Abstract
A dual beam pump/probe technique has been used with a 585-nm probe wavelength to obtain maximal resonance enhancement of the Raman lines of bathorhodopsin in a photostationary steady-state mixture at -160°C. These studies show that bathorhodopsin has a protonated Schiff base vibration at 1657 cm-1 which shifts upon deuteration to 1625 cm-1. Within our experimental error (±2 cm-1) these frequencies are identical to those observed in rhodopsin and isorhodopsin. These effects show that the strength of the C=N bond and the degree of protonation of the Schiff base nitrogen are the same in bathorhodopsin, rhodopsin, and isorhodopsin. The implication of these results for the structure of the retinal chromophore in bathorhodopsin are discussed. The resonance Raman spectrum of pure bathorhodopsin has been generated by accurately subtracting the residual contributions of rhodopsin and isorhodopsin from spectra of the low temperature photostationary mixture. Bathorhodopsin is found to have lines at 853, 875, 920, 1006, 1166, 1210, 1278, 1323, 1536, and 1657 cm-1. Also, by using an intensified vidicon detector, we have observed Raman scattering from bathorhodopsin at room temperature by generating a photostationary steady state with pulsed laser excitation. At room temperature the three characteristic lines of bathorhodopsin are found at 858, 873, and 920 cm-1. The fact that the frequencies of these bathorhodopsin lines are nearly identical at both temperatures implies that the retinal conformation in bathorhodopsin formed at -160°C is the same as that formed at room temperature.
Keywords: retinal conformations, visual excitation, kinetics, multichannel detection
Full text
PDF
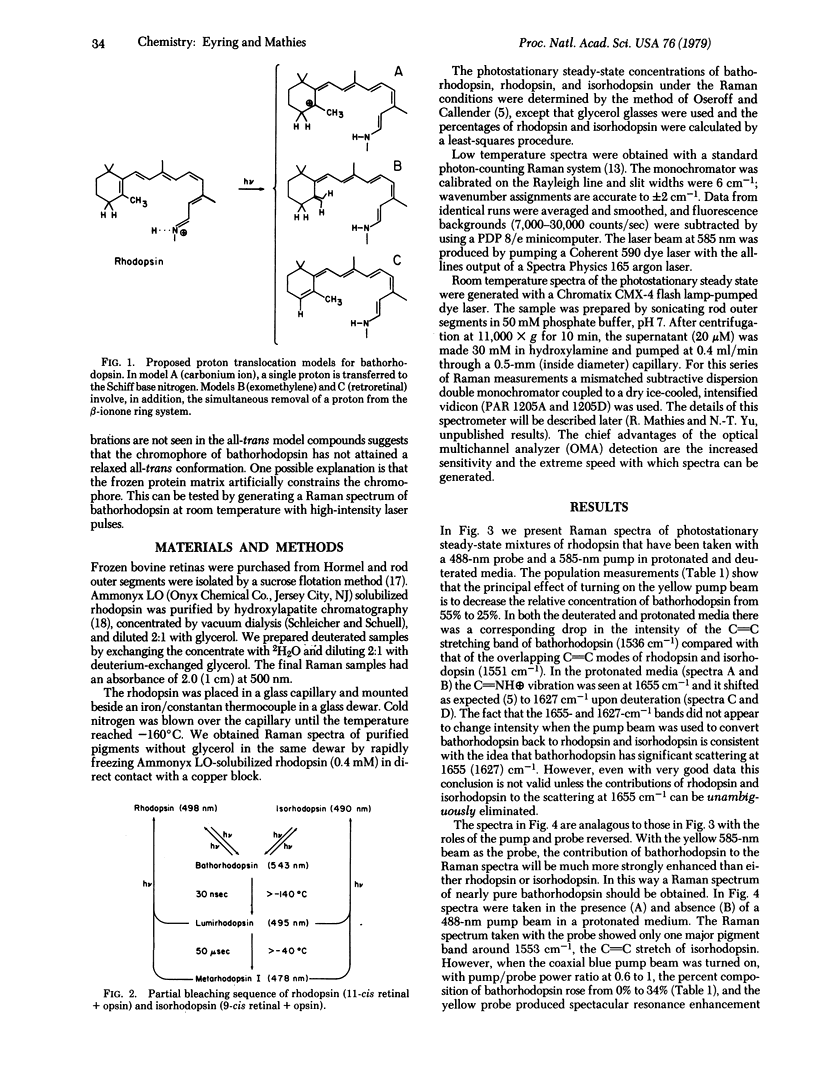
