Abstract
The spinal cord of rats contains the sexually dimorphic motoneurons of the spinal nucleus of the bulbocavernosus (SNB). In males, SNB dendrites fail to grow after castration, but androgen or estrogen treatment supports dendritic growth in castrated males. Estrogenic support of SNB dendrite growth is mediated by estrogen receptors (ER) in the target muscle. ERα expression in cells lacking a basal lamina (referred to as “extra-muscle fiber cells”) of the SNB target musculature coincides with the period of estrogen-dependent SNB dendrite growth. In the SNB target muscle, extra-muscle fiber ERα expression declines with age and is typically absent after postnatal (P) day 21 (P21). Given that estradiol downregulates ERα in skeletal muscle, we tested the hypothesis that depleting gonadal hormones would prevent the postnatal decline in ERα expression in the SNB target musculature. We castrated male rats at P7 and assessed ERα immunolabeling at P21; ERα expression was significantly greater in castrated males compared with normal animals. Because ERα expression in SNB target muscles mediates estrogen-dependent SNB dendrogenesis, we further hypothesized that the castration-induced increase in muscle ERα would heighten the estrogen sensitivity of SNB dendrites. Male rats were castrated at P7 and treated with estradiol from P21 to P28; estradiol treatment in castrates resulted in dendritic hypertrophy in SNB motoneurons compared with normal males. We conclude that early castration results in an increase in ERα expression in the SNB target muscle, and this upregulation of ERα supports estrogen sensitivity of SNB dendrites, allowing for hypermasculinization of SNB dendritic arbors.
Indexing terms: gonadal hormones, motoneurons, dendrites, spinal cord, rat
INTRODUCTION
The lumbar spinal cord of rats contains a sexually dimorphic population of motoneurons, the spinal nucleus of the bulbocavernosus (SNB), also known as the dorsomedial nucleus (DM; Schrøder, 1980). In male rats, the SNB consists of ~200 motoneurons that innervate the perineal muscles bulbocavernosus (BC), levator ani (LA) and the anal sphincter (Breedlove and Arnold, 1980; Schrøder, 1980; McKenna and Nadelhaft, 1986). The BC/LA muscles attach to the base of the penis, and are necessary for successful copulation and insemination (Sachs, 1982; Hart and Melese-D'Hospital, 1983). In female rats, the perineal musculature is absent or greatly reduced and the SNB consists of only ~60 motoneurons that predominantly innervate the external anal sphincter (Hayes, 1965; Čihák et al., 1970; McKenna and Nadelhaft, 1986; Tobin and Joubert, 1991). The morphology of SNB motoneurons is also sexually dimorphic, and SNB somata of adult females are typically half as large as those of adult males (Breedlove and Arnold, 1981; McKenna and Nadelhaft, 1986).
The dimorphisms in the SNB neuromuscular system result from the actions of gonadal steroids, operating both developmentally and in adulthood. During perinatal development, androgens are responsible for the prevention of normally occurring motoneuron death (Nordeen et al., 1985), somal growth (Lee et al., 1989; Goldstein et al., 1990; Goldstein and Sengelaub, 1992), perineal musculature retention (Čihák et al., 1970), and neuromuscular synapse elimination (Jordan et al., 1989a; Jordan et al., 1989b). Conversion of testosterone to estrogenic metabolites does not appear to be involved in these androgen-dependent events, and treatment with estradiol has no effect on SNB motoneuron survival (Breedlove et al., 1982; Goldstein et al., 1990), somal growth (Breedlove et al., 1982; Goldstein and Sengelaub, 1994; Burke et al., 1997; Hebbeler et al., 2002), target muscle retention (Breedlove et al., 1982; Burke et al., 1997), or neuromuscular synapse elimination (Jordan et al., 1995).
Conversely, dendritic development in the SNB is regulated by both androgens and estrogens. During typical development, SNB dendrites grow profusely during the first 4 postnatal weeks (Goldstein et al., 1990). This growth is steroid-dependent: In males castrated 7 days after birth, dendrites fail to grow beyond their precastration lengths, whereas dendritic lengths of castrated males receiving testosterone replacement are not different from those of normal males by 4 weeks of age (Goldstein et al., 1990). Treatment with either of testosterone’s metabolites, dihydrotestosterone or estradiol, partially supports dendritic growth (Goldstein and Sengelaub 1994), but combined treatment with both metabolites is as effective as testosterone in fully supporting dendritic growth (Goldstein and Sengelaub, 1994). Estrogenic support of postnatal dendritic growth is mediated by estrogen action at the neuromuscular periphery, as local blockade of estrogen receptors (ERs) with tamoxifen implants at the SNB target musculature during the period of dendritic growth results in significantly decreased dendritic lengths, but placing similar implants at the scapula (to rule out systemic effects) has no effect (Nowacek and Sengelaub, 2006). Treating castrated males with estradiol implants at the BC muscle supports SNB dendrite growth, indicating that estradiol action at the periphery during the early postnatal period is critical for SNB dendrite growth (Nowacek and Sengelaub, 2006). Together, these data demonstrate that estrogen-dependent SNB dendrogenesis occurs through estradiol action at ERs in the SNB target muscle.
Whereas estradiol promotes SNB dendrite growth during early development, it is ineffective in supporting dendritic morphology in adulthood (Verhovshek et al., 2010a). Recent work has demonstrated that ERα expression in BC extra-muscle fiber cells coincides with the period for estrogen-dependent dendrite growth in the SNB, and potentially mediates the estrogen sensitivity of SNB morphology (Rudolph and Sengelaub, 2013). ERα expression in extra-muscle fiber cells of the SNB target muscle could regulate the estrogen sensitivity of SNB dendrites: extra-muscle fiber ERα is high during the first few weeks of life, declining through P21, and is reduced or absent in adulthood, when SNB dendrites are unresponsive to estrogens. Treatment of castrated males with estradiol restricted to the period of elevated ERα expression in BC extra-muscle fiber cells (P7–P21) results in full masculinization of SNB dendritic morphology at P28 (Rudolph and Sengelaub, 2013). Therefore, it appears that ERα expression in extra-muscle fiber cells is critical for estrogen-regulated masculinization of SNB motoneuron morphology during development. Similarly, the masculinization of SNB motoneuron number is thought to be mediated by hormone action at non-muscle fiber cells in the target musculature (Niel et al., 2009). These data indicate a clear role for gonadal hormone receptor expression in extra-muscle fiber cells of the SNB target musculature in the developmental masculinization of SNB motoneurons.
The developmental expression patterns of ERα in BC muscle coincide with the transient period of estrogen sensitivity of SNB dendrites (Rudolph and Sengelaub, 2013). However, it is unclear why levels of ERα in extra-muscle fiber cells decrease during the postnatal period. It is possible that ERα in BC extra-muscle fiber cells is downregulated by gonadal hormones. In female rat dorsal root ganglia, ER mRNA levels are low during proestrus (when circulating estrogens are elevated; Sohrabji et al., 1994). Levels of ER mRNA are high after removal of estrogens by ovariectomy, and estrogen treatment of ovariectomized animals reduces ER mRNA to levels of those found in proestrus animals (Sohrabji et al., 1994). Similarly, ovariectomy-induced increases in ERα mRNA and protein occur in skeletal muscles of female mice (Baltgavis et al., 2010). ERα mRNA in soleus and extensor digitorum longus muscles and ERα protein in tibialis anterior muscle increase significantly after castration, and either acute (48 hour) or chronic (21 day) estradiol treatment in castrates returns muscle ERα levels to those of gonadally intact animals (Baltgavis et al., 2010). Together, these data suggest that developmental downregulation of extra-muscle fiber cell ERα in the SNB target muscle is potentially caused by the presence of gonadal hormones. We hypothesized that castration would result in an attenuation of the developmental decrease in ERα in the SNB target musculature. In Experiment 1, we castrated male rats on P7 and measured ERα immunolabeling in the BC muscle at P21 and compared this immunolabeling to that of gonadally intact P21 males.
Experiment 1
MATERIALS AND METHODS
Animals
Untimed pregnant rat dams (Sprague-Dawley, Harlan, Indianapolis, IN) were maintained on a 12-h light, 12-h dark cycle, with unlimited access to food and water. When pregnant females gave birth (day of birth = P1), pups were sexed and litters were culled to eight pups when necessary, retaining males preferentially. One group of males remained gonadally intact (n = 6) and another group was bilaterally castrated under isofluorane anesthesia at P7 (n = 6). All procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Bloomington Institutional Animal Care and Use Committee.
