Abstract
The cuff of the endotracheal tube (ETT) is designed to provide a seal within the airway, allowing airflow through the ETT but preventing passage of air or fluids around the ETT. Deliberate or inadvertent movement of the ETT may affect cuff pressure or shift folds in the cuff, mobilizing pooled secretions. When this seal is compromised, microaspirations contaminated with gastric contents or bacterially colonized oral secretions can occur that leave the patient susceptible to a host of problems, such as hypoxia, pneumonitis, and respiratory infections. These complications are costly in terms of morbidity and mortality, as well as hospital expense. We will discuss the role of the ETT cuff in microaspiration and identify potential directions for future research to improve outcomes in mechanically ventilated patients.
Keywords: Cuff leak, Cuff pressure, Endotracheal tube, Microaspiration, Ventilator-associated pneumonia
Leakage of fluid around the cuff of the endotracheal tube (ETT) into the airway is a potentially serious form of microaspiration. The cuff is designed to provide a seal with the airway, allowing airflow through the ETT but preventing passage of air or fluids around the ETT. When this seal is compromised, microaspirations contaminated with gastric contents or bacterially colonized oral secretions can occur that leave the patient susceptible to a host of problems, such as hypoxia, pneumonitis, and respiratory infections.
Hypoxic events have been shown to accompany aspiration in mechanically ventilated patients.1 Pneumonitis and ventilator-associated tracheobronchitis (VAT) can also result from aspiration of gastric contents.
VAT has been suggested to be an early stage of the ventilator-associated pneumonia (VAP) pathway.2 VAP is associated with increased duration of mechanical ventilation (MV), intensive care unit (ICU) length of stay (LOS), mortality, and overall hospital costs.3, 4
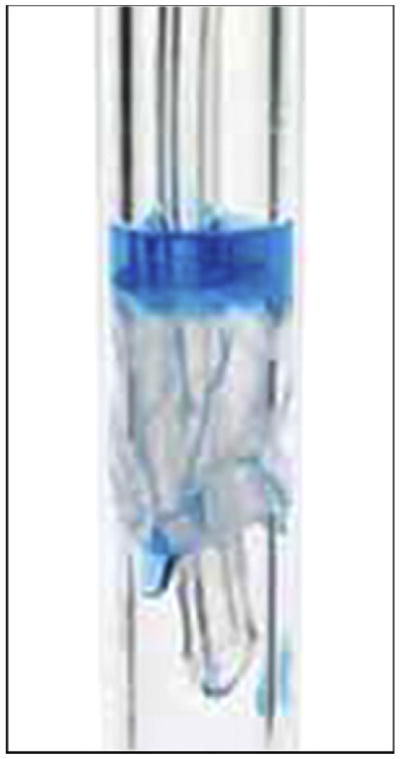
There are many factors that contribute to the development of VAP as a result of microaspiration, including the presence (or absence) of accumulated subglottic secretions above the cuff (Figure 1) and the inability of the ETT cuff to maintain a seal within the airway.5 This article will discuss the role of the ETT cuff in microaspiration and identify potential directions for future research to improve outcomes in mechanically ventilated patients.
Figure 1.
Pooled secretions above the cuff leaking into the airway. Image used by permission from Nellcor Puritan Bennett LLC, Boulder, Colorado, doing business as Covidien.
Materials and Methods
Microaspiration: Relationship to Ventilator-Associated Tracheobronchitis and Pneumonia
VAT is not uncommon among intubated acutely ill patients, 6 with a 2010 review finding an incidence as high as 11.5%.7 VAT represents an intermediate process between lower respiratory tract colonization and VAP.8 In the most recently published guidelines of the American Thoracic Society and the Infectious Disease Society of America on health care-associated pneumonia, 9 VAT is regarded as a type of lower respiratory tract infection and as an alternative diagnosis for patients with possible VAP. VAT is defined using the following criteria: fever (>38°C) with no other recognizable cause, purulent sputum production, positive culture of respiratory specimen at significant threshold, and no radiographic signs of new pneumonia.10 This infection is characterized by lower respiratory tract inflammation and increased sputum production that result in weaning difficulties and longer duration of MV.10 VAT is often the precursor to VAP. In a randomized controlled trial conducted by Nseir et al, 11 58 patients with clinically diagnosed VAT were randomly assigned to routine treatment or antibiotic therapy. A significant reduction in ICU mortality (18% vs 47%; P = .047) and VAP (13% vs 47%; P = .011) was identified in the antibiotic treatment group, indicating that intervention early in this pathway may prevent the development of VAP.
