Abstract
Background
The prevalence of atrial fibrillation (AF) continues to increase; however, there are limited data describing the division of care among practitioners in the community and whether care differs depending on provider specialty.
Methods and Results
Using the Outcomes Registry for Better Informed Treatment of AF (ORBIT‐AF) Registry, we described patient characteristics and AF management strategies in ambulatory clinic practice settings, including electrophysiology (EP), general cardiology, and primary care. A total of 10 097 patients were included; of these, 1544 (15.3%) were cared for by an EP provider, 6584 (65.2%) by a cardiology provider, and 1969 (19.5%) by an internal medicine/primary care provider. Compared with those patients who were cared for by cardiologists or internal medicine/primary care providers, patients cared for by EP providers were younger (median age, 73 years [interquartile range, IQR, 64, 80 years, Q1, Q3] versus 75 years [IQR, 67, 82 years] for cardiology and versus 76 years [IQR, 68, 82 years] for primary care). Compared with cardiology and internal medicine/primary care providers, EP providers used rhythm control (versus rate control) management more often (44.2% versus 29.7% and 28.8%, respectively, P<0.0001; adjusted odds ratio [OR] EP versus cardiology, 1.66 [95% confidence interval, CI, 1.05 to 2.61]; adjusted OR for internal medicine/primary care versus cardiology, 0.91 [95% CI, 0.65 to 1.26]). Use of oral anticoagulant therapy was high across all providers, although it was higher for cardiology and EP providers (overall, 76.1%; P=0.02 for difference between groups).
Conclusions
Our data demonstrate important differences between provider specialties, the demographics of the AF patient population treated, and treatment strategies—particularly for rhythm control and anticoagulation therapy.
Keywords: antithrombotic therapy, atrial fibrillation, ORBIT‐AF, outpatient, provider, specialty
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, with a lifetime prevalence of one fourth of patients >40 years old.1 With an aging population and increasing prevalence of known risk factors,2 the predominance of AF is increasing in the Western world.3–4 In addition to impairing quality of life,5–6 AF also increases the risk of stroke and thromboembolism,7 potentially leading to permanent disability and death.8 As a result, the appropriate management of this disease, including stroke prevention, is critically important. Previous studies in heart failure9–14 and myocardial infarction (MI)15–16 have shown that processes of care and outcomes improve when patients are cared for by a specialist versus a nonspecialist. Nevertheless, no AF data currently exist regarding differences in patient characteristics, treatment strategies, and treatment patterns across care settings.
We used the Outcomes Registry for Better Quality of Care in the Treatment of AF (ORBIT‐AF) database to compare patient characteristics and treatment strategies according to provider specialty (electrophysiology [EP] versus cardiology versus primary care/internal medicine).
Methods
Data Sources and Study Population
The ORBIT‐AF Registry is a multicenter, prospective, outpatient database of incident and prevalent AF. The registry is a nationwide collaboration of health care providers, including internists, primary care physicians, cardiologists, and EPs across the United States. Site investigators enrolled consecutive patients with AF who were ≥18 years of age, had electrocardiographic evidence of AF, were able to provide informed consent, and were able to adhere to follow‐up criteria. Patients were excluded who had a life expectancy of <6 months or were diagnosed with AF secondary to an easily reversible condition. The primary source of data was a patient's medical record, which was entered into a Web‐based case report form. The rationale and design of ORBIT‐AF have been published previously.17 For this analysis, we included all patients in ORBIT‐AF with available baseline data who were enrolled from June 29, 2010, to August 9, 2011. A total of 174 sites contributed to the ORBIT‐AF baseline data. The current analysis was approved by the Duke University Institutional Review Board.
Definition of Provider Type
Provider specialty was the variable of interest for this analysis and was based on the specialty of the provider who resided at the specific enrollment site (EP, cardiology, internal medicine/primary care, or neurology). In addition, provider specialty was also captured for all types of providers who followed the care of the patient, including ascertainment of multiple providers (EP, cardiology, internal medicine/primary care, and neurology).
Atrial Fibrillation and Treatment Strategies
The type of AF was classified according to treatment guidelines as first detected, paroxysmal, persistent, or permanent (long‐standing persistent).18–20 The following treatment strategies were compared across provider specialty: rhythm versus rate control, prior use of cardioversion, antiarrhythmic drugs, warfarin, and surgical interventions (AF radiofrequency ablation or surgical Maze procedure). Pharmacotherapy was also compared according to provider specialty, drug class, and individual antiarrhythmic and antithrombotic agents. Contraindications for oral anticoagulant therapy included both absolute and relative contraindications and were captured via checkboxes on the ORBIT‐AF data form.
