Abstract
Purpose: Burn injury is associated with early apoptotic death of T cells. Insulin-like growth factor-1 (IGF-I) is able to protect T cells from apoptosis. Association of IGF-I with its IGFBP (Binding Protein)-1 limits its bioavailability and serine phosphorylation of IGFBP-1 lowers this further because of an increased affinity for IGF-I. The level of phosphorylated IGFBP-1 has been shown to increase in pediatric burn patients. Thus we hypothesized that a longitudinal study of burn patients would demonstrate 1) increased IGFBP-1 levels, 2) increased IGFBP-1 phosphorylation and 3) decreased IGF-I levels over time. Methods: We conducted a prospective observational study in adult burn patients admitted to UNC Jaycee Burn Center. Plasma levels of insulin, insulin-like growth factor 1 (IGF-I) and insulin-like growth factor binding protein 1 (IGGBP-1) were measured on admission up to 10 days post admission. ELISA was used to measure serum levels of insulin, IGF-I and IGFBP-1. Serine phosphorylation of IGFBP-1 was measured by Western blot with and without the incubation of calf intestinal phosphatase (CIP). Significant findings: There was a significant positive correlation of increasing %TBSA burn and increasing levels of serum IGFBP-1 from admittance blood draws. Levels of IGF-I also decreased with increasing Total Body Surface Area (TBSA, p<0.05). In patients studied longitudinally (n=84) we found that IGFBP-1 levels are significantly (p<0.05) increased 1-72 hours post burn (mean±SEM serum concentration; burn=172±23 ng/mL, normal=13±3 ng/mL) and that levels of IGF-I are reduced. IGFBP-1 is serine phosphorylated in burn patients. In patients surviving past 72 hours IGFBP-1 remained phosphorylated over the study period. Conclusions: IGFBP-1 and its serine phosphorylation regulate and limit IGF-I bioavailability. Our results suggest that increases in IGFBP-1 and persistent serine phosphorylation of IGFBP-1 correlate with the severity of burn injury, and may contribute to burn-associated T cell apoptosis and subsequent immune dysfunction by reducing the bioavailability of this important cell survival factor.
Keywords: Insulin-like growth factor, insulin-like growth factor binding protein-1, trauma, burns
Introduction
The leading causes of death from serious burn injury following resuscitation are sepsis and multiple system organ failure secondary to profound immune dysfunction [1]. Burn is associated with apoptosis of lymphocytes in the thymus and in the peripheral lymphoid organs [2,3]. The consequence of these events is suppression of the inflammatory immune response in burn patients, thereby leading to an increased susceptibility to infection. Insulin-like Growth factor-1 (IGF-I) has been shown to be immunomodulatory primarily via its ability to act as a potent stimulator of T-cell survival and function. The IGFs (IGF-I and II) are 7.5 kDa conserved secreted polypeptides with 70% homology with each other and 50% with insulin [4,5]. They are known to have diverse effects including metabolic insulin-like effects and effects on cell growth, differentiation and survival acting in an endocrine, autocrine and paracrine manner. Specifically, in the case of T cells IGF-I has been shown to protect activated T cells from apoptosis [6,7].
IGF Binding Protein (IGFBP)-1 is the major modulator of the bioactivity of free IGF-I. The majority of IGF-I in the circulation is associated with IGFBP-3 [4,5]. Serine phosphorylation of IGFBP-1 increases its affinity for IGF-I [8], thereby sequestering IGF-I away from its receptor and reducing its bioactivity [9]. It has been suggested that a sustained high serum concentration of IGFBP-1 can predict poor outcome from sepsis and critical illness [10-13]. It has been shown in critically ill patients and in mouse models that the amount of phosphorylated IGFBP-1 in the circulation also increases [9,14]. Burn injury has been associated with a similar decrease in IGF-I levels and an increase in IGFBP-1 levels in burn patients [15-17]. These changes in the IGF-I axis were found to persist for the 40 days studied post-burn, but phosphorylation status has not been reported.
Given the potential role of IGF-I as a T cell survival factor this decrease in IGF-I may contribute to the loss of T cell function. Clinical studies in which IGF-I (associated with its principal binding protein IGFBP-3 which prolongs its half-life and reduces associated side effects, notably hypoglycemia) was administered to burn patients showed improvement in outcome [15,18]. An alteration in the cytokine profile of patients following IGF-I/IGFBP-3 administration was observed suggesting that the positive effects of IGF-I were mediated at least in part through its ability to modify the immune response [16,19].
We therefore hypothesized that the decrease in IGF and increase in IGFBP-1 levels after burn is associated with IGFBP-1 phosphorylation (Figure 1). To test this, we obtained peripheral blood from burn patients admitted to the Jaycee Burn Center within the first 24 hours after injury and daily thereafter up to 10 days post burn. We used ELISA to measure the serum levels of IGF-I and IGFBP-1. We also characterized the phosphorylation status of IGFBP-1 within each sample by calf intestinal phosphatase (CIP) treatment followed by Western blot. We observed 1) increased IGFBP-1 levels, 2) decreased IGF-I levels over time and 3) increased and persistent IGFBP-1 phosphorylation.
Figure 1.