Immunohistochemistry
Immunohistochemistry was performed according to the methods used previously (Rudolph and Sengelaub, 2013). At P21 animals were weighed, given an overdose of urethane (0.5 ml/100 g of body weight), and perfused transcardially with 0.9% saline followed by cold 4% paraformaldehyde in 0.1M phosphate buffer (pH = 7.4). The BC/LA muscles were removed and postfixed in the same fixative for 24 h and transferred to 30% sucrose in 0.1M phosphate buffer for a minimum of 24 h of cryoprotection. The BC/LA was cut horizontally into 12 µm sections on a cryostat at −16°C. Sections were thaw-mounted onto gelatin-coated slides, and stored at −16°C. For immunohistochemical processing, slides were brought to room temperature, and rinsed 3 × 5 min in phosphate buffered saline (PBS, pH 7.4). After rinsing, a hydrophobic border (Super Pap Pen, Ted Pella, Inc., Redding, CA) was created around each tissue section. All incubations occurred in a humidified chamber and were performed by pipetting 200 µl of solution onto each section.
Sections were incubated for 45 min at room temperature in blocking solution containing 10% normal goat serum (NGS; Vector Laboratories, Inc., Burlingame, CA) and 0.2% Triton X-100 in PBS. Sections were then incubated for 48 h at 4°C in 4% NGS in PBS containing a purified polyclonal antibody directed against the last 15 amino acids of the C-terminus of rat ERα (C1355, 1:1000; Millipore, Temecula, CA; this antibody does not cross-react with ERβ). After primary ERα incubation, sections were rinsed in PBS and incubated for 2 h at room temperature in 4% NGS in PBS containing a conjugated secondary antibody (goat anti-rabbit Alexa Fluor 488 F(ab’)2 fragments (H+L), 1:200; Invitrogen, Eugene, OR). Sections were then rinsed in PBS, and incubated in a 4% NGS in PBS solution containing a mouse monoclonal antibody for basal lamina (D18 supernatant, 1:50; Developmental Studies Hybridoma Bank, Iowa City, IA) for 12–18 h at 4°C. Following this incubation, sections were rinsed in PBS and incubated for 2 h at room temperature in a secondary antibody (goat anti-mouse IgG-TRITC, 1:200; Sigma-Aldrich, Oakville, Ontario, Canada). Sections were then rinsed in PBS, briefly air dried, and coverslipped with aqueous mounting fluid (Vectashield HardSet Mounting Medium; Vector Laboratories, Inc.). To control for nonspecific staining, control sections incubated without both primary antibodies were generated and demonstrated no immunostaining.
Microscopic Analysis
Because all of our previous developmental work on SNB motoneuron dendritic growth, hormone sensitivity, critical period effects, and the characterization of developmental changes in ERα was done in BC-projecting motoneurons, the current analysis was limited to the BC muscle. Sections were first viewed under epifluorescent illumination to visualize basal lamina staining. The BC is a relatively complex muscle, with muscle fibers traveling in several different directions, resulting in fields containing both cross- and longitudinally-sectioned muscle fibers (Fargo et al., 2003); fields containing large numbers of basal lamina-stained muscle fibers in cross-section were selected for analysis. Using Stereo Investigator (MBF Bioscience, Inc., Williston, VT), muscle fibers were sampled systematically at 2200× (final magnification on display), by superimposing a grid on the tissue image (e.g., 75 µm × 75 µm square frame) and sampling all fibers within the frame; only fields containing a minimum of 23 muscle fibers were examined.
To quantify ERα immunostaining, muscle fibers were visualized under FITC fluorescence and images were captured for subsequent analysis. Areas that appeared to be labeled relative to background (see below) were identified as either in-fiber or extra-fiber depending on the location of ERα label relative to the basal lamina stain. One field per section and 1–2 sections per animal were analyzed; overall, an average 76.5 muscle fibers per animal were traced. For each analyzed tissue section, the mean and standard deviation of muscle fiber FITC luminance values were calculated and a minimum labeling criterion of the mean plus two standard deviations was established for each section. Cells that exceeded this criterion were identified as ERα-positive and were categorized as either ERα-positive muscle fibers (if surrounded by a basal lamina) or ERα-positive extra-muscle fiber cells. We sampled equivalent numbers of muscle fibers across groups and expressed ERα-positive muscle fibers as a percentage of the sample. ERα-positive extra-muscle fiber cells were identified only on the basis of ERα labeling, and their numbers were expressed as a density per unit area or as a ratio relative to the number of muscle fibers sampled. The percentage of ERα-positive muscle fibers and density of ERα-positive extra-muscle fiber cells per section were analyzed using one-way ANOVAs with Fisher’s LSD post-hoc tests. Differences were considered significant if p < 0.05.
RESULTS
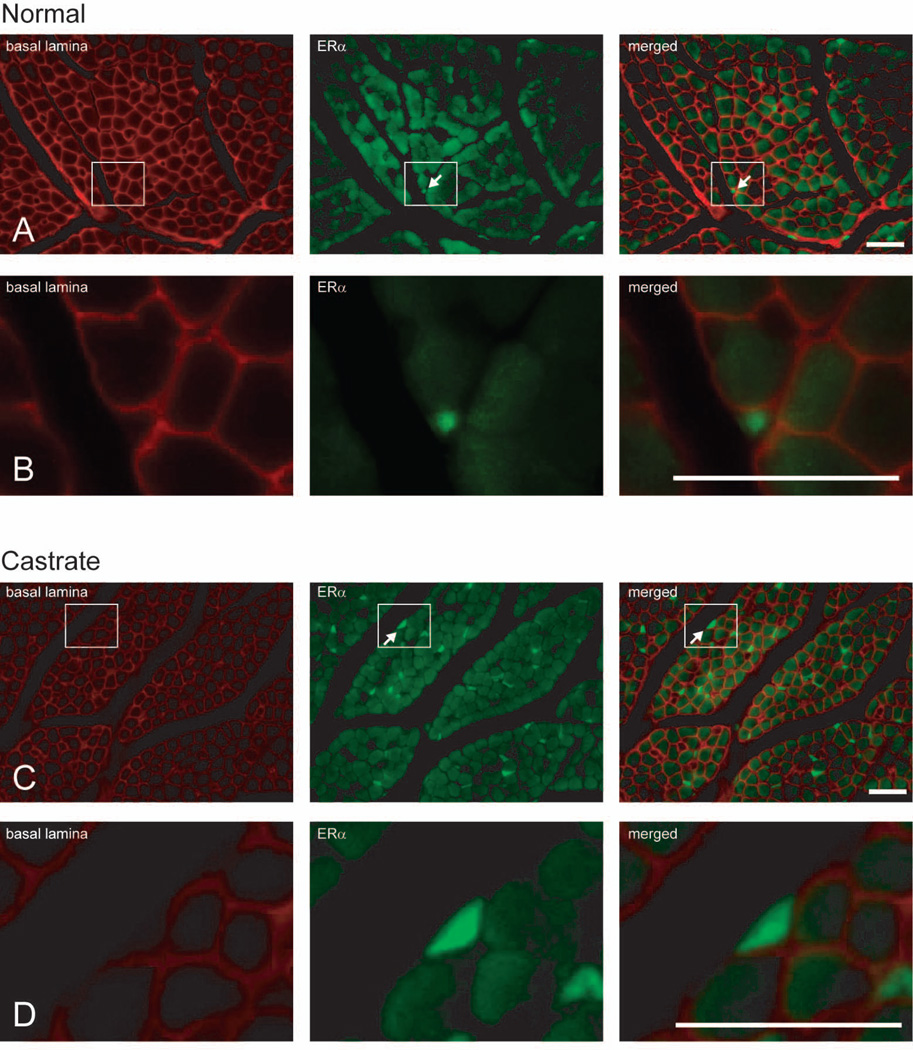
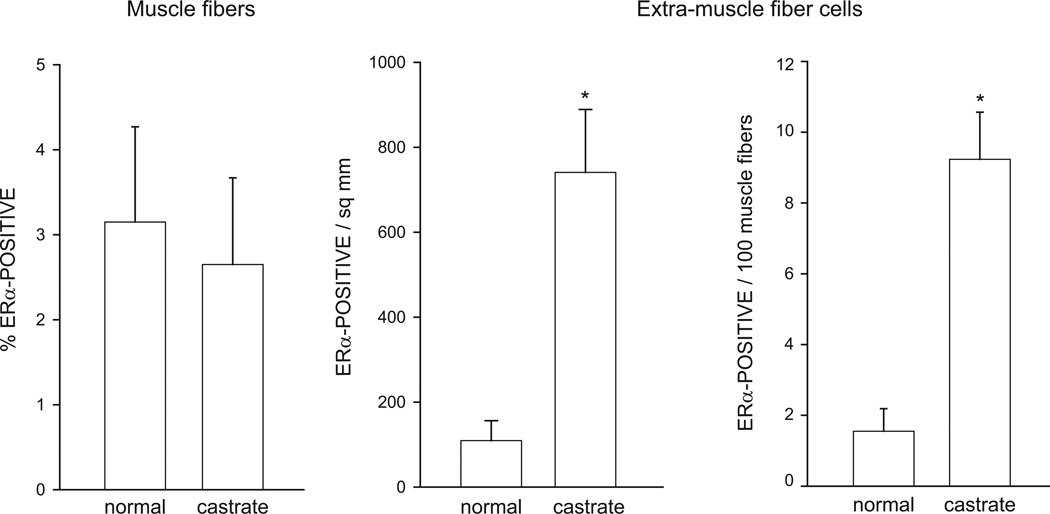
As reported previously (Rudolph and Sengelaub, 2013), ERα immunolabeling was present in both muscle fibers (label contained within the basal lamina stain) and extra-muscle fiber cells (label outside basal lamina stain; Fig. 1). The average number of muscle fibers analyzed per animal did not differ between groups [F(1,10) = 0.25, n.s.]. For each animal, the number of labeled muscle fibers was expressed as a percentage of total muscle fibers sampled. The percentage of ERα-positive muscle fibers did not differ between normal (3.15 ± 1.12%) and castrated males [2.65 ± 1.02%; F(1,10) = 0.11, n.s.; Fig. 2].
