Abstract
Background
Nuclear receptor Rev‐erbα plays important roles in circadian clock timing, lipid metabolism, adipogenesis, and vascular inflammation. However, the role of Rev‐erbα in atherosclerotic lesion development has not been assessed in vivo.
Methods and Results
The nuclear receptor Rev‐erbα was knocked down in mouse haematopoietic cells by means of shRNA‐lentiviral transduction, followed by bone marrow transplantation into LDL receptor knockout mice. The Rev‐erbα protein in peripheral macrophage was reduced by 70% as compared to control mice injected with nontargeting shRNA lentivirus‐transduced bone marrow. A significant increase in atherosclerotic lesions was observed around the aorta valves as well as upon en face aorta analysis of Rev‐erbα knock‐down bone marrow recipients (P<0.01) as compared to the control mice, while plasma cholesterol, phospholipid, and triacylglycerol levels were not affected. Overexpression of Rev‐erbα in bone marrow mononuclear cells decreased inflammatory M1 while increasing M2 macrophage markers, while Rev‐erbα knock down increased the macrophage inflammatory phenotype in vitro and in vivo. Furthermore, treatment of differentiating macrophages with the Rev‐erbα ligand heme promoted expression of antiinflammatory M2 markers.
Conclusions
These observations identify hematopoietic cell Rev‐erbα as a new modulator of atherogenesis in mice.
Keywords: atherosclerosis, macrophages, Rev‐erbα
Introduction
Atherosclerosis is a complex and chronic inflammatory disease of the vessel walls. A crucial step preceding the formation of atherosclerotic lesions is monocyte migration into the arterial wall and their differentiation into macrophages.1 Macrophages are important organizers of plaque inflammatory responses.2 Monocytes are heterogeneous cells, which, depending on the microenvironment, differentiate into distinct macrophage subsets (M1 and M2).3 Recent studies have revealed that the heterogeneity of macrophages may confer both atherogenic and atheroprotective properties.4–6 M1 macrophages activated by interferon (IFNγ) or lipopolysaccharide (LPS) are powerful effectors that can kill microorganisms and secrete proinflammatory cytokines such as tumor necrosis factor α (TNFα), interleukin‐6 (IL‐6), and interleukin‐12 (IL‐12),7–8 which have generally considered atherogenic functions. M2 macrophages polarized by interleukin‐4 (IL‐4) or interleukin‐13 (IL‐13) reduce the inflammatory response and produce antiinflammatory factors such as interleukin‐10 (IL‐10) and transforming growth factor β. M2 macrophages are thus able to remove metabolic debris, enhance angiogenesis, facilitate tissue remodeling and repair,9 and likely act in an atheroprotective fashion. This balance in macrophage M1/M2 activity is suggested to play an important role in the pathogenesis of atherosclerosis.10–11
Rev‐erbα (Nr1D1) is a member of the nuclear receptor (NR) superfamily.12 The NR1D1 gene is located on chromosome 17, and is transcribed from the antisense strand of the thyroid receptor α gene (TRα). Due to the lack of a ligand, Rev‐erbα was considered as an “orphan receptor” until 2007, when Raghuram et al found protoporphyrin or heme as ligands of Rev‐erbα and Rev‐erbβ.13–14 Rev‐erbα is expressed in a variety of tissues and cell types such as the heart, liver, adipose tissue, skeletal muscle, vascular smooth muscle cells (VSMCs), and macrophages.12,15–16 Recent studies have confirmed that Rev‐erbα is not only an important component of the biological clock, but also a regulator of energy balance, inflammation, and immunity, and may thus play an important role in metabolic and cardiovascular diseases.17–18
Emerging evidence suggests that Rev‐erbα affects the pathogenesis of atherosclerosis. Rev‐erbα has been implicated in the development of atherosclerosis by decreasing plasma triglyceride‐rich lipoproteins.19 In human macrophages, Rev‐erbα inhibits the induction of Toll‐like receptor 4, thereby reducing cytokine production induced by LPS, which prompts Rev‐erbα antiinflammatory functions and a potential atheroprotective action.20 Furthermore, Rev‐erbα may exert atheroprotective functions through negative regulation of plasminogen activator inhibitor (PAI)‐1, an important inhibitor of the fibrinolytic cascade that promotes the development of atherothrombosis. Taken together, Rev‐erbα regulates plasma glucose and lipid metabolism as well as inflammationm and may thus affect the development of atherosclerosis. However, its role in atherosclerosis has not been assessed in vivo.
In the present study, we investigate the consequences of macrophage Rev‐erbα knock‐down in the development of atherosclerotic lesions in LDLr−/− mice using the bone marrow transplantation technique and elucidate the possible underlying molecular mechanisms.
Methods
Animals
All animal experiments were approved by the Ethics Committee for Animal Experiments of Jinan University and performed in compliance with Chinese government guidelines. C57BL/6 mice were obtained from Animal Center of Guangdong Province. Homozygous LDL receptor knockout (LDLr−/−; C57BL/6) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) as mating pairs and bred at the Animal Centre of Jinan University. Mice were housed in sterilized filter‐top cages and given unlimited access to food and water with 12/12 dark/light cycles. Mice were maintained on sterilized regular chow, containing 4.3% (w/w) fat and no cholesterol (No. 201, Baiyun animal diet factory, Guangzhou, China), or fed a semisynthetic Western‐type diet, containing 15% (w/w) fat and 0.25% (w/w) cholesterol (No. 202, Baiyuan animal diet factory). Drinking water was supplied with antibiotics (83 mg/L ciprofloxacin and 67 mg/L polymyxin B sulphate) and 6.5 g/L sucrose.
Irradiation and Bone Marrow Transplantation
To induce bone marrow aplasia, female C57BL/6 mice (8 weeks of age) were exposed to a single dose of 9 Gy (0.19 Gy/minute, 200 kV, 4 mA) total body irradiation, using a γ‐ray source (Jixing Group) with a 6‐mm aluminium filter. The mice were then fed chow diet for 8 weeks. Bone marrow cell suspensions were isolated from C57BL/6 mice by flushing the femurs and tibias with PBS. Single‐cell suspensions were prepared by passing the cell mass through a cell strainer with 27 pond needle, and bone marrow cells (1.0×107) were transduced with either H1.shNT or H1.shRev‐erbα lentivirus in the presence of 10 μg/mL DEAE‐dextran (multiplicity of infection [moi]=15). Eighteen hours posttransduction, the cells sorted by flow cytometry; GFP‐positive cells were collected and injected into the tail vein of the irradiated recipients (1x107 cells/mouse, n=10 mice per group).