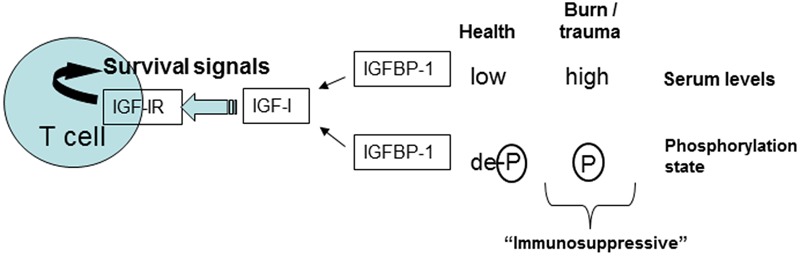
A model of the changes that occur in the insulin-like growth factor 1 (IGF-I) axis in burn patients. Phosphorylation of IGF binding protein 1 (IGFBP-1) would occur in burn patients which increases binding affinity to IGF-I. IGF-I is a well characterized T cell survival factor, therefore in the presence of elevated IGFBP-1 and reduced IGF-I levels, the immunosuppressive state of the adaptive immune system would be significantly increased.
Materials and methods
Patients
With the approval of the University of North Carolina Healthcare Internal Review Board (IRB), samples were collected from excess serum originally obtained for clinical use. The Internal Review board approved the waiver of consent as the study did not incur additional risks and patient specimens were systematically de-identified. All patients admitted to the Jaycee Burn Center were eligible to be included in the study. Patients enrolled in the study had peripheral blood samples collected within 24 hours of admission and were followed up to 10 days post admission.
Data on the total body surface area (TBSA) burn, presence of inhalational injury and patient age on admission were collected from patient charts. A total of 84 patients were included in the study. These patients were arranged into 4 groups in respect to burn size. This included a group of burn patients with TBSA burn injury of 1-15%, 16-30%, 31-50% and 51-95% (Table 1).
Table 1.
Patient characteristics. Data on the total body surface area (TBSA) burn, presence of inhalational injury and patient age on admission were collected from patient charts. A total of 84 patients were included in the study. These patients were arranged into 4 groups in respect to burn size. Mean age and TBSA are shown ± Standard Deviation from the mean
| Burn Injury TBSA (%) | Number of patients | Mean age (years) | Mean TBSA (%) | Inhalation injury (# of patients) |
|---|---|---|---|---|
| 1-15 | 37 | 36±18.2 | 7±4.3 | 0 |
| 16-30 | 10 | 32±26.3 | 21±4.6 | 2 (10%) |
| 31-50 | 19 | 44±14.0 | 41±6.1 | 8 (42%) |
| 51-95 | 18 | 37±20.2 | 68±12.3 | 15 (83%) |
Insulin/insulin-like growth factor/insulin-like growth factor binding protein axis measurements
Excess serum samples were obtained from burn patients within 24 hours of injury and daily thereafter up to 10 days. Insulin, insulin-like growth factor I (IGF-I) and insulin-like growth factor binding protein 1 (IGFBP-1) concentrations were measured by enzyme-linked immunosorbent assays (ELISA, Diagnostic Systems Laboratories, Webster, TX, USA). Serum analyzed included burn patient specimens and normal controls. Normal controls were obtained from healthy volunteers.
Measurements of serine phosphorylation of insulin-like growth factor binding protein 1
The degree of IGFBP-1 serine phosphorylation was assessed by non-denaturing and non-reducing SDS PAGE immunoblotting of patient serum with anti-IGFBP-1 antibody before and after treatment with 50U calf intestinal phosphatase (CIP), as detailed by Sakai et al [8,14]. PAGE was performed in the absence of SDS in order to alter protein migration on the basis of molecular charge as well as mass, thus permitting separation of phosphorylated from non-phosphorylated IGFBP-1. In addition, the pH during the resolving phase of electrophoresis was lowered to 8.3. Gels were analyzed for a decrease in IGFBP-1 migration after CIP-treatment indicating IGFBP-1 phosphorylation [8].
Study end point
Patient blood samples were included in the study up to 10 days post admission or patient demise.
Murine model of burn injury and thymocyte culture
Wild-type C57BL/6 female 18-20 gram, 6 week old mice underwent 20% TBSA contact burn procedure as we have detailed previously [2,20]. Mice were anesthetized with isoflurane and received a full-contact burn using a copper rod, heated in boiling water, applied to the animal’s dorsum/flank for 10 seconds. Four applications produced an approximately 20% TBSA full-thickness burn. After burn injury, we resuscitated mice with an intraperitoneal injection of lactated Ringers solution (0.1 ml/g body weight) and gave buprenorphine (2 mg/kg body weight) for pain control immediately after injury and placed mice in separate cages. The mice were given 0.02 mg/cc of morphine sulfate (4 mg/kg/day) in drinking water. Sham-treated control groups of mice were also setup; these mice received all of the above treatments, with the exception of application the heated rod. The burn procedure was followed according to institutionally approved protocols performed in accordance with NIH guidelines.
We isolated thymocytes at 6 hours post burn from each mouse and placed thymocytes in culture (1x106 cells/ml in 2 ml of RPMI 1640 medium plus 1% FCS, antibiotics and glutamine) in the absence or presence of 100 ng/ml IGF-I. Eighteen hours after burn, apoptosis was assessed by staining with anti-CD8 (APC), anti-CD3 (FITC), Annexin V conjugated to PE and 7-Aminoactinomycin D (7AAD) in PBS containing calcium. The degree of apoptosis was assessed by four color flow cytometry, quantifying the percentage of CD3+CD3+T cells that are apoptotic (defined by 7AAD-,lo Annexin V+ staining).
Statistical analyses
Variables were recorded upon admission and up to 10 days afterward. Patient age and TBSA burn in each TBSA group was reported as a mean. Categorical data which included the presence of inhalation injury was reported as total numbers of patients and percentages of each TBSA group.