Statistical Analysis
Baseline characteristics were compared using the Kruskal–Wallis test for continuous variables and the χ2 test for categorical variables. We calculated the congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA or thromboembolism (CHADS2); the congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA or thromboembolism, vascular disease, age 65 to 74 years, sex category (CHA2DS2‐VASc); and the anticoagulation and risk factors in atrial fibrillation (ATRIA) prediction scores (as previously described)18–19,21 to assess the predicted risk of downstream stroke and subsequent bleeding. The CHADS2 and CHA2DS2‐VASc scores were calculated and presented for each group to compare the baseline risk of thromboembolic events influencing treatment strategy.19 To illustrate the use of oral anticoagulation by provider type, we report the proportion of patients treated with oral anticoagulation (warfarin or dabigatran) according to CHA2DS2‐VASc scores and by provider type. We have only included patients without contraindications for treatment.
We assessed the effect of provider specialty (using cardiology as the reference group) on utilization of select treatment strategies using hierarchical multivariable logistic regression, with the following care processes as outcomes: rhythm control (versus rate), prior cardioversion, dabigatran (versus warfarin), oral anticoagulant, antiplatelet therapy, and anticoagulant clinic use to monitor international normalized ratio (INR) values. The hierarchical logistic regression model included patient‐level adjustment variables and a random intercept to account for provider variability.
The variables included for adjustment are age, female, race/ethnicity, payer/insurance, alcohol abuse, cognitive impairment/dementia, chronic obstructive pulmonary disease (COPD), dialysis, diabetes, heart failure, hypertension, significant valvular disease, obstructive sleep apnea, osteoporosis, peripheral vascular disease, prior stroke or transient ischemic attack (TIA), prior MI, gastrointestinal bleed, sinus node dysfunction, smoking status, thyroid disease, type of AF, heart rate, systolic blood pressure (BP), diastolic BP, interventricular conduction, left atrial diameter, left ventricular ejection fraction (LVEF) dysfunction, creatinine clearance (estimated using the Cockcroft–Gault formula), hematocrit, prior warfarin therapy, contraindication to anticoagulation, or currently on warfarin, dabigatran, or antiplatelet therapy (aspirin, clopidogrel, prasugrel, or ticagrelor). This list was modified according to the treatment strategies examined. All continuous variables were examined for linearity, and the specific treatment strategy and nonlinear variables were fit with linear splines where appropriate.
Missing data (all the adjustment variables <2% except for creatinine clearance [8%], hematocrit [11%], % LVEF dysfunction [14%], and left atrial diameter [27%]) were multiply imputed. Final estimates and standard errors reflect the combined analysis over 5 imputed data sets. Adjusted associations of provider specialty with the care processes are displayed as odds ratios (ORs) with 95% confidence intervals (95% CIs). P<0.05 was considered statistically significant. All analyses were performed using SAS software (versions 9.2 and 9.3; SAS Institute, Cary, NC).
Results
A total of 10 097 outpatient AF patients were included in this study; baseline patient characteristics stratified according to provider specialty are shown in Table 1. Electrophysiologists were the primary AF care providers for 15.3% of patients, general cardiologists for 65.2%, and internal medicine/primary care physicians for 19.5%. Overall, 42.7% of patients saw 1 provider exclusively, 47.7% saw 2, and 9.2% saw >2 specialty providers. Among those seen primarily by an internal medicine/primary care provider, 47.4% also received care from another provider (ie, EP or cardiologist). The proportion of patients seeing more than 1 provider was higher among patients seen primarily by a cardiologist (60.0% of these patients received care from other providers) or an electrophysiologist (57.2% of these patients received care from other providers).