Figure 1.
Digital light micrographs of cross-sections of BC muscle in normal (A, B) and castrated (C, D) male rats at P21; higher magnification views (B, D) of indicated areas. Each row shows the same tissue section viewed under different epifluorescent illumination, demonstrating immunolabeling for basal lamina only (red; left), ERα only (green; middle), and the merged image showing combined basal lamina and ERα immunolabeling (right). Arrows indicate ERα-positive extra-muscle fiber cells; note the absence of basal lamina staining of the indicated extra-muscle fiber cells in rows B, D. Scale bars = 25 µm.
Figure 2.
The percentage of ERα-positive muscle fibers (left), density of ERα-positive extra-muscle fiber cells (middle), and ratio of ERα-positive extra-muscle fiber cells to muscle fibers in the BC muscle at P21 in normal males and males castrated at P7. Bar heights represent means ± SEM for six animals per group. * denotes significantly different from normal males.
There was a significant main effect of castration on the density of ERα-positive extra-muscle fiber cells. ERα expression in extra-muscle fiber cells was significantly greater in castrated animals (740.74 ± 148.15 per mm2) compared to that of normal animals (109.26 ± 46.83 per mm2); [F(1,10) = 16.52, p < 0.05; Fig. 2]. Similarly, the number of ERα-positive extra-muscle fiber cells relative to the number of muscle fibers sampled was significantly increased in castrated animals (8.67 ± 1.25 per 100 muscle fibers) compared to that of normal animals (1.47 ± 0.60 per 100 muscle fibers); [F(1,10) = 27.17, p < 0.01; Fig. 2]. Changes in androgens during the postnatal period examined in this experiment have no effect on muscle weight (Hebbeler and Sengelaub, 2003; Experiment 2, current study) or fiber number in the SNB target musculature (Tobin and Joubert, 1991). Thus the increase in ERα-positive extra-muscle fiber cells as a function of area or relative to the number of muscle fibers sampled is not likely a result of changes in BC/LA size or fiber number.
Experiment 2
In Experiment 1, we found that the density of ERα-positive BC extra-muscle fiber cells at P21 was significantly greater in animals castrated at P7 compared with normal males. Based on these results, and earlier data demonstrating that extra-muscle fiber ERα expression coincides with the critical period for estrogen-dependent SNB dendrite growth, we hypothesized that the elevated ERα expression in BC extra-muscle fiber cells in castrated animals would result in heightened estrogen sensitivity of SNB dendrites. To test this hypothesis, in Experiment 2 we assessed SNB dendritic morphology at P28 in normal males, untreated castrates, castrates treated with estradiol implants from P21 to P28, and normal males treated with estradiol implants from P21 to P28.
MATERIALS AND METHODS
Animals
Untimed pregnant rat dams (Sprague-Dawley; Harlan, Indianapolis, IN) were maintained on a 12-h light, 12-h dark cycle, with unlimited access to food and water. Litters were culled to eight pups when necessary, retaining males preferentially. Males were bilaterally castrated under isofluorane anesthesia at P7. One group of castrates had estradiol implants placed at the left BC muscle at P21, remaining until P28 (n = 7); another group of castrates remained untreated (n = 7). Age-matched normal males were also included, one group was left untreated (n = 5) and another group received estradiol implants at the left BC muscle from P21 to P28 (n = 5; overall n = 24). All procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Bloomington Institutional Animal Care and Use Committee.
Implants
To restrict hormone treatment to the SNB target musculature, we used our previous method (Nowacek and Sengelaub, 2006; Rudolph and Sengelaub, 2013) to deliver estradiol locally to the BC muscle. This treatment produces alterations in motoneuron morphology that can be directly ascribed to local rather than systemic effects; furthermore, the treatment has no effect on the typical development of SNB motoneurons, and no effect on dendritic labeling or tracer transport (Nowacek and Sengelaub, 2006; Rudolph and Sengelaub, 2013). Small (0.85 mm3) implants impregnated with estradiol (Steraloids, Newport, RI) were constructed by mixing crystalline estradiol with Silastic adhesive (Dow-Corning, Auburn, MI) and compressing the mixture between two glass slides (lightly greased with petroleum jelly) separated by a spacer (0.85 mm thick), and allowed to cure. The cured Silastic was cut into 1 × 1 mm pieces (total volume 0.85 mm3) and coated on five sides with acrylic. The amount of estradiol in each implant (0.1 mg) was similar to that used previously; this dosage does not produce systemic effects, and supports SNB dendritic growth (Nowacek and Sengelaub, 2006).
Histochemistry
Horseradish peroxidase conjugated to the cholera toxin β subunit (BHRP; List Biological, Campbell, CA) was used to retrogradely label SNB motoneurons innervating the BC muscle. Previous studies have demonstrated that BHRP labeling permits sensitive detection and quantitative analysis of SNB somal and dendritic morphologies (Kurz et al., 1986; Goldstein and Sengelaub, 1990; Hebbeler and Sengelaub, 2003). SNB motoneuron morphology was examined at P28 (when SNB dendritic length is normally maximal; Goldstein et al., 1990). At P26, animals were anesthetized with isoflurane, the perineal muscles exposed, and BHRP unilaterally injected (0.5 µl; 0.2% solution) into the left BC muscle, the same muscle for those males that received muscle implants. Forty-eight h after BHRP injection, a period that ensures optimal labeling of SNB motoneurons (Kurz et al., 1986; Goldstein et al., 1990), animals were given an overdose of urethane (0.5 ml/100 g body weight) and perfused intracardially with saline followed by cold 1% paraformaldehyde/1.25% glutaraldehyde fixative. Lumbar spinal cords were removed, postfixed in the same fixative for 5 h, and transferred to sucrose phosphate buffer (10% w/v, pH = 7.4) overnight for cryoprotection. Spinal cord segments were then embedded in gelatin, frozen, and sectioned transversely at 40 µm; all sections were collected into four alternate series. For visualization of BHRP, the tissue was immediately reacted using a modified tetramethyl benzidine protocol (TMB; Mesulam, 1982), mounted on gelatin-coated slides, counterstained with thionin, and cover-slipped with Permount. BC/LA muscles were removed at perfusion and weighed to evaluate potential treatment effects on gross muscle development.
Motoneuron Somata
The number of BHRP-filled motoneurons was assessed in all sections through the entire rostrocaudal extent of the SNB for all animals. Counts of labeled motoneurons in the SNB were made under brightfield illumination, where somata and nuclei could be visualized and cytoplasmic inclusion of BHRP reaction product confirmed. Estimates of the total number of labeled SNB motoneurons were obtained using the optical dissector method yielding an unbiased count of SNB motoneurons (Raouf et al., 2000). Counts were made at 500× motoneuron somata could be easily visualized in multiple focal planes and labeled somata in the first focal plane (i.e., “tops”) were not counted. For each animal, counts were derived from sections spaced at 160 µm intervals uniformly distributed through the entire rostrocaudal extent of the SNB. Estimates of the total number of labeled SNB motoneurons were then obtained by correcting for percentage of the tissue sampled.
The cross-sectional soma area of BHRP-labeled motoneurons was measured in an average of 22.63 motoneurons for each animal using a video-based morphometry system (Stereo Investigator; MBF Bioscience, Inc.) at a final magnification of 1350×. Soma areas within each animal were averaged for statistical analysis. The optical density of labeled somata was also measured under brightfield illumination to confirm equivalence of BHRP labeling density.