Insulin, IGF-I and IGFBP-1 were measured and the Pearson correlation coefficient was used to determine the coefficient of determination (r2) for the serum concentrations compared to the extent of the burn injury as determined by measuring the TBSA. A two way ANOVA was used to assess continuous data at different time points after burn injury and after thymocyte culture with or without IGF-1. P<0.05 was considered statistically significant. All significance tests were two-tailed. Data was analyzed using the GraphPad Prism version 4 software (GraphPad Software Inc., 2003, San Diego, CA, USA).
Results
Levels of IGF-I and IGFBP-1 correlate with TBSA on admittance
Blood samples were drawn on admittance and analyzed by ELISA for insulin, IGF-I and IGFBP-1. There was no correlation between serum insulin levels and burn size on admittance (Figure 2A). There was a significant positive correlation of increasing burn size and increasing levels of serum IGFBP-1 at admittance blood draws (n=84, Figure 2B, coefficient of determination (r2)=0.42). As predicted, levels of IGF-I decreased with increasing burn size (p<0.05) (n=84, Figure 2C, coefficient of determination (r2)=0.2). These data suggest that burn injury does decrease the level of available IGF-I in the circulation of patients that this happens early after burn and is dependent on the total burn size.
Figure 2.
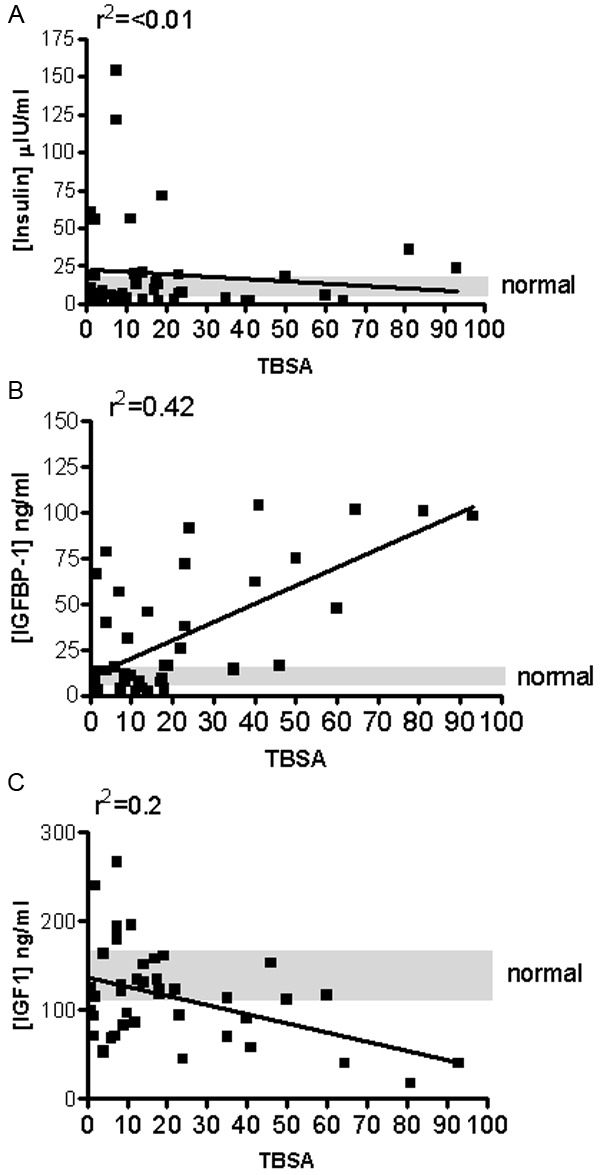
Levels of IGF-I and IGFBP-1, but not insulin, correlate with burn Total Body Surface Area (TBSA) on admittance. Blood samples were drawn on admittance and analyzed by ELISA for A: insulin, B: IGF-I and C: IGFBP-1 (n=84). Serum was analyzed from normal controls. Normal controls were obtained from healthy volunteers (n=16, range is indicated by grey shading). r2 indicates the coefficient of determination
Levels of IGF-I and IGFBP-1 in longitudinal samples
Blood samples were drawn daily after admittance. Levels of serum IGF-I and IGFBP-1 were assayed using commercial ELISA. We stratified patients according to their burn size (TBSA 1-15% (n=37), 16-30% (n=10), 31-50% (n=19) or 51-95% (n=18) and compared their level of IGF-I and IGFBP-1 to normal serum levels (Figure 3). The level of IGF-I was significantly lower only in the 31-50% (Figure 3C) and 51-95% (Figure 3D) groups for the whole study time (up to 10 days post burn) compared to normal levels (170±62 ng/mL). In contrast, the level of IGF-I in the 16-30% TBSA group was only significantly lower at the day 1 post burn. IGFBP-1 levels were significantly increased 1-10 days post burn in all burn patients compared to normal IGFBP-1 levels (13±3 ng/mL), with the exception of the 1-15% group in which the levels of IGFBP-1 became significant only from day 3 post burn and on. The highest serum concentrations of IGFBP-1 were found in the 51-95% TBSA burn patients (Figure 3D).
Figure 3.