VAP is defined as a pulmonary infection that occurs 48 hours or more after endotracheal intubation (in patients with no evidence of pneumonia at the time of intubation).9 Microaspiration of subglottic contents is a contributing factor to the development of VAP.5 During MV, oral secretions move from the oral cavity and pool above the ETT cuff. Colonization of these secretions with pathogenic organisms is nearly unavoidable, and the relationship with microaspiration of these secretions and VAP is well established.12-15 Of patients requiring MV, up to 8% to 28% develop VAP.16, 17 Studies have shown that patients with VAP have significantly longer ICU LOS, 18 an additional $40,000 in hospital costs,4, 19 and mortality rates up to 76% versus 32% compared with MV patients without VAP.4, 20, 21 With increased overall costs and mortality rates, it is critical to identify all factors potentially contributing to VAP to reduce risk. Healthy People 2020 (archived health status objective 14-20e) includes a goal to reduce the incidence of VAP in ICU patients by at least 10%.22 The American Thoracic Society update of research priorities for nursing addresses an “emphasis on nursing interventions that decrease the incidence of ventilator-associated pneumonia.”23 In addition, the 5 Million Lives Campaign to reduce ICU mortality, initiated by the Institute for Healthcare Improvement, has at its core, interventions to reduce VAP, that is, the VAP bundle.24 This continued focus on VAP prevention illustrates the importance of interventions aimed at reducing risks.
Factors Affecting Microaspiration
Subglottic Secretions
One of the primary preventive measures for VAP includes reducing the risk of aspirating oral secretions by preventing accumulation of bacteria-laden secretions above the ETT cuff or minimizing leakage between the cuff and the tracheal wall. Four randomized prospective studies have evaluated the effect of removal of the secretions via intermittent or continuous aspiration of subglottic secretions (CASS) on the development of VAP.12, 13, 15, 25 In all 4 studies, the incidence of VAP was reduced. In 101 randomized patients, Yang et al26 demonstrated that those with CASS had significantly lower morbidity of VAP (25% vs 46.5%, P = .032) and delay of onset of VAP (7.3 ± 4.2 days vs 5.1 ± 3.0 days, P = .100). In another randomized study that spanned 2 years and included 714 patients undergoing cardiac surgery, subglottic secretion removal demonstrated a significant reduction in the incidence of VAP, ICU LOS, antibiotic use, and overall mortality.27 Evidence showing that removal of subglottic secretions reduces the incidence of VAP suggests that reducing microaspiration is an appropriate and effective method to also reduce VAP.
Endotracheal Tube Cuff Pressure
To prevent potential microaspirations around the ETT cuff, maintenance of adequate cuff pressure is essential.28-30 Although sealing the airway around the ETT cuff by high inflation pressure would be effective, high pressures threaten perfusion and integrity of the tracheal mucosa.28, 31 Excessive pressure may compromise the microcirculation of the tracheal mucosa and cause ischemic lesions.29, 32 ETT cuff pressure is recommended to be maintained within 20 to 30 cm H2O to provide an adequate seal without compromising mucosal perfusion.5, 33 However, insufficient cuff pressure impedes ventilation with positive pressure and may allow the passage of subglottic secretions.34 Rello et al35 analyzed the effect of cuff pressure on the development of VAP in 83 patients undergoing CASS and showed that CASS failure (when subglottic secretions were not continuously removed) and persistent intracuff pressures less than20cmH2Owere associated with a significantly increased risk of pneumonia. They demonstrated that leakage around the cuff is the risk factor of greatest importance for VAP within the first 8 days of MV.
Valencia and colleagues36 assessed the efficacy of an automatic ETT cuff pressure control device in optimizing cuff pressures and preventing VAP development. MV patients (n=142) with no prior diagnosis of pneumonia were randomly assigned to the treatment group (continuous cuff pressure control) or control group (routine ETT cuff care), with the end point being the incidence of VAP. Investigators were able to maintain ETT cuff pressures within target range (20-30 cm H2O) in approximately 80% of the treatment group. Only .7% of the treatment group patients had cuff pressures less than 20 cm H2O compared with approximately 45.3% in the control group (P=.001). Although investigators did not demonstrate a statistically significant difference in the pneumonia rate between the groups (29% control vs 22% automatic cuff), a few possible explanations exist for this finding.