Dendritic Length
For each animal, dendritic lengths in a single representative set of alternate sections were measured under darkfield illumination. Beginning with the first section in which BHRP-labeled fibers were present, labeling through the entire rostrocaudal extent of the SNB dendritic field was assessed in every other section (320 µm apart) in three dimensions using a computer-based morphometry system (Neurolucida; MBF Bioscience, Inc.; final magnification 250×) to yield both composite illustrations of the arbor and measurements of individual fiber lengths. All BHRP-labeled fibers were drawn regardless of location, size, or contiguity with labeled cell bodies to ensure a complete assessment of dendritic length. Because the entire rostrocaudal range of the SNB dendritic field in each animal was sampled, this method allows for a complete assessment of SNB dendrites in both the transverse and horizontal planes. Average dendritic arbor per labeled motoneuron was estimated by summing the measured dendritic lengths of the series of sections, multiplying by two to correct for sampling, then dividing by the total number of labeled motoneurons in that series. This method does not attempt to assess the actual total dendritic length of labeled motoneurons (Kurz et al., 1991), but has been shown to be a sensitive and reliable indicator of changes in dendritic morphology in normal development (Goldstein et al., 1990), after hormonal or surgical manipulation (Kurz et al., 1986; Goldstein et al., 1990; Kurz et al., 1991; Goldstein and Sengelaub, 1994; Goldstein et al., 1996; Hays et al., 1996; Hebbeler and Sengelaub, 2003), due to dendritic interactions (Goldstein et al., 1993), or after NMDA receptor blockade (Hebbeler et al., 2002). Potential redistributions of dendrites as well as the comparability of BHRP labeling across treatment groups were assessed using previously described methods (Rudolph and Sengelaub, 2013).
Statistical analysis consisted of analyses of variance (one- or two-way with repeated measures) followed by appropriate planned comparisons (Fisher’s Protected LSD) as described below. Digital light micrographs were obtained using an MDS 290 digital camera system (Eastman Kodak Company, Rochester, NY). Brightness and contrast of these images were adjusted in Adobe PhotoShop (Adobe Systems, Inc., San Jose, CA).
RESULTS
BC/LA Muscle Weight
Body weights differed across groups [F(3,17) = 3.89, p < 0.05], being largest in normal males and castrated males (LSD, n.s.) and smaller in estradiol-treated animals (LSD, n.s.; Table 1). BC/LA muscle weight showed an identical pattern of differences [F(3,17) = 19.47, p < 0.01; Table 1]. Differences in muscle weight remained after correcting for differences in body weight [raw muscle weight / body weight) × 100]: BC/LA muscle weights showed a significant effect of group [F(3,17) = 22.94, p < 0.01; normal males = 0.100 ± 0.002, castrates = 0.094 ± 0.004, castrates treated with estradiol implants from P21–P28 = 0.072 ± 0.002, normal males treated with estradiol from P21–P28 = 0.077 ± 0.004]. The corrected weights of BC/LA muscles from animals treated with estradiol implants were significantly lower than those of normal and castrated males (LSDs, p < 0.01).
Table 1.
Raw body and muscle weights and number of BHRP-labeled motoneurons (Means ± SEM) in normal males, castrated males, castrated males treated with estradiol from P21–P28, and normal males treated with estradiol from P21–P28
| Normal | Castrate | Castrate + E21–28 | Normal +E21–28 | |
|---|---|---|---|---|
| Body weight (g) | 89.40 ± 5.86 | 100.25 ± 4.73 | 85.57 ± 2.07† | 79.40 ± 4.18† |
| BC/LA weight (g) | 0.091 ± 0.007 | 0.094 ± 0.005 | 0.061 ± 0.001*† | 0.060 ± 0.002*† |
| BHRP-labeled motoneurons | 60.00 ± 11.38 | 63.43 ± 7.58 | 60.57 ± 10.16 | 60.00 ± 19.8 |
Significantly different from normal males
Significantly different from castrated males
Morphometry
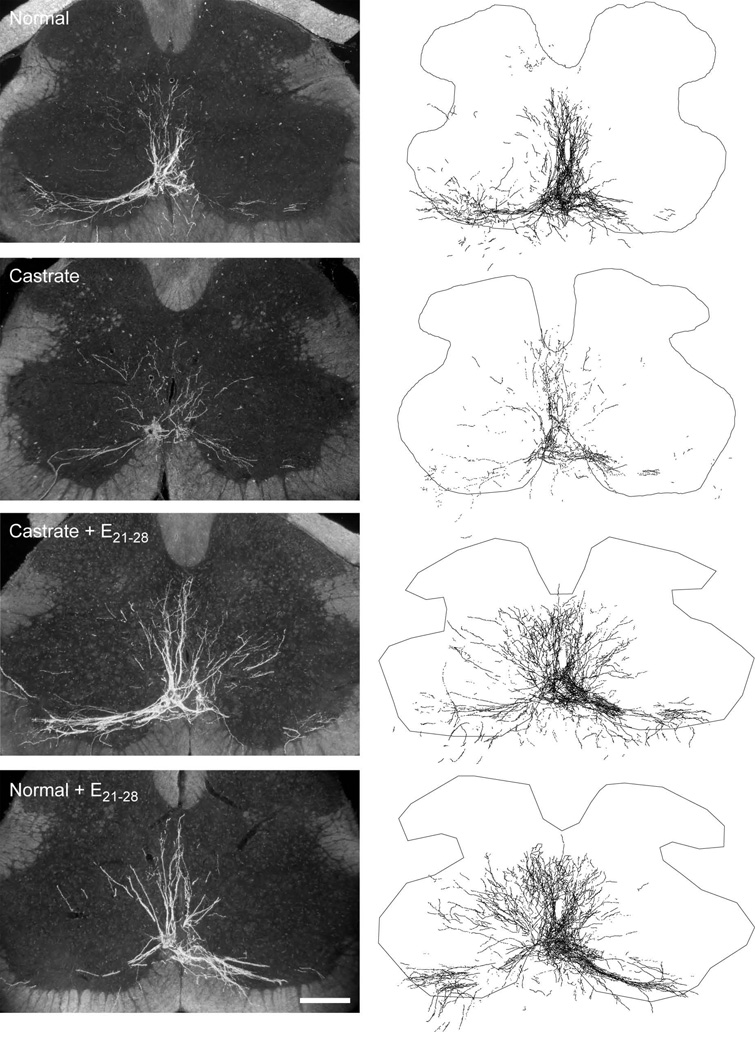
Injections of BHRP into the left BC successfully labeled ipsilateral SNB motoneurons in all animals in a manner consistent with previous studies (Kurz et al., 1986; Goldstein and Sengelaub, 1990; Goldstein and Sengelaub, 1994; Burke et al., 1997; Burke et al., 1999). SNB motoneurons displayed their characteristic multipolar morphologies, with dendritic arbors projecting ventrolaterally, dorsomedially, and across the midline into the area of the contralateral SNB (Fig. 3). The number of BHRP-labeled SNB motoneurons (61.17 ± 5.59) did not differ across groups [F(3,20) = 0.02, n.s.; Table 1].
Figure 3.
(Left) Darkfield digital micrographs of transverse sections through the lumbar spinal cord of a normal male (top), an untreated castrate (second from top), a castrate treated with an estradiol implant from P21–P28 (Castrate + E21–28; second from bottom), and a normal male treated with an estradiol implant from P21–P28 (Normal + E21–28; bottom) after BHRP injection into the left BC muscle at P28. (Right) Computer-generated composites of BHRP-labeled somata and processes drawn at 320 µm intervals through the entire rostrocaudal extent of the SNB; these composites were selected as they are representative of their respective group average dendritic lengths. Scale bar = 250 µm.
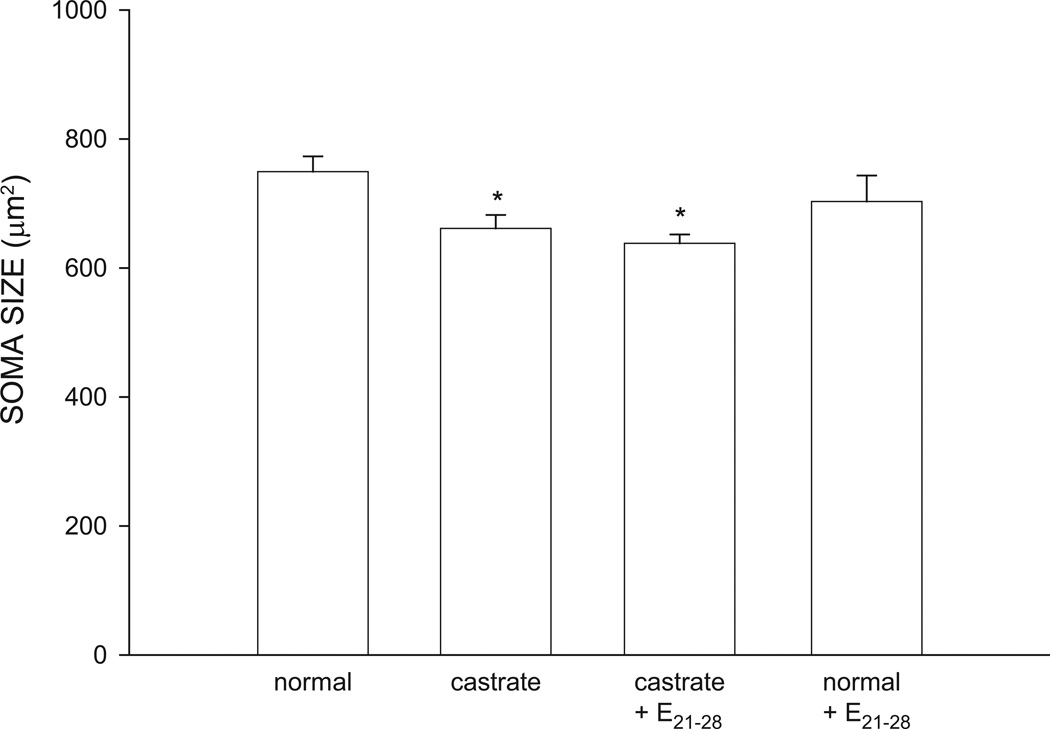
Soma Area
The size of SNB somata at P28 differed significantly across groups [F(3,20) = 3.90, p < 0.05; Fig. 4]. The mean cross-sectional area of SNB somata in normal males (749.30 ± 23.78 µm2) was typical. The somata of untreated castrates (661.15 ± 21.11 µm2, reduced 12%), or castrates treated with estradiol implants from P21–P28 (638.18 ± 13.77 µm2, reduced 15%) were both significantly smaller than those of normal males (LSDs, p < 0.05). The size of SNB somata of normal males treated with estradiol from P21–P28 (702.92 ± 40.43 µm2) were not different than that of untreated normal males (LSD; n.s.).