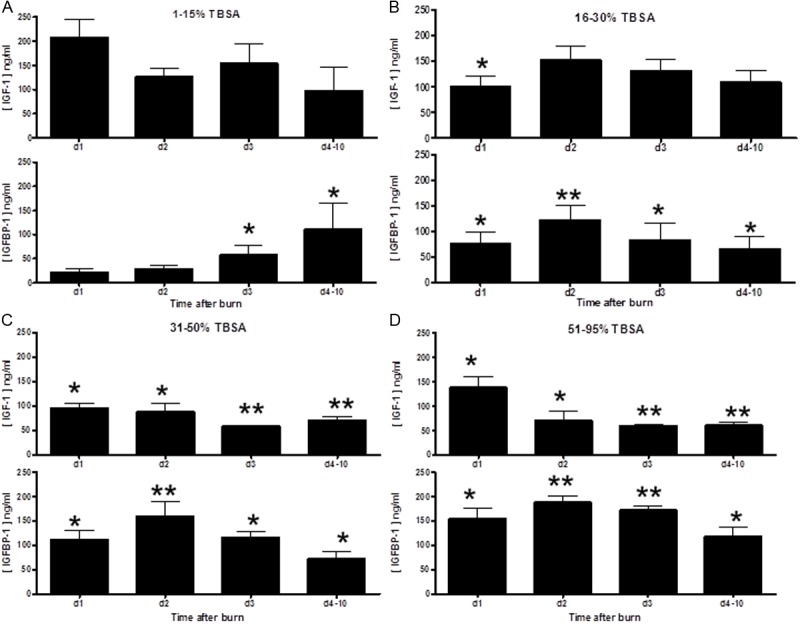
Longitudinal levels of IGF-I are significantly decreased and IGFBP-1 levels are significantly increased after burn injury compared to normal serum and is dependent on burn TBSA. Blood samples were drawn daily (d1=day 1, d2=day 2, d3=day 3, day 4-10 represent a sample taken within the day 4-10 period) after admittance and analyzed by ELISA for IGF-I and IGFBP-1. Serum was analyzed from normal controls. Patients were stratified according to their burn TBSA (A: 1-15%, (n=37), B: 16-30% (n=10), C: 31-50% (n=19) or D: 51-95% (n=18) and levels of IGF-I and IGFBP-1 were compared to normal serum levels. Statistical significance is indicated by *, p<0.05; **, p<0.005 by two way ANOVA.
These data demonstrate that the level of IGF-I is significantly decreased and IGFBP-1 level is significantly increased after burn injury compared to normal serum. These changes in the IGF-1/IGBP-1 axis are dependent on the size of the burn injury.
IGFBP-1 is serine phosphorylated after burn injury
We hypothesized that not only the levels of IGF-I and IGFBP-1 would be altered after burn injury, but that phosphorylation of IGFBP-1 would also occur in burn patients. Immunoblotting with anti-IGFBP-1 revealed that the level of IGFBP-1 in serum from a pool of normal serum is almost undetectable whereas IGFBP-1 can be seen in the sera from burn patients (Figure 4). The quantity of IGFBP-1 was increased with higher burn area (representative bands in Figure 4 from 84, 43 and 15% TBSA patients). This increase in IGFBP-1 is consistent with ELISA measurements. We ran phosphorylation analyses on all the samples. When this serum was treated with CIP there was a marked shift in the molecular mass of the IGFBP-1 indicating that the IGFBP-1 in the sera from the burn patients was heavily serine phosphorylated. We consistently observed 100% phosphorylation of IGFBP-1 in burn patients and remained fully phosphorylated (Figure 5) until the end of the study.
Figure 4.
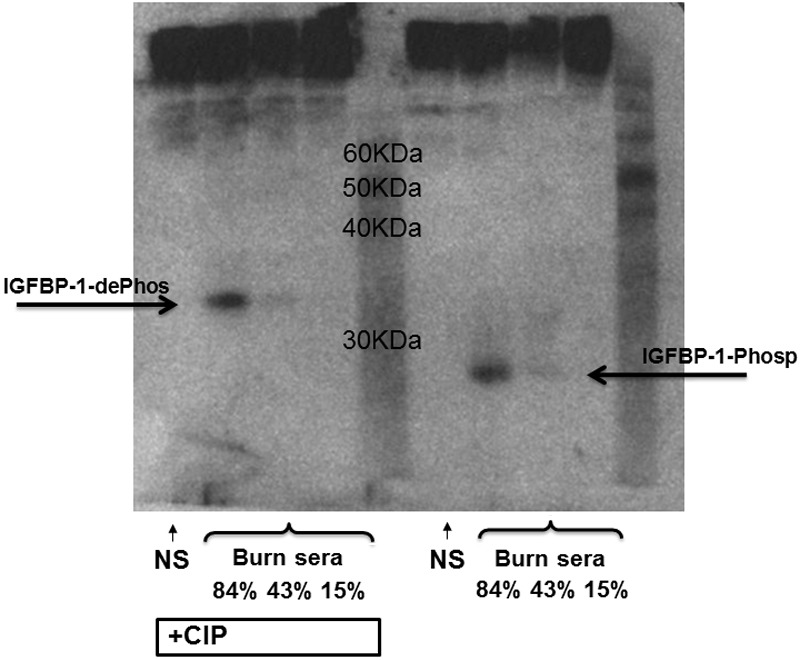
IGFBP-1 is serine phosphorylated after burn injury. Serum from normal (NS) or two burn patients (84% or 43% burn TBSA), with (+CIP) or without CIP exposure, was immunoblotted with anti-IGFBP-1 antibody. IGFBP-1 and molecular mass-shifted phosphorylated-IGFBP-1 are represented by the labels.
Figure 5.