Table 1.
Baseline Characteristics According to Provider Specialty
| Variable | Overall (n=10 097) | Internal Medicine/Primary Care (n=1969) | Cardiology (n=6584) | Electrophysiology (n=1544) | P Value |
|---|---|---|---|---|---|
| Age, median (Q1, Q3) | 75 (67, 82) | 76 (68, 82) | 75 (67, 82) | 73 (64, 80) | <0.0001 |
| Female (%) | 4274 (42.3) | 879 (44.6) | 2778 (42.2) | 617 (40.0) | 0.0192 |
| Race/ethnicity, n (%) | <0.0001 | ||||
| Black | 505 (5.0) | 119 (6.0) | 349 (5.3) | 37 (2.4) | |
| Hispanic | 421 (4.2) | 65 (3.3) | 349 (5.3) | 7 (0.5) | |
| White | 9008 (89.2) | 1755 (89.1) | 5763 (87.5) | 1490 (96.5) | |
| Other* | 144 (1.4) | 24 (1.2) | 110 (1.7) | 10 (0.7) | |
| Payer/insurance, n (%) | <0.0001 | ||||
| Medicaid | 468 (4.6) | 118 (6.0) | 314 (4.8) | 36 (2.3) | |
| Medicare | 6562 (65.0) | 1282 (65.1) | 4286 (65.1) | 994 (64.4) | |
| Private | 2580 (25.6) | 470 (23.9) | 1666 (25.3) | 444 (28.8) | |
| Military | 109 (1.1) | 11 (0.6) | 72 (1.1) | 26 (1.7) | |
| Other* | 284 (2.8) | 64 (3.3) | 185 (2.8) | 35 (2.3) | |
| None | 91 (0.9) | 24 (1.2) | 59 (0.9) | 8 (0.5) | |
| CHADS2 score, mean (SD) | 2.3 (1.3) | 2.4 (1.3) | 2.3 (1.3) | 2.1 (1.3) | <0.0001 |
| CHA2DS2‐VASc score, mean (SD) | 3.9 (1.8) | 4.0 (1.8) | 3.9 (1.8) | 3.6 (1.8) | <0.0001 |
| ATRIA score, mean (SD) | 2.8 (2.0) | 2.9 (2.0) | 2.9 (2.0) | 2.3 (1.9) | <0.0001 |
| Cognitive impairment/dementia, n (%) | 309 (3.1) | 118 (6.0) | 171 (2.6) | 20 (1.3) | <0.0001 |
| COPD, n (%) | 1652 (16.4) | 372 (18.9) | 1060 (16.1) | 220 (14.3) | 0.0007 |
| Dialysis, n (%) | 127 (1.3) | 21 (1.1) | 91 (1.4) | 15 (1.0) | 0.2981 |
| Diabetes, n (%) | 2970 (29.4) | 617 (31.3) | 1947 (29.6) | 406 (26.3) | 0.0045 |
| Heart failure, n (%) | 3277 (32.5) | 571 (29.0) | 2181 (33.1) | 525 (34.0) | 0.0010 |
| Hypertension, n (%) | 8378 (83.0) | 1669 (84.8) | 5516 (83.8) | 1193 (77.3) | <0.0001 |
| Significant valvular disease, n (%)* | 2570 (25.5) | 439 (22.3) | 1676 (25.5) | 455 (29.5) | <0.0001 |
| Peripheral vascular disease, n (%) | 1345 (13.3) | 282 (14.3) | 872 (13.2) | 191 (12.4) | 0.2290 |
| Prior stroke or TIA, n (%) | 1521 (15.1) | 342 (17.4) | 974 (14.8) | 205 (13.3) | 0.0020 |
| Prior MI, n (%) | 1597 (15.8) | 281 (14.3) | 1092 (16.6) | 224 (14.5) | 0.0147 |
| Sinus node dysfunction, n (%) | 1767 (17.5) | 229 (11.6) | 1263 (19.2) | 275 (17.8) | <0.0001 |
| Current smoker (among smokers), n (%) | 584 (12.0) | 124 (13.3) | 370 (11.7) | 90 (11.3) | 0.0251 |
| Type of AF, n (%) | <0.0001 | ||||
| First detected | 475 (5.0) | 120 (6.1) | 324 (4.9) | 31 (2.0) | |
| Paroxysmal | 5093 (50.0) | 1028 (52.2) | 3172 (48.2) | 893 (57.8) | |
| Persistent | 1695 (16.8) | 290 (14.7) | 1080 (16.4) | 325 (21.1) | |
| Permanent | 2820 (27.9) | 528 (26.8) | 1997 (30.3) | 295 (19.1) | |
| Heart rate, median (Q1, Q3) | 70 (63, 80) | 71 (64, 78) | 70 (63, 80) | 70 (61, 79) | 0.0027 |
| Systolic BP, median (Q1, Q3) | 126 (116, 138) | 125 (118, 136) | 126 (116, 138) | 122 (112, 136) | <0.0001 |
| Diastolic BP, median (Q1, Q3) | 72 (66, 80) | 72 (66, 80) | 72 (66, 80) | 72 (66, 80) | 0.3139 |
| LBBB, n (%) | 397 (3.9) | 92 (4.7) | 243 (3.7) | 62 (4.0) | <0.0001 |
| LVEF <30%, n (%) | 423 (4.2) | 49 (2.5) | 276 (4.2) | 98 (6.4) | 0.0001 |
| Creatinine clearance (mL/min per 1.73 m2), median (Q1, Q3) | 70 (50, 97) | 66 (49, 94) | 69 (50, 96) | 76 (54, 104) | <0.0001 |
Q1, Q3 indicates quartiles 1, 3; CHADS2, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA or thromboembolism; CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA or thromboembolism, vascular disease, age 65 to 74 years, sex category; ATRIA, anticoagulation and risk factors in atrial fibrillation; COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack; MI, myocardial infarction; AF, atrial fibrillation; BP, blood pressure; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction.
Other includes American Indian/Alaskan native, Asian, Native Hawaiian or Pacific Native, and other.
Other includes state‐specific plan (non‐Medicaid) and other insurance.
Any moderate to severe valvular insufficiency or stenosis.
