Abstract
Background
Controversy persists regarding the optimal revascularization strategy for diabetic patients with multivessel coronary artery disease (MVD). Coronary artery bypass grafting (CABG) has been compared with percutaneous coronary intervention (PCI) using drug‐eluting stents (DES) in recent randomized controlled trials (RCTs).
Methods and Results
RCTs comparing PCI with DES versus CABG in diabetic patients with MVD who met inclusion criteria were analyzed (protocol registration No. CRD42013003693). Primary end point (major adverse cardiac events) was a composite of death, myocardial infarction, and stroke at a mean follow‐up of 4 years. Analyses were performed for each outcome by using risk ratio (RR) by fixed‐ and random‐effects models. Four RCTS with 3052 patients met inclusion criteria (1539 PCI versus 1513 CABG). Incidence of major adverse cardiac events was 22.5% for PCI and 16.8% for CABG (RR 1.34, 95% CI 1.16 to 1.54, P<0.0001). Similar results were obtained for death (14% versus 9.7%, RR 1.51, 95% CI 1.09 to 2.10, P=0.01), and MI (10.3% versus 5.9%, RR 1.44, 95% CI 0.79 to 2.6, P=0.23). Stroke risk was significantly lower with DES (2.3% versus 3.8%, RR 0.59, 95% CI 0.39 to 0.90, P=0.01) and subsequent revascularization was several‐fold higher (17.4% versus 8.0%, RR 1.85, 95% CI 1.0 to 3.40, P=0.05).
Conclusions
These data demonstrate that CABG in diabetic patients with MVD at low to intermediate surgical risk (defined as EUROSCORE <5) is superior to MVD PCI with DES. CABG decreased overall death, nonfatal myocardial infarction, and repeat revascularization at the expense of an increase in stroke risk.
Keywords: CABG, diabetes, multivessel disease, PCI
Introduction
Patients with diabetes mellitus and coronary artery disease (CAD) often have severe and diffuse atherosclerotic involvement of multiple epicardial coronary arteries.1 Accordingly, a large proportion of diabetic patients require multivessel revascularization, either percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG).2 In an overall population, trials have suggested similar outcomes in patients with low‐ to intermediate‐risk SYNTAX Score with multivessel disease.3 However, in the diabetic subgroup, older trials dating back several decades have suggested improved outcomes with CABG versus PCI.4 A meta‐analysis of randomized controlled trials (RCTs) of either balloon angioplasty or bare metal stents (BMS) versus CABG has demonstrated superior outcomes with CABG,5–6 largely related to the higher rate of in‐stent restenosis and subsequent target vessel revascularization with PCI.5 Drug‐eluting stents (DES) have shown consistent and robust efficacy in reducing in‐stent restenosis and target vessel revascularization to single digits, even in the diabetic subgroup, without any effect on mortality and myocardial infarction (MI) rates compared with BMS.6
Recent observational studies and a meta‐analysis of these studies have suggested comparable outcomes of multivessel PCI with DES and CABG in diabetic patients.7–8 Whereas observational data provide a “real world” perspective, these studies have fundamental limitations of selection bias for treatment allocation and confounding inherent to the observational nature of the study. RCTs are the benchmark for comparing the efficacy of treatments for a clinical condition.9 In this context, long‐term results of RCTs comparing multivessel PCI with DES versus CABG in diabetic patients have recently been published. A quantitative evaluation and synthesis of this information are essential in elucidating the optimal and most durable revascularization strategy in this patient group. We performed a comprehensive meta‐analysis of these RCTs.
Methods
We developed a prospective protocol (registration No. CRD42013003693) detailing the specific objectives, criteria for study selection, approach to assess study quality, outcomes, and statistical methods. This protocol was approved and registered at PROSPERO,10–11 the international database of prospectively registered systematic reviews (managed by the Center for Reviews and Dissemination).
Search Strategy
We performed a literature search from January 2003 to March 2013 using PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Google Scholar, and Internet‐based sources of information on clinical trials (www.clinicaltrials.gov,www.tctmd.com,www.cardiosource.com). The Medical Subject Heading terms “coronary artery bypass grafting,” “randomized controlled trials,” “percutaneous coronary intervention,” “drug eluting stents,” “multi‐vessel disease,” and “diabetes” were used without language restrictions. Bibliographies of relevant studies and the “Related Articles” link in PubMed were used to identify additional studies. Published abstracts from annual meetings of the American College of Cardiology, American Heart Association, European Society of Cardiology, Trans Catheter Therapeutics, Society of Coronary Angiography and Intervention, and Euro Percutaneous Coronary Revascularization were reviewed, and if an abstract could be attached to a published article, that article was evaluated. However, in the absence of a full‐length refereed publication, abstracts were excluded. Using this methodology, the only abstract used was the 5‐year follow‐up data of the CARDia (Coronary Artery Revascularisation in Diabetes) trial including full‐text of the presentation slides at the 2012 European Society of Cardiology Scientific Sessions. Reference lists of review articles and cited articles were used to locate additional studies. For trials that were reported in >1 publication, we extracted data from the most complete publication and used other publications to clarify data. Publications of the same trials, summarizing results at different lengths of follow‐up, were also included to assess the temporal trends in outcomes between the 2 revascularization strategies.