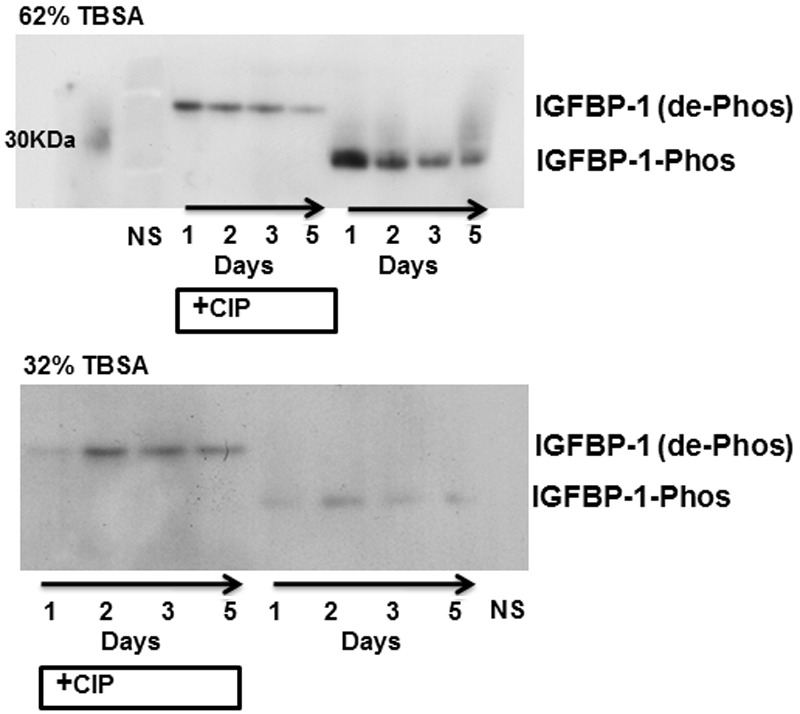
IGFBP-1 remains serine phosphorylated for at least 5 days after burn injury. Serum from normal (NS) and longitudinal samples from two burn patients (62% or 32% burn TBSA), with (+CIP) or without CIP exposure, was immunoblotted with anti-IGFBP-1 antibody. IGFBP-1 and molecular mass-shifted phosphorylated-IGFBP-1 are represented by the labels.
IGF-I can act to prevent thymocyte apoptosis caused by burn injury
In order to demonstrate that IGF-I can be beneficial in reducing burn injury-induced apoptosis of immune T cells, we utilized a mouse model of burn injury. Wildtype female C57/BL6 mice received either a 20% TBSA or sham treatment as we have described previously [2,20]. We typically observe induction of thymic apoptosis in vivo between 12 and 24 hours after burn [2]. As can be seen in Figure 6, apoptosis of thymocytes from burn mice occurs even after the cells are isolated from the mouse and placed in culture. This “programmed” death is reversed by addition of IGF-I to the thymocyte culture. Addition of IGF-I returned levels of apoptosis to that of thymocytes from sham mice. This suggests that elevated levels IGF-I is able to rescue T cells from burn-mediated apoptosis.
Figure 6.
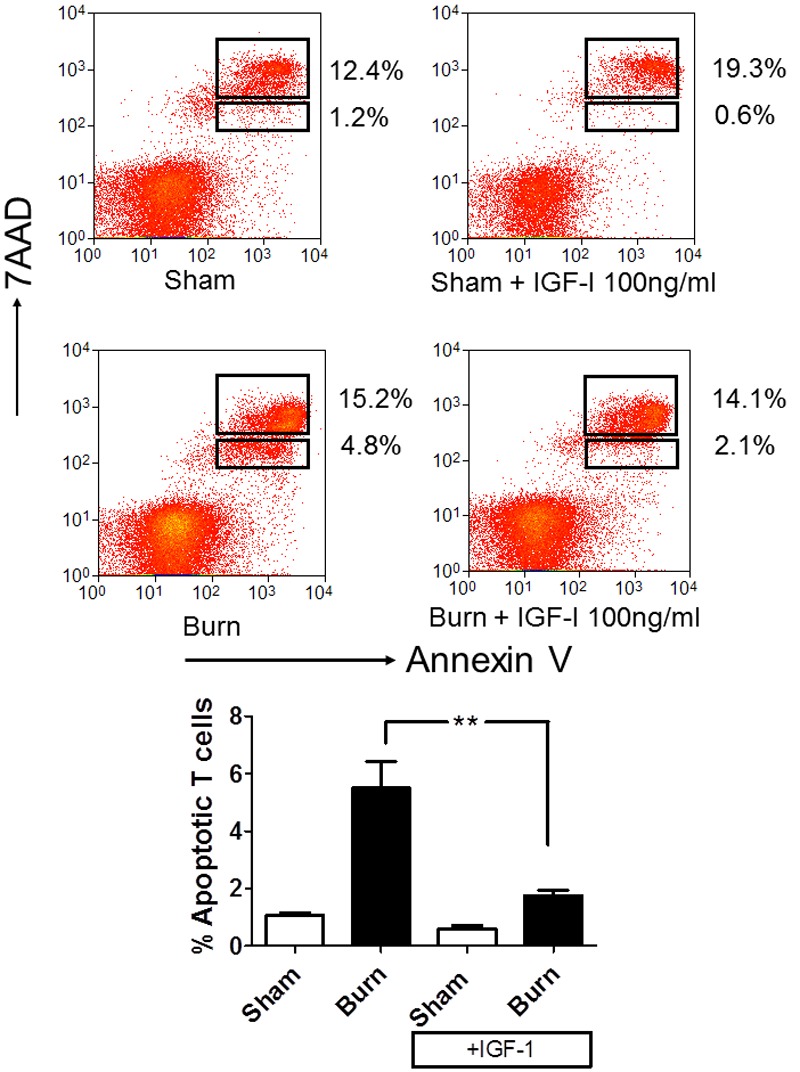
IGF-I can act to prevent thymocyte apoptosis caused by burn injury. Wild-type female C57/BL6 mice received either a 20% TBSA or sham treatment (n=6/group). Thymocytes were harvested at 6 hours post burn from each mouse and placed thymocytes in culture in the absence or presence of 100 ng/ml IGF-I. Eighteen hours after burn, apoptosis was assessed by staining with anti-CD8 (APC), anti-CD3 (FITC), Annexin V conjugated to PE and 7-Aminoactinomycin D (7AAD) in PBS containing calcium. The degree of apoptosis was assessed by four color flow cytometry, quantifying the percentage of CD8+CD3+T cells that are apoptotic (defined by 7AAD-,lo Annexin V+ staining). Top panel, representative flow cytometry of each group. Bottom Panel, collated groups, plotting percentage of CD8+CD3+T cells that were apoptotic, where statistical significance is indicated by **, p<0.005 by two way ANOVA.