Automatic devices might generally maintain cuff pressure well, but the response time during a coughing episode might be too slow and allow a compromise in airway seal for just milliseconds.36 This can lead to the unnoticed microaspiration that occurs with constant cuff pressures. Although 20 cm H2O is the commonly used pressure to provide sufficient sealing with minimal damage to mucosa, as changes occur in the airway (with movement or coughing), a constant pressure may not be adequate to ensure a constant seal. Chadha et al37 showed in a randomized controlled study in a porcine model (n = 10) that dynamic cuff pressure modulation (varying with inspiration and expiration) significantly reduced laryngotracheal mucosal injury while providing a more consistent seal with the trachea. These findings suggest that research into the effects of movement on ETT cuff pressure in MV humans is warranted.
Sole and colleagues38 demonstrated that ETT cuff pressures change over time by measuring cuff pressures of 23 intubated patients at 4-hour intervals over a 12-hour shift. All patients showed statistically significant decreases in cuff pressure at each measurement interval (4, 8, and 12 hours), with many experiencing clinically significant (>10%) decreases in pressure.38 In a multisite survey, Sole and colleagues39 found that ETT cuff pressures are typically checked, according to institutional policies, only at change of shift (every 8 to 12 hours) or as indicated by suspicion of cuff leak. Frequently estimated by palpation of the pilot balloon, ETT cuff pressure is often significantly lower than estimated by caregivers.40 These periods of insufficient pressure leave the patient susceptible to microaspiration, because secretions pooled on top of the ETT cuff may move past it. Movement of the ETT may affect cuff pressure enough to provide this avenue into the lungs.
In a 2011 crossover design study including 32 orally intubated patients, Sole and colleagues41 used alarms as triggers for intervention to correct out of range cuff pressures. They found that 51.7% of cuff pressures were out of range during the control period compared with 11.1% during the intervention (P < .001). Sole and colleagues38, 42 have shown that continuous cuff pressure monitoring is possible and may be necessary to consistently monitor and maintain optimal cuff pressure.37, 41
Folds in Endotracheal Tube Cuff
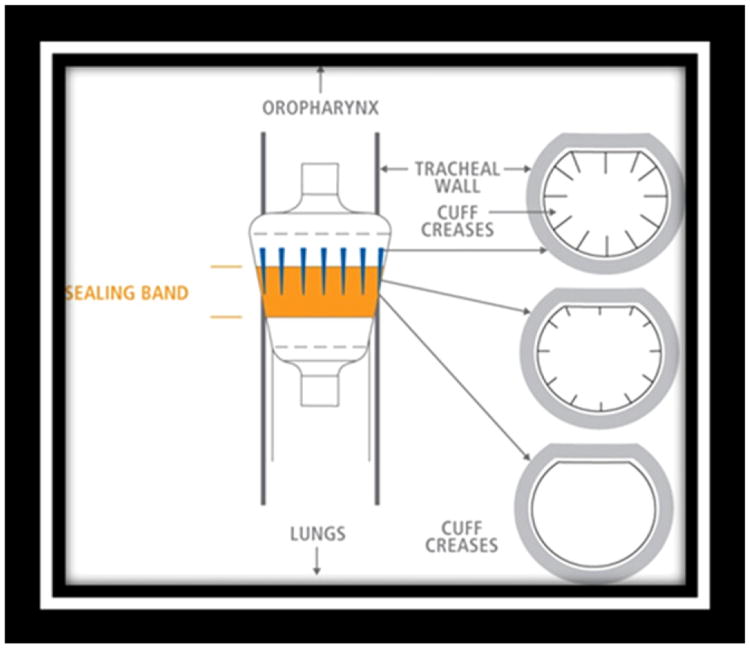
The ETT cuff is designed to provide enough pressure to seal the airway without causing perfusion problems in the tracheal mucosa. The cuff itself is made of a pliable medical grade polyvinylchloride (PVC) and forms to the airway as it is inflated.43 Because patients have physiologic and anatomic differences, it can require different amounts of air injected to reach target ETT cuff pres-sure.Asaresult, the cuff is inflated in a slightly different configuration with a potentially different volume of air with each ventilated patient. This high-volume, low-pressure (HVLP) “one size fits all” PVC cuff leaves room for an incompletely inflated cuff that, while inflated to recommended pressures, can create channels or passageways for secretions to circumvent the ETT cuff (Figure 2).43 The leakage of secretions despite using clinically appropriate ETT cuff pressure has been hypothesized to be related to the formation of folds in the cuff that allow longitudinal leakage.43-46 Movement of the ETT may exacerbate the effect of these folds, shifting them and mobilizing pooled oral secretions.
