Abstract
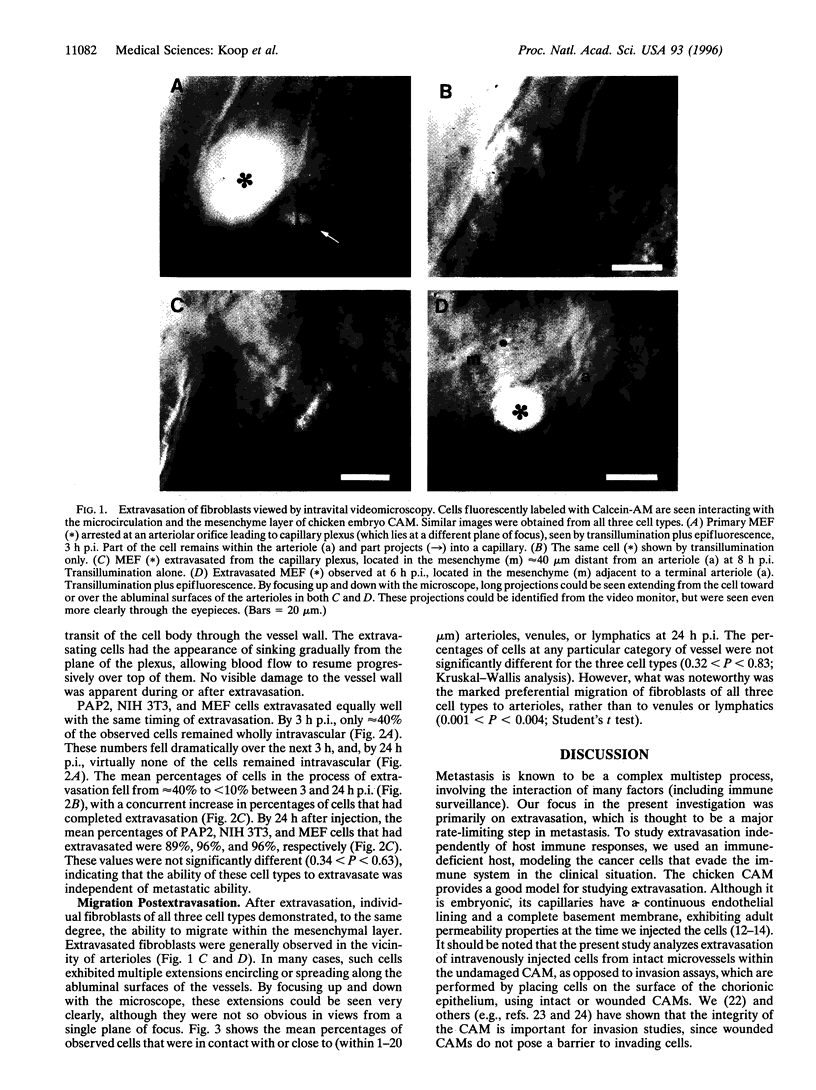
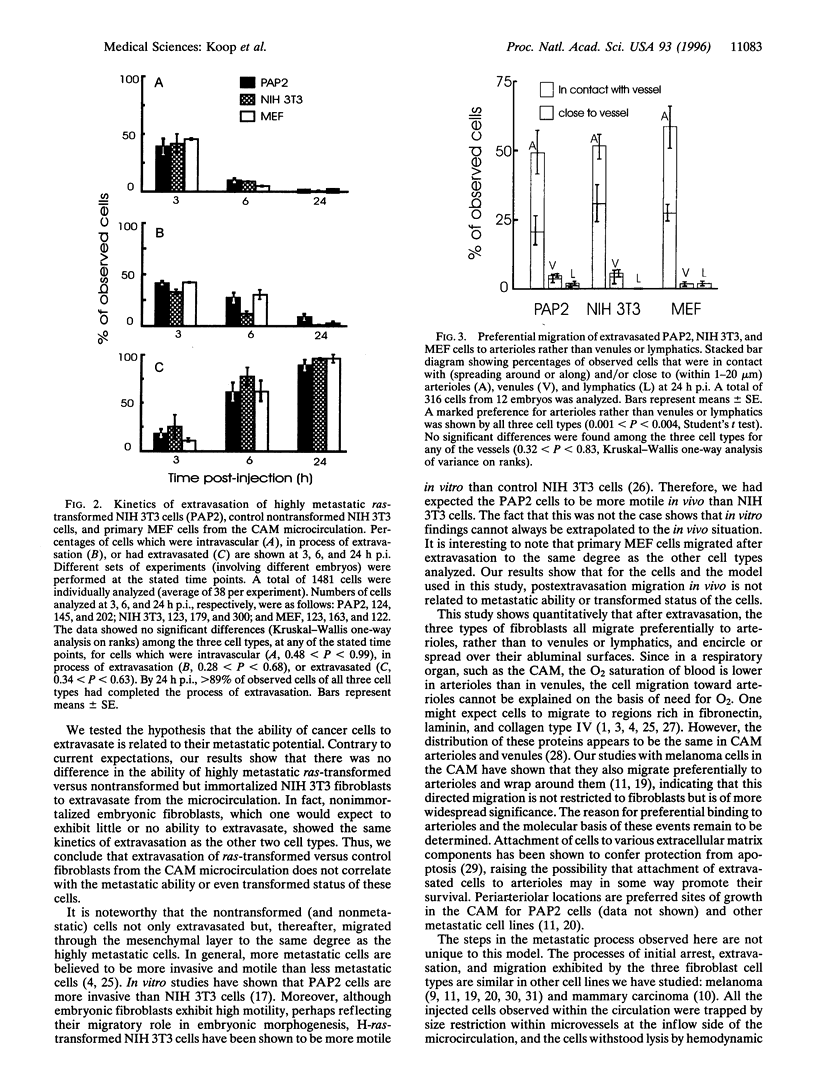
Escape of cancer cells from the circulation (extravasation) is thought to be a major rate-limiting step in metastasis, with few cells being able to extravasate. Furthermore, highly metastatic cells are believed to extravasate more readily than poorly metastatic cells. We assessed in vivo the extravasation ability of highly metastatic ras-transformed NIH 3T3 cells (PAP2) versus control nontumorigenic nontransformed NIH 3T3 cells and primary mouse embryo fibroblasts. Fluorescently labeled cells were injected intravenously into chicken embryo chorioallantoic membrane and analyzed by intravital videomicroscopy. The chorioallantoic membrane is an appropriate model for studying extravasation, since, at the embryonic stage used, the microvasculature exhibits a continuous basement membrane and adult permeability properties. The kinetics of extravasation were assessed by determining whether individual cells (n = 1481) were intravascular, extravascular, or in the process of extravasation, at 3, 6, and 24 h after injection. Contrary to expectations, our results showed that all three cell types extravasated with the same kinetics. By 24 h after injection > 89% of observed cells had completed extravasation from the capillary plexus. After extravasation, individual fibroblasts of all cell types demonstrated preferential migration within the mesenchymal layer toward arterioles, not to venules or lymphatics. Thus in this model and for these cells, extravasation is independent of metastatic ability. This suggests that the ability to extravasate in vivo is not necessarily predictive of subsequent metastasis formation, and that postextravasation events may be key determinants in metastasis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong P. B., Quigley J. P., Sidebottom E. Transepithelial invasion and intramesenchymal infiltration of the chick embryo chorioallantois by tumor cell lines. Cancer Res. 1982 May;42(5):1826–1837. [PubMed] [Google Scholar]
- Bondy G. P., Wilson S., Chambers A. F. Experimental metastatic ability of H-ras-transformed NIH3T3 cells. Cancer Res. 1985 Dec;45(12 Pt 1):6005–6009. [PubMed] [Google Scholar]
- Chambers A. F., Denhardt G. H., Wilson S. M. ras-transformed NIH 3T3 cell lines, selected for metastatic ability in chick embryos, have increased proportions of p21-expressing cells and are metastatic in nude mice. Invasion Metastasis. 1990;10(4):225–240. [PubMed] [Google Scholar]
- Chambers A. F., MacDonald I. C., Schmidt E. E., Koop S., Morris V. L., Khokha R., Groom A. C. Steps in tumor metastasis: new concepts from intravital videomicroscopy. Cancer Metastasis Rev. 1995 Dec;14(4):279–301. doi: 10.1007/BF00690599. [DOI] [PubMed] [Google Scholar]