The overall median age of the cohort was 75 years (67, 82 [Q1, Q3] years), and 42.3% were women. The median CHADS2 score was 2 (1, 3 [Q1, Q3]). Patients who were cared for by an EP were more often younger, male, white, and privately insured and had fewer comorbidities than patients cared for by cardiologists or internal medicine/primary care physicians. Systolic BP was lower and creatinine clearance was higher in the EP patient group compared with the other groups (Table 1).
Figure 1 displays the unadjusted CHADS2 scores categorized by type of provider; when compared with the other groups, patients cared for by EP providers had the lowest CHADS2 scores, whereas patients cared for by internal medicine/primary care providers had the highest scores. This relationship was similar for the CHA2DS2‐VASc scores. When compared with those cared for by EP providers, a higher proportion of patients with permanent AF were cared for by internal medicine/primary care providers (Figure 2). Electrophysiology providers had significantly higher proportions of paroxysmal and persistent AF patients compared with the other groups (P<0.001). In addition, the use of oral anticoagulants differed by provider, as shown in Figure 3. The overall use of warfarin or dabigatran was 73.6% for internal medicine/primary care providers, 76.7% for cardiology providers, and 76.8% for EP (P=0.02 for difference between groups). Electrophysiology providers also prescribed warfarin or dabigatran more often than the other providers for patients with a CHA2DS2‐VASc ≥2.
Figure 1.

CHADS2 risk scores according to provider specialty. This figure displays the distribution of CHADS2 risk score by provider specialty. CHADS2 indicates congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA or thromboembolism; EP, electrophysiology; TIA, transient ischemic attack.
Figure 2.

Type of atrial fibrillation distribution according to provider specialty. The distribution of AF type by provider specialty (first detected, paroxysmal, persistent, or permanent) is shown. AF indicates atrial fibrillation; EP, electrophysiology.
Figure 3.
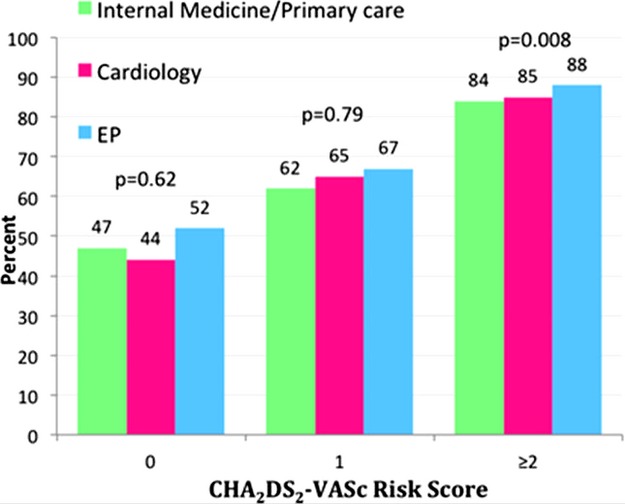
Use of oral anticoagulation (warfarin or dabigatran) according to CHA2DS2‐VASc score and provider specialty. This figure displays the distribution of oral anticoagulation use by provider specialty. CHA2DS2‐VASc indicates congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA or thromboembolism, vascular disease, age 65 to 74 years, sex category; AF, atrial fibrillation; EP, electrophysiology; TIA, transient ischemic attack.
Management of Atrial Fibrillation by Provider: Unadjusted Results
Table 2 shows how patients' AF was managed according to provider. Electrophysiology providers employed rate control in 55% of patients (with the remainder of their patients on rhythm control), whereas cardiology and internal medicine/primary care providers employed a rate control strategy in 70% of patients. Rhythm control was used in 44% of AF patients treated by EP providers versus approximately 30% and 29% of those treated by a cardiologist and internal medicine/primary care physician, respectively. Similarly, 42% of the EP provider patients had previously received cardioversion versus 29% of cardiology provider patients and 26% of internal medicine/primary care provider patients. Overall, EP providers used more catheter ablations and surgical interventions (P<0.0001).
Table 2.