Atherosclerotic Lesion Analysis
Atherosclerotic plaque burden in the aorta (aortic root to the iliac bifurcation) was determined by oil red O staining. Transplanted LDLr−/− mice were anesthetized by intraperitoneal injection of 3 mg of xylozine and 3 mg of ketamine after 11 weeks on the Western‐type diet, and blood samples were collected via heart puncture for lipid profile analyses. The left ventricle of the heart was perfused first with PBS and then with a fixative solution (4% paraformaldehyde, 5% sucrose, 50 mmol/L EDTA, pH 7.4). After removal of outside connecting tissue and fat, the aorta was dissected from the aortic root to the iliac artery under the dissection microscope, cut open longitudinally in situ, and immersed in the fixative for 12 hours before being rinsed with 1× PBS and stained with oil red O (Sigma). Stained aortas were washed in 60% isopropanol and imaged with a Nikon CoolPix digital camera. Quantification of the percentage of aortic surface area occupied by oil red O positive plaque was performed using digital image analysis (Simple PCI, C‐Imaging). The heart and proximal portion of aortic root were also fixed and embedded in OCT. Subsequently, the aortic root area was sliced. The atherosclerotic lesion areas in oil red O‐stained cryostat sections of the aortic root were quantified using the Leica image analysis system, consisting of a Leica DMRE microscope coupled to a video camera and Leica Qwin Imaging software (Leica Ltd.). Mean lesion area (in μm2) was calculated from 10 oil red O‐stained sections, starting at the appearance of the tricuspid valves. Plaque necrotic areas were quantified from the average of 6 sections per mouse, spaced 30 μm apart, by measuring the area of hematoxylin and eosin‐negative acellular and anuclear white areas. For macrophage and smooth muscle staining, the sections were heated in an EDTA solution and endogenous peroxidase was blocked using hydrogen peroxide and methanol. Sections were blocked in 2% BSA/PBS for 30 minutes and incubated with the macrophage marker Moma‐2 (1:25, Serotec Inc.) and α‐smooth muscle actin (1:80, Dako Cytomation Inc.) antibodies overnight. Subsequently, the sections were incubated with biotinylated secondary antibodies for 30 minutes followed by 30‐minute incubation with ABComplex/HRP. Finally, the sections were stained with 3, 30‐diaminobenzidine (DAB) and counterstained with hematoxylin. The resulting slides were mounted under glass coverslip and analyzed by light microscope. All quantifications were done blinded by computer‐aided morphometric analysis using the Leica image analysis system.
Isolation and Culture of Bone Marrow‐Derived Macrophages
Mononuclear phagocyte progenitor cells derived from femoral and tibial bone marrow were propagated in the presence of M‐CSF. This macrophage growth factor secreted by L929 cells was used in the form of L929 cell‐conditioned medium. The progenitor cells proliferate and differentiate through monoblast, promonocyte, and monocyte stages before maturing to macrophages. For the observation of Rev‐erbα knock‐down effect on monocyte differentiation, bone marrow cell suspensions were isolated from C57Bl/6 mice by flushing the femurs and tibias with PBS. Single‐cell suspensions were prepared by passing the cell mass through a cell strainer with 27 pond needle, and bone marrow cells (1.0×107) were transduced with either H1.shNT or H1.shRev‐erbα lentivirus in the presence of 10 μg/mL DEAE‐dextran (multiplicity of infection [moi]=15). Eighteen hours posttransduction, the cells were sorted by flow cytometry, and GFP‐marker‐positive cells with green were collected and cultured at a density of 2×106 cells/well in six‐well plastic culture dishes for 6 days with the macrophage growth factor from L929 cells. Alternatively differentiated macrophages (M2) were obtained by stimulating monocytes during differentiation with human IL‐4 (15 ng/mL). M1 macrophage differentiation was induced by incubating the monocytes with LPS (100 ng/mL). The effect of Rev‐erbα overexpression on monocyte differentiation was observed by carrying out the same protocol as above. Heme was added at 20 μmol/L in the differentiation medium.
Western Blotting
Protein specimens for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) were prepared by homogenizing C57BL/6 bone marrow cells, peripheral blood monocytes, and peritoneal macrophages, macrophages isolated from lentivirus‐shNT, or lentivirus‐shRev‐erbα BM transplantation recipient mice after 11 weeks on the Western‐type diet in 250 mmol/L Tris‐HCl, pH 6.8, 8% SDS, protease inhibitor cocktail (Roche Diagnostics). The crude extracts were cleared by centrifugation at 16 000g for 3 minutes, and protein concentration of the supernatant was determined by the DC assay (BioRad). Equal amounts of the proteins were electrophoresed and Western blotted using the mouse monoclonal primary antibody against Rev‐erbα (AT3093a, Abgent Biotech). The bound antibodies were visualized by using horseradish peroxidase‐conjugated goat antimouse IgG (Bio‐rad) and enhanced chemiluminescence (ECL; GE Healthcare).
Analysis of Plasma Lipids
Total plasma cholesterol (Kit 1489232, Roche Diagnostics GmbH), choline‐containing phospholipids (Kit 990‐54009, Wako Chemicals GmbH) and triglycerides (Kit 1488872, Roche Diagnostics) were measured using enzymatic methods.
Quantification of Macrophage mRNAs
Total RNA was isolated from myeloid cell‐derived macrophages and peritoneal macrophages from experimental animals with the RNeasy Mini kit (Qiagen) according to the manufacturer's instructions. The RNA (2 μg) was treated with DNase I (Promega) in the presence of RNase Inhibitor (Promega) and reverse‐transcribed by using Superscript II (Invitrogen) and random hexamer primers (Applied Biosystems). Each RNA sample was amplified in triplicate for the genes of interest and two housekeeping markers, 36B4 and 18S rRNA, on a 7000 Sequence Detection System (Applied Biosystems) by using SYBR‐green (ABgene). Sequences of the primers used are listed in Table S1. The threshold was set in the linear range of fluorescence, and a threshold cycle (Ct) was measured for each well. Data were analyzed as described previously.21
Immunohistochemical Analysis
Human atherosclerotic plaques were removed from patients eligible for surgical carotid endarterectomy. The carotid arteries were fixed with paraformaldehyde and embedded in paraffin. Paraffin‐embedded arteries were cross‐sectioned into 4‐μm thick pieces, dewaxed, rehydrated, and pretreated by boiling in 0.01 mol/L citrate buffer (pH 6.0) for 10 minutes in a microwave oven (750 W). Sections were incubated with the mouse monoclonal antihuman Rev‐erbα antibodies (1:150) overnight. Subsequently, the sections were incubated with biotinylated secondary antibodies for 30 minutes, followed by 30 minutes incubation with ABComplex/HRP. Finally, the sections were stained with 3, 30‐diaminobenzidine (DAB) and counterstained with hematoxylin. The slides were mounted under glass coverslips and analyzed by light microscope. For immunofluorescence double staining, the sections were blocked with 1% BSA in PBS after microwave antigen retrieval and incubated with the primary antibodies (mouse anti–Rev‐erbα [AT3093a, Abgent Biotech]. and macrophage specific rabbit anti‐CD68 [16192‐1‐AP, Proteintech Group Inc]) diluted in 1% (w/v) BSA in PBS for 45 minutes at room temperature. After copious washing in PBS, the sections were incubated with fluorescently labeled secondary antibodies diluted in 1% (w/v) BSA in PBS for 30 minutes at 37°C (Cy3 conjugated goat antirabbit IgG [Proteintech Group Inc] for detection of CD68 antibodies; Alexa Fluor 488 conjugated goat anti‐mouse IgG [Invitrogen] for detection of Rev‐erbα antibodies). The sections were counterstained with 4′,6‐diamidino‐2‐phenylindole (Sigma‐Aldrich), mounted in fluorescence mounting medium (Invitrogen), and analyzed with a laser scanning confocal microscope (Zeiss LSM 510 Meta).