Figure 2.
Microchannels form as the result of variations in patient airways. A polyurethane cuff designed to eliminate leakage through these folds is shown. Image used by permission from Nellcor Puritan Bennett LLC, Boulder, Colorado, doing business as Covidien.
The elimination of leakage around the cuff has been achieved with the use of lubricating gels that fill the cuff channels.47, 48 Blunt and colleagues47 placed dye in the subglottic space and observed for leakage into the tracheobronchial tree in 2 different studies. In their in vitro model, leakage was 0% for cuffs treated with water-soluble gel and 100% for non-treated cuffs. In a double-blind randomized study in anesthetized patients (n=36), the lubricated group had 11% leakage and the non-lubricated group had 83% leakage (P < .0001).
Another study targeting leakage around the ETT was conducted by Young and colleagues.49 They used the dye-leakage method to test a low-volume, low-pressure (LVLP) ETT cuff in a rigid tracheal model, an in vitro pig trachea model, and a randomized controlled trial of 38 anesthetized patients. Leakage was 0% for the rigid tracheal model and the pig trachea model in the LVLP cuffs, whereas it was 100% and 79%, respectively, in the standard HVLP cuffs. In the patient trials, leakage was 5% in the LVLP group and 67% in the HVLP group.49 Because the LVLP cuff requires a lower volume, it theoretically does not form the longitudinal folds that an HVLP cuff does when inflated to seal, whereas it provides reliable pressure against the tracheal wall. However, it has not, been demonstrated whether these improvements in ETT cuff seal will lower the incidence of VAP.
The use of different types of ETT cuffs has also been investigated to reduce the incidence of cuff folds. A variety of different materials and designs have been investigated, including latex, silicone, and poly-urethane. Several studies have shown that polyurethane cuffs are superior to conventional PVC cuffs in the prevention of microaspiration.50-53
Endotracheal Tube Movement
Securement of the ETT is generally driven by institutional policy. The ETT is frequently secured with surgical tape that holds the tube at a specific depth, but does little to limit lateral movement of the tube around that site. Multiple studies have attempted to determine which method of securement is ideal, 54-57 but there is little evidence that any particular device or technique is superior to any other.58 Overall, the data suggest that it is less expensive, more convenient, and just as effective to use standard white surgical tape to secure the ETT as any other alternative method.54, 56 Unfortunately, none of these studies has examined the amount or effect of lateral and longitudinal movement of the ETT based on securement method.
When nursing care is administered, the ETT is often moved deliberately or inadvertently. The ETT is deliberately moved periodically to prevent breakdown of oral mucosa and often repositioned to improve access when providing patient care and to facilitate effective oral care. The ETT also moves when the patient is turned (manually by nursing staff or automatically by specialty mattress/bed), when the patient moves independently, or when the ventilator tubing is disturbed. Although the tube is secured well enough to maintain the depth of intubation, anecdotal evidence suggests that nurses often provide patient care without extra attention paid to lateral and rotational movement of the ETT tube. Kim et al, 59 using a fiberoptic bronchoscope, observed movement of the ETT tip in 24 anesthetized adults during flexion, extension, and rotation of the head. Although Kim et al documented measurements only related to distance from the carina, they demonstrated clear movement of the ETT during these positional changes.59 It is possible that this movement affects cuff seal, although this has not been specifically investigated. Because movement of the ETT, both deliberate and inadvertent, is frequent and often not considered during routine care, closer investigation of the potential impact of ETT movement is warranted.