Discussion
In this study, we evaluated the relationship between burn injury and the IGF-I/IGFBP-1 axis. Using unidentified burn patients of all ages, we discovered a direct correlation with the TBSA burn injury and IGFBP-1 at the time of admission, but an inverse relationship with IGF-I and the TBSA burn injury. We observed that these relationships continued at 10 days after injury, our end-point. Size of the burn injury appears to play an influential role in the homeostasis of the IGF-I/IGFBP-1 axis. While these data concur with earlier studies, we demonstrate here that IGFBP-1 in burn patients is heavily phosphorylated. Increased IGFBP-1 phosphorylation increases its affinity for IGF-1, likely reducing its bioavailability. These data suggest that the increase in IGFBP-1 phosphorylation coincides with the reduction of serum IGF-I seen in burn patients and therefore reduces the survival benefit of IGF-1.
Elevated levels of IGF-I provide a survival benefit in critically ill patients, and improve survival in sepsis [10,11,21,22]. IGF-I is also significantly decreased in non-survivors of multi-organ failure [22-24]. The mechanism for this is thought to be that IGF-I improves survival of immune effector cells such as T cells [7,25,26] and other multiple immune cell types [27,28]. IGF-I activates specific signaling pathways including Akt and Jun N-terminal kinases which in turn promotes T cell survival from Fas-induced cell death [7]. We have presented data here demonstrating in vitro IGF-1 can protect against prior in vivo burn-induced thymic lymphocyte apoptosis, a well-established consequence of burn injury, mainly mediated by glucocorticoid release. We have published that burn mice given the glucocorticoid inhibitor mifepristone do not exhibit systemic T cell apoptosis, and their immune function is restored [2]. The data presented here gives rationale to study whether this effect can be optimized with depleted levels of IGFBP-1.
The IGF-1 axis has critical roles in cell survival as seen in multiple organ systems and particularly the immune system, and modulating the IGF-I axis appears to offer many potential benefits to burn patients [15,17,29-32]. Nonetheless, it appears that treatment with IGF-I alone does not offer substantial therapeutic benefit [33,34]. It cannot be understated that there is a complex interaction between IGF-I and its binding proteins [11]. Administration of IGF-I along with IGFBP-3 has been shown to restore pro-inflammatory and anti-inflammatory homeostasis, improve renal and hepatic function and increase peripheral muscle protein synthesis [16,34,35]. IGFBP-3 is the principal binding protein of IGF-I, and is essential in enhancing the half-life of IGF-I [11]. The insulin binding proteins can act independently of IGF with undesirable effects especially in critically ill patients [36].
This study provides a possible mechanism for the reduced bioavailability of IGF-1 after burn injury. The rapid and sustained phosphorylation of IGFBP-1 after burn injury represents an important biological finding. Further work will be required to identify ligands, and if phosphorylated IGFBP-1 is competing with IGFBP-3 for IGF-1, this provides rationale for disrupting this interaction to provide a possible immunotherapeutic increasing IGF-1 availability. Identification of kinases that control IGFBP-1 phosphorylation is also a target of investigation. We hypothesize that phosphorylated IGFBP-1 may also become localized to the cell surface or extracellular matrix to deliver IGF-1. This may provide a novel mechanism for studies in which IGFBP-1 deficiency both by antibody treatment and in IGFBP-1 knockout mice increased Fas-induced hepatocyte apoptosis [37] where IGBP-1 has a protective anti-apoptotic effect.
There are some limitations to this study which primarily involve the complexity of the IGF/IGFBP axis and the contribution of other metabolic factors. Although the size of burn injury coincides with an increase in IGFBP-1, we cannot claim a direct relationship between these two variables as other variables such as age, depth of injury and presence of co-morbidities cannot be adjusted for in our statistical analysis. We also recognize that hormone levels fluctuate widely within a 24-hour period following injury [22]. Nevertheless we attempted to limit the effect of temporal variations by collecting samples at similar time points each day. Patient outcomes and mortality are not included in this study due to the restricted access of comprehensive patient data. Additionally we cannot include information pertaining to pre-existing conditions or gender. However, our results still support a prognostic role for phosphorylated IGFBP-1 in burn patients and a potential molecular mechanism for immune dysfunction after burn.