Statistical Analysis
The Kolmogorov‐Smirnov test was used to determine whether each variable had a normal distribution. When data were normally distributed and variances were similar across comparison groups, the statistical significance of data between the groups was assessed by analysis of variance (ANOVA). Except for the date on determination of lesion area between the groups, statistical significance was estimated by Wilcoxon rank‐sum test. All values are reported as mean±SEM. Analyses were performed using the SPSS (version 16.0; APSS), statistical package and the P<0.05 values were regarded as statistically significant.
Results
Increased Atherosclerotic Lesions in LDL Receptor Deficient Mice With Haematopoietic Nuclear Receptor Rev‐erbα Knock‐Down
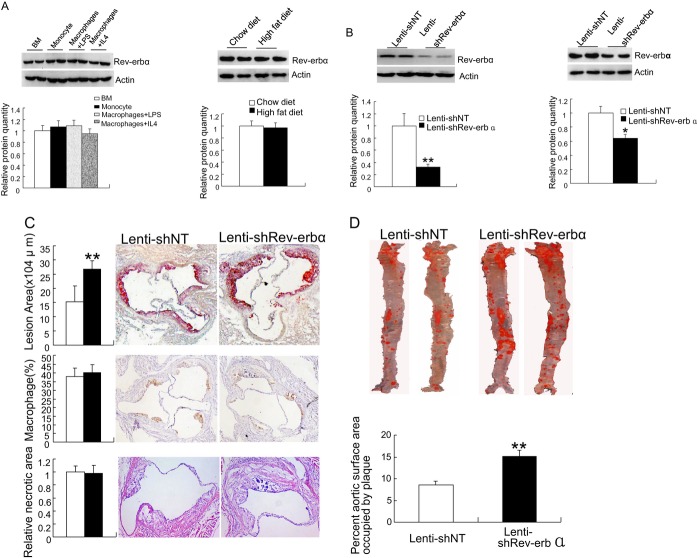
The expression of Rev‐erbα protein in mouse bone marrow, peripheral blood monocytes, and peripheral macrophages stimulated with LPS or IL‐4 was monitored by Western blotting analysis and showed the presence of Rev‐erbα in all of these cell populations (Figure 1A, left panel). Moreover, we assessed the expression of Rev‐erbα in LDLr−/− mice fed normal chow or a high fat diet, and found no diet‐dependency of Rev‐erbα protein expression in bone marrow cells or peritoneal macrophages (Figure 1A, right panel). To study the effects of Rev‐erbα on the development of atherosclerosis, wild‐type C57BL/6 mouse bone marrow was transduced with lentivirus‐shRev‐erbα or a nontargeting shRNA lentivirus (lentivirus‐shNT), followed by transplantation into LDLr−/− mice. To induce atherosclerotic lesion development, the transplanted mice were allowed to recover for 8 weeks and then fed a Western‐type diet for 11 weeks, followed by analysis of atherosclerotic lesion size in the aortic root and in the aorta en face. Efficacy of the Rev‐erbα knock‐down was assessed in peripheral macrophages of the animals after 11 weeks of Western‐type diet, by Western blotting. The results (Figure 1B, left panel) showed >70% reduction of Rev‐erbα protein in the peripheral macrophages of mice injected with lentivirus‐shRev‐erbα–transduced bone marrow as compared to that of the control mice injected with lentivirus‐shNT–transduced bone marrow. Knock‐down of Rev‐erbα in bone marrow cells resulted in a significant increase in aortic root atherosclerotic lesion size from 17.2x104 μm2 in control mice to 26.46x104 μm2 in Rev‐erbα knock‐down mice (P<0.01, n=10, Figure 1C). Knock‐down of Rev‐erbα also led to a significant increase in atherosclerotic plaque burden compared with the control mice by en face analysis, with an approximately 70% increase in lesion area (Figure 1D). Histological analysis of aortic root lesions revealed no significant differences in macrophages (Figure 1C, middle panel) or necrotic area (Figure 1C, bottom panel) in plaques of shNT and shRev‐erbα bone marrow recipient mice. In summary, bone marrow Rev‐erbα knock‐down resulted in increased atherosclerotic lesion size, identifying Rev‐erbα in haematopoietic cell lineages as an antiatherogenic factor.
Figure 1.
Increased atherogenesis in mice upon haematopoietic Rev‐erbα knock‐down. A, (left panel) Western blot analysis of Rev‐erbα protein expression in C57BL/6 bone marrow cells (BM), peripheral blood monocytes, and macrophages differentiated in the presence of IL‐4 or LPS (identified at the top) (15 μg total protein/lane), ß‐actin blot is shown as a loading control, the bar diagram presents the relative protein quantity (bottom); (right panel), Western blot analysis of Rev‐erbα protein expression in bone marrow cells from C57BL/6 mice fed with high fat or chow diet (top), the bar diagram presents the relative protein quantity (bottom). The data represent mean±SEM (n=3). B, The efficiency of Rev‐erbα knock‐down in peritoneal macrophages of bone marrow recipient LDLr−/− mice after the 11‐week Western‐type diet. Peritoneal cells from recipients of Lentivirus‐shNT and Lentivirus‐shRev‐erbα bone marrow are shown (left, top), and quantification of the Western blot signals for Rev‐erbα (left, bottom; n=6); The right panel shows the efficiency of Rev‐erbα knock‐down after the 16 weeks Western‐type diet and quantification of the protein level (n=3); statistical analysis was performed by Kolmogorov‐Smirnov test followed by two‐factor ANOVA, *P<0.05 and **P<0.01. C, Analysis of atherosclerotic lesions in the aortic root after BMT, 8‐week recovery and 11‐week Western‐type diet. Aortic sections from shNT→LDLr−/− (Lenti‐shNT) or shRev‐erbα→LDLr−/− mice (Lenti‐shRev‐erbα) were stained with oil red O (up panel). Quantification of the lesion area (top, left panel) was performed by using Leica Qwin Imaging software as described in Methods. The mean area of lipid staining per section from 10 sections was determined for each mouse. The statistical significance of the differences between the groups was estimated by Wilcoxon rank‐sum test. The data represent mean±SEM (n=10, **P<0.01). Aortic root sections from LDLr−/− mice transplanted with shNT (Lenti‐shNT) or shRev‐erbα (Lenti‐shRev‐erba) bone marrow were subjected to histochemical analysis. Images of representative sections of aortic roots stained for macrophage Moma‐2 (middle panel). Representative sections of hematoxylin and eosin (HE)‐stained aortic root sections from Lenti‐shNT and Lenti‐shRev‐erba mice (bottom panel) are shown. Magnification, ×100. D, Representative aortas displayed en face; atherosclerotic lesions of aortas from shNT→LDLr−/− (Lenti‐shNT) or shRev‐erbα→LDLr−/− mice (Lenti‐shRev‐erbα) were stained with oil Red O. Quantification of plaque burden expressed as the percentage of aortic surface area (bottom panel). The statistical significance of the differences between the groups was assessed by Wilcoxon rank‐sum test. The data represent mean±SEM; (n=10/group; **P<0.01). IL‐4 indicates interleukin‐4; LPS, lipopolysaccharide; LDLr−/− mice, homozygous LDL receptor knockout mice; Lentivirus‐shNT, non‐targeting shRNA lentivirus; ANOVA, analysis of variance; BM, bone marrow cells.