Conclusions
It is clear that microaspiration continues to be a significant contributor to the development of VAP despite advances in the design and care of the ETT cuff. Although continuous removal of bacteria-laden subglottic secretions has been shown to reduce the incidence of VAP, complete removal of all potential pathogens is difficult, and removal of subglottic secretion is not included in VAP prevention bundles. It has been demonstrated that maintenance of ETT cuff pressures in the recommended target range is, at times, not adequate to prevent microaspirations. Changes in cuff pressure occur and can affect the ETT cuff's ability to maintain a seal against the tracheal wall, increasing the risk of aspirating oral secretions. Determining the role of the ETT cuff in microaspiration is the first step toward understanding how movement of the ETT itself may affect the risk of microaspiration and the subsequent development of VAP.
Directions for Future Research
Identifying and understanding actions that increase a patient's risk of life-threatening complications is only the first step in reducing risk. Research to describe the types of ETT movement and the nursing care that affects movement is currently under way. Further research into oral care interventions that reduce the level of colonization of oral secretions and studies to determine the best method of ETT securement to reduce lateral movement are areas of potential interest. Using ETTs with cuffs made of various materials or studies that test methods to reduce the impact of folds in the cuff are possible avenues to reach better patient outcomes. Future research may be directed toward nursing interventions that minimize the impact of ETT movement. Asweunderstand moreabout the impact of nursing interventions on the movement of the ETT, we can direct future research specifically to address improvement in patient outcomes.
References
- 1.Young P, Wyncoll D. PEEP is protective against pulmonary microaspiration. Crit Care Med. 2009;37:380–2. doi: 10.1097/CCM.0b013e31819325cc. [DOI] [PubMed] [Google Scholar]
- 2.Craven DE, Hjalmarson KI. Ventilator-associated tracheobronchitis and pneumonia: thinking outside the box. Clin Infect Dis. 2010;51(Suppl 1):S59–66. doi: 10.1086/653051. [DOI] [PubMed] [Google Scholar]
- 3.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33:1266–71. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 4.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33:2184–93. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- 5.Acka O. Endotracheal tube cuff leak: Can optimum managment of cuff pressure prevent pneumonia? Crit Care Med. 2007;36:1624–6. doi: 10.1097/01.CCM.0000267654.98551.B3. [DOI] [PubMed] [Google Scholar]
- 6.Craven DE. Ventilator-associated tracheobronchitis (VAT): questions, answers, and a new paradigm? Crit Care. 2008;12:157. doi: 10.1186/cc6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrafiotis M, Siempos II, Falagas ME. Frequency, prevention, outcome and treatment of ventilator-associated tracheobronchitis: systematic review and meta-analysis. Respir Med. 2010;104:325–36. doi: 10.1016/j.rmed.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Dallas J, Skrupky L, Abebe N, Boyle WA, III, Kollef MH. Ventilator-associated tracheobronchitis in a mixed surgical and medical ICU population. Chest. 2011;139:513–8. doi: 10.1378/chest.10-1336. [DOI] [PubMed] [Google Scholar]
- 9.American Thoracic Society and Infectious Disease Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-assocated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 10.Nseir S, Ader F, Marquette CH. Nosocomial tracheobronchitis. Curr Opin Infect Dis. 2009;22:148–53. doi: 10.1097/QCO.0b013e3283229fdb. [DOI] [PubMed] [Google Scholar]