Atrial Fibrillation Management According to Provider Specialty
| Variable | Overall (n=10 097), n (%) | Internal Medicine/Primary Care (n=1969), n (%) | Cardiology (n=6584), n (%) | Electrophysiology (n=1544), n (%) | P Value |
|---|---|---|---|---|---|
| Rate control | 6859 (67.9) | 1388 (70.5) | 4615 (70.1) | 856 (55.4) | <0.0001 |
| Rhythm control | 3202 (31.7) | 566 (28.8) | 1953 (29.7) | 683 (44.2) | |
| Prior cardioversion | 3037 (30.1) | 503 (25.6) | 1887 (28.7) | 647 (41.9) | <0.0001 |
| 1 | 1790 (58.9) | 313 (62.2) | 1138 (60.3) | 339 (52.4) | <0.0001 |
| 2 | 726 (23.9) | 118 (23.5) | 448 (23.7) | 160 (24.7) | |
| ≥3 | 513 (16.9) | 72 (14.3) | 294 (15.6) | 147 (22.7) | |
| Prior antiarrhythmic drug therapy | 4573 (45.3) | 847 (43.0) | 2824 (42.9) | 902 (58.4) | <0.0001 |
| 1 | 2939 (64.3) | 568 (67.1) | 1788 (63.3) | 583 (64.6) | <0.0001 |
| 2 | 923 (20.2) | 159 (18.8) | 549 (19.4) | 215 (23.8) | |
| ≥3 | 368 (8.1) | 71 (8.4) | 203 (7.2) | 94 (10.4) | |
| Prior warfarin therapy | 8256 (81.8) | 1532 (77.8) | 5403 (82.1) | 1321 (85.6) | <0.0001 |
| Catheter ablation of AF | 552 (5.5) | 100 (5.1) | 320 (4.9) | 132 (8.6) | <0.0001 |
| Surgical/hybrid maze | 194 (1.9) | 32 (1.6) | 104 (1.6) | 58 (3.8) | <0.0001 |
| AV node ablation | 220 (2.2) | 34 (1.7) | 142 (2.2) | 44 (2.9) | 0.0778 |
| Rate control agents | |||||
| Beta‐blocker | 6449 (63.9) | 1161 (59.0) | 4270 (64.9) | 1018 (65.9) | <0.0001 |
| Dihydropyridine CCB | 1391 (13.8) | 315 (16.0) | 884 (13.4) | 192 (12.4) | 0.0034 |
| Digoxin | 2361 (23.4) | 445 (22.6) | 1609 (24.4) | 307 (19.9) | 0.0005 |
| Antiarrhythmic drug therapy | 2895 (28.7) | 480 (24.4) | 1785 (27.1) | 630 (40.8) | <0.0001 |
| Amiodarone | 1002 (9.9) | 167 (8.5) | 625 (9.5) | 210 (13.6) | <0.0001 |
| Dronedarone | 466 (4.6) | 68 (3.5) | 320 (5.0) | 78 (5.1) | 0.0231 |
| Dofetilide | 190 (1.9) | 21 (1.1) | 93 (1.4) | 76 (4.9) | <0.0001 |
| Flecainide | 290 (2.9) | 55 (2.8) | 145 (2.2) | 90 (5.8) | <0.0001 |
| Propafenone | 236 (2.3) | 47 (2.4) | 154 (2.3) | 35 (2.3) | 0.9714 |
| Sotalol | 618 (6.1) | 90 (4.6) | 398 (6.0) | 130 (8.4) | <0.0001 |
| Other* | 131 (1.3) | 36 (1.8) | 77 (1.2) | 18 (1.2) | 0.0665 |
| Antithrombotic therapy (any use) | 9579 (94.9) | 1833 (93.1) | 6254 (95.0) | 1492 (96.6) | <0.0001 |
| Aspirin | 4452 (44.1) | 745 (37.8) | 2946 (44.7) | 761 (49.3) | <0.0001 |
| Clopidogrel | 712 (7.1) | 105 (5.3) | 494 (7.5) | 113 (7.3) | 0.0040 |
| Prasugrel | 14 (0.1) | 3 (0.2) | 9 (0.1) | 2 (0.1) | 0.9810 |
| Dabigatran | 496 (4.9) | 71 (3.6) | 352 (5.4) | 73 (4.7) | 0.0070 |
| Warfarin | 7198 (71.3) | 1381 (70.1) | 4705 (71.5) | 1112 (72.0) | 0.4116 |
| Combination antithrombotic therapy | |||||
| Aspirin and warfarin | 2556 (25.3) | 373 (18.9) | 1732 (26.3) | 451 (29.2) | <0.0001 |
| Aspirin and dabigatran | 155 (1.5) | 18 (0.9) | 108 (1.6) | 29 (1.9) | 0.0353 |
| Aspirin, clopidogrel, and warfarin | 165 (1.6) | 20 (1.0) | 122 (1.9) | 23 (1.5) | 0.0330 |
| Clopidogrel and warfarin | 334 (3.3) | 44 (2.2) | 230 (3.5) | 60 (3.9) | 0.0092 |
| Clopidogrel and dabigatran | 13 (0.1) | 3 (0.2) | 9 (0.1) | 1 (0.06) | 0.7371 |
| Aspirin, clopidogrel, and dabigatran | 3 (0.03) | 1 (0.05) | 1 (0.02) | 1 (0.06) | 0.4962 |
| Contraindications to anticoagulation | 1407 (13.9) | 295 (15.0) | 915 (13.9) | 197 (12.8) | 0.1667 |
| Management in anticoagulation clinic | 3112 (43.2) | 459 (33.2) | 2009 (42.7) | 644 (57.9) | <0.0001 |
AF indicates atrial fibrillation; AV, arterioventricular; CCB, calcium channel blocker.
Other includes disopyramide, ranolazine, quinidine, and other antiarrhythmic medications.