Data Extraction
We extracted and presented data according to the Providing Innovative Service Models and Assessment (PRISMA) criteria.12 Studies were selected and data were extracted independently by 2 reviewers (A.H. and N.G.). Disagreements were resolved by consensus. Studies were evaluated carefully for duplicate or overlapping data. Clinical variables included age, gender, diabetes mellitus, hypertension, left ventricle ejection fraction (LVEF) and duration of clinical follow‐up. Raw data obtained from source information of the individual studies were used for all analyses. We also used the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions for our meta‐analysis.13
Selection Criteria
Eligible trials had to meet the following criteria: (1) RCTs and prespecified RCT subanalyses comparing multivessel PCI with DES with CABG in diabetics and (2) reporting outcomes of death, MI, stroke, and repeat revascularization. The primary end point was a composite of death, nonfatal MI, and stroke (major adverse cardiac events [MACE]) as defined in the primary trials. Separate analysis was performed for individual end points of death, cardiovascular death, MI, stroke, and repeat revascularization.
Statistical Methods
We used the risk ratio (RR) with 95% CIs as the metric of choice for all outcomes. Categorical variables were reported as percentages and continuous variables as mean±standard deviation (SD). Weighted means were used for the pooled estimates of continuous variables. The pooled RR was calculated with the DerSimonian–Laird method for random effects.14 For all the treatment effects that were statistically significant, we determined the absolute risk reduction (ARR) or the absolute risk increase and the corresponding number needed to treat (NNT) or number needed to harm (NNH). To assess heterogeneity across trials, we used the Cochran Q via a Mantel–Haenszel test based on the pooled RR. Heterogeneity was also assessed by means of the I2 statistic as proposed by Higgins et al15 (determining the variance across groups as a result of heterogeneity instead of chance). Based on the I2 statistic, values of 25%, 50%, and 75% were considered as yielding low, moderate, and high heterogeneity, respectively.15–16 Results were considered statistically significant at P<0.05. A funnel plot and the adjusted rank correlation test were used to assess for publication bias with respect to the primary outcome of interest (MACE). With use of a funnel plot, the RR was plotted on a logarithmic scale against its corresponding SE for each study. In the absence of publication bias, one would expect studies of all sizes to be scattered equally right and left of the line showing the pooled estimate of natural log RR. Begg's and the weighted regression test of Egger (P<0.05) were also used to assess publication bias.17 Sensitivity analysis was performed by evaluating the influence of removing individual studies on the pooled RR. Statistical analyses were performed with Revman software version 5.2.0 and Comprehensive Meta analysis (Biostat).
Meta Regression Analysis
Meta regression analyses18 were performed to evaluate the comparative effectiveness of CABG versus PCI as a function of time in relation to the end points of MACE, all‐cause mortality, MI, and repeat revascularization.
Results
Four randomized trials comparing PCI with DES and CABG in diabetic patients with multivessel CAD met inclusion criteria (Figure 1).19–25 Characteristics of trials and study participants are summarized in Table 1. Tables S1 and S2 summarize the study quality and key selection criteria of the included trials, respectively. The VA CARDS (Coronary Artery Revascularization in Diabetes) trial was severely underpowered and had to be terminated early because of recruitment issues.24
Figure 1.
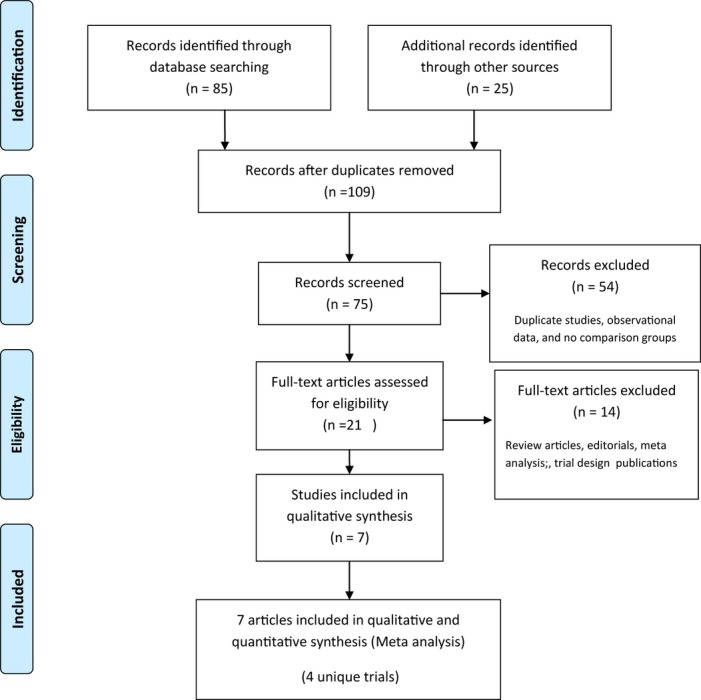
Study selection—flowchart depicts the selection of studies for inclusion in the meta‐analysis.