- 11.Nseir S, Favory R, Jozefowicz E, Decamps F, Dewavrin F, Brunin G, et al. Antimicrobial treatment for ventilator-associated tracheobronchitis: a randomized, controlled, multicenter study. Crit Care. 2008;12:R62. doi: 10.1186/cc6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahul P, Auboyer C, Jospe R, Ros A, Guerin C, el Khouri Z, et al. Prevention of nosocomialpneumonia in intubated patients: respective role of mechanical subglottic secretions drainage and stress ulcer prophylaxis. Intensive Care Med. 1992;18:20–5. doi: 10.1007/BF01706421. [DOI] [PubMed] [Google Scholar]
- 13.Smulders K, van der Hoeven H, Weers-Pothoff I, Vandenbroucke-Grauls C. A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation. Chest. 2002;121:858–62. doi: 10.1378/chest.121.3.858. [DOI] [PubMed] [Google Scholar]
- 14.Torres A, Serra-Batlles J, Ros E, Piera C, Puig de la Bellacasa J, Cobos A, et al. Pulmonary aspiration of gastric contents in patients receiving mechanical ventilation: the effect of body position. Ann Intern Med. 1992;116:540–3. doi: 10.7326/0003-4819-116-7-540. [DOI] [PubMed] [Google Scholar]
- 15.Valles J, Artigas A, Rello J, Bonsoms N, Fontanals D, Blanch L, et al. Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia. Ann Intern Med. 1995;122:179–86. doi: 10.7326/0003-4819-122-3-199502010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Chevret S, Hemmer M, Carlet J, Langer M. Incidence and risk factors of pneumonia acquired in intensive care units. Results from a multicenter prospective study on 996 patients European Cooperative Group on Nosocomial Pneumonia. Intensive Care Med. 1993;19:256–64. doi: 10.1007/BF01690545. [DOI] [PubMed] [Google Scholar]
- 17.National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;19:470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 18.Milbrandt EB, Kersten A, Rahim MT, Dremsizov TT, Clermont G, Cooper LM, et al. Growth of intensive care unit resource use and its estimated cost in Medicare. Crit Care Med. 2008;36:2504–10. doi: 10.1097/CCM.0b013e318183ef84. [DOI] [PubMed] [Google Scholar]
- 19.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care–associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53(RR-3):1–36. [PubMed] [Google Scholar]
- 20.Cook DJ, Kollef MH. Risk factors for ICU-acquired pneumonia. JAMA. 1998;279:1605–6. doi: 10.1001/jama.279.20.1605. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest. 2001;120:555–61. doi: 10.1378/chest.120.2.555. [DOI] [PubMed] [Google Scholar]
- 22.HealthyPeople.gov: Health-Care Associated Infections. Available at: http://www.healthypeople.gov/2010/document/html/objectives/14-20.htm.
- 23.Larson JL, Ahijevych K, Gift A, Hoffman L, Janson SL, Lanuza DM, et al. American Thoracic Society statement on research priorities in respiratory nursing. Am J Respir Crit Care Med. 2006;174:471–8. doi: 10.1164/rccm.200409-1300ST. [DOI] [PubMed] [Google Scholar]
- 24.Institute for Healthcare Improvement: Campaign-Overview. Available at: http://www.ihi.org/knowledge/Pages/Tools/HowtoGuidePreventVAP.aspx.
- 25.Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–8. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 26.Yang CS, Qiu HB, Zhu YP, Huang YZ, Xu XT, Gao L. Effect of continuous aspiration of subglottic secretions on the prevention of ventilator-associated pneumonia in mechanically ventilated patients: a prospective, randomized, controlled clinical trial. Zhonghua Nei Ke Za Zhi. 2008;47:625–9. [PubMed] [Google Scholar]
- 27.Bouza E, Perez MJ, Munoz P, Rincon C, Barrio JM, Hortal J. Continuous aspiration of subglottic secretions in the prevention of ventilator-associated pneumonia in the postoperative period of major heart surgery. Chest. 2008;134:938–46. doi: 10.1378/chest.08-0103. [DOI] [PubMed] [Google Scholar]