- Chambers A. F., Schmidt E. E., MacDonald I. C., Morris V. L., Groom A. C. Early steps in hematogenous metastasis of B16F1 melanoma cells in chick embryos studied by high-resolution intravital videomicroscopy. J Natl Cancer Inst. 1992 May 20;84(10):797–803. doi: 10.1093/jnci/84.10.797. [DOI] [PubMed] [Google Scholar]
- Chambers A. F., Shafir R., Ling V. A model system for studying metastasis using the embryonic chick. Cancer Res. 1982 Oct;42(10):4018–4025. [PubMed] [Google Scholar]
- Hill S. A., Wilson S., Chambers A. F. Clonal heterogeneity, experimental metastatic ability, and p21 expression in H-ras-transformed NIH 3T3 cells. J Natl Cancer Inst. 1988 Jun 1;80(7):484–490. doi: 10.1093/jnci/80.7.484. [DOI] [PubMed] [Google Scholar]
- Koop S., Khokha R., Schmidt E. E., MacDonald I. C., Morris V. L., Chambers A. F., Groom A. C. Overexpression of metalloproteinase inhibitor in B16F10 cells does not affect extravasation but reduces tumor growth. Cancer Res. 1994 Sep 1;54(17):4791–4797. [PubMed] [Google Scholar]
- Koop S., MacDonald I. C., Luzzi K., Schmidt E. E., Morris V. L., Grattan M., Khokha R., Chambers A. F., Groom A. C. Fate of melanoma cells entering the microcirculation: over 80% survive and extravasate. Cancer Res. 1995 Jun 15;55(12):2520–2523. [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Stetler-Stevenson W. G. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991 Sep 15;51(18 Suppl):5054s–5059s. [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- MacDonald I. C., Schmidt E. E., Morris V. L., Chambers A. F., Groom A. C. Intravital videomicroscopy of the chorioallantoic microcirculation: a model system for studying metastasis. Microvasc Res. 1992 Sep;44(2):185–199. doi: 10.1016/0026-2862(92)90079-5. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Bowden G. T., Krieg P., Fürstenberger G., Briand J. P., Leroy P., Breathnach R. The mRNA coding for the secreted protease transin is expressed more abundantly in malignant than in benign tumors. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9413–9417. doi: 10.1073/pnas.83.24.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsky W. L., Chen W. T. Proteases of cell adhesion proteins in cancer. Semin Cancer Biol. 1993 Aug;4(4):251–258. [PubMed] [Google Scholar]
- Morris V. L., Koop S., MacDonald I. C., Schmidt E. E., Grattan M., Percy D., Chambers A. F., Groom A. C. Mammary carcinoma cell lines of high and low metastatic potential differ not in extravasation but in subsequent migration and growth. Clin Exp Metastasis. 1994 Nov;12(6):357–367. doi: 10.1007/BF01755879. [DOI] [PubMed] [Google Scholar]