Burn injury greatly impacts the IGF-I/IGFBP-1 axis. We demonstrate that IGF-I and IGFBP-1 directly correlate with the severity of burn injury. IGF-I is reduced with increasing TBSA% burn injury, while IGFBP-1 steadily increases along with increasing TBSA% burn injury. IGFBP-1 is heavily phosphorylated in burn patients early after injury which likely contributes to the reduction of circulating IGF-I. Longitudinal outcome studies will be necessary in order to confirm the prognostic value of the serum admission levels of IGF-I/IGFBP-1 on the overall outcomes for burn patients.
Disclosure of conflict of interest
None.
References
- 1.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–244. doi: 10.1016/s1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 2.Maile R, Barnes CM, Nielsen AI, Meyer AA, Frelinger JA, Cairns BA. Lymphopenia-Induced Homeostatic Proliferation of CD8+ T Cells Is a Mechanism for Effective Allogeneic Skin Graft Rejection following Burn Injury. J Immunol. 2006;176:6717–6726. doi: 10.4049/jimmunol.176.11.6717. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan IB, Maile R, Frelinger JA, Fair JH, Meyer AA, Cairns BA. The effect of burn injury on CD8+ and CD4+ T cells in an irradiation model of homeostatic proliferation. J Trauma. 2006;61:1062–1068. doi: 10.1097/01.ta.0000195984.56153.21. [DOI] [PubMed] [Google Scholar]
- 4.Clemmons DR. Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol Cell Endocrinol. 1998;140:19–24. doi: 10.1016/s0303-7207(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 5.Clemmons DR. IGF binding proteins and their functions. Mol Reprod Dev. 1993;35:368–374. doi: 10.1002/mrd.1080350409. discussion 374-365. [DOI] [PubMed] [Google Scholar]
- 6.Walsh PT, O’Connor R. The insulin-like growth factor-I receptor is regulated by CD28 and protects activated T cells from apoptosis. Eur J Immunol. 2000;30:1010–1018. doi: 10.1002/(SICI)1521-4141(200004)30:4<1010::AID-IMMU1010>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Walsh PT, Smith LM, O'Connor R. Insulin-like growth factor-1 activates Akt and Jun N-terminal kinases (JNKs) in promoting the survival of T lymphocytes. Immunology. 2002;107:461–471. doi: 10.1046/j.1365-2567.2002.01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones JI, D'Ercole AJ, Camacho-Hubner C, Clemmons DR. Phosphorylation of insulin-like growth factor (IGF)-binding protein 1 in cell culture and in vivo: effects on affinity for IGF-I. Proc Natl Acad Sci U S A. 1991;88:7481–7485. doi: 10.1073/pnas.88.17.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coverley JA, Baxter RC. Phosphorylation of insulin-like growth factor binding proteins. Mol Cell Endocrinol. 1997;128:1–5. doi: 10.1016/s0303-7207(97)04032-x. [DOI] [PubMed] [Google Scholar]
- 10.Hunninghake GW, Doerschug KC, Nymon AB, Schmidt GA, Meyerholz DK, Ashare A. Insulin-like growth factor-1 levels contribute to the development of bacterial translocation in sepsis. Am J Respir Crit Care Med. 2010;182:517–25. doi: 10.1164/rccm.200911-1757OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mesotten D, Van den Berghe G. Changes within the GH/IGF-I/IGFBP axis in critical illness. Crit Care Clin. 2006;22:17–28. v. doi: 10.1016/j.ccc.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Mesotten D, Wouters PJ, Peeters RP, Hardman KV, Holly JM, Baxter RC, Van den Berghe G. Regulation of the somatotropic axis by intensive insulin therapy during protracted critical illness. J Clin Endocrinol Metab. 2004;89:3105–3113. doi: 10.1210/jc.2003-032102. [DOI] [PubMed] [Google Scholar]
- 13.Van den Berghe G. Endocrine evaluation of patients with critical illness. Endocrinol Metab Clin North Am. 2003;32:385–410. doi: 10.1016/s0889-8529(03)00005-7. [DOI] [PubMed] [Google Scholar]
- 14.Sakai K, D’Ercole AJ, Murphy LJ, Clemmons DR. Physiological differences in insulin-like growth factor binding protein-1 (IGFBP-1) phosphorylation in IGFBP-1 transgenic mice. Diabetes. 2001;50:32–38. doi: 10.2337/diabetes.50.1.32. [DOI] [PubMed] [Google Scholar]
- 15.Jeschke MG, Barrow RE, Herndon DN. Recombinant human growth hormone treatment in pediatric burn patients and its role during the hepatic acute phase response. Crit Care Med. 2000;28:1578–1584. doi: 10.1097/00003246-200005000-00053. [DOI] [PubMed] [Google Scholar]
- 16.Jeschke MG, Barrow RE, Suzuki F, Rai J, Benjamin D, Herndon DN. IGF-I/IGFBP-3 equilibrates ratios of pro- to anti-inflammatory cytokines, which are predictors for organ function in severely burned pediatric patients. Mol Med. 2002;8:238–246. [PMC free article] [PubMed] [Google Scholar]
- 17.Lang CH, Fan J, Frost RA, Gelato MC, Sakurai Y, Herndon DN, Wolfe RR. Regulation of the insulin-like growth factor system by insulin in burn patients. J Clin Endocrinol Metab. 1996;81:2474–2480. doi: 10.1210/jcem.81.7.8675563. [DOI] [PubMed] [Google Scholar]