- 28.Craven DE, Steger KA. Epidemiology of nosocomial pneumonia. New perspectives on an old disease. Chest. 1995;108(2 Suppl):1S–16S. doi: 10.1378/chest.108.2_supplement.1s. [DOI] [PubMed] [Google Scholar]
- 29.Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheotomy. A prospective study of 150 critically ill adult patients. Am J Med. 1981;70:65–76. doi: 10.1016/0002-9343(81)90413-7. [DOI] [PubMed] [Google Scholar]
- 30.Estes RJ, Meduri GU. The pathogenesis of ventilator-associated pneumonia: I.Mechanisms of bacterial transcolonization and airway inoculation. Intensive Care Med. 1995;21:365–83. doi: 10.1007/BF01705418. [DOI] [PubMed] [Google Scholar]
- 31.Greene KE, Peters JI. Pathophysiology of acute respiratory failure. Clin Chest Med. 1994;15:1–12. [PubMed] [Google Scholar]
- 32.Knowlson GT, Bassett HF. The pressures exerted on the trachea by endotracheal inflatable cuffs. Br J Anaesth. 1970;42:834–7. doi: 10.1093/bja/42.10.834. [DOI] [PubMed] [Google Scholar]
- 33.Nseir S, Brisson H, Marquette CH, Chaud P, DiPompeo C, Diarra M, et al. Variations in endotracheal cuff pressure in intubated critically ill patients: prevalence and risk factors. Eur J Anaesthesiol. 2009;26:229–34. doi: 10.1097/eja.0b013e3283222b6e. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez P, Ferrer M, Torres A. Prevention measures for ventilator-associated pneumonia: a new focus on the endotracheal tube. Curr Opin Infect Dis. 2007;20:190–7. doi: 10.1097/QCO.0b013e328014daac. [DOI] [PubMed] [Google Scholar]
- 35.Rello J, Sonora R, Jubert P, Artigas A, Rue M, Valles J. Pneumonia in intubated patients: role of respiratory airway care. Am J Respir Crit Care Med. 1996;154:111–5. doi: 10.1164/ajrccm.154.1.8680665. [DOI] [PubMed] [Google Scholar]
- 36.Valencia M, Ferrer M, Farre R, Navajas D, Badia JR, Nicolas JM, et al. Automatic control of tracheal tube cuff pressure in ventilated patients in semirecumbent position: a randomized trial. Crit Care Med. 2007;35:1543–9. doi: 10.1097/01.CCM.0000266686.95843.7D. [DOI] [PubMed] [Google Scholar]
- 37.Chadha NK, Gordin A, Luginbuehl I, Patterson G, Campisi P, Taylor G, et al. Automated cuff pressure modulation: a novel device to reduce endotracheal tube injury. Arch Otolaryngol Head Neck Surg. 2011;137:30–4. doi: 10.1001/archoto.2010.228. [DOI] [PubMed] [Google Scholar]
- 38.Sole ML, Aragon D, Bennett M, Johnson RL. Continuous measurement of endotracheal tube cuff pressure: how difficult can it be? AACN Adv Crit Care. 2008;19:235–43. doi: 10.1097/01.AACN.0000318126.79630.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sole ML, Byers JF, Ludy JE, Zhang Y, Banta CM, Brummel K. A multisite survey of suctioning techniques and airway management practices. Am J Crit Care. 2003;12:220–30. [PubMed] [Google Scholar]
- 40.Janossy KM, Pullen J, Young D, Bell G. The effect of pilot balloon design on estimation of safe tracheal tube cuff pressure. Anaesthesia. 2010;65:785–91. doi: 10.1111/j.1365-2044.2010.06413.x. [DOI] [PubMed] [Google Scholar]
- 41.Sole ML, Su X, Talbert S, Penoyer DA, Kalita S, Jimenez E, et al. Evaluation of an intervention to maintain endotracheal tube cuff pressure within therapeutic range. Am J Crit Care. 2011;20:109–18. doi: 10.4037/ajcc2011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sole ML, Penoyer DA, Su X, Jimenez E, Kalita SJ, Poalillo E, et al. Assessment of endotracheal cuff pressure by continuous monitoring: a pilot study. Am J Crit Care. 2009;18:133–43. doi: 10.4037/ajcc2009441. [DOI] [PubMed] [Google Scholar]
- 43.Young PJ, Rollinson M, Downward G, Henderson S. Leakage of fluid past the tracheal tube cuff in a benchtop model. Br J Anaesth. 1997;78:557–62. doi: 10.1093/bja/78.5.557. [DOI] [PubMed] [Google Scholar]
