Abstract
Background
The purpose of this study was to define the incidence, imaging characteristics, natural history, and prognostic implication of corticospinal tract Wallerian degeneration (CST‐WD) in spontaneous intracerebral hemorrhage (ICH) using serial MR imaging.
Methods and Results
Consecutive ICH patients with supratentorial ICH prospectively underwent serial MRIs at 2, 7, 14, and 21 days. MRIs were analyzed by independent raters for the presence and topographical distribution of CST‐WD on diffusion‐weighted imaging (DWI). Baseline demographics, hematoma characteristics, ICH score, and admission National Institute of Health Stroke Score (NIHSS) were systematically recorded. Functional outcome at 3 months was assessed by the modified Rankin Scale (mRS) and the motor‐NIHSS. Twenty‐seven patients underwent 93 MRIs; 88 of these were serially obtained in the first month. In 13 patients (48%), all with deep ICH, CST‐WD changes were observed after a median of 7 days (interquartile range, 7 to 8) as reduced diffusion on DWI and progressed rostrocaudally along the CST. CST‐WD changes evolved into T2‐hyperintense areas after a median of 11 days (interquartile range, 6 to 14) and became atrophic on MRIs obtained after 3 months. In univariate analyses, the presence of CST‐WD was associated with poor functional outcome (ie, mRS 4 to 6; P=0.046) and worse motor‐NIHSS (5 versus 1, P=0.001) at 3 months.
Conclusions
Wallerian degeneration along the CST is common in spontaneous supratentorial ICH, particularly in deep ICH. It can be detected 1 week after ICH on DWI and progresses rostrocaudally along the CST over time. The presence of CST‐WD is associated with poor motor and functional recovery after ICH.
Keywords: diffusion‐weighted imaging, intracerebral hemorrhage, magnetic resonance imaging, natural history, prognosis, wallerian degeneration
Introduction
Wallerian degeneration (WD) refers to the pathological process of secondary degeneration of axons and myelin sheaths that occurs distal to the site of acute injury to the nerve cell body or proximal axon. It was first described histopathologically by Waller in 1850.1 In the brain, WD occurs after a variety of insults including ischemic stroke, multiple sclerosis, traumatic brain injury, and intracerebral hemorrhage (ICH).2–7
Wallerian degeneration in the brain is most commonly identified along the corticospinal tract (CST) but can also occur along other white matter tracts including the corpus callosum.8–9 Histopathologically, WD begins in the first week after an acute brain injury and progresses through several pathologically distinct stages over the next 3 to 6 months.10 In its early stage, cessation of membrane energy‐dependent transport leads to cytotoxic edema and increased intracellular water. Newer magnetic resonance imaging (MRI) sequences such as diffusion‐weighted imaging (DWI) and diffusion tensor imaging (DTI) are exquisitely sensitive to altered tissue water content and can therefore identify WD quite early.11–15 The early stage of CST‐WD can be detected on DWI as areas of reduced diffusion along the CST‐WD and on T2 and FLAIR (fluid attenuated inversion recovery) as hyperintense (bright) areas in the same location.2,5–6,13
Spontaneous ICH affects about 1 million people worldwide annually and is associated with a higher mortality and worse functional outcome than ischemic stroke. Functional outcome after stroke is highly dependent on the degree of motor recovery. Early identification of patients who are likely to have limited motor recovery is useful, not only for prognostication, but also for appropriate patient selection into neurorecovery and acute rehabilitation trials. Histologically, WD of the white matter tracts in adults is irreversible because of limited neuronal capacity for repair and regeneration.10–11 Therefore, an association of CST‐WD with poor motor recovery is plausible, and CST‐WD may in fact serve as a surrogate imaging marker for poor functional recovery after ICH, similar to what is known in pediatric and ischemic stroke patients.16–22 To our knowledge, the natural history and prognostic implications of CST‐WD in ICH patients have not been systematically studied.
In this report, we describe the incidence, MR imaging characteristics, temporal profile, and prognostic implication of CST‐WD in a prospective cohort of 27 consecutive patients with spontaneous supratentorial ICH using serial multimodality MRI. We hypothesized that patients with MRI evidence of CST‐WD would be more likely to have poor motor recovery and worse functional outcomes than patients without CST‐WD.
Methods
After our institutional review board approval, consecutive patients with supratentorial spontaneous ICH of ≥5 and <100 cm3 with symptom onset <24 hours before admission and a Glasgow Coma Scale (GCS) score of ≥6 were prospectively enrolled after obtaining informed consent from a patient or designated surrogate.23 Exclusion criteria included the inability to undergo MRI because of metallic objects or unstable medical condition, systemic disease with a life expectancy <3 months, hematoma evacuation, recombinant factor VIIa administration, intraventricular hemorrhage (IVH) with a Graeb score of ≥8 and ICH due to known or suspected coagulopathy or underlying structural lesion.