Plasma Lipids Are Not Modulated by Haematopoietic Rev‐erbα Knock‐Down
Next, we assessed the potential influence of Rev‐erbα knock‐down on plasma lipids. Our data showed no significant difference in plasma total cholesterol, triglyceride, or choline‐containing phospholipid concentrations between the mice injected with lentivirus‐shRev‐erbα–transduced bone marrow and the control mice (Table).
Table 1.
Plasma Lipid Values of the NT→LDLr−/− and Reverb α→LDLr−/− Mice
| TC mmol/L | TG mmol/L | PL mmol/L | ||
|---|---|---|---|---|
| NT→LDLr−/− | B | 7.2±0.19* | 1.93±0.17 | 5.0±0.17 |
| A | 23±1.35 | 3.2±0.31 | 8.21±0.30 | |
| Reverbα→LDLr−/− | B | 7.7±0.28 | 1.77±0.15 | 5.5±0.15 |
| A | 25.01±1.44 | 3.3±0.37 | 8.41±0.32 | |
TC indicates total cholesterol; TG, Triglyceride; PL, choline‐containing phospholipids; B, before Western‐type diet; A, after Western‐type diet.
The values represent mean±SEM (NT→LDLr−/−, n=10; Reverbα→LDLr−/−, n=10).
Rev‐erbα Promotes the Appearance of an Anti‐Inflammatory M2 Macrophage Like Phenotype
The mechanism underlying the elevated atherosclerotic lesion area upon haematopoietic Rev‐erbα knock‐down was assessed. An emerging concept is that a shift of macrophage polarity plays an important role in atherosclerosis,10 M1 being effective in secreting proinflammatory cytokines whereas M2 dampen the inflammatory response by producing antiinflammatory factors. M1 and M2 are the two extremes, macrophage polarization being continuous as can be monitored using a series of markers. For example, M1 macrophages express more inducible nitric oxide synthase (iNOS) while M2 macrophages express more Chitinase 3‐like proteins (YM1/2), arginase 1 (arg 1) and the mannose receptor (MR). Therefore, we used reverse transcriptase quantitative PCR (qPCR) to measure the expression of M1/M2 molecular markers in macrophages differentiated from the bone marrow of transplantation recipient mice. The data (Figure 2) revealed that when bone marrow cells were differentiated into macrophages by stimulation with IL‐4, Rev‐erbα knock‐down resulted in reduced expression levels of all M2 markers (arg1, MR, YM1/2) as compared to differentiated macrophages from the control animals. By contrast, LPS stimulation of Rev‐erbα knock‐down macrophages resulted in an increased expression of the M1 marker iNOS (Figure 2). To assess the impact of Rev‐erbα overexpression on macrophage markers, we next prepared a lentivirus expressing the Rev‐erbα cDNA (Lentivirus‐Rev‐erbα) and overexpressed the nuclear receptor in mononuclear cells from bone marrow of wild type C57BL/6 mice. Rev‐erbα overexpression resulted, upon IL‐4 stimulation, in increased expression levels of all M2 markers (arg1, MR, YM1/2). By contrast, LPS stimulation of these cells resulted in a decreased expression of the M1 marker iNOS (Figure 3). mRNA levels of other M1 macrophage markers, such as TNFα, IL‐1, IL‐6, IL‐12, MCP‐1, and CCL5, tended to increase after LPS stimulation of the Rev‐erbα knock‐down macrophages, whereas they displayed a tendency toward reduction in Rev‐erbα overexpressing cells (Figure S1). The results indicated that Rev‐erbα overexpression skews monocyte differentiation toward the M2 macrophage subtype (Figure 3).
Figure 2.
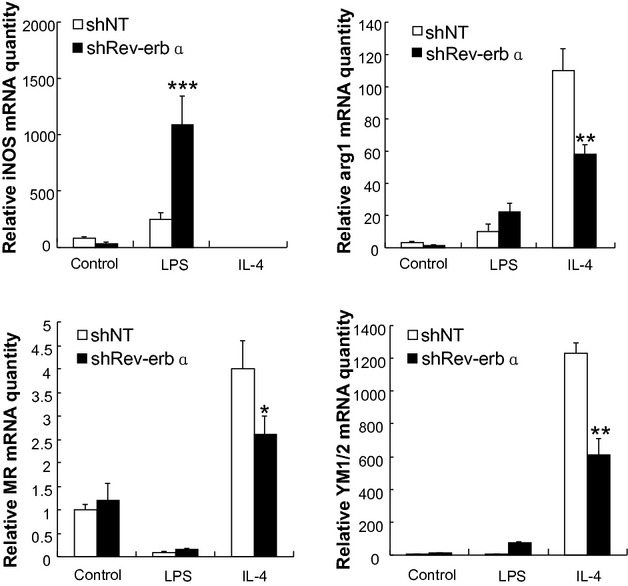
Rev‐erbα knock‐down in myeloid cells impairs alternative macrophage activation. Monocytes from the bone marrow infected by Lentivirus‐shNT or Lentivirus‐shRev‐erbα were differentiated into macrophages in the absence or presence of LPS or IL‐4. The expression levels of M1 (iNos) and M2 (arg1, MR and Ym1/2) macrophage markers were measured by qPCR. The bars represent the mean±SEM from at least three separate experiments each analyzed in triplicate; statistical analysis was performed by Kolmogorov‐Smirnov test followed by two‐factor ANOVA, *P<0.05, ** P<0.01 and *** P<0.001. iNos indicates inducible nitric oxide synthase; arg1, arginase 1; Ym1/2, Chitinase 3‐like proteins; MR, mannose receptor. Lentivirus‐shNT, non‐targeting shRNA lentivirus; LPS, lipopolysaccharide; IL‐4, interleukin‐4; qPCR, quantitative polymerase chain reaction; ANOVA, analysis of variance.