- Morris V. L., MacDonald I. C., Koop S., Schmidt E. E., Chambers A. F., Groom A. C. Early interactions of cancer cells with the microvasculature in mouse liver and muscle during hematogenous metastasis: videomicroscopic analysis. Clin Exp Metastasis. 1993 Sep;11(5):377–390. doi: 10.1007/BF00132981. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Schmidt E. E., Koop S., MacDonald I. C., Grattan M., Khokha R., McLane M. A., Niewiarowski S., Chambers A. F., Groom A. C. Effects of the disintegrin eristostatin on individual steps of hematogenous metastasis. Exp Cell Res. 1995 Aug;219(2):571–578. doi: 10.1006/excr.1995.1266. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Molecular mechanisms of cancer metastasis: tumor and host properties and the role of oncogenes and suppressor genes. Curr Opin Oncol. 1991 Feb;3(1):75–92. [PubMed] [Google Scholar]
- Nicolson G. L. Organ specificity of tumor metastasis: role of preferential adhesion, invasion and growth of malignant cells at specific secondary sites. Cancer Metastasis Rev. 1988 Jun;7(2):143–188. doi: 10.1007/BF00046483. [DOI] [PubMed] [Google Scholar]
- Ossowski L. In vivo invasion of modified chorioallantoic membrane by tumor cells: the role of cell surface-bound urokinase. J Cell Biol. 1988 Dec;107(6 Pt 1):2437–2445. doi: 10.1083/jcb.107.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo V., DeFouw D. O. Macromolecular selectivity of chick chorioallantoic membrane microvessels during normal angiogenesis and endothelial differentiation. Tissue Cell. 1993 Dec;25(6):847–856. doi: 10.1016/0040-8166(93)90033-h. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Reed J. C. Anchorage dependence, integrins, and apoptosis. Cell. 1994 May 20;77(4):477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V. Cancer metastasis: experimental approaches, theoretical concepts, and impacts for treatment strategies. Adv Cancer Res. 1985;43:1–73. doi: 10.1016/s0065-230x(08)60942-2. [DOI] [PubMed] [Google Scholar]
- Smejda Haug F. M. Heavy metals in the brain. A light microscope study of the rat with Timm's sulphide silver method. Methodological considerations and cytological and regional staining patterns. Adv Anat Embryol Cell Biol. 1973;47(4):1–71. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Aznavoorian S., Liotta L. A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Stracke M. L., Aznavoorian S. A., Beckner M. E., Liotta L. A., Schiffmann E. Cell motility, a principal requirement for metastasis. EXS. 1991;59:147–162. doi: 10.1007/978-3-0348-7494-6_10. [DOI] [PubMed] [Google Scholar]
- Tuck A. B., Wilson S. M., Khokha R., Chambers A. F. Different patterns of gene expression in ras-resistant and ras-sensitive cells. J Natl Cancer Inst. 1991 Apr 3;83(7):485–491. doi: 10.1093/jnci/83.7.485. [DOI] [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Wilson B. Motility of rasH oncogene transformed NIH-3T3 cells. Invasion Metastasis. 1986;6(6):335–346. [PubMed] [Google Scholar]
- Weiss L. Metastatic inefficiency. Adv Cancer Res. 1990;54:159–211. doi: 10.1016/s0065-230x(08)60811-8. [DOI] [PubMed] [Google Scholar]