Clinical Data
Patients were medically managed according to the contemporaneous AHA guidelines for ICH management.24 Demographic and laboratory data were collected prospectively. Patients' GCS, ICH score, and National Institute of Health Stroke Score (NIHSS) were collected on admission. Functional outcome scales including modified Rankin Scale (mRS), Barthel index (BI), extended Glasgow outcome scale (eGOS), and NIHSS were prospectively collected at 3 months by clinic follow‐up or telephone interview.
Imaging Protocol
All patients underwent a baseline noncontrast head CT. Serial MR imaging was performed at prespecified time intervals on a 1.5‐T GE Signa Horizon NV/i scanner (EXCITE III) equipped with cardiac‐enhanced gradients (40 mT/m): 48±12 hours, 7±1 days, 14±2 days, and, when feasible, 21±3 days after ICH onset. MRI scans were characterized by the following parameters: T1 localizer, axial T2, gradient recall echo (GRE; repetition time/echo time=550/30 ms, 24 contiguous sections, 256×256 matrix, field of view=24 cm, 5‐/1.5‐mm slice thickness/gap), spin echo echo‐planar imaging DWI (256×256 acquisition matrix; field of view=24 cm; 5‐/1.5‐mm slice thickness/gap; 20 to 23 contiguous sections; x, y, and z axes averaged; b=0 and 1000 seconds/mm2; repetition time/echo time=6000/72 ms) with corresponding apparent diffusion coefficient (ADC) maps, fast spin‐echo FLAIR imaging (repetition time/echo time=8802/120 ms, 24 contiguous sections, 512×512 matrix, field of view=24 cm, slice thickness per gap=5/1.5 mm), 3‐dimensional time‐of‐flight MR angiography and T1 postgadolinium sequences.
Image Analysis
Hematoma volumes were measured on the admission CT and the FLAIR sequence of the initial MRI with strong correlation between these volumes (r2=0.93).23 Hematomas were classified as lobar or deep. Two experienced and board‐certified neuroradiologists and 2 board‐certified stroke neurologists independently reviewed DWI, ADC, T1, T2, FLAIR, and GRE sequences on all MRIs for the presence of CST‐WD along the posterior limb of the internal capsule, cerebral peduncle, pons, and medulla. Signal changes on DWI attributed to WD were defined as areas of reduced diffusion (hyperintensity on b1000 and decreased intensity on ADC), on ≥2 consecutive levels (eg, posterior limb of internal capsule and peduncle or peduncle and pons) along the CST, in the absence of hemorrhage on the corresponding CT slices, FLAIR, T1, and GRE sequences. The DWI and ADC sequences were evaluated together, similar to what is practiced in routine clinical MRI interpretation. When there was ICH extension into the posterior limb of the internal capsule, WD was identified along the CST caudal to the ICH extension. Perihematomal edema was distinguished from WD by the absence of restricted diffusion on ADC in the former. The timing, appearance, and duration of persistence of CST‐WD‐related signal changes on DWI, ADC, T2, and/or FLAIR were recorded for each patient. The corpus callosum was also evaluated for WD changes using similar definitions on DWI/ADC. When available, late (>3 months) MRIs were also reviewed for atrophy in the same areas that previously demonstrated WD‐related restricted diffusion or T2 changes in the CST‐WD or corpus callosum.
Outcomes
Poor functional outcome was categorized as follows: mRS, 4 to 6 versus 0 to 3; BI, <60 versus ≥60; eGOS, <4 versus ≥4; NIHSS, 0 to 8 versus 9 to 19 versus ≥20; and motor‐NIHSS scores, 3 or 4 on either arm or leg versus 0 to 2 on both arm and leg.18
Statistical Analysis
Interrater reliability for the presence of WD was assessed with a kappa statistic. A kappa >0.8 was considered almost perfect or excellent.25 The Mann–Whitney U test was used to compare medians, the t test to compare means, and Fisher's exact test to compare proportions. Associations between functional outcome and common ICH outcome predictors including age, ICH location, ICH volume, history of hypertension, IVH, admission GCS, admission NIHSS, ICH score and CST‐WD were tested. All statistical tests were 2 tailed, and the level of significance was defined at α<0.05. IBM SPSS version 20 was used for statistical analysis.
Results
Demographics
Twenty‐seven patients with spontaneous supratentorial ICH were included in this study from 32 eligible patients. Reasons for exclusion were: 1 each due to spinal deformity precluding MR imaging, surgical hematoma evacuation due to deterioration, and withdrawal of care <7 days and 2 due to hospital discharge <7 days. Of these 5 excluded patients, 3 had lobar ICH. The excluded patients were similar to our final cohort, with a mean age of 64±18 years and a median ICH score of 1 (0.75 to 3).