Figure 4.
Soma areas of SNB motoneurons at P28 for normal males, untreated castrates (castrate), castrates treated with estradiol implants from P21–P28 (castrate + E21–28), and normal males treated with estradiol implants from P21–P28 (normal + E21–28). Bar heights represent means ± SEM for four to seven animals per group. * denotes significantly different from normal males.
Dendritic Length
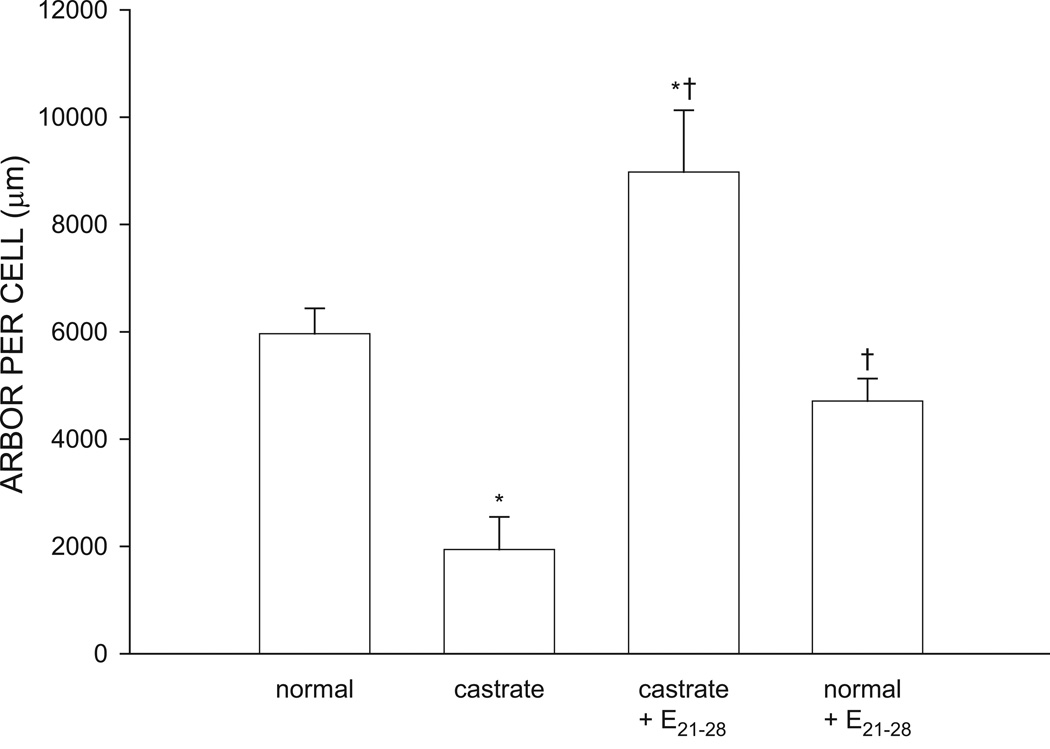
The overall length of SNB dendrites significantly differed across groups [F(3, 20) = 14.97, p < 0.01; Fig. 5]. SNB dendritic length in normal males was typical (5963.02 ± 473.75 µm), reflecting the exuberant growth that occurs over the first four postnatal weeks. Dendritic lengths in castrates were approximately 67% shorter (1943.61 ± 604.17 µm) than those of normal males (LSD, p < 0.05). Castrates treated with estradiol from P21–P28 had SNB dendritic arbors (8978.28 ± 1150.46) that were significantly greater than those of normal males and untreated castrates (LSD, p < 0.05). Dendritic lengths of normal males treated with estradiol from P21–P28 (4708.33 ± 420.29 µm) were no different from those of untreated normal males (LSD, n.s.).
Figure 5.
Dendritic lengths expressed as length of arbor per labeled SNB motoneuron at P28 for normal males, untreated castrates (castrate), castrates treated with estradiol implants from P21–P28 (castrate + E21–28), and normal males treated with estradiol implants from P21–P28 (normal + E21–28). Bar heights represent means ± SEM for four to seven animals per group. * denotes significantly different from normal males; † indicates significantly different from castrated males.
Dendritic Distribution
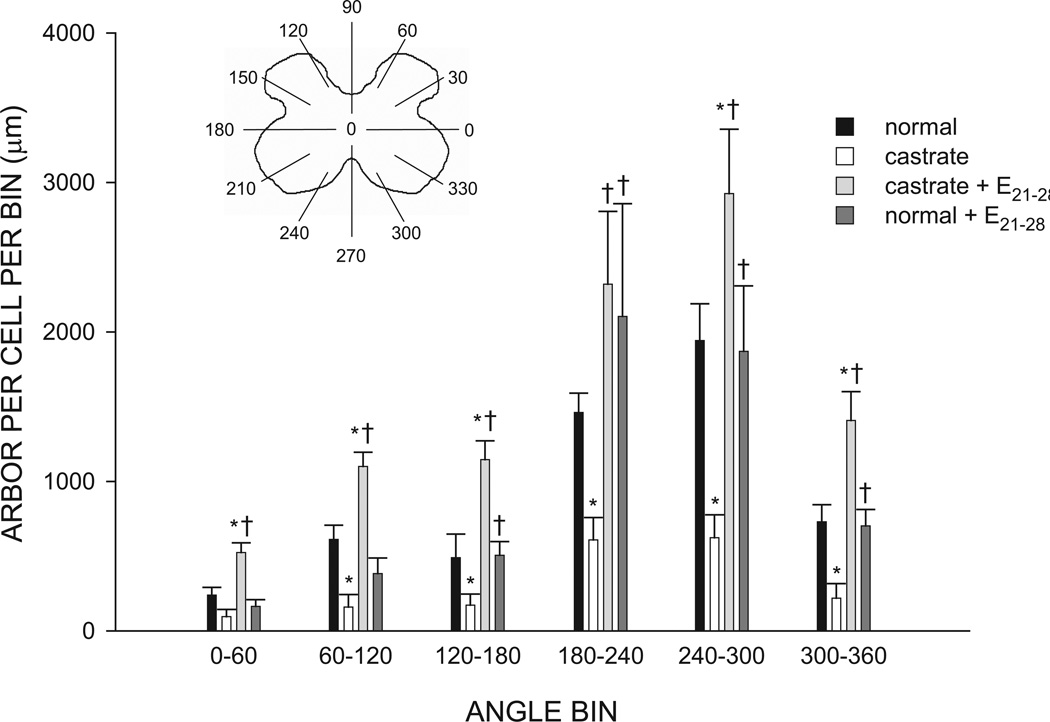
The SNB dendritic arbor of normal males is radially organized but not uniformly distributed, with over 50% of the arbor concentrated ventrolaterally between 180 and 300° (Goldstein et al., 1993). The distribution of SNB dendrites showed an effect of group [repeated measures F(3,209) = 13.03, p < 0.01], as well as the typical significant effect of location [repeated measures F(11,209) = 42.04, p < 0.01; Fig. 6], and a group by location interaction [F(33,209) = 3.12, p < 0.01]. Castration not only reduced overall dendritic length, but it did so throughout the arbor, resulting in amounts of dendritic material per bin ranging from 26 to 42% of normal male values [F(1,10) = 23.31, p < 0.05]. Treatment of castrates with estradiol from P21–P28 not only prevented reductions in dendrite lengths [F(1,11) = 35.40, p < 0.05], but resulted in dendritic hypertrophy throughout the arbor [F(1,9) = 8.30, p < 0.05], with dendritic arbors of P21–P28 estradiol-treated castrates ranging from 152–234% of those of normal males. In normal males, SNB dendritic distribution was not affected by estradiol treatment from P21–P28 [F(1,8) = 0.04, n.s.].