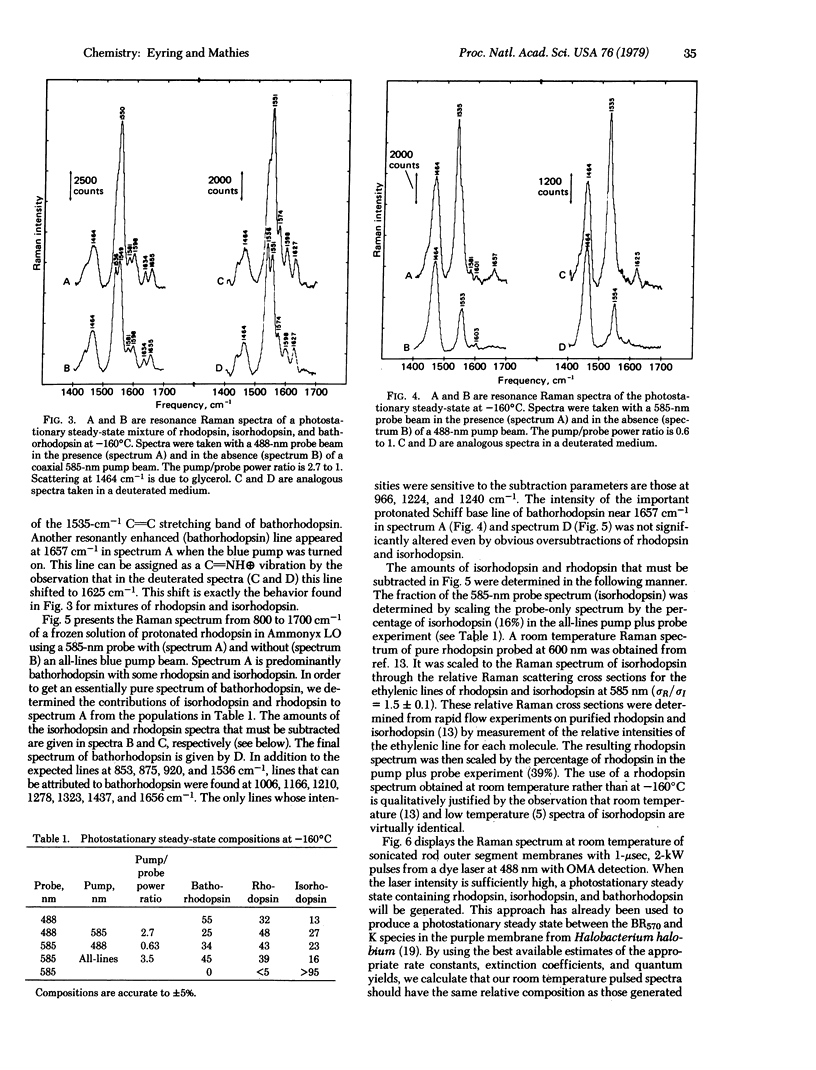
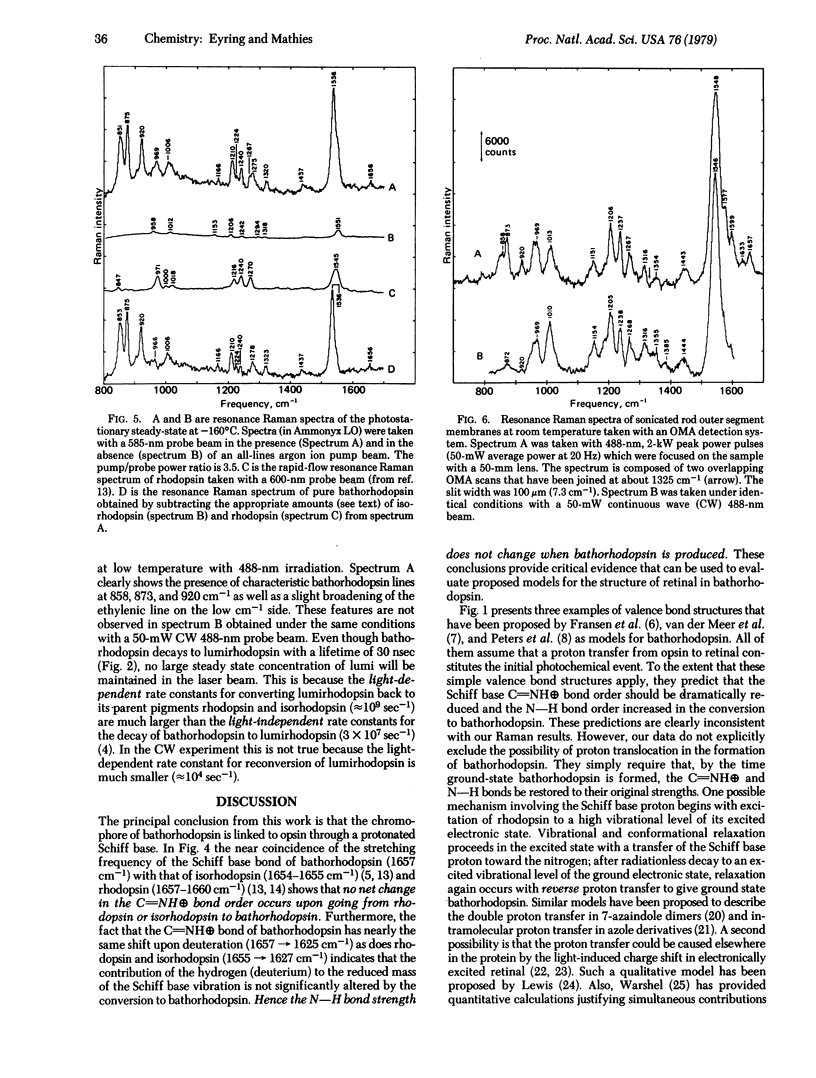

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Zuckerman D. M., Lamola A. A., Jovin T. M. Rhodopsin. Purification and recombination with phospholipids assayed by the metarhodopsin I leads to metarhodopsin II transition. Biochemistry. 1974 Aug 13;13(17):3448–3458. doi: 10.1021/bi00714a005. [DOI] [PubMed] [Google Scholar]
- Busch G. E., Applebury M. L., Lamola A. A., Rentzepis P. M. Formation and decay of prelumirhodopsin at room temperatures. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2802–2806. doi: 10.1073/pnas.69.10.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callender R. H., Doukas A., Crouch R., Nakanishi K. Molecular flow resonance Raman effect from retinal and rhodopsin. Biochemistry. 1976 Apr 20;15(8):1621–1629. doi: 10.1021/bi00653a005. [DOI] [PubMed] [Google Scholar]
- Doukas A. G., Aton B., Callender R. H., Ebrey T. G. Resonance Raman studies of bovine metarhodopsin I and metarhodopsin II. Biochemistry. 1978 Jun 13;17(12):2430–2435. doi: 10.1021/bi00605a028. [DOI] [PubMed] [Google Scholar]
- Fransen M. R., Luyten W. C., Van Thuijl J., Jansen P. A., Van Breugel P. J., Daemen F. J. Structure of the chromophoric group in bathorhodopsin. Nature. 1976 Apr 22;260(5553):726–727. [PubMed] [Google Scholar]
- Goldschmidt C. R., Ottolenghi M., Korenstein R. On the primary quantum yields in the bacteriorhodopsin photocycle. Biophys J. 1976 Jul;16(7):839–843. doi: 10.1016/S0006-3495(76)85732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. H., Monger T. G., Alfano R. R., Aton B., Callender R. H. Cis-trans isomerisation in rhodopsin occurs in picoseconds. Nature. 1977 Sep 8;269(5624):179–180. doi: 10.1038/269179a0. [DOI] [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Lipid requirements for Rhodopsin regenerability. Biochemistry. 1973 Oct 23;12(22):4517–4523. doi: 10.1021/bi00746a033. [DOI] [PubMed] [Google Scholar]