A total of 93 MRIs with DWIs were available for analysis, of which 88 were obtained in the first month after ICH at the following times (median [interquartile range]): MRI #1 (n=27) at 29 hours (17.5 to 43.8 hours), MRI #2 (n=27) at 7.2 days (6.6 to 8.5 days), MRI #3 (n=22) at 13.9 days (13 to 16 days), and MRI #4 (n=12) at 22.1 days (19.7 to 24.1 days). Some of the reasons for not getting the third and/or fourth MRIs were hospital discharge (n=11), death within the first month (n=1), withdrawal of consent (n=1), and refusal to undergo further MRIs (n=2). Five late MRIs (3 deep and 2 lobar ICH) were obtained, of which 4 were at 3 months and 1 was at 2 years. Patients who had only 2 MRIs were similar to those who received >2 MRIs with the exception of admission NIHSS (5 versus 12, P=0.07). All studies were of adequate quality for interpretation.
All patients were diagnosed as having a primary supratentorial ICH after a standardized diagnostic workup that included a detailed medical history, laboratory work including toxicology, and an echocardiogram. MR angiography was performed in all patients. Contrast angiography or CTA was performed in selected patients on the basis of clinical suspicion. Seventeen patients (63%) had deep ICH (Table 1). The topography of the lobar hematomas was temporoparietal (4), frontal (2), parieto‐occipital (2), and parietal (2). Extension into the motor cortex was seen in the 3 patients with either a parietal or frontal hematoma.
Table 1.
Baseline Demographics of 27 ICH Patients Grouped by Those With and Without Corticospinal Tract Wallerian Degeneration on MRI
| Patient Characteristics | WD in the CST (n=13) | No WD in the CST (n=14) | P Value |
|---|---|---|---|
| Age (y), mean | 55 | 65 | 0.082 |
| Women | 31% | 29% | 1 |
| Deep ICH, n=17 | 100% | 29% | <0.0001 |
| ICH etiology | |||
| Hypertension | 100% | 64% | 0.041 |
| Cerebral amyloid angiopathy | 21% | ||
| Unknown | 15% | ||
| Median ICH volume (cc) | 31 (18 to 50) | 25 (16 to 52) | 0.65 |
| IVH extension | 46% | 7% | 0.03 |
| Location of WD | |||
| Internal capsule | 11 (85%) | ||
| Cerebral peduncle | 13 (100%) | ||
| Pons | 6 (46%) | ||
| Medulla | 2 (15%) | ||
| Corpus callosum* | 3 (21%) | ||
| Admission GCS, median (IQR) | 12 (9.5 to 15) | 15 (13 to 15) | 0.44 |
| Admission NIHSS, median (IQR) | 17 (12 to 22) | 8 (6 to 12) | 0.002 |
| No. of MRIs/patient, median (IQR) | 3 (3 to 4) | 3 (2 to 4) | 0.28 |
| Timing of MRIs, median (IQR) | |||
| MRI #1 (in hours), n=27 | 29 (18 to 41) | 32 (17 to 46) | 0.68 |
| MRI #2 (in days), n=27 | 7 (6.8 to 8.1) | 7 (6 to 9) | 1.0 |
| MRI #3 (in days), n=22 | 14 (13 to 15) | 15 (14 to 18) | 0.13 |
| MRI #4 (in days), n=12 | 21 (19 to 23) | 25 (21 to 26) | 0.15 |
| Three‐month outcome, median (IQR) | |||
| NIHSS | 10 (8 to 11) | 5 (2 to 19) | 0.17 |
| NIHSS (0 to 8/9 to 19/≥20) | 31%/69%/0% | 71%/7%/21% | 0.001 |
| Modified Rankin score | 4 (4 to 4) | 3 (2 to 5) | 0.42 |
| Modified Rankin 4 to 6 | 85% | 43% | 0.046 |
| eGOS ≥4 | 2 (17%) | 8 (57%) | 0.051 |
| Barthel index | 30 (20 to 50) | 73 (0 to 100) | 0.25 |
| Barthel index <60 | 10 (77%) | 7 (50%) | 0.24 |
| Motor‐NIHSS | 5 (3 to 6) | 1 (0 to 2) | 0.001 |
WD indicates Wallerian degeneration; CST, corticospinal tract; ICH, intracerebral hemorrhage; MRI, magnetic resonance imaging; IVH, intraventricular hemorrhage; GCS, Glasgow Coma Scale; NIHSS, National Institute of Health Stroke Score; eGOS, extended Glasgow outcome scale; DWI, diffusion‐weighted imaging; ADC, apparent diffusion coefficient.
Three patients with lobar ICH (2 parieto‐occipital and 1 parietal) had DWI/ADC changes of presumed wallerian degeneration in the splenium of the corpus callosum.
Spatial Profile of Corticospinal Tract Wallerian Degeneration
Thirteen patients (48%) exhibited CST‐WD. Interrater reliability for the presence of CST‐WD on DWI/ADC was excellent, with a kappa statistic of 0.82 (95% CI, 0.76 to 0.88) and an absolute agreement of 91%. All 13 patients with CST‐WD had deep ICH versus 29% (4 of 14) of those without CST‐WD (P<0.0001). Of the 17 patients with deep ICH, 13 (76%) had CST‐WD. Extension of deep ICH into the posterior limb of the internal capsule was seen in 13 patients (76%), of whom 9 (69%) had CST‐WD below the caudal extent of ICH extension. CST‐WD was seen at the following levels in order of frequency: ipsilateral cerebral peduncle (n=13), posterior limb of the internal capsule (n=11), pons (n=6), and medulla (n=2) (Figure 1).