Figure 6.
(Inset) Schematic drawing of spinal gray matter divided into radial sectors for measure of SNB dendritic distribution. Length per radial bin of SNB dendrites at P28 for normal males, untreated castrates (castrate), castrates treated with estradiol implants from P21–P28 (castrate + E21–28), and normal males treated with estradiol from P21–P28 (normal + E21–28). For graphical purposes, dendritic length measures have been collapsed into six bins of 60° each. Bar heights represent means ± SEM for four to seven animals per group. * denotes significantly different from normal males; † indicates significantly different from castrated males.
Dendritic Extent
The distance spanned by SNB dendrites in the rostrocaudal axis showed a difference across groups [F(3,20) = 3.61, p < 0.05; normal males = 2720.00 ± 208.61 µm, castrates = 2377.14 ± 161.62 µm, castrated treated with estradiol from P21–P28 = 2971.43 ± 109.62 µm, normal males treated with estradiol from P21–P28 = 2976.00 ± 148.38 µm]. This effect of group was due to decreases in rostrocaudal extent of dendrites in castrates; rostrocaudal extent did not differ between normal males and estradiol treated animals [F(2,14) = 0.87, n.s.]. The radial extent of BHRP labeling showed an effect of group [F(3,19) = 3.79, p < 0.05]. The effect of group was due to decreases in radial extent in castrates; radial extent did not differ between normal males and estradiol-treated animals [F(2,13) = 2.73, n.s.].
DISCUSSION
Immunolabeling for ERα in the BC muscle at P21 in castrated and gonadally intact males revealed ERα expression in muscle fibers and extra-muscle fiber cells. While the percentage of ERα-positive muscle fibers was unaltered by castration, the density of extra-muscle fiber cells immunostained for ERα was significantly greater in castrates compared with gonadally intact males. The castration-induced increase in muscle ERα appears to have functional consequences for SNB morphology, as 7 days of estradiol treatment was sufficient not only to masculinize SNB dendrites at P28, but cause dendritic growth that exceeded that of normal males. These data demonstrate that gonadal hormones regulate ERα expression in the SNB target musculature, and suggest that the density of ERα in BC extra-muscle fiber cells determines the estrogen sensitivity of SNB dendrites. Furthermore, to our knowledge, this is the first demonstration that the timing of a sensitive period in sexual differentiation can be regulated by gonadal hormones.
ERα Immunolabeling
It has been known for some time that the BC/LA binds estrogens (Dube et al., 1976), and more recently that the BC muscle expresses ERα (Rudolph and Sengelaub, 2013). In our previous study describing the developmental expression of ERα in the BC muscle, we found that ERα immunolabeling in BC muscle fibers did not change across ages (Rudolph and Sengelaub, 2013). The current results demonstrate that ERα expression in BC muscle fibers at P21 is unaltered by castration on P7. This constancy in ERα expression in BC muscle fibers, regardless of age or hormone treatment, suggests that the temporally restricted effects of estrogen on SNB motoneuron morphology are not mediated by muscle fiber ERα.
Our previous work showed that ERα expression in extra-muscle fiber cells is high during the early postnatal period, decreases around P21 and is absent in adulthood (Rudolph and Sengelaub, 2013). The current study suggests a potential explanation for the downregulation of ERα-positive extra-muscle fiber cells in the BC, the presence of gonadal hormones. Consistent with the present data, in dorsal root ganglia and skeletal muscles, ER mRNA and ERα mRNA and protein are upregulated after castration, and estradiol treatment in castrates decreases ER levels to those found in gonadally intact animals (Baltgavis et al., 2010; Sohrabji et al., 1994). Future studies should determine if treatment of castrates with estradiol (or androgenic gonadal hormones) prevents the castration-induced upregulation in extra-muscle fiber ERα.
It is possible that other ER isoforms, including ERβ play a role in the estrogenic effects on SNB motoneuron dendrites. In other skeletal muscles, however, ERα appears to be the predominant form of receptor. Skeletal muscle ERα expression is 180 times greater than that of ERβ in humans (Wiik et al., 2003). In mice, ERα expression is more abundant than ERβ (Baltgavis et al., 2010), and in some cases ERβ levels are undetectable (Couse et al., 1997). Furthermore, ERβ in mouse skeletal muscle was not affected by ovariectomy with or without estradiol replacement (Baltgavis et al., 2010).
Target Musculature
BC/LA muscle development requires androgens (Čihák et al., 1970; Tobin and Joubert, 1991). During the first postnatal days the number of myotubes, mononucleate cells, and muscle fibers show an androgen-dependent increase, and androgen exposure during this time is sufficient to maintain BC/LA development through P28 (Hebbeler and Sengelaub, 2003; Tobin and Joubert, 1991). In the present study, BC/LA weights at P28 males castrated at P7 did not differ from those of normal males. Aromatase inhibition in gonadally intact males also does not affect BC/LA muscle growth, demonstrating that androgen-dependent BC/LA development is not mediated by the conversion of testosterone to its estrogenic metabolites (Burke et al., 1999). While androgens promote BC/LA development, estradiol does not appear to have any trophic effects on the BC/LA (Jordan et al., 1995). In fact, estrogen treatment in castrated males inhibits prepubertal increases in BC/LA weight (Hebbeler and Sengelaub, 2003), and BC/LA weight at P28 is decreased in castrated males given estradiol implants at the BC muscle compared to gonadally intact animals (Nowacek and Sengelaub, 2006). In the present study, BC/LA weights in normal animals treated with estradiol at the muscle from P21–P28 were also smaller than those of untreated normal males. Based on these data, it appears that estradiol not only fails to support normal BC/LA growth, but can produce atrophic effects in gross muscle morphology. Interestingly, while estradiol induces muscle atrophy in gonadally intact males, their SNB dendritic lengths develop normally, and castrated males treated with estradiol at the muscle show SNB dendritic hypertrophy despite having reduced BC/LA weights. This dissociation suggests that the estrogenic support of dendritic growth is not dependent on muscle size per se, but is potentially mediated by other cellular functions of muscle cells or other cell types in the neuromuscular periphery (Nowacek and Sengelaub, 2006).
Motoneuron Morphology
Somata
In normal males, most SNB somal growth occurs during the first four postnatal weeks, and by P28 SNB somata are approximately 75% of their adult sizes (Goldstein et al., 1990). Similar to the BC/LA, SNB somal growth is androgen-dependent (Breedlove and Arnold, 1983a, b; Goldstein and Sengelaub, 1990; Goldstein and Sengelaub, 1994): blocking estrogen synthesis with aromatase inhibition does not alter normal SNB somal growth (Burke et al., 1999) and treating castrates with estradiol through injections or implants fails to masculinize SNB somata (Breedlove et al., 1982; Goldstein and Sengelaub, 1994; Hebbeler et al., 2002; Nowacek and Sengelaub, 2006; Rudolph and Sengelaub, 2013). Consistent with previous findings, in the present study, treatment of castrates with estradiol failed to support SNB somal growth.
Dendrites
In untreated castrates, SNB dendritic lengths were significantly reduced compared with normal animals, replicating previous findings (Goldstein et al., 1990; Goldstein and Sengelaub, 1994; Nowacek and Sengelaub, 2006; Rudolph and Sengelaub, 2013). Our results also confirm the SNB target musculature as the site of action for estrogen-dependent dendrite growth during the early postnatal period (Nowacek and Sengelaub, 2006; Rudolph and Sengelaub, 2013). Previous work has demonstrated that the BC/LA binds estrogens (Dube et al., 1976) and extra-muscle fiber cells in the BC have been shown to express ERα in a developmentally transient fashion (Rudolph and Sengelaub, 2013). The present study further demonstrates that ERα expression in BC extra-muscle fiber cells is also hormone-sensitive. Further, extra-muscle fiber ERα expression in the SNB target muscle coincides with the critical period for estrogen-dependent SNB dendrite growth, suggesting that this type of ERα expression could be important in mediating estrogen sensitivity of SNB motoneuron dendrites (Rudolph and Sengelaub, 2013). In this study, we show that castration results in a robust upregulation of extra-muscle fiber ERα at P21, and treating castrates with estradiol when ERα is upregulated in extra-muscle fiber cells results in SNB dendritic hypertrophy, despite the limited period of estradiol treatment. In fact, given that estradiol can downregulate its own receptor quite rapidly (Baltgavis et al., 2010), the estrogen-treated castrated animals may have had elevated ERα for an even shorter period during the estradiol treatment. Dendrite lengths of castrates treated with estradiol from P21 to P28 were significantly greater than those of normal males, and this enhanced dendritic growth occurred throughout the arbor. The general hyper-responsiveness of SNB dendrites in castrates to estradiol treatment starting at P21 likely reflects the increase in extra-muscle fiber ERα expression in the SNB target muscle, as previous work has demonstrated that hormone receptor density in skeletal muscle fibers confers hormone sensitivity to motoneuron dendrites (Huguenard et al., 2011). Changes in ERα density in the target musculature would necessarily affect SNB motoneurons as a whole, resulting in the increases in dendritic lengths throughout the arbor we observed. Furthermore, given that alterations in afferent input can result in highly localized changes in SNB dendritic morphology (Hebbeler and Sengelaub, 2003), it is unlikely that the estrogen effects we observed were mediated by changes in specific estrogen-sensitive afferents.