Table 2 also displays the pharmacologic therapies for AF according to provider type. Overall, class III antiarrhythmic drugs (amiodarone, dronedarone, dofetilide, and sotalol) were the most prevalently used agents for all providers. Electrophysiology providers were more likely to use antiarrhythmic drugs, as well as antithrombotic agents (overall P<0.01). Patients cared for by EP providers were more likely to have warfarin management from an anticoagulation clinic (58% versus 43% for cardiology and versus 33% for internal medicine/primary care), although the number of patients with contraindications to anticoagulation was similar across the provider groups (P=0.17).
Relationship Between Provider Specialty and Treatment Strategies: Adjusted Results
Table 3 shows the results for the adjusted association of provider specialty and the various treatment strategies. After adjusting for case mix, patients seen by an EP provider were more likely to be on rhythm control when compared with patients seen by a cardiology provider (adjusted OR, 1.66; 95% CI, 1.05 to 2.61; P=0.0301). Also, patients seen by an EP provider were more likely to have prior cardioversions than patients seen by a cardiology provider (adjusted OR, 1.39; 95% CI, 1.03 to 1.87; P=0.0292).
Table 3.
Adjusted Relationship Between Specialty of Care Provider and Treatment Strategies
| Care Process | Provider Specialty | Adjusted OR | Lower (95% CI for OR) | Upper (95% CI for OR) | P Value | Global P Value |
|---|---|---|---|---|---|---|
| Rhythm control | EP (vs cardiology) | 1.66 | 1.05 | 2.61 | 0.0301 | 0.0470 |
| FP/IM (vs cardiology) | 0.91 | 0.65 | 1.26 | 0.5512 | — | |
| Prior cardioversion | EP (vs cardiology) | 1.39 | 1.03 | 1.87 | 0.0292 | 0.0231 |
| FP/IM (vs cardiology) | 0.88 | 0.70 | 1.10 | 0.2699 | — | |
| Oral anticoagulant (warfarin or dabigatran) | EP (vs cardiology) | 1.25 | 0.71 | 2.19 | 0.4350 | 0.1500 |
| FP/IM (vs cardiology) | 0.73 | 0.49 | 1.09 | 0.1233 | — | |
| Dabigatran vs warfarin | EP (vs cardiology) | 1.11 | 0.49 | 2.50 | 0.8023 | 0.2395 |
| FP/IM (vs cardiology) | 0.61 | 0.32 | 1.13 | 0.1179 | — | |
| Antiplatelet* | EP (vs cardiology) | 1.34 | 0.93 | 1.92 | 0.1132 | <0.0001 |
| FP/IM (vs cardiology) | 0.60 | 0.47 | 0.79 | 0.0002 | — | |
| Anticoagulant clinic* | EP (vs cardiology) | 3.51 | 0.90 | 13.67 | 0.0705 | 0.0577 |
| FP/IM (vs cardiology) | 0.60 | 0.23 | 1.60 | 0.3097 | — |
OR indicates odds ratio; CI, confidence interval; EP, electrophysiology; FP, family practice; IM, internal medicine.
Aspirin, clopidogrel, prasugrel.
Analyses only among those on warfarin (n=7176).
Discussion
This study examined differences in patient characteristics, type of AF, and treatment strategies among a large sample of outpatients who received care among a diverse group of provider specialties. Our analysis yielded 3 major findings. First, EP providers tended to care for those AF patients who were younger, healthier, white, at lower risk, and more likely to have paroxysmal or persistent AF. Second, EP providers were more likely to initiate rhythm control strategies, including antiarrhythmic therapy and/or ablation therapy, than those treated by cardiologists or internal medicine/primary care physicians. Finally, warfarin treatment was consistently high across providers, although internal medicine providers tended to use fewer antiplatelet agents and novel anticoagulant therapy than did the EP and cardiology providers.
Two previous studies examined AF care patterns among cardiology providers as part of the AF: Focus on Effective Clinical Treatment Strategies (AFFECTS) Registry.22–23 Patients enrolled in the AFFECTS Registry were treated with rhythm control in two thirds of the cases, whereas only one third of patients were treated with rhythm control in the ORBIT‐AF Registry. Furthermore, use of anticoagulants was slightly higher in the ORBIT‐AF Registry at baseline (76% versus 64%), although the 64% at baseline in the AFFECTS Registry increased to 73% at the end of the study period. We found similar rates of warfarin utilization among the 3 provider groups but different rates of overall anticoagulation use, with cardiology and EP providers prescribing warfarin or dabigatran more often than the internal medicine providers. These differences did not persist after multivariable adjustment, although the results suggested a trend. We also assessed provider‐level treatment guideline adherence for anticoagulation therapy singly according to CHA2DS2‐VASc score. Patients with a contraindication for oral anticoagulation were excluded, and the results showed that EP providers were more prone to employing anticoagulation treatment compared with cardiology providers and internal medicine/general practitioner providers for CHA2DS2‐VASc scores ≥2. For CHA2DS2‐VASc scores of 0 and 1, EP providers again tended to prescribe anticoagulants more often, but this did not reach statistical significance. Hence, EP providers are seemingly more aggressive in terms of stroke prophylaxis, and our results suggest that they adhere better to treatment guidelines compared with cardiology and internal medicine/general practitioner providers. Similarly, EP providers in the ORBIT‐AF Registry were more likely to select a rhythm control strategy than were the cardiology providers. This association persisted after accounting for type of AF and other patient characteristics. Overall, EP providers elected to prescribe stroke prevention prophylaxis and attempted to maintain sinus rhythm more frequently than other providers, yet the relative differences were moderate.