Figure 1.
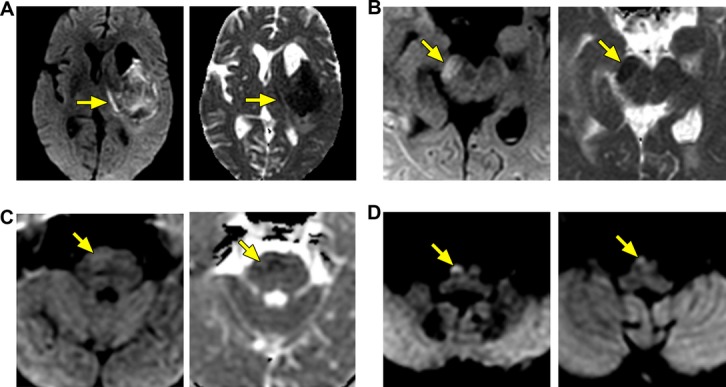
Wallerian degeneration (WD) in the corticospinal tract. Diffusion‐weighted imaging (DWI) and corresponding apparent diffusion coefficient (ADC) maps in 3 patients with WD along the corticospinal tract. Early on, WD appears bright on DWI and dark on ADC (ie, reduced diffusion). WD along the corticospinal tract, typically observed in deep hemorrhages, is shown in the internal capsule (A), the cerebral peduncle (B), the pons (C), and the medullary pyramid (D).
Temporal Profile of Corticospinal Tract Wallerian Degeneration
Wallerian degeneration was not observed on the initial MRI in 6 of the 13 patients (46%) who eventually showed CST‐WD. The DWI changes of CST‐WD were first identified in the posterior limb of the ipsilateral internal capsule at a median of 7 days (interquartile range [IQR], 7 to 8 days), followed by the cerebral peduncle at a median of 7 days (IQR, 4 to 8 days) and in the medulla at around 2 weeks (IQR, 14 and 16 days) (Figure 2). In patients with CST‐WD identified at multiple levels, changes in the caudal portions of the CST were observed later (median, 8 days; IQR, 7 to 10 days) than in the proximal portions (median, 7 days; IQR, 5 to 8 days).
Figure 2.
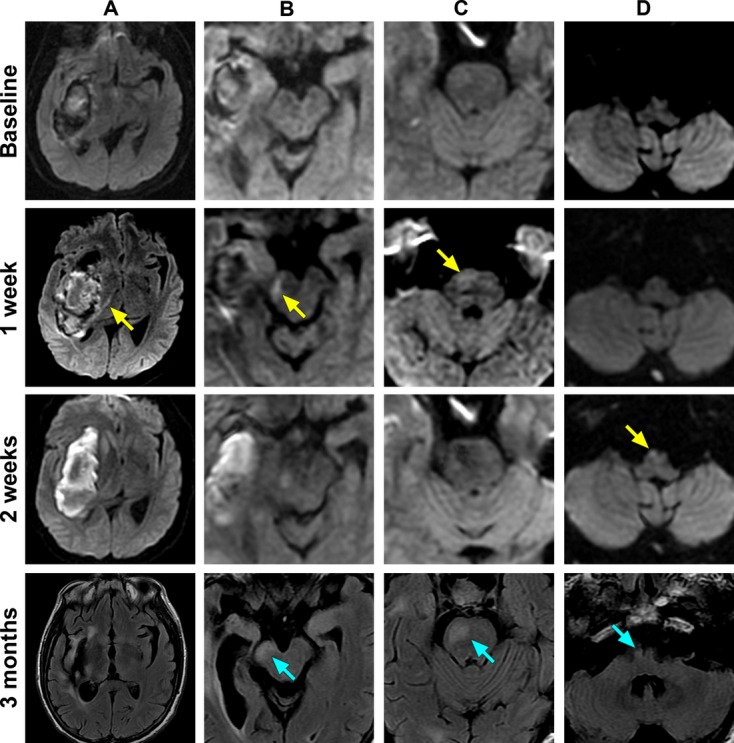
Spatial and temporal evolution of Wallerian degeneration in a single patient. Serial diffusion‐weighted imaging magnetic resonance images (MRIs) are shown for a single patient with a putaminal hemorrhage, obtained 2 days, 1 week, and 2 weeks after symptom onset. Corresponding fluid attenuated inversion recovery (FLAIR) sequences are shown at 3 months. A, Internal capsule. B, Cerebral peduncle. C, Pons. D, Medulla. Restricted diffusion appears along the ipsilateral corticospinal tract at 1 week in the posterior limb of the internal capsule, cerebral peduncle, and pons (arrows). At 2 weeks restricted diffusion appears in the medullary pyramid (arrow). At 3 months, areas that previously showed restricted diffusion are hyperintense on FLAIR and have undergone atrophy (arrows).