Comparability of HRP Labeling
Previous studies have demonstrated that neither axonal transport of BHRP (Leslie et al., 1991) nor dendritic transport as demonstrated by the rostrocaudal or mediolateral extent of dendritic labeling (Kurz et al., 1990; Goldstein and Sengelaub, 1994) is affected by hormone levels. In the present study, the possibility that hormone manipulations could affect retrograde transport is an important consideration, as such artifact could potentially result in apparent alterations in dendritic morphology. No difference in rostrocaudal extents of SNB dendrites were observed, but radial extents in untreated castrates were smaller than those of normal males. This result most likely reflects the attenuated growth of SNB dendrites rather than a transport artifact, which (because rostrocaudal extent was not affected) would necessarily have had to occur selectively in the transverse plane. In contrast, no differences in either rostrocaudal or radial extents of dendrites were observed between normal males and estradiol-treated castrates, indicating that the ability of SNB dendrites to transport BHRP out to the most distal, highest order branches was not affected in these groups. Thus, we believe the dendritic labeling across groups was comparable, allowing direct comparisons of dendritic length and distribution across groups.
Mechanisms of Target-Mediated Dendritic Growth
Hormone receptors in the SNB target muscle are important for the development of many features of the SNB system. SNB motoneurons are spared from ontogenetic cell death perinatally by androgen action at androgen receptors (ARs) in the SNB target muscle (Freeman et al., 1996). ERs in the SNB target muscle mediate the estrogen-dependent SNB dendrite growth (Nowacek and Sengelaub, 2006), a developmentally restricted process that coincides with ERα expression in extra-muscle fiber cells in the BC muscle (Rudolph and Sengelaub, 2013). Furthermore, there is evidence of a causal relationship between hormone receptor expression in the target musculature and the degree of hormone sensitivity of the innervating motoneurons. In rats, dendritic arbors of quadriceps motoneurons are androgen-insensitive, and castration with or without testosterone treatment does not affect dendrite lengths of these motoneurons (Huguenard et al., 2011). When ARs are overexpressed in the skeletal muscle fibers of transgenic rats, dendrites of quadriceps motoneurons become androgen-sensitive: castration results in dendritic atrophy, and dendritic lengths of castrates return to normal lengths with testosterone treatment (Huguenard et al., 2011). The present study provides a developmental example of the relationship between changes in hormone receptor density in the target muscles of motoneurons and corresponding changes in hormone sensitivity of the dendrites of innervating motoneurons. We have previously demonstrated that the density of ERα expression in extra-muscle fiber cells in the BC muscle coincides with the period of estrogen sensitivity for SNB dendrites (Rudolph and Sengelaub, 2013). In this study, we show that a castration-induced increase in extra-muscle fiber ERα in the developing SNB target muscle results in SNB dendrites that respond to brief estradiol treatment with robust dendritic hypertrophy. Together, these data suggest that in multiple neuromuscular systems, the hormonal sensitivity of motoneuron dendrites is controlled by hormone receptor density in the target muscle.
For estrogen-dependent dendrite growth in the SNB, the relationship between ERα density in the target musculature and estrogen sensitivity of SNB dendrites appears to involve ERα expression in a non-muscle fiber cell type. ERα expression in muscle fibers does not change across development (Rudolph and Sengelaub, 2013) or in response to removal of gonadal hormones by castration (present study). However, ERα expression in extra-muscle fiber cells in the SNB target muscle coincides with the period of SNB dendrite sensitivity and is also regulated by gonadal hormones. These data suggest that these ERα -expressing extra-muscle fiber cells have a critical role in the estrogen sensitivity of SNB motoneuron dendritic morphology during development. Additionally, non-muscle fiber cells in the SNB target musculature have been suggested to be a critical mediator of androgen-dependent perinatal development of SNB cell number (Niel et al., 2009).
It is clear that an extra-muscle fiber cell type in the SNB target muscle is critical for the masculinization of SNB morphology during development, but the specific identity of this cell type is unknown. While the apparent lack of a basal lamina rules out several cell types (e.g., muscle fibers), other candidates (e.g., fibroblasts) remain. Further, it is not known how estradiol promotes SNB dendrite growth through ERα in extra-muscle fiber cells in the target musculature. A likely mechanism is through the regulation of trophic factors that influence dendritic length. For example, in adulthood, androgens regulate brain-derived neurotrophic factor (BDNF) in SNB motoneurons (Ottem et al., 2007; Verhovshek et al., 2010b), as well as in their target musculature (Verhovshek et al., 2010b), and androgen action at the target musculature regulates BDNF protein in SNB motoneurons (Verhovshek and Sengelaub, 2013). The regulation of BDNF by androgen is critical for SNB dendritic morphology: supplying BDNF to cut axons after peripheral axotomy restores soma size (Yang and Arnold, 2000) and dendritic length (Yang et al., 2004), while castration results in elevated muscle BDNF levels and dendritic atrophy which can be prevented by blockade of BDNF signaling (Verhovshek and Sengelaub, 2010). Estradiol has been shown to regulate BDNF levels in vitro (Krizsan-Agbas et al., 2003) and in vivo (Solum and Handa, 2002), and BDNF-ERα coexpression occurs in developing neurons, providing a clear mechanism for estrogen regulation of BDNF (Solum and Handa, 2002). Together, these data suggest that during the early postnatal period, estrogen-dependent SNB dendrite growth could occur through an estrogen-BDNF interaction in the SNB target muscle, and a target-mediated regulation of BDNF levels could be driving changes in SNB dendritic morphology.
Acknowledgments
We thank Drs. Greg Demas and Cara Wellman for their helpful comments on the manuscript. The D18 antibody developed by J.R. Sanes was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Supported by NIH-NICHD HD35315 to D.R.S.
