information. Additional variables not available on the birth certificate or medical record, such as stress, social support, poverty, and unintended pregnancy, could be explored in analyses of the Pregnancy Risk Assessment and Monitoring System.
Finally, our analysis was only representative of the disparity in Hawai’i. Although the majority of Native Hawaiians live in Hawai’i,16 a national analysis could be conducted in the coming decade when all states have implemented the 2003 revision of the birth certificate, which collects multiple race and other variables in a comparable format. Granular racial/ethnic data that identifies Native Hawaiians separately from other Pacific Islander groups is required by all federally sponsored surveys in section 4302 of the Affordable Care Act and marks a major advance in the ability to identify, monitor, and address racial/ethnic disparities with appropriate specificity and uniformity.58 Nonetheless, small sample sizes still impede the examination of Native Hawaiians in many national health surveys.
Conclusions
Our findings regarding the determinants of excess infant mortality among Native Hawaiians suggest several avenues for preventive action and further research. Reducing educational inequality, perhaps through reform initiatives and home-visiting programs, would go furthest in addressing a fundamental cause of multiple health outcomes. Additional strategies to address specific health risks related to postneonatal mortality, particularly safe sleep practices and smoking, are also warranted. Further efforts to link birth certificate and hospital discharge records and to analyze Pregnancy Risk Assessment and Monitoring System data would yield more information on the contribution of additional risk factors to preterm birth and neonatal mortality, such as chronic conditions, obesity, and other social determinants beyond education.
Acknowledgments
We thank the Hawai’i Pregnancy Risk Assessment and Monitoring System coordinator, Emily Roberson, MPH, for furnishing an unpublished estimate referenced in the Discussion section, and Michael Kogan, PhD, for providing helpful comments on an earlier version of the article.
Human Participant Protection
Ethics review was not required because the data did not include personal identifiers.
References
- 1. Native American Programs Act of 1974, PL 93-638, as amended. Indian Health Care Improvement Act, PL 93-644.
- 2.Busch J, Easa D, Grandinetti A, Mor J, Harrigan R. Healthy people in Hawaii?: an overview of ethnic health disparities in Hawaii for the Healthy People 2010 initiative targeted health concerns. Hawaii Med J. 2003;62(1):10–14. [PubMed] [Google Scholar]
- 3.Mau MK, Sinclair K, Saito EP, Baumhofer KN, Kaholokula JK. Cardiometabolic health disparities in native Hawaiians and other Pacific Islanders. Epidemiol Rev. 2009;31:113–129. doi: 10.1093/ajerev/mxp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panapasa SV, Mau MK, Williams DR, McNally JW. Mortality patterns of Native Hawaiians across their lifespan: 1990–2000. Am J Public Health. 2010;100(11):2304–2310. doi: 10.2105/AJPH.2009.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Center for Health Statistics. User Guide to the 2010 Natality Public Use File. Hyattsville, MD: National Center for Health Statistics; 2012. [Google Scholar]
- 6.Mathews TJ, Menacker F, MacDorman MF. Infant mortality statistics from the 2002 period: linked birth/infant death data set. Natl Vital Stat Rep. 2004;53(10):1–29. [PubMed] [Google Scholar]
- 7.Kieffer EC, Mor JM, Alexander GR. Native Hawaiian birth weight and infant mortality: is birth in Hawai’i protective? Asian Am Pac Isl J Health. 1996;4(4):343–351. [PubMed] [Google Scholar]
- 8.Kieffer EC, Alexander GR, Mor JM. Pregnancy outcomes of Pacific Islanders in Hawaii. Am J Epidemiol. 1995;141(7):674–679. doi: 10.1093/oxfordjournals.aje.a117484. [DOI] [PubMed] [Google Scholar]
- 9.Singh GK, Yu SM. Pregnancy outcomes among Asian Americans. Asian Am Pac Isl J Health. 1993;1(1):63–78. [PubMed] [Google Scholar]
- 10.Kieffer EC, Mor JM, Alexander GR. The perinatal and infant health status of Native Hawaiians. Am J Public Health. 1994;84(9):1501–1504. doi: 10.2105/ajph.84.9.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baruffi G, Alexander G, Novotny R. Causes of infant mortality in a multiethnic population. Asia Pac J Public Health. 1990;4(2–3):145–150. doi: 10.1177/101053959000400312. [DOI] [PubMed] [Google Scholar]
- 12.Schempf AH, Branum AM, Lukacs SL, Schoendorf KC. The contribution of preterm birth to the Black–White infant mortality gap, 1990 and 2000. Am J Public Health. 2007;97(7):1255–1260. doi: 10.2105/AJPH.2006.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarnizo-Herreño CC, Wehby GL. Explaining racial/ethnic disparities in children’s dental health: a decomposition analysis. Am J Public Health. 2012;102(5):859–866. doi: 10.2105/AJPH.2011.300548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson K, Posner SF, Biermann J et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55(RR-6):1–23. [PubMed] [Google Scholar]
- 15.Michels KB, Rosner BA. Data trawling: to fish or not to fish. Lancet. 1996;348(9035):1152–1153. doi: 10.1016/S0140-6736(96)05418-9. [DOI] [PubMed] [Google Scholar]
- 16. US Census Bureau. QT-P9 race reporting for the Native Hawaiian and other Pacific Islander population by selected categories: 2010 Census summary file 1. Available at: http://factfinder2.census.gov. Accessed June 4, 2012.