Imaging Characteristics of Corticospinal Tract Wallerian Degeneration
Corticospinal tract WD was first appreciated as high signal intensity (ie, bright) on DWI and as corresponding hypointensity (ie, dark) on ADC. Reduced diffusion on ADC lasted for a median of 11 days (IQR, 8 to 12 days) before becoming hyperintense. T2/FLAIR showed hyperintensity in the same areas at a median of 11 days (IQR, 6 to 14 days) in 12 of 13 patients. None of the patients in this cohort had CST‐WD identified first on T2/FLAIR in the later MRIs without it being already evident on DWI/ADC.
Three patients with deep ICH who had acute CST‐WD DWI changes had a follow‐up MRI at >3 months. In these patients, there was persistent T2 hyperintensity, particularly in the cerebral peduncle with ipsilateral peduncular atrophy.
Corpus Callosum Wallerian Degeneration
Three patients with lobar ICH (2 parieto‐occipital and 1 parietal) had reduced diffusion on DWI/ADC at a median of 9 days in the splenium of the corpus callosum (Figure 3). One of these patients had an MRI at >3 months, and this showed persistent T2 hyperintensity in the splenium with splenial atrophy.
Figure 3.
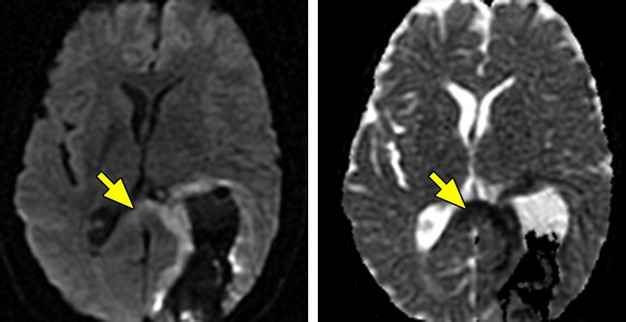
Wallerian degeneration in the corpus callosum. Diffusion‐weighted imaging (DWI) and corresponding apparent diffusion coefficient (ADC) map in a patient with a large parieto‐occipital lobar intracerebral hemorrhage, showing reduced diffusion (bright on DWI and dark on ADC) in the splenium of the corpus callosum from Wallerian degeneration.
Functional Outcome in Patients With Corticospinal Tract Wallerian Degeneration
At the 3‐month follow‐up, patients with CST‐WD had a trend (though not statistically significant in this sample size) for worse median BI scores (30 [20 to 68] versus 72 [33 to 98], P=0.251) and a higher proportion of patients with BI <60 (77% versus 50%, P=0.236). Motor‐NIHSS was significantly different between groups (5 versus 1, P=0.001). The results of univariate analyses are shown in Table 2. The presence of CST‐WD was associated with worse functional outcomes including mRS 4 to 6 (85% versus 43%, P=0.046) and eGOS ≥4 (17% versus 57%, P=0.051).
Table 2.
Univariate Analyses of ICH‐Related Factors and Functional Outcome Measures
| Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|
| eGOS | mRS | BI | NIHSS Motor Score >2 in Any Limb | |||||
| Predictor | eGOS ≥4, n=10 | eGOS 0 to 3, n=16 | mRS 4 to 6, n=17 | mRS 0 to 3, n=10 | BI <60, n=17 | BI 65 to 100, n=10 | Present, n=8 | Absent, n=16 |
| CST‐WD | 2 (20) | 10 (63)* | 11 (65) | 2 (20)* | 10 (59) | 3 (30) | 7 (88) | 6 (38)* |
| HTN | 6 (60) | 15 (94)* | 16 (94) | 6 (60)* | 16 (94) | 6 (60)* | 8 (100) | 11 (69) |
| Age, y | 61±17 | 58±13 | 60±12 | 60±17 | 60±12 | 60±17 | 58±15 | 60±13 |
| Location, deep | 4 (40) | 12 (75) | 13 (77) | 4 (40) | 13 (77) | 4 (40) | 7 (88) | 8 (50) |
| ICH volume (cc), median (IQR) | 37 (17 to 52) | 20 (14 to 30) | 20 (12 to 54) | 36 (20 to 52) | 21 (12 to 54) | 36 (18 to 52) | 20 (12 to 36) | 52 (37 to 78)* |
| ICH score, median (IQR) | 1.5 (1 to 2) | 0.5 (0 to 1)* | 1 (0 to 1.5) | 1 (1 to 2) | 1 (0 to 1.5) | 1 (1 to 2) | 1 (0 to 1.75) | 2 (1 to 2.75)* |
Age compared using t test; ICH volume and ICH score, Mann–Whitney test; CST‐WD, HTN, and location, Fisher's exact test. ICH indicates intracerebral hemorrhage; eGOS, extended Glasgow outcome scale; mRS, modified Rankin Scale; BI, Barthel index; NIHSS, National Institute of Health Stroke Score; CST‐WD, corticospinal tract Wallerian degeneration; HTN, hypertension.