Figure 3.
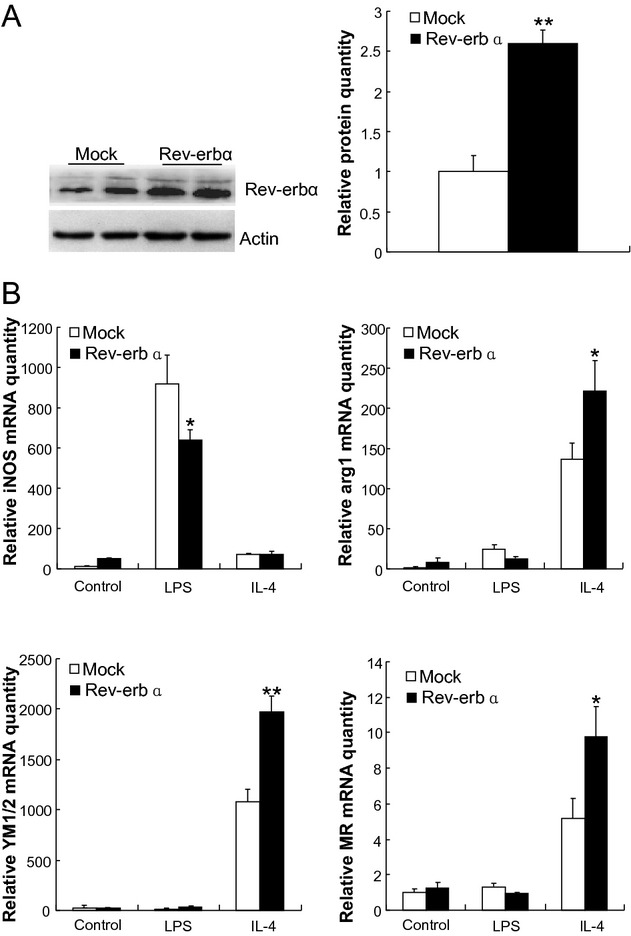
Overexpression of Rev‐erbα in myeloid cells enhances alternative macrophage activation. Monocytes from the bone marrow of wild type C57BL/6 mice were tranduced by Lentivirus‐Rev‐erbα for overexpression of Rev‐erbα or by control Lentivirus (Mock). A, Western blot analysis of overexpression level of Rev‐erbα protein, and the bar diagram presents the relative protein quantity. B, Macrophage differentiation was induced in the absence or presence of LPS or IL‐4. The expression levels of M1 (iNos) and M2 (arg1, MR and Ym1/2; y‐axes) macrophage markers were measured by qPCR. The bars represent the mean±SEM from at least three separate experiments each analyzed in triplicate; statistical analysis was performed by Kolmogorov‐Smirnov test followed by two‐factor ANOVA, *P<0.05 and ** P<0.01. iNos indicates inducible nitric oxide synthase; arg1, arginase 1; Ym1/2, chitinase‐like proteins, MR, mannose receptor. LPS, lipopolysaccharide; IL‐4, interleukin‐4; qPCR, quantitative polymerase chain reaction; ANOVA, analysis of variance
Rev‐erbα Knock‐Down Impairs Alternative Macrophage Activation in Vivo
To explore the effect of Rev‐erbα on M1‐type or alternative macrophage activation in vivo, we injected LPS or IL4, respectively, in shNT→LDLr−/− (Lenti‐shNT) or shRev‐erbα→LDLr−/− mice (Lenti‐shRev‐erbα) mice which were fed Western‐type diet. We found that peritoneal CD80+ macrophages were increased significantly with LPS stimulation in shRev‐erbα→LDLr−/− mice, and a clear elevation of iNOS (M1 marker) mRNA was detected in the peritoneal macrophages. The peritoneal CD206+ macrophages monitored after IL4 injection showed a dramatic decrease in shRev‐erbα→LDLr−/− mice, and the M2 marker mRNAs including arg1, Ym1/2, and MR (CD206) were significantly reduced. The data indicated that Rev‐erbα enhances M2 macrophages differentiation in vivo (Figure 4).
Figure 4.
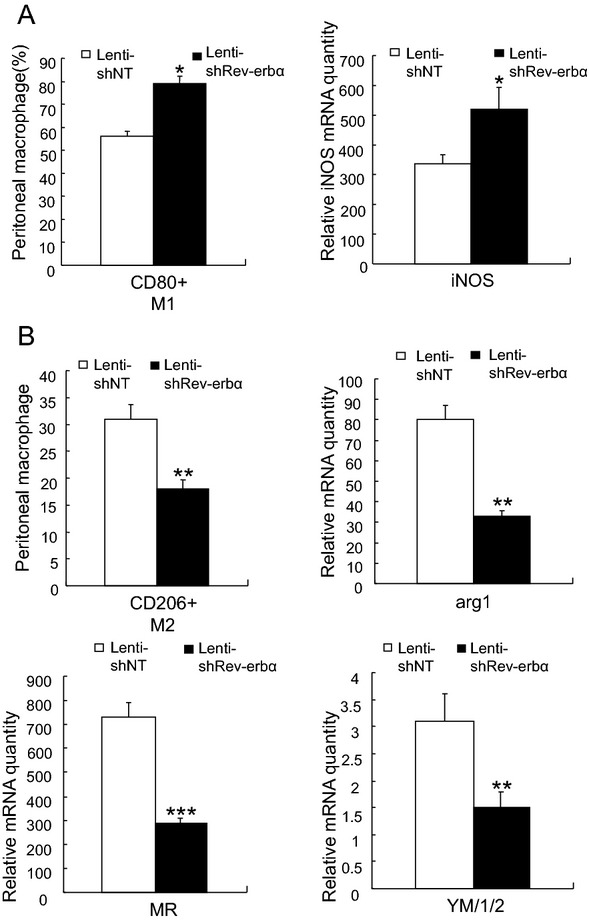
Rev‐erbα knock‐down impairs alternative macrophage activation in vivo. A. shNT→LDLr−/− (Lenti‐shNT) or shRev‐erbα→LDLr−/− (Lenti‐shRev‐erbα) mice were fed an atherogenic diet for 6 weeks and challenged with lipopolysaccharide (LPS) at 30 mg/30 g body weight for 3 days. Peritoneal cells were stained with antibodies against CD80 (M1) and identified by flow cytometry. The values represent the percentages of individual macrophage subtypes (left panel). Peritoneal macrophages were isolated from the mice and the expression levels of a M1 macrophage marker (iNos, inducible nitric oxide synthase) were measured by qPCR. The bars represent the mean±SEM from at least three separate experiments each analyzed in triplicate; statistical analysis was performed by Kolmogorov‐Smirnov test followed by two‐factor ANOVA,*P<0.05. B. shNT→LDLr−/− (Lenti‐shNT) or shRev‐erbα→LDLr−/− (Lenti‐shRev‐erbα) mice were fed an atherogenic diet for 6 weeks and challenged with IL‐4 at 500 ng/30 g body weight for 2 days. Peritoneal cells were stained with antibodies against CD206 (M2) and identified by flow cytometry. The values represent the percentages of individual macrophage subtypes. Peritoneal macrophages were isolated from the mice and the expression levels of M2 macrophages markers were measured by qPCR. The bars represent the mean±SEMfrom at least three separate experiments each analyzed in triplicate; statistical analysis was performed by Kolmogorov‐Smirnov test followed by two‐factor ANOVA, **P<0.01 and ***P<0.001. arg1 indicates arginase 1; Ym1/2, chitinase‐like proteins, MR, mannose receptor. LDLr−/−, mice homozygous LDL receptor knockout mice; Lentivirus‐shNT, non‐targeting shRNA lentivirus; qPCR, quantitative polymerase chain reaction; ANOVA, analysis of variance; IL‐4, interleukin‐4.