- Hubbard R., Kropf A. THE ACTION OF LIGHT ON RHODOPSIN. Proc Natl Acad Sci U S A. 1958 Feb;44(2):130–139. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G., Honig B., Ottolenghi M. Temperature and wavelength effects on the photochemistry of rhodopsin, isorhodopsin, bacteriorhodopsin and their photoproducts. Nature. 1977 Dec 8;270(5637):540–542. doi: 10.1038/270540a0. [DOI] [PubMed] [Google Scholar]
- Kropf A. Is proton transfer the initial photochemical process in vision? Nature. 1976 Nov 4;264(5581):92–94. doi: 10.1038/264092a0. [DOI] [PubMed] [Google Scholar]
- Lewis A. The molecular mechanism of excitation in visual transduction and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):549–553. doi: 10.1073/pnas.75.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathies R., Freedman T. B., Stryer L. Resonance Raman studies of the conformation of retinal in rhodopsin and isorhodopsin. J Mol Biol. 1977 Jan 15;109(2):367–372. doi: 10.1016/s0022-2836(77)80040-5. [DOI] [PubMed] [Google Scholar]
- Mathies R., Oseroff A. R., Stryer L. Rapid-flow resonance Raman spectroscopy of photolabile molecules: rhodopsin and isorhodopsin. Proc Natl Acad Sci U S A. 1976 Jan;73(1):1–5. doi: 10.1073/pnas.73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathies R., Stryer L. Retinal has a highly dipolar vertically excited singlet state: implications for vision. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2169–2173. doi: 10.1073/pnas.73.7.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseroff A. R., Callender R. H. Resonance Raman spectroscopy of rhodopsin in retinal disk membranes. Biochemistry. 1974 Sep 24;13(20):4243–4248. doi: 10.1021/bi00717a027. [DOI] [PubMed] [Google Scholar]