Provider specialty‐specific data for AF quality of care are sparse. In prior studies of heart failure management, patients treated by cardiologists tended to have higher resource utilization and better short‐term outcomes compared with other provider types.9–14 Atrial fibrillation is a chronic condition with substantial morbidity and has similarities as well as clinical overlap with both heart failure and the chronic phase of MI/stable angina, so it is not surprising that we observed similar management trends in our study. In general, patients cared for by EP providers received more rhythm control than rate control. We also observed that newer drugs, such as dabigatran, were used more often by EP and cardiology providers. In addition, we observed an interesting difference in patient race: white patients generally received more specialty care than patients of other races. Finally, patients in the EP group had more private health insurance and were less likely to have no health insurance than those in the other groups. This could easily influence the reference patterns and patient profiles in the groups. Importantly, we accounted for this in our models, and our findings in Table 3 were independent of this observed difference.
Modern systems of health care are moving more toward multispecialty care and “disease‐state” centers. Accountable care organizations incorporate multiple specialties and providers into the parallel treatment of patients. In clinical practice, AF patients often require multiple specialty care providers. Although the differences between providers are relatively small, future studies should examine if these differences in therapeutic management are associated with different/improved outcomes.
This was an observational study and had several limitations. First, we used data from a voluntary clinical registry, so extrapolation to other care settings and providers should be done cautiously. Second, the provider specialty reported was the specialty of the clinic administrator and not of the individual clinician treating the patient. Third, we selected the types of practices participating in ORBIT‐AF; therefore, these results may not be fully representative of the overall distribution of provider specialties caring for AF in community practice. Finally, we do not have any information regarding prior patient physician contact and possible treatments for AF. In turn, information regarding the specific reason for AF care referral was not obtainable.
In conclusion, we found that in this large registry of outpatient AF patients, the delivering care provider's specialty was associated with differences in patient demographics, as well as in the care provided. Electrophysiologists were more likely to employ stroke prevention and rhythm control treatment strategies for their patients, whereas the patients of internal medicine/family practice providers received fewer antithrombotic agents. Patients cared for by internal medicine/family medicine providers were generally older. In light of our study's findings, appropriately risk‐adjusted outcomes data for comparison of various providers should be developed. These data should include net benefit estimations including thrombosis, as well as bleeding risks.
Sources of Funding
This work was supported by an award from the American Heart Association‐Pharmaceutical Roundtable and David and Stevie Spina.
Disclosures
Dr Fosbol, Ms Holmes, Dr Thomas, and Dr Gersh have no relevant disclosures to report. Dr Piccini reports being a consultant/on the advisory board for Forest Laboratories (modest), Medtronic (modest), and Johnson & Johnson (significant); and receiving research grants from Johnson & Johnson (significant). Dr Reiffel reports being a consultant/on the advisory board for Boehringer Ingelheim (modest) and Sanofi Aventis (modest). R. M. Mills and K. Mahaffey: TBD. Dr Kowey reports being a consultant/on the advisory board for Boehringer Ingelheim, Johnson & Johnson, Portola, Merck, BMS, Pfizer, AstraZeneca, Sanofi Aventis, and Daiichi‐Sankyo (all modest). Dr Peterson reports receiving research grants from Johnson & Johnson and Eli Lilly (both significant); being a consultant/on the advisory board for Merck, Johnson & Johnson, Pfizer, and Sanofi‐Aventis (all modest).
Acknowledgments
The authors thank Erin LoFrese, MS, for her editorial contributions to this article. Ms LoFrese did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted. The authors also thank the ORBIT‐AF investigators and patients.