Heme Primes Monocyte Differentiation Into M2 Macrophages in Vitro
To study the potential effect of the Rev‐erbα ligand heme on macrophage polarization markers, bone marrow cells from wild type C57BL/6 mice were differentiated into macrophages in the presence of heme (20 μmol/L). The data (Figure 5, left panels) indicated that heme increased all M2 markers (arg1, MR, YM1/2) while Rev‐erbα knock‐down abolished the effect of heme (Figure 5, right panels). These results suggested that heme stimulation promotes a similar macrophage phenotype as Rev‐erbα overexpression, lending further support to the suggested role of Rev‐erbα in macrophage function.
Figure 5.
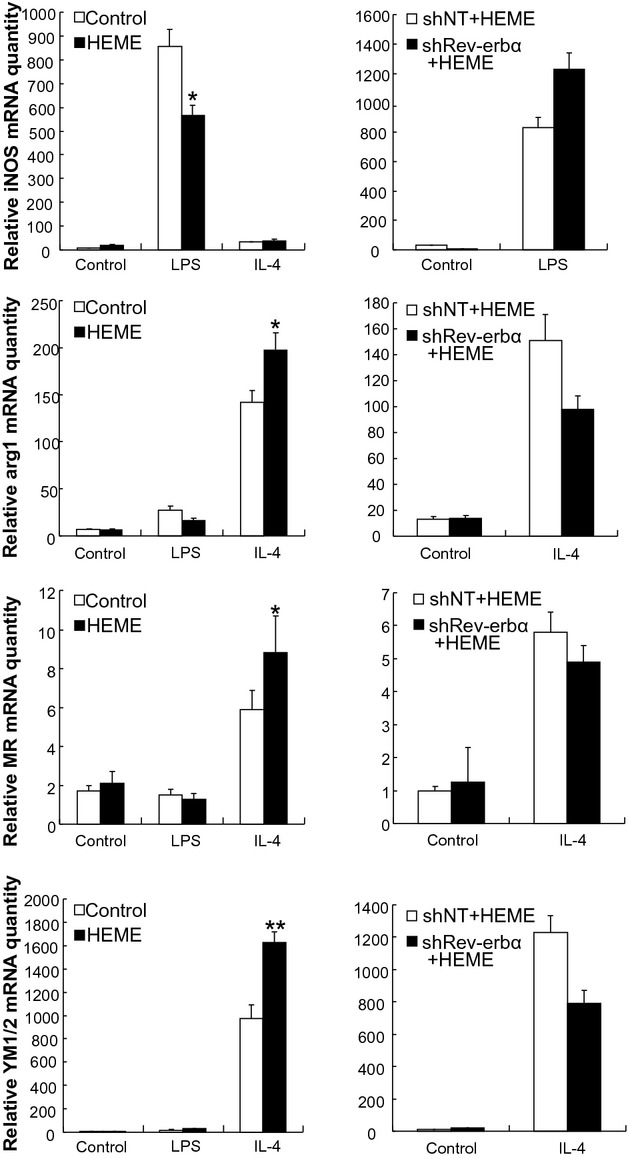
Heme induces alternative macrophage activation. Monocytes from the bone marrow of wild type C57BL/6 mice (left panels) or from that infected by Lentivirus‐shNT or Lentivirus‐shRev‐erbα (right panels) were differentiated into macrophages in the absence or presence of LPS or IL‐4, or of 20 μmol/L heme. The expression levels of M1 macrophage (iNos) and M2 (arg1, MR and Ym1/2, y‐axes) macrophage markers were measured by qPCR. The bars represent the mean±SEM from at least three separate experiments each analyzed in triplicate; statistical analysis was performed by Kolmogorov‐Smirnov test followed by two‐factor ANOVA,*P<0.05 and**P<0.01. iNos indicates inducible nitric oxide synthase; arg1, arginase 1; Ym1/2, chitinase‐like proteins, MR, mannose receptor. Lentivirus‐shNT, non‐targeting shRNA lentivirus; LPS, lipopolysaccharide; IL‐4, interleukin‐4; qPCR, quantitative polymerase chain reaction; ANOVA, analysis of variance.
Rev‐erbα is Present in the Macrophages of Human Atherosclerotic Plaques
To assess the presence of Rev‐erbα in human carotid atherosclerotic plaques, immunohistochemical analysis of Rev‐erbα in plaque sections was performed. Significant Rev‐erbα immunoreactivity was observed in the intimal region of the atherosclerotic vessel wall (Figure 6A). Immunofluorescence microscopy analysis showed the colocalization of Rev‐erbα staining with that of CD68, identifying the Rev‐erbα positive cells as macrophages (Figure 6B). This result was confirmed by staining sections of carotid lesions from several additional patients (Figure S2).
Figure 6.