P value for CST‐WD/eGOS is 0.051 and for HTN/eGOS is 0.055.
Indicates difference in outcomes is significant at the P<0.05 level for the respective comparisons.
Discussion
In this report we describe the incidence, spatial and temporal evolution, and prognostic significance of CST‐WD in 27 patients with supratentorial primary ICH who underwent serial MR imaging at predefined times during the first month after ICH. We observed that CST‐WD was detected on DWI/ADC as early as the first week after ICH, particularly in the rostral portion of the CST in 48% of our cohort, all of whom had deep ICH. Most importantly, the presence of CST‐WD was associated with poor functional outcome on the 3‐month mRS.
The CST‐WD was evident on DWI/ADC only in patients with deep ICH. The association of CST‐WD with IVH is likely because of this being a surrogate maker for deep ICH. Although it would have been reasonable a priori to exclude patients with ICH topography away from the CST or the motor cortex because of a low chance of damaging CST motor fibers, we chose not to do this, as the data on who develops CST‐WD after ICH are scarce. For example, lobar ICH, despite being of reasonable size and extending into the motor cortex in 3 of our patients, did not produce CST‐WD changes on DWI/ADC in our cohort. This observation differs from observations in acute ischemic stroke involving the MCA territory, where CST‐WD has been reported even with moderate‐sized strokes.13,18 We hypothesize that the lobar ICHs in our cohort (median volume, 44 cc) may not have been extensive enough to injure a sufficient number of cell bodies/axons to produce signal changes on DWI/ADC in the CST. Alternatively, we may have missed subtle changes that may be detected by a more sensitive technique such as DTI. The importance of location is underscored by the finding that ICH volume itself was not associated with CST‐WD.
Wallerian degeneration of the splenium has been described in the literature from pathological studies and DTI in patients with destructive lesion of the cerebral hemispheres (eg, middle cerebral artery stroke).26–27 We postulate that the splenial reduced diffusion in the acute stage after lobar ICH is consistent with WD of the corticocallosal fibers that connect the temporal and parietal lobes to the splenium. This again underscores the importance of the location of ICH (ie, deep) in causing CST‐WD.
We used routine DWI/ADC sequences to detect CST‐WD (as opposed to using, for example, DTI or quantitative ADC analyses), as this sequence is readily available on routine clinical MRIs and can be interpreted by clinicians without requiring sophisticated quantitation of ADC parameters.18 The DWI/ADC changes of CST‐WD were easy to detect visually and highly reproducible among independent raters.
The relative straightforwardness of visually detecting CST‐WD in ICH attests to the exquisite sensitivity of DWI to cytotoxic edema occurring in early WD (within the first week) from focal axonal swelling because of cessation of energy‐dependent transport.11,28–29 In a recent article, a decrease in axonal neurofilament staining due to WD was seen as early as 1 to 3 days along the ipsilateral CST with a corresponding DWI hyperintensity/ADC reduction in a rat model of unilateral cortical/subcortical ischemic insult.29 This supports the notion that early DWI/ADC changes along the CST do indeed correspond to early stages of WD. The variation in reports in the literature of CST‐WD producing reduced versus facilitated diffusion on DWI/ADC is likely a result of variability in timing of the MRI.30–32
It is important that CST‐WD changes on DWI in patients with spontaneous ICH are not confused with ischemia, tissue compression, or spread of perihematomal edema along the white matter tracts.33 The DWI changes along a well‐defined tract (ie, CST),34 nonconformation to a single vascular territory, and the rostrocaudal progression along the CST over time should help to avoid misinterpretation.
In neonatal and adult ischemic stroke, early CST DWI changes correlate with the degree of long‐term motor and functional recovery.16–22,16–36 Similarly, in our ICH cohort, the presence of CST‐WD was associated with worse 3‐month motor outcome on motor‐NIHSS and worse functional outcome on mRS and eGOS. Given the sample size in this cohort, we are unable to definitively conclude whether these associations are independent of known ICH prognostic factors such as age and hematoma volume.
A potential limitation of this study is also the nonavailability of all 4 MRIs in all patients. Five patients had only the first 2 MRIs (3 lobar, 2 deep), but there was no difference in the timing of these MRIs from that of the rest of the cohort. We acknowledge that we may have missed DWI changes in CST‐WD or the corpus callosum, particularly if the early DWI changes were subtle.7 We think that this scenario is less likely because we did not observe any CST‐WD changes on T2/FLAIR in the rest of the patients who had more longitudinal MRIs without already having identified these changes initially on DWI. Further, of the 5 patients who had only 2 MRIs, 3 had lobar ICH. As mentioned previously, none of the lobar ICH had CST‐WD despite extension of the ICH in the motor cortex. However, we may have missed corpus callosum WD in the lobar ICH patients with only 2 MRIs.