References
- 1.Lloyd‐Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004; 110:1042-1046 [DOI] [PubMed] [Google Scholar]
- 2.Olesen JB, Lip GY, Lane DA. An epidemic of atrial fibrillation? Europace. 2011; 13:1059-1060 [DOI] [PubMed] [Google Scholar]
- 3.Stefansdottir H, Aspelund T, Gudnason V, Arnar DO. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace. 2011; 13:1110-1117 [DOI] [PubMed] [Google Scholar]
- 4.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006; 114:119-125 [DOI] [PubMed] [Google Scholar]
- 5.Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM, Camm J, Akhtar M, Luderitz B. The impairment of health‐related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000; 36:1303-1309 [DOI] [PubMed] [Google Scholar]
- 6.Singh SN, Tang XC, Singh BN, Dorian P, Reda DJ, Harris CL, Fletcher RD, Sharma SC, Atwood JE, Jacobson AK, Lewis HD, Jr, Lopez B, Raisch DW, Ezekowitz MD. Quality of life and exercise performance in patients in sinus rhythm versus persistent atrial fibrillation: a Veterans Affairs Cooperative Studies Program Substudy. J Am Coll Cardiol. 2006; 48:721-730 [DOI] [PubMed] [Google Scholar]
- 7.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991; 22:983-988 [DOI] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998; 98:946-952 [DOI] [PubMed] [Google Scholar]
- 9.Auerbach AD, Hamel MB, Davis RB, Connors AF, Jr, Regueiro C, Desbiens N, Goldman L, Califf RM, Dawson NV, Wenger N, Vidaillet H, Phillips RS. Resource use and survival of patients hospitalized with congestive heart failure: differences in care by specialty of the attending physician. SUPPORT investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med. 2000; 132:191-200 [DOI] [PubMed] [Google Scholar]
- 10.Boom NK, Lee DS, Tu JV. Comparison of processes of care and clinical outcomes for patients newly hospitalized for heart failure attended by different physician specialists. Am Heart J. 2012; 163:252-259 [DOI] [PubMed] [Google Scholar]
- 11.Foody JM, Rathore SS, Wang Y, Herrin J, Masoudi FA, Havranek EP, Krumholz HM. Physician specialty and mortality among elderly patients hospitalized with heart failure. Am J Med. 2005; 118:1120-1125 [DOI] [PubMed] [Google Scholar]
- 12.Jong P, Gong Y, Liu PP, Austin PC, Lee DS, Tu JV. Care and outcomes of patients newly hospitalized for heart failure in the community treated by cardiologists compared with other specialists. Circulation. 2003; 108:184-191 [DOI] [PubMed] [Google Scholar]
- 13.Philbin EF, Jenkins PL. Differences between patients with heart failure treated by cardiologists, internists, family physicians, and other physicians: analysis of a large, statewide database. Am Heart J. 2000; 139:491-496 [DOI] [PubMed] [Google Scholar]
- 14.Reis SE, Holubkov R, Edmundowicz D, McNamara DM, Zell KA, Detre KM, Feldman AM. Treatment of patients admitted to the hospital with congestive heart failure: specialty‐related disparities in practice patterns and outcomes. J Am Coll Cardiol. 1997; 30:733-738 [DOI] [PubMed] [Google Scholar]
- 15.Birkhead JS, Weston C, Lowe D. Impact of specialty of admitting physician and type of hospital on care and outcome for myocardial infarction in England and Wales during 2004‐5: observational study. BMJ. 2006; 332:1306-1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jollis JG, Peterson ED, Nelson CL, Stafford JA, DeLong ER, Muhlbaier LH, Mark DB. Relationship between physician and hospital coronary angioplasty volume and outcome in elderly patients. Circulation. 1997; 95:2485-2491 [DOI] [PubMed] [Google Scholar]
- 17.Piccini JP, Fraulo ES, Ansell JE, Fonarow GC, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Kong MH, Lopes RD, Mills RM, Peterson ED. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT‐AF. Am Heart J. 2011; 162:606.e1-612.e1 [DOI] [PubMed] [Google Scholar]
- 18.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006; 114:e257-e354 [DOI] [PubMed] [Google Scholar]
- 19.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001; 285:2864-2870 [DOI] [PubMed] [Google Scholar]
- 20.Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, Pollack CV, Jr, Peterson ED, Alexander KP. Baseline risk of major bleeding in non‐ST‐segment‐elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) Bleeding Score. Circulation. 2009; 119:1873-1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Dimarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D, Prystowsky EN, Damiano R, Jr, Jackman WM, Marchlinski F, McCarthy P. 2012 HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012; 9:632.e21-696.e21 [DOI] [PubMed] [Google Scholar]
- 22.Reiffel JA, Kowey PR, Myerburg R, Naccarelli GV, Packer DL, Pratt CM, Reiter MJ, Waldo AL. Practice patterns among United States cardiologists for managing adults with atrial fibrillation (from the AFFECTS Registry). Am J Cardiol. 2010; 105:1122-1129 [DOI] [PubMed] [Google Scholar]
- 23.Kowey PR, Reiffel JA, Myerburg R, Naccarelli GV, Packer DL, Pratt CM, Reiter MJ, Waldo AL. Warfarin and aspirin use in atrial fibrillation among practicing cardiologist (from the AFFECTS Registry). Am J Cardiol. 2010; 105:1130-1134 [DOI] [PubMed] [Google Scholar]