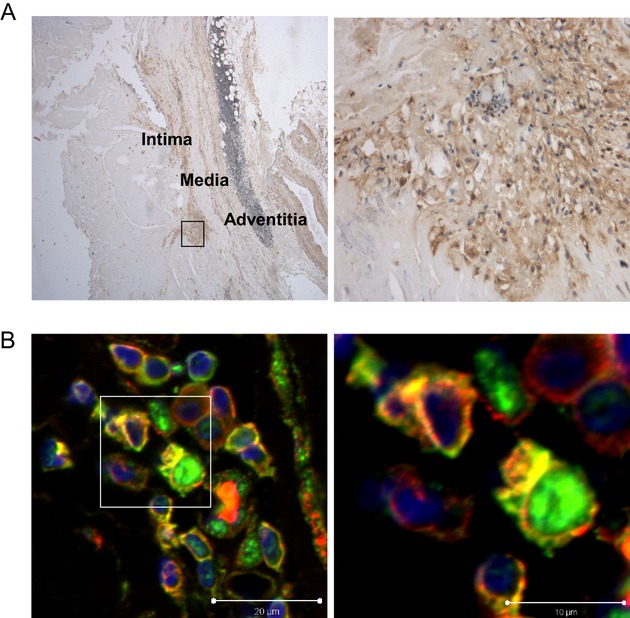
Rev‐erbα protein is present in the macrophages of human carotid artery atherosclerotic lesions. Human carotid artery sections were subjected to immunohistochemistry using mouse monoclonal anti‐human Rev‐erbα. A, Rev‐erbα immunoreactivity in a representative plaque (40×) (left panels), and a high‐magnification view of the same image (400×) (right panels) illustrating the cellular nature of the staining. B, Immunofluoresence staining identifying the Rev‐erbα positive cells as macrophages. A representative section of a human carotid plaque stained by using primary antibodies against Rev‐erbα (green) and the macrophage‐specific marker CD68 (red). Colocalization of the two markers is shown in yellow (left panels), and a higher magnification view of the same immunofluorescence image (right panels).
Discussion
The present study demonstrates that haematopoietic Rev‐erbα knock‐down increases the size of atherosclerotic lesions in LDLr−/− mouse, thus providing evidence for an antiatherogenic role of macrophage Rev‐erbα. The possible molecular mechanisms through which Rev‐erbα could influence lesion development were evaluated. Our results in vitro and in vivo strongly suggest that Rev‐erbα promotes the differentiation of macrophages towards the M2 phenotype.
Atherosclerosis is a chronic inflammatory disease manifested as vessel wall lesions1 in which macrophages play key roles in the inflammatory response and lipids accumulation.2 Heterogeneity of circulating monocytes and the functional diversity of macrophages likely influence plaque formation and phenotype.22–23 Our in vitro data and experiments in live mice provide the first evidence that Rev‐erbα regulates the differentiation of macrophages, resulting in a more antiinflammatory M2 macrophage state. We therefore find it likely that Rev‐erbα may also affect the development of atherosclerosis by enhancing the antiinflammatory function of macrophages in the large vessel wall. Furthermore, Rev‐erbα acts as an integrator of circadian rhythms and metabolism including that of lipids.18,24 It downregulates expression of the apolipoprotein CIII component of triglyceride‐rich lipoproteins which are associated with increased risk of cardiovascular disease 19,25–26 in human hepatoblastoma cells. Interestingly, in our study, no significant difference was observed in plasma total cholesterol, triglyceride, or choline‐containing phospholipid concentrations between the recipients of the Rev‐erbα knock‐down and control bone marrow. The liver is the major organ controlling lipoprotein metabolism, so it is thus not surprising that Rev‐erbα knock‐down in macrophages had no significant influence on plasma lipids. Based on the present results, we therefore hypothesize that the observed impact of Rev‐erbα on M2 monocyte polarization may be an important factor contributing to the increase of atherosclerotic lesion size in recipients of Rev‐erbα deficient bone marrow.
A large body of data confirms the close relation between cardiovascular disease and biological rhythms. A shift of sleep‐wake rhythms is an independent risk factor of cardiovascular disease.27–29 In addition, according to clinical observations, atherosclerosis‐based cardiovascular disease deaths occur mostly in the morning,30–31 indicating a link between CVD and the biological clock. Our results suggested that changes of hematopoietic Rev‐erbα expression are associated with macrophage polarization and cytokine expression in vivo. Rev‐erbα repress IL10 expression by direct binding to the IL10 promoter,32 and the recent data of Gibbs et al.33 which demonstrate that Rev‐erbα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines, together identify a potential mechanistic connection between biological rhythms and atherosclerosis, its complications and clinical manifestations. Our study reveals for the first time that a clock gene affects the balance of M1/M2 markers, and may thus provide new clues for elucidation of the mechanisms underlying the connections of CVD with biological rhythms.
The detailed mechanism through which Rev‐erbα affects monocyte differentiation into M2 macrophages is thus far unknown. Peroxisome proliferator‐activated receptor γ (PPARγ) is another nuclear receptor with antiinflammatory properties,34–35 and it is reported to enhance the differentiation of monocytes into an M2 state.36 Interestingly, PPARα and γ are capable of binding a RevDR‐2 sequence located in the Rev‐erbα gene promoter, hence activating its transcription.37 Whether the PPARs and Rev‐erbα operate in concert to regulate monocyte differentiation is therefore an important topic for future investigation.
Given the multiple roles of Rev‐erbα in lipid metabolism, adipogenesis, and vascular wall pathophysiology, Rev‐erbα represents a promising target for the treatment of metabolic diseases and atherosclerosis.38 Identification of synthetic ligands which can be pharmacologically used, provides a means of controlling Rev‐erbα function.17 Recently, heme and synthetic small molecular ligands for Rev‐erbα were shown to be effective modulators of adipogenesis.39 Our observation that heme mimics the impact of Rev‐erbα overexpression in promoting M2 monocyte polarization suggests that Rev‐erbα agonists may also be potential tools to combat atherosclerosis.
In conclusion, our data show that haematopoietic Rev‐erbα knock‐down increases the development of atherosclerotic lesions LDLr−/− mice, demonstrating an anti‐atherogenic role of Rev‐erbα. Moreover, we suggest that promotion of monocyte polarization into a M2 macrophage phenotype contributes to the effect of Rev‐erbα.
Sources of Funding
This work was supported by grants from State Key Development Program (973) for Basic Research of China (grant 2012CB517502 to Dr Yan), National Nature Science Foundation of China (grant 30971104 to Dr Yan) the Fundamental Research Funds for the Central Universities, China (grant 21610609 to Dr Yan), the Guangdong Natural Science Fund (grant 32210029 to Dr Yan), an unrestricted Astra‐Zeneca/CMN ITMO Grant to Dr Staels), the Sigrid Juselius Foundation, and the Novo Nordisk Foundation (Dr Olkkonen).
Disclosures
None.