- Peters K., Applebury M. L., Rentzepis P. M. Primary photochemical event in vision: proton translocation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3119–3123. doi: 10.1073/pnas.74.8.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem L., Bruckmann P. Conversion of a photon to an electrical signal by sudden polarisation in the N-retinylidene visual chromophore. Nature. 1975 Dec 11;258(5535):526–528. doi: 10.1038/258526a0. [DOI] [PubMed] [Google Scholar]
- Sulkes M., Lewis A., Lemley A. T., Cookingham R. Modeling the resonance Raman spectrum of a metarhodopsin: implications for the color of visual pigments. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4266–4270. doi: 10.1073/pnas.73.12.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968 Aug 24;219(5156):800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- Warshel A. Charge stabilization mechanism in the visual and purple membrane pigments. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2558–2562. doi: 10.1073/pnas.75.6.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A. Interpretation of resonance Raman spectra of biological molecules. Annu Rev Biophys Bioeng. 1977;6:273–300. doi: 10.1146/annurev.bb.06.060177.001421. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA T., WALD G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963 Mar 30;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]
- van der Meer K., Mulder J. J., Lugtenburg J. A new facet in rhodopsin photochemistry. Photochem Photobiol. 1976 Oct;24(4):363–367. doi: 10.1111/j.1751-1097.1976.tb06837.x. [DOI] [PubMed] [Google Scholar]