- 17.Hawai’i Health Data Warehouse. Health reports and data: maternal and child health, infant health. Available at: http://www.hhdw.org. Accessed December 18, 2012.
- 18.Sorensen CA, Wood B, Prince EW. Race and ethnicity data: developing a common language for public health surveillance in Hawaii. Californian J Health Promot. 2003;1(special issue):91–104. [Google Scholar]
- 19.International Classification of Diseases, 10th Revision. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 20.MacDorman MF, Mathews TJ. Understanding Racial and Ethnic Disparities in U.S. Infant Mortality Rates. Hyattsville, MD: National Center for Health Statistics; 2011. NCHS data brief 74. [PubMed] [Google Scholar]
- 21.Sappenfield WM, Peck MG, Gilbert CS, Haynatzka VR, Bryant T., 3rd Perinatal periods of risk: analytic preparation and phase 1 analytic methods for investigating feto-infant mortality. Matern Child Health J. 2010;14(6):838–850. doi: 10.1007/s10995-010-0625-4. [DOI] [PubMed] [Google Scholar]
- 22.Sappenfield WM, Peck MG, Gilbert CS, Haynatzka VR, Bryant T., 3rd Perinatal periods of risk: phase 2 analytic methods for further investigating feto-infant mortality. Matern Child Health J. 2010;14(6):851–863. doi: 10.1007/s10995-010-0624-5. [DOI] [PubMed] [Google Scholar]
- 23.Wise PH. The anatomy of a disparity in infant mortality. Annu Rev Public Health. 2003;24:341–362. doi: 10.1146/annurev.publhealth.24.100901.140816. [DOI] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics. ICD–10 cause-of-death lists for tabulating mortality statistics, effective 1999. Hyattsville, MD: Public Health Service; 1999. NCHS instruction manual, part 9. [Google Scholar]
- 25.Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–1573. doi: 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- 26.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2007 period linked birth/infant death data set. Natl Vital Stat Rep. 2011;59(6):1–30. [PubMed] [Google Scholar]
- 27.Shapiro-Mendoza CK, Tomashek KM, Anderson RN, Wingo J. Recent national trends in sudden, unexpected infant deaths: more evidence supporting a change in classification or reporting. Am J Epidemiol. 2006;163(8):762–769. doi: 10.1093/aje/kwj117. [DOI] [PubMed] [Google Scholar]
- 28.Kitagawa EM. Components of a difference between two rates. J Am Stat Assoc. 1955;50(272):1168–1194. [Google Scholar]
- 29.Callaghan WM, Dietz PM. Differences in birth weight for gestational age distributions according to the measures used to assign gestational age. Am J Epidemiol. 2010;171(7):826–836. doi: 10.1093/aje/kwp468. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson JO, Robinson P, Bluthenthal RN. A multilevel decomposition approach to estimate the role of program location and neighborhood disadvantage in racial disparities in alcohol treatment completion. Soc Sci Med. 2007;64(2):462–476. doi: 10.1016/j.socscimed.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 31.O’Donnell O, van Doorslaer E, Wagstaff A, Lindelow M. Explaining Differences Between Groups: Oaxaca Decomposition. Analyzing Health Equity Using Household Survey Data: A Guide to Techniques and Their Implementation. Washington, DC: World Bank; 2008. pp. 147–157. [Google Scholar]
- 32.Hargraves JL, Hadley J. The contribution of insurance coverage and community resources to reducing racial/ethnic disparities in access to care. Health Serv Res. 2003;38(3):809–829. doi: 10.1111/1475-6773.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wooldridge JM. Linear Probability Model for Binary Response. Econometric Analysis of Cross Section and Panel Data. 2nd ed. Cambridge, MA: MIT Press; 2010. pp. 562–565. [Google Scholar]
- 34.Jann B. The Blinder-Oaxaca decomposition for linear regression models. Stata J. 2008;8(4):453–479. [Google Scholar]
- 35.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;(spec no):80–94. [PubMed] [Google Scholar]
- 36.Zabin LS. Addressing adolescent sexual behavior and childbearing: self-esteem or social change? Womens Health Issues. 1994;4(2):92–97. doi: 10.1016/s1049-3867(05)80042-8. [DOI] [PubMed] [Google Scholar]
- 37. US Dept of Education. Race to the Top fund. Available at: http://www2.ed.gov/programs/racetothetop/index.html. Accessed June 4, 2012.