- 44.Pavlin EG, VanNimwegan D, Hornbein TF. Failure of a high-compliance low-pressure cuff to prevent aspiration. Anesthesiology. 1975;42:216–9. doi: 10.1097/00000542-197502000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Ouanes I, Lyazidi A, Danin PE, Rana N, Di Bari A, Abroug F, et al. Mechanical influences on fluid leakage past the tracheal tube cuff in a benchtop model. Intensive Care Med. 2011;37:695–700. doi: 10.1007/s00134-011-2145-0. Epub 2011 Feb 12. [DOI] [PubMed] [Google Scholar]
- 46.Seegobin RD, van Hasselt GL. Aspiration beyond endotracheal cuffs. Can Anaesth Soc J. 1986;33(3 Pt 1):273–9. doi: 10.1007/BF03010737. [DOI] [PubMed] [Google Scholar]
- 47.Blunt MC, Young PJ, Patil A, Haddock A. Gel lubrication of the tracheal tube cuff reduces pulmonary aspiration. Anesthesiology. 2001;95:377–81. doi: 10.1097/00000542-200108000-00019. [DOI] [PubMed] [Google Scholar]
- 48.Dave MH, Koepfer N, Madjdpour C, Frotzler A, Weiss M. Tracheal fluid leakage in benchtop trials: comparison of static versus dynamic ventilation model with and without lubrication. J Anesth. 2010;24:247–52. doi: 10.1007/s00540-010-0871-z. [DOI] [PubMed] [Google Scholar]
- 49.Young PJ, Pakeerathan S, Blunt MC, Subramanya S. A low-volume, low-pressure tracheal tube cuff reduces pulmonary aspiration. Crit Care Med. 2006;34:632–9. doi: 10.1097/01.CCM.0000201406.57821.5B. [DOI] [PubMed] [Google Scholar]
- 50.Lorente L, Lecuona M, Jimenez A, Mora ML, Sierra A. Influence of an endotracheal tube with polyurethane cuff and subglottic secretion drainage on pneumonia. Am J Respir Crit Care Med. 2007;176:1079–83. doi: 10.1164/rccm.200705-761OC. [DOI] [PubMed] [Google Scholar]
- 51.Miller MA, Arndt JL, Konkle MA, Chenoweth CE, Iwashyna TJ, Flaherty KR, et al. A polyurethane cuffed endotracheal tube is associated with decreased rates of ventilator-associated pneumonia. J Crit Care. 2011;26:280–6. doi: 10.1016/j.jcrc.2010.05.035. Epub 2010 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nseir S, Zerimech F, De JJ, Alves I, Balduyck M, Durocher A. Impact of polyurethane on variations in tracheal cuff pressure in critically ill patients: a prospective observational study. Intensive Care Med. 2010;36:1156–63. doi: 10.1007/s00134-010-1892-7. [DOI] [PubMed] [Google Scholar]
- 53.Poelaert J, Depuydt P, De WA, Van d V, Herck I, Blot S. Polyurethane cuffed endotracheal tubes to prevent early postoperative pneumonia after cardiac surgery: a pilot study. J Thorac Cardiovasc Surg. 2008;135:771–6. doi: 10.1016/j.jtcvs.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 54.Barnason S, Graham J, Wild MC, Jensen LB, Rasmussen D, Schulz P, et al. Comparison of two endotracheal tube securement techniques on unplanned extubation, oral mucosa, and facial skin integrity. Heart Lung. 1998;27:409–17. doi: 10.1016/s0147-9563(98)90087-5. [DOI] [PubMed] [Google Scholar]
- 55.Carlson J, Mayrose J, Krause R, Jehle D. Extubation force: tape versus endotracheal tube holders. Ann Emerg Med. 2007;50:686–91. doi: 10.1016/j.annemergmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Farbod F, Tuli P, Robertson BF, Jackson IT. Endotracheal tube fixation methods for optimal stability: a comparison of adhesive tape, suture, and tape-suture fixation. J Craniofac Surg. 2010;21:1250–1. doi: 10.1097/SCS.0b013e3181e20860. [DOI] [PubMed] [Google Scholar]
- 57.Kaplow R, Bookbinder M. A comparison of four endotracheal tube holders. Heart Lung. 1994;23:59–66. [PubMed] [Google Scholar]
- 58.Gardner A, Hughes D, Cook R, Henson R, Osborne S, Gardner G. Best practice in stabilisation of oral endotracheal tubes: a systematic review. Aust Crit Care. 2005;18. 158:160–5. doi: 10.1016/s1036-7314(05)80029-3. [DOI] [PubMed] [Google Scholar]
- 59.Kim JT, Kim HJ, Ahn W, Kim HS, Bahk JH, Lee SC, et al. Head rotation, flexion, and extension alter endotracheal tube position in adults and children. Can J Anaesth. 2009;56:751–6. doi: 10.1007/s12630-009-9158-y. [DOI] [PubMed] [Google Scholar]