- 18.Jeschke MG, Barrow RE, Mlcak RP, Herndon DN. Endogenous anabolic hormones and hypermetabolism: effect of trauma and gender differences. Ann Surg. 2005;241:759–767. doi: 10.1097/01.sla.0000161028.43338.cd. discussion 767-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeschke MG, Einspanier R, Klein D, Jauch KW. Insulin attenuates the systemic inflammatory response to thermal trauma. Mol Med. 2002;8:443–450. [PMC free article] [PubMed] [Google Scholar]
- 20.Cairns BA, Maile R, Buchanan I, Pilati D, Deserres S, Collins EJ, Frelinger JA, Meyer AA. CD8 T cells express a T-helper 1-like phenotype after burn injury. Surgery. 2001;130:210–216. doi: 10.1067/msy.2001.115835. [DOI] [PubMed] [Google Scholar]
- 21.Ashare A, Nymon AB, Doerschug KC, Morrison JM, Monick MM, Hunninghake GW. Insulin-like growth factor-1 improves survival in sepsis via enhanced hepatic bacterial clearance. Am J Respir Crit Care Med. 2008;178:149–157. doi: 10.1164/rccm.200709-1400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharshar T, Bastuji-Garin S, Polito A, De Jonghe B, Stevens RD, Maxime V, Rodriguez P, Cerf C, Outin H, Touraine P, Laborde K Groupe de Réflexion et d’Etude des Neuromyopathies En Réanimation. Hormonal status in protracted critical illness and in-hospital mortality. Crit Care. 2011;15:R47. doi: 10.1186/cc10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardelis JG, Hatzis TD, Stamogiannou LN, Dona AA, Fotinou AD, Brestas PS, Constantopoulos AG. Activity of the growth hormone/insulin-like growth factor-I axis in critically ill children. J Pediatr Endocrinol Metab. 2005;18:363–372. doi: 10.1515/jpem.2005.18.4.363. [DOI] [PubMed] [Google Scholar]
- 24.Marquardt DJ, Knatz NL, Wetterau LA, Wewers MD, Hall MW. Failure to recover somatotropic axis function is associated with mortality from pediatric sepsis-induced multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2010;11:18–25. doi: 10.1097/PCC.0b013e3181b06046. [DOI] [PubMed] [Google Scholar]
- 25.Johnson EW, Jones LA, Kozak RW. Expression and function of insulin-like growth factor receptors on anti-CD3-activated human T lymphocytes. J Immunol. 1992;148:63–71. [PubMed] [Google Scholar]
- 26.Kooijman R, Coppens A. Insulin-like growth factor-I stimulates IL-10 production in human T cells. J Leukoc Biol. 2004;76:862–7. doi: 10.1189/jlb.0404248. [DOI] [PubMed] [Google Scholar]
- 27.Alpdogan O, Muriglan SJ, Kappel BJ, Doubrovina E, Schmaltz C, Schiro R, Eng JM, Greenberg AS, Willis LM, Rotolo JA, O’Reilly RJ, van den Brink MR. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation. 2003;75:1977–1983. doi: 10.1097/01.TP.0000070167.81584.A2. [DOI] [PubMed] [Google Scholar]
- 28.Liu E, Law HK, Lau YL. Insulin-like growth factor I promotes maturation and inhibits apoptosis of immature cord blood monocyte-derived dendritic cells through MEK and PI 3-kinase pathways. Pediatr Res. 2003;54:919–925. doi: 10.1203/01.PDR.0000088067.04673.1B. [DOI] [PubMed] [Google Scholar]
- 29.Gauglitz GG, Williams FN, Herndon DN, Jeschke MG. Burns: where are we standing with propranolol, oxandrolone, recombinant human growth hormone, and the new incretin analogs? Curr Opin Clin Nutr Metab Care. 2011;14:176–81. doi: 10.1097/MCO.0b013e3283428df1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 31.Jeschke MG, Klein D, Herndon DN. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann Surg. 2004;239:553–560. doi: 10.1097/01.sla.0000118569.10289.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nygren J, Sammann M, Malm M, Efendic S, Hall K, Brismar K, Ljungqvist O. Distributed anabolic hormonal patterns in burned patients: the relation to glucagon. Clin Endocrinol (Oxf) 1995;43:491–500. doi: 10.1111/j.1365-2265.1995.tb02622.x. [DOI] [PubMed] [Google Scholar]
- 33.Langouche L, Van den Berghe G. Glucose metabolism and insulin therapy. Crit Care Clin. 2006;22:119–129. vii. doi: 10.1016/j.ccc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Chung TP, Laramie JM, Meyer DJ, Downey T, Tam LH, Ding H, Buchman TG, Karl I, Stormo GD, Hotchkiss RS, Cobb JP. Molecular diagnostics in sepsis: from bedside to bench. J Am Coll Surg. 2006;203:585–598. doi: 10.1016/j.jamcollsurg.2006.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeschke MG, Herndon DN, Vita R, Traber DL, Jauch KW, Barrow RE. IGF-I/BP-3 administration preserves hepatic homeostasis after thermal injury which is associated with increases in no and hepatic NF-kappa B. Shock. 2001;16:373–379. doi: 10.1097/00024382-200116050-00009. [DOI] [PubMed] [Google Scholar]
- 36.Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278:E967–976. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- 37.Leu JI, Crissey MA, Taub R. Massive hepatic apoptosis associated with TGF-beta1 activation after Fas ligand treatment of IGF binding protein-1-deficient mice. J Clin Invest. 2003;111:129–139. doi: 10.1172/JCI16712. [DOI] [PMC free article] [PubMed] [Google Scholar]