- 38.US Dept of Health and Human Services. Home visiting evidence of effectiveness. 2011 Available at: http://homvee.acf.hhs.gov. Accessed June 5, 2012. [Google Scholar]
- 39.Office of the Surgeon General. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Rockville, MD: Public Health Service; 2006. [PubMed] [Google Scholar]
- 40. Hawai’i State Dept of Health. Pregnancy Risk Assessment Monitoring System (PRAMS) data, 2009. Available at: http://healthuser.hawaii.gov/health/family-child-health/mchb/programs/prams.html. Accessed June 4, 2012.
- 41. Centers for Disease Control and Prevention. State Tobacco Activities Tracking and Evaluation (STATE) System. Available at: http://www.cdc.gov/tobacco/statesystem. Accessed June 4, 2012.
- 42.Adams EK, Markowitz S, Kannan V, Dietz PM, Tong VT, Malarcher AM. Reducing prenatal smoking: the role of state policies. Am J Prev Med. 2012;43(1):34–40. doi: 10.1016/j.amepre.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. State Medicaid coverage for tobacco-dependence treatments—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(41):1340–1343. [PubMed] [Google Scholar]
- 44.Schempf A, Hayes D, Fuddy L. Infant Sleep Position Fact Sheet. Honolulu, HI: Hawai’i Dept of Health; 2011. [Google Scholar]
- 45.American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116(5):1245–1255. doi: 10.1542/peds.2005-1499. [DOI] [PubMed] [Google Scholar]
- 46.Task Force on Sudden Infant Death Syndrome. Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128(5):1030–1039. doi: 10.1542/peds.2011-2284. [DOI] [PubMed] [Google Scholar]
- 47.National SUID/SIDS Resource Center. What works: changing knowledge and behavior to reduce sudden unexpected infant death. Available at: http://www.sidscenter.org/whatworks.html. Accessed April 2, 2012.
- 48.National Conference of State Legislatures. Sudden infant death syndrome laws. Available at: http://www.ncsl.org/issues-research/health/sudden-infant-death-syndrome-laws.aspx. Accessed April 2, 2012. [Google Scholar]
- 49. Native Hawaiian Health Care Act: PL 100-579; 1988.
- 50.Papa Ola Lokahi. Native Hawaiian Health Care Systems. Available at: http://www.papaolalokahi.org/?page_id=23. Accessed June 4, 2012. [Google Scholar]
- 51.Northam S, Knapp TR. The reliability and validity of birth certificates. J Obstet Gynecol Neonatal Nurs. 2006;35(1):3–12. doi: 10.1111/j.1552-6909.2006.00016.x. [DOI] [PubMed] [Google Scholar]
- 52.Schoendorf KC, Branum AM. The use of United States vital statistics in perinatal and obstetric research. Am J Obstet Gynecol. 2006;194(4):911–915. doi: 10.1016/j.ajog.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention. Maternal, pregnancy, and birth characteristics of Asians and Native Hawaiians/Pacific Islanders—King County, Washington, 2003–2008. MMWR Morb Mortal Wkly Rep. 2011;60(7):211–213. [PubMed] [Google Scholar]
- 54.Feigal D, Hayes D, Zeng X, Roberson E, Shor R, Fuddy L. Maternal Diabetes and Pregnancy Fact Sheet. Honolulu, HI: Family Health Services Division, Hawai’i Dept of Health; 2010. [Google Scholar]
- 55.Hayes D, Shor R, Roberson E, Fuddy L. Maternal High Blood Pressure and Pregnancy Fact Sheet. Honolulu, HI: Family Health Services Division, Hawai’i Dept of Health; 2010. [Google Scholar]
- 56.Zeng X, Hayes D, Shor R, Feigal D, Roberson E, Fuddy L. Preconception Overweight/Obesity and Pregnancy Fact Sheet. Honolulu, HI: Family Health Services Division, Hawai’i Dept of Health; 2010. [Google Scholar]
- 57.Chen A, Feresu SA, Fernandez C, Rogan WJ. Maternal obesity and the risk of infant death in the United States. Epidemiology. 2009;20(1):74–81. doi: 10.1097/EDE.0b013e3181878645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.US Dept of Health and Human Services. Reducing health disparities with improved data collection: new refined data standards for race, ethnicity, sex, primary language, and disability status. Available at: http://minorityhealth.hhs.gov/templates/content.aspx?ID=9232&lvl=2&lvlID=208. Accessed June 4, 2012.