The strengths of our study are its prospective design with systematic, serial MR imaging in patients with primary ICH who are likely to survive to hospital discharge. Limitations include the modest sample size, fewer MRIs after the first 2 weeks, the lack of quantitative MRI analyses, and the absence of concurrent pathology. Despite these limitations, we found that CST‐WD can be easily detected by routine MRI 1 week after spontaneous ICH, and this is associated with poor motor and functional recovery after ICH.
Conclusions
In summary, CST‐WD is common after primary supratentorial ICH, especially in those with deep ICH, and can be readily detected early, by the end of the first week on routine DWI/ADC sequences. In adult ICH, CST‐WD is an underappreciated finding. The presence of CST‐WD is a marker for poor motor recovery and functional outcome, and its routine detection in ICH patients may facilitate prognostication and patient selection for neuroprotection and rehabilitation trials. Future, larger studies will be needed to determine if CST‐WD as detected on routine MRI offers independent prognostic information for functional outcome.
Sources of Funding
This study was supported by the Neurocritical Care Society Career Development Fellowship in Cerebrovascular Disease and Neurotrauma (to Dr Venkatasubramanian), NIH 2RO1 NS 034866‐08 (to Dr Wijman), the Stanford School of Medicine Medical Scholars program (to Dr Kleinman), and PDL Biopharma, Inc (to Dr Wijman).
Disclosures
None.
Acknowledgments
Statistical analyses were performed by Michael Mlynash (MS Epidemiology).
References
- 1.Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog and observations of the alterations produced thereby in the structure of their primitive fibre. Philos Trans R Soc. 1850; 140:423-429 [Google Scholar]
- 2.Sawlani V, Gupta R, Singh M, Kohli A. MRI demonstration of wallerian degeneration in various intracranial lesions and its clinical implications. J Neurol Sci. 1997; 146:103-108 [DOI] [PubMed] [Google Scholar]
- 3.Ajilogba K, Rao P. Diffusion‐weighted imaging of wallerian degeneration in non‐accidental head injury. Pediatr Radiol. 2006; 36:1326. [DOI] [PubMed] [Google Scholar]
- 4.Cobb S, Mehringer C. Wallerian degeneration in a patient with schilder disease: MR imaging demonstration. Radiology. 1987; 162:521-522 [DOI] [PubMed] [Google Scholar]
- 5.Orita T, Tsurutani T, Izumihara A, Kajiwara K. Early, evolving wallerian degeneration of the pyramidal tract in cerebrovascular diseases: MR study. J Comput Assist Tomogr. 1994; 18:943-946 [DOI] [PubMed] [Google Scholar]
- 6.Uchino A, Imada H, Ohno M. MR imaging of wallerian degeneration in the human brain stem after ictus. Neuroradiology. 1990; 32:191-195 [DOI] [PubMed] [Google Scholar]
- 7.Kuhn M, Johnson K, Davis K. Wallerian degeneration: evaluation with MR imaging. Radiology. 1988; 168:199-202 [DOI] [PubMed] [Google Scholar]
- 8.Davidoff R. The pyramidal tract. Neurology. 1990; 40:332-339 [DOI] [PubMed] [Google Scholar]
- 9.Waragai M, Watanabe H, Iwabuchi S. The somatotopic localisation of the descending cortical tract in the cerebral peduncle: a study using MRI of changes following wallerian degeneration in the cerebral peduncle after a supratentorial vascular lesion. Neuroradiology. 1994; 36:402-404 [DOI] [PubMed] [Google Scholar]
- 10.McCaman R, Robins E. Quantitative biochemical studies of wallerian degeneration in the peripheral and central nervous systems. I. Chemical constituents. J Neurochem. 1959; 5:19-42 [Google Scholar]
- 11.DeWitt L, Kistler J, Miller D, Richardson EJ, Buonanno F. NMR‐neuropathologic correlation in stroke. Stroke. 1987; 18:342-351 [DOI] [PubMed] [Google Scholar]
- 12.Kuhn M, Mikulis D, Ayoub D, Kosofsky B, Davis K, Taveras J. Wallerian degeneration after vertebral infarction: evaluation with sequential MR imaging. Radiology. 1989; 172:179-182 [DOI] [PubMed] [Google Scholar]
- 13.Uchino A, Sawada A, Takase Y, Egashira R, Kudo S. Transient detection of early wallerian degeneration on diffusion‐weighted MRI after an acute cerebrovascular accident. Neuroradiology. 2004; 46:183-188 [DOI] [PubMed] [Google Scholar]
- 14.Werring D, Toosy A, Clark C, Parker G, Barker G, Miller D. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry. 2000; 69:269-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomalla G, Glauche V, Koch M, Beaulieu C, Weiller C, Rother J. Diffusion tensor imaging detects early wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004; 22:1767-1774 [DOI] [PubMed] [Google Scholar]