References
- 1.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999; 340:115-126 [DOI] [PubMed] [Google Scholar]
- 2.Shibata N, Glass CK. Macrophages, oxysterols and atherosclerosis. Circ J. 2010; 74:2045-2051 [DOI] [PubMed] [Google Scholar]
- 3.Chinetti‐Gbaguidi G, Staels B. Macrophage polarization in metabolic disorders: functions and regulation. Curr Opin Lipidol. 2011; 22:365-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011; 12:204-212 [DOI] [PubMed] [Google Scholar]
- 5.Shimada K. Immune system and atherosclerotic disease: heterogeneity of leukocyte subsets participating in the pathogenesis of atherosclerosis. Circ J. 2009; 73:994-1001 [DOI] [PubMed] [Google Scholar]
- 6.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev. 2008; 8:802-815 [DOI] [PubMed] [Google Scholar]
- 7.Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Lander ES, Young RA. Human macrophage activation programs induced by bacterial pathogens. Proc Natl Acad Sci USA. 2002; 99:1503-1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boldrick JC, Alizadeh AA, Diehn M, Dudoit S, Liu CL, Belcher CE, Botstein D, Staudt LM, Brown PO, Relman DA. Stereotyped and specific gene expression programs in human innate immune responses to bacteria. Proc Natl Acad Sci USA. 2002; 99:972-977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon S. Alternative activation of macrophages. Nat Rev. 2003; 3:23-35 [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009; 29:1419-1423 [DOI] [PubMed] [Google Scholar]
- 11.Lefebvre P, Chinetti G, Staels B. Nur77turing macrophages in atherosclerosis. Circ Res. 2012; 110:375-377 [DOI] [PubMed] [Google Scholar]
- 12.Lazar MA, Hodin RA, Darling DS, Chin WW. A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c‐erbA alpha transcriptional unit. Mol Cell Biol. 1989; 9:1128-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F. Identification of heme as the ligand for the orphan nuclear receptors REV‐ERBalpha and REV‐ERBbeta. Nat Struct Mol Biol. 2007; 14:1207-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Rev‐erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007; 318:1786-1789 [DOI] [PubMed] [Google Scholar]
- 15.Barish GD, Downes M, Alaynick WA, Yu RT, Ocampo CB, Bookout AL, Mangelsdorf DJ, Evans RM. A nuclear receptor atlas: macrophage activation. Mol Endocrinol. 2005; 19:2466-2477 [DOI] [PubMed] [Google Scholar]
- 16.Migita H, Morser J, Kawai K. Rev‐erbalpha upregulates NF‐kappaB‐responsive genes in vascular smooth muscle cells. FEBS Lett. 2004; 561:69-74 [DOI] [PubMed] [Google Scholar]
- 17.Burris TP. Nuclear hormone receptors for heme: REV‐ERBalpha and REV‐ERBbeta are ligand‐regulated components of the mammalian clock. Mol Endocrinol. 2008; 22:1509-1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duez H, Staels B. Rev‐erb‐alpha: an integrator of circadian rhythms and metabolism. J Appl Physiol. 2009; 107:1972-1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coste H, Rodriguez JC. Orphan nuclear hormone receptor Rev‐erbalpha regulates the human apolipoprotein CIII promoter. J Biol Chem. 2002; 277:27120-27129 [DOI] [PubMed] [Google Scholar]
- 20.Fontaine C, Rigamonti E, Pourcet B, Duez H, Duhem C, Fruchart JC, Chinetti‐Gbaguidi G, Staels B. The nuclear receptor Rev‐erbalpha is a liver X receptor (LXR) target gene driving a negative feedback loop on select LXR‐induced pathways in human macrophages. Mol Endocrinol. 2008; 22:1797-1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaffl MW. A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res. 2001; 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly‐6Chi monocytes dominate hypercholesterolemia‐associated monocytosis and give rise to macrophages in atheromata. J Clin Investig. 2007; 117:195-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Investig. 2007; 117:185-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramakrishnan SN, Muscat GE. The orphan Rev‐erb nuclear receptors: a link between metabolism, circadian rhythm and inflammation? Nucl Recept Signal. 2006; 4:e009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raspe E, Duez H, Mansen A, Fontaine C, Fievet C, Fruchart JC, Vennstrom B, Staels B. Identification of Rev‐erbalpha as a physiological repressor of apoC‐III gene transcription. J Lipid Res. 2002; 43:2172-2179 [DOI] [PubMed] [Google Scholar]
- 26.Raspe E, Duez H, Gervois P, Fievet C, Fruchart JC, Besnard S, Mariani J, Tedgui A, Staels B. Transcriptional regulation of apolipoprotein C‐III gene expression by the orphan nuclear receptor RORalpha. J Biol Chem. 2001; 276:2865-2871 [DOI] [PubMed] [Google Scholar]
- 27.Lund J, Arendt J, Hampton SM, English J, Morgan LM. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J Endocrinol. 2001; 171:557-564 [DOI] [PubMed] [Google Scholar]
- 28.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Speizer FE, Hennekens CH. Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995; 92:3178-3182 [DOI] [PubMed] [Google Scholar]
- 29.Knutsson A, Hallquist J, Reuterwall C, Theorell T, Akerstedt T. Shiftwork and myocardial infarction: a case‐control study. Occup Environ Med. 1999; 56:46-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987; 75:131-138 [DOI] [PubMed] [Google Scholar]
- 31.Tanaka A, Kawarabayashi T, Fukuda D, Nishibori Y, Sakamoto T, Nishida Y, Shimada K, Yoshikawa J. Circadian variation of plaque rupture in acute myocardial infarction. Am J Cardiol. 2004; 93:1-5 [DOI] [PubMed] [Google Scholar]
- 32.Chandra V, Mahajan S, Saini A, Dkhar HK, Nanduri R, Raj EB, Kumar A, Gupta P. Human IL10 repression by Reverb alpha ameliorates Mycobacterium tuberculosis clearance. J Biol Chem. 2013; 288:10692-10702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon AS. The nuclear receptor REV‐ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. 2012; 109:582-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Straus DS, Glass CK. Anti‐inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007; 28:551-558 [DOI] [PubMed] [Google Scholar]
- 35.Marx N, Kehrle B, Kohlhammer K, Grub M, Koenig W, Hombach V, Libby P, Plutzky J. PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation‐associated arteriosclerosis. Circ Res. 2002; 90:703-710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti‐Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti‐inflammatory properties. Cell Metab. 2007; 6:137-143 [DOI] [PubMed] [Google Scholar]
- 37.Fontaine C, Dubois G, Duguay Y, Helledie T, Vu‐Dac N, Gervois P, Soncin F, Mandrup S, Fruchart JC, Fruchart‐Najib J, Staels B. The orphan nuclear receptor Rev‐Erbalpha is a peroxisome proliferator‐activated receptor (PPAR) gamma target gene and promotes PPARgamma‐induced adipocyte differentiation. J Biol Chem. 2003; 278:37672-37680 [DOI] [PubMed] [Google Scholar]
- 38.Duez H, Staels B. Rev‐erba: a potential target for the treatment of circadian disorders. Heart Metab. 2009; 44:21-24 [Google Scholar]
- 39.Kumar N, Solt LA, Wang Y, Rogers PM, Bhattacharyya G, Kamenecka TM, Stayrook KR, Crumbley C, Floyd ZE, Gimble JM, Griffin PR, Burris TP. Regulation of adipogenesis by natural and synthetic REV‐ERB ligands. Endocrinology. 2010; 151:3015-3025 [DOI] [PMC free article] [PubMed] [Google Scholar]