REFERENCES
- Baltgavis KA, Greising SM, Warren GL, Lowe DA. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS One. 2010;5(4):e10164. doi: 10.1371/journal.pone.0010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. I. Complete Demasculinization of the male rat spinal nucleus of the bulbocavernosus using the anti-androgen flutamide. J Neurosci. 1983a;3:417–423. doi: 10.1523/JNEUROSCI.03-02-00417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. II. Sensitive periods for the androgen-induced masculinization of the rat spinal nucleus of the bulbocavernosus. J Neurosci. 1983b;3:424–432. doi: 10.1523/JNEUROSCI.03-02-00424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Jacobson CD, Gorski RA, Arnold AP. Masculinization of the female rat spinal cord following a single neonatal injection of testosterone propionate but not estradiol benzoate. Brain Res. 1982;237:173–181. doi: 10.1016/0006-8993(82)90565-0. [DOI] [PubMed] [Google Scholar]
- Burke KA, Kuwajima M, Sengelaub DR. Aromatase inhibition reduces dendritic growth in a sexually dimorphic rat spinal nucleus. J Neurobiol. 1999;38:301–312. [PubMed] [Google Scholar]
- Burke KA, Widows MR, Sengelaub DR. Synergistic effects of testosterone metabolites on the development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. J Neurobiol. 1997;33:1–10. doi: 10.1002/(sici)1097-4695(199707)33:1<1::aid-neu1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Čihák R, Gutmann E, Hanzlíková V. Involution and hormone-induced persistence of the M. sphincter (levator) ani in female rats. J Anat. 1970;106:93–110. [PMC free article] [PubMed] [Google Scholar]
- Couse JK, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- Dube JY, Lesage R, Tremblay RR. Androgen and estrogen binding in rat skeletal and perineal muscles. Can J Biochem. 1976;54:50–55. doi: 10.1139/o76-008. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Foster AM, Harty MW, Sengelaub DR. Estrogen alters excitability but not morphology of a sexually dimorphic neuromuscular system in adult rats. J Neurobiol. 2003;56:66–77. doi: 10.1002/neu.10224. [DOI] [PubMed] [Google Scholar]
- Freeman L, Watson N, Breedlove SM. Androgen spares androgen-insensitive motoneurons from apoptosis in the spinal nucleus of the bulbocavernosus in rats. Horm Behav. 1996;30:424–433. doi: 10.1006/hbeh.1996.0047. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Kalkbrenner AE, Sengelaub DR. Changes in dendritic morphology of rat spinal motoneurons during development and after unilateral target deletion. Dev Brain Res. 1993;73:151–163. doi: 10.1016/0165-3806(93)90133-u. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Sengelaub DR. Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J Neurosci. 1990;10:935–946. doi: 10.1523/JNEUROSCI.10-03-00935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LA, Mills AC, Sengelaub DR. Motoneuron development after deafferentation. 1. Dorsal rhizotomy does not alter growth in the spinal nucleus of the bulbocavernosus (SNB) Dev Brain Res. 1996;91:11–19. doi: 10.1016/0165-3806(95)00150-6. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR. Hormonal control of neuron number in sexually dimorphic spinal nuclei of the rat. IV. Masculinization of the spinal nucleus of the bulbocavernosus with testosterone metabolites. J Neurobiol. 1990;21:719–730. doi: 10.1002/neu.480210506. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR. Timing and duration of dihydrotestosterone treatment affect the development of motoneuron number and morphology in a sexually dimorphic rat spinal nucleus. J Comp Neurol. 1992;326:147–157. doi: 10.1002/cne.903260113. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR. Differential effects of dihydrotestosterone and estrogen on the development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. J Neurobiol. 1994;25:878–892. doi: 10.1002/neu.480250711. [DOI] [PubMed] [Google Scholar]
- Hart BL, Melese-D'Hospital PY. Penile mechanisms and the role of the striated penile muscles in penile reflexes. Physiol Behav. 1983;31:807–813. doi: 10.1016/0031-9384(83)90277-9. [DOI] [PubMed] [Google Scholar]
- Hayes KJ. So-called levator ani of rat. Acta Endocrinol (Copenh) 1965;48:337–347. doi: 10.1530/acta.0.0480337. [DOI] [PubMed] [Google Scholar]
- Hays TC, Goldstein LA, Mills AC, Sengelaub DR. Motoneuron development after deafferentation. 2. Dorsal rhizotomy does not block estrogen-supported growth in the dorsolateral nucleus (DLN) Dev Brain Res. 1996;91:20–28. doi: 10.1016/0165-3806(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Sengelaub DR. Development of a sexually dimorphic neuromuscular system in male rats after spinal transection: Morphologic changes and implications for estrogen sites of action. J Comp Neurol. 2003;467:80–96. doi: 10.1002/cne.10911. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Verhovshek T, Sengelaub DR. N-methyl-D-aspartate receptor blockade inhibits estrogenic support of dendritic growth in a sexually dintorphic rat spinal nucleus. J Comp Neurol. 2002;451:142–152. doi: 10.1002/cne.10347. [DOI] [PubMed] [Google Scholar]
- Huguenard A, Fernando SM, Monks DA, Sengelaub DR. Overexpression of androgen receptor in target musculature confers androgen sensitiviry to motoneuron dendrites. Endocrinology. 2011;152:639–650. doi: 10.1210/en.2010-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CL, Letinsky MS, Arnold AP. The role of gonadal hormones in neuromuscular synapse elimination in rats. I. Androgen delays the loss of multiple innervation in the levator ani muscle. J Neurosci. 1989a;9:229–238. doi: 10.1523/JNEUROSCI.09-01-00229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CL, Letinsky MS, Arnold AP. The role of gonadal hormones in neuromuscular synapse elimination in rats. II. Multiple innervation persists in the adult levator ani muscle after juvenile androgen treatment. J Neurosci. 1989b;9:239–247. doi: 10.1523/JNEUROSCI.09-01-00239.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CL, Watamura S, Arnold AP. Androgenic, not estrogenic, steroids alter neuromuscular synapse elimination in the rat levator ani. Dev Brain Res. 1995;84:225–232. doi: 10.1016/0165-3806(94)00175-y. [DOI] [PubMed] [Google Scholar]
- Krizsan-Agbas D, Pedchenko T, Hasan W, Smith PG. Oestrogen regulates sympathetic neurite outgrowth by modulating brain derived neurotrophic factor synthesis and release by the rodent uterus. Eur J Neurosci. 2003;18(10):2760–2768. doi: 10.1111/j.1460-9568.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Bowers CA, Sengelaub DR. Morphology of rat spinal motoneurons with normal and hormonally altered specificity. J Comp Neurol. 1990;292:638–650. doi: 10.1002/cne.902920412. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Brewer RG, Sengelaub DR. Hormonally mediated plasticity of motoneuron morphology in the adult rat spinal cord: a cholera toxin-HRP study. J Neurobiol. 1991;22:976–988. doi: 10.1002/neu.480220909. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jordan CL, Arnold AP. Critical period for androgenic regulation of soma size of sexually dimorphic motoneurons in rat lumbar spinal cord. Neurosci Lett. 1989;98:79–84. doi: 10.1016/0304-3940(89)90377-7. [DOI] [PubMed] [Google Scholar]
- Leslie M, Forger NG, Breedlove SM. Does androgen affect axonal transport of cholera toxin HRP in spinal motoneurons? Neurosci Lett. 1991;126:499–514. doi: 10.1016/0304-3940(91)90553-6. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- Mesulam M. Tracing neural connections with horseradish peroxidase. Chichester: Wiley; 1982. p. 251. [Google Scholar]
- Niel L, Shah AH, Lewis GA, Mo K, Chatterjee D, Fernando SM, Hong MH, Chang WY, Vollmayr P, Rosen J, Miner JN, Monks DA. Sexual differentiation of the spinal nucleus of the bulbocavernosus is not mediated solely by androgen receptors in muscle fibers. Endocrinology. 2009;150:3207–3213. doi: 10.1210/en.2008-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Nowacek AS, Sengelaub DR. Estrogenic support of motoneuron dendritic growth via the neuromuscular periphery in a sexually dimorphic motor system. J Neurobiol. 2006;66:962–976. doi: 10.1002/neu.20274. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Beck LA, Jordan CL, Breedlove SM. Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology. 2007;148:3655–3665. doi: 10.1210/en.2007-0308. [DOI] [PubMed] [Google Scholar]
- Raouf S, Van Roo B, Sengelaub D. Adult plasticity in hormone-sensitive motoneuron morphology: Methodological/behavioral confounds. Horm Behav. 2000;38:210–221. doi: 10.1006/hbeh.2000.1620. [DOI] [PubMed] [Google Scholar]
- Rudolph LM, Sengelaub DR. Critical period for estrogen-dependent motoneuron dendrite growth is coincident with ERα expression in target musculature. Dev Neurobiol. 2013;73(1):72–84. doi: 10.1002/dneu.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil. 1982;66:433–443. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- Schrøder H. Organization of the motoneurons innervating the pelvic muscles of the rat. J Comp Neurol. 1980;192:567–587. doi: 10.1002/cne.901920313. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda R, Toran-Allerand C. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum D, Handa R. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin C, Joubert Y. Testosterone-induced development of the rat levator ani muscle. Dev Biol. 1991;146:131–138. doi: 10.1016/0012-1606(91)90453-a. [DOI] [PubMed] [Google Scholar]
- Verhovshek T, Buckley KE, Sergent MA, Sengelaub DR. Testosterone metabolites differentially maintain adult morphology in a sexually dimorphic neuromuscular system. Dev Neurobiol. 2010a;70(4):206–221. doi: 10.1002/dneu.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhovshek T, Cai Y, Osborne MC, Sengelaub DR. Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Endocrinology. 2010b;151:253–261. doi: 10.1210/en.2009-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhovshek T, Sengelaub DR. Trophic effects of brain-derived neurotrophic factor blockade in an androgen-sensitive neuromuscular system. Endocrinology. 2010;151:5337–5348. doi: 10.1210/en.2010-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhovshek T, Sengelaub DR. Androgen action at the target musculature regulates brain-derived neurotrophic factor protein in the spinal nucleus of the bulbocavernosus. Dev Neurobiol. 2013;73(8):587–598. doi: 10.1002/dneu.22083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiik A, Glenmark B, Ekman M, Esbjornsson-Liljedahl M, Johansson O, Bodin K, Enmark E, Jansson E. Oestrogen receptor beta is expressed in adult human skeletal muscle both at the mRNA and protein level. Acta Physiol Scand. 2003;179:381–387. doi: 10.1046/j.0001-6772.2003.01186.x. [DOI] [PubMed] [Google Scholar]
- Yang LY, Arnold AP. Interaction of BDNF and testosterone in the regulation of adult perineal motoneurons. J Neurobiol. 2000;44(3):308–319. doi: 10.1002/1097-4695(20000905)44:3<308::aid-neu2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Yang LY, Verhovshek T, Sengelaub DR. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145(1):161–168. doi: 10.1210/en.2003-0853. [DOI] [PubMed] [Google Scholar]