- 16.Cho S, Kim S, Choi S, Kang J, Lee C, Byun W. Motor outcome according to diffusion tensor tractography findings in the early stage of intracerebral hemorrhage. Neurosci Lett. 2007; 421:142-146 [DOI] [PubMed] [Google Scholar]
- 17.De Vries L, Van der Grond J, Van Haastert I, Groenendaal F. Prediction of outcome in new‐born infants with arterial ischaemic stroke using diffusion‐weighted magnetic resonance imaging. Neuropediatrics. 2005; 36:12-20 [DOI] [PubMed] [Google Scholar]
- 18.DeVetten G, Coutts S, Hill M, Goyal M, Eesa M, O'Brien B. Acute corticospinal tract wallerian degeneration is associated with stroke outcome. Stroke. 2010; 41:751-756 [DOI] [PubMed] [Google Scholar]
- 19.Domi T, deBever G, Shroff M, Kouzmitcheva E, MacGregor D, Kirton A. Corticospinal tract pre‐wallerian degeneration: a novel outcome predictor for pediatric stroke on acute MRI. Stroke. 2009; 40:780-787 [DOI] [PubMed] [Google Scholar]
- 20.Fumeya H, Hideshima H. Early MR abnormality indicating functional recovery from spontaneous intracerebral hemorrhage. Neurol Med Chir. 1991; 31:650-653 [DOI] [PubMed] [Google Scholar]
- 21.Kirton A, Shroff M, Visvanathan T, deVeber G. Quantified corticospinal tract diffusion restriction predicts neonatal stroke outcome. Stroke. 2007; 38:974-980 [DOI] [PubMed] [Google Scholar]
- 22.Puig J, Pedraza S, Blasco G, Daunis‐i‐Estadella J, Prats A, Prados F. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol. 2010; 31:1324-1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatasubramanian C, Mlynash M, Finley‐Cauldfield A, Eyngorn I, Kalimuthu R, Snider R. Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke. 2010; 42:73-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broderick J, Adams HJ, Barsan W, Feinberg W, Feldman E, Grotta J. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999; 30:905-915 [DOI] [PubMed] [Google Scholar]
- 25.Landis R, Koch G. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159-174 [PubMed] [Google Scholar]
- 26.DeLa Coste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. J Neuropathol Exp Neurol. 1985; 44:578-591 [DOI] [PubMed] [Google Scholar]
- 27.Gupta RK, Saksena S, Hasan KM, Agarwal A, Haris M, Pandey CM, Narayana PA. Focal wallerian degeneration of the corpus callosum in large middle cerebral artery stroke: serial diffusion tensor imaging. J Magn Reson Imaging. 2006; 24:549-555 [DOI] [PubMed] [Google Scholar]
- 28.Daniel P, Strich S. Histological observations on wallerian degeneration in the spinal cord of the baboon, Papio papio. Acta Neuropathol. 1969; 12:314-328 [DOI] [PubMed] [Google Scholar]
- 29.Lama S, Qiao M, Kirton A, Sun S, Cheng E, Foniok T, Tuor UI. Imaging corticospinal degeneration in neonatal rats with unilateral cerebral infarction. Exp Neurol. 2011; 228:192-199 [DOI] [PubMed] [Google Scholar]
- 30.Sylaja R, Goyal M, Watson T, Hill M. Wallerian‐like degeneration after ischemic stroke revealed by diffusion‐weighted imaging. Can J Neurol Sci. 2007; 34:243-244 [DOI] [PubMed] [Google Scholar]
- 31.Kang D, Chu K, Yoon B, Song I, Chang K, Roh J. Diffusion‐weighted imaging in wallerian degeneration. J Neurol Sci. 2000; 178:167-169 [DOI] [PubMed] [Google Scholar]
- 32.Groenendaal F, Benders M, De Vries L. Pre‐wallerian degeneration in the neonatal brain following perinatal cerebral hypoxia‐ischemia demonstrated with MRI. Semin Perinatol. 2006; 30:146-150 [DOI] [PubMed] [Google Scholar]
- 33.Wijdicks E. Neurological picture. Acute brainstem displacement without uncal herniation and posterior cerebral artery injury. J Neurol Neurosurg Psychiatry. 2008; 79:744. [DOI] [PubMed] [Google Scholar]
- 34.Pujol J, Marti‐Vilalta J, Junque C, Vendrell P, Fernandez J, Capdevila A. Wallerian degeneration of the pyramidal tract in capsular infarction studied by magnetic resonance imaging. Stroke. 1990; 21:404-409 [DOI] [PubMed] [Google Scholar]
- 35.Orita T, Tsurutani T, Izumihara A, Kajiwara K, Matsunaga T. Pyramidal tract wallerian degeneration and correlated symptoms in stroke. Eur J Radiol. 1994; 18:26-29 [DOI] [PubMed] [Google Scholar]
- 36.Sonoda S, Tsubahara A, Saito M, Chino N. Extent of pyramidal tract wallerian degeneration in the brain stem on MRI and degree of motor impairment after supratentorial stroke. Disabil Rehabil. 1992; 14:89-92 [DOI] [PubMed] [Google Scholar]