Table 1.
Characteristics of Included Trials and Participants
| Study Name | SYNTAX Trial19 | CARDia Study20 | FREEDOM Trial23 | VA CARDS24 |
|---|---|---|---|---|
| Study type | Subgroup analysis of RCT (noninferiority) | RCT (noninferiority) | RCT (superiority) | RCT |
| Study criteria | De novo LM and/or 3VD randomized to PCI or CABG | Diabetics with MVD or ostial/proximal LAD | Diabetics with MVD >70% in ≥2 major epicardial vessels in ≥2 separate coronary artery territories | Diabetics with MVD including the LAD or isolated proximal LAD |
| Total No. of PCI patients | 231 | 256 | 953 | 101 |
| Total No. of CABG patients | 221 | 254 | 947 | 97 |
| Recruitment period | 2005–2007 | 2002–2007 | 2005–2010 | 2006–2010 |
| Age, y | 65.4±9.2 | 64±8.7 | 63.1±9.1 | 62±7 |
| Female, n | 131 (29%) | 132 (25.9%) | 544 (28.6%) | 2 (1%) |
| Current smoker, n | 71 (15.8%) | 122 (23.1%) | 298 (15.6%) | 48(24%) |
| Prior MI, n | 143 (32%) | N/A | 487 (25.6%) | 63(32%) |
| LVEF, n | 13 (2.9%)* | 4 (0.8%)* | 32 (1%)* | 32(16%)* |
| Mean SYNTAX Score | 29±11.2 | N/A | 26.2±8.2 | 22.1±9 |
| Mean euroSCORE | 4.0±2.7 | N/A | 2.8±2.5 | N/A |
| Mean HbA1c, % | >7* | 7.9±1.5 | 7.8±1.7 | 7.9±1.7 |
| Insulin use, n (%) | 40.3% | 192 (37.6%) | 615 (32.4%) | 93 (47%) |
| Mean follow‐up period, y | 5 | 5.1 (interquartile range 3.8 to 5.4) | 3.8 (range 2.5 to 4.9) | 2 |
| Follow‐up completeness | 92.2% CABG; 98% PCI | 95% CABG; 96.8% PCI | 94.9% CABG; 91% PCI | 100% |
| LIMA | N/A | 94% | 94.4% | 100% |
| No. of grafts | N/A | 2.9 | 2.9±0.8 | N/A |
| No. of stents | N/A | 3.6 | 3.5±1.4 | N/A |
| Total length of stent, (mean)* | 88.6±49.0 mm | 71 mm | 26.1+14.2 mm | N/A |
| Patients with 3VD | 71% | 65% | 83% | N/A |
| Patients on DAPT at 1 y | 71.8% | 50.9% | 90% | N/A |
| Patients with DES | 100% | 69% | 100% | 95.5% |
| Screened population meeting eligibility criteria and eventually randomized | 41% of screened patients were eligible and enrolled | N/A | 10% of screened population met eligibility criteria and 5.7% of screened population was ultimately randomized | 9% of screened population met eligibility criteria and 35% of eligible patients were enrolled |
RCT indicates randomized controlled trial; LM, left main; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft surgery; MVD, multivessel coronary artery disease; LAD, left anterior descending coronary artery; HbA1c, glycated hemoglobin; LIMA, left internal mammary artery; MI, myocardial infarction; N/A, not available; LVEF, left ventricular ejection fraction; DAPT, dual antiplatelet therapy; DES, drug‐eluting stents.
LVEF <30%.
LVEF <40%.
Fifty‐seven percent of patient had HbA1c >7%.
PCI arm only.
There were a total of 3052 patients (1539 patients in the PCI arm and 1513 patients in the CABG arm). There were no differences (PCI versus CABG) in the weighted mean age (63.4 years versus 63.1 years), males (74.7% versus 74%), current smokers (18.3% versus 18.5%), mean time since diagnosis of diabetes (10.5 years versus 10.4 years), and insulin use (35.6% versus 34.4%). Weighted mean follow‐up duration was 4 years (range 1 to 5 years).
Outcomes
Clinical End Points
RRs and 95% CIs for clinical follow‐up are presented in Figures 2 through 6.
Figure 2.

A, Major adverse cardiac events (MACE)—Percutaneous coronary intervention (PCI) vs coronary artery bypass graft surgery (CABG) for the risk of MACE. The Forest plot depicts the individual trials and subtotal risk ratios and 95% CIs comparing the outcome of MACE for PCI vs CABG. B, Publication bias—Funnel plot for assessment of publication bias of trials comparing PCI with CABG for the end point of MACE. The circles correspond to the treatment effects from individual trials, the central line shows the summary estimate, and the diagonal lines show the expected 95% CIs around the summary estimate. There is no evident asymmetry of the points in relation to the summary estimate that might indicate a relevant publication bias. MH indicates Mantel–Haenszel.
Figure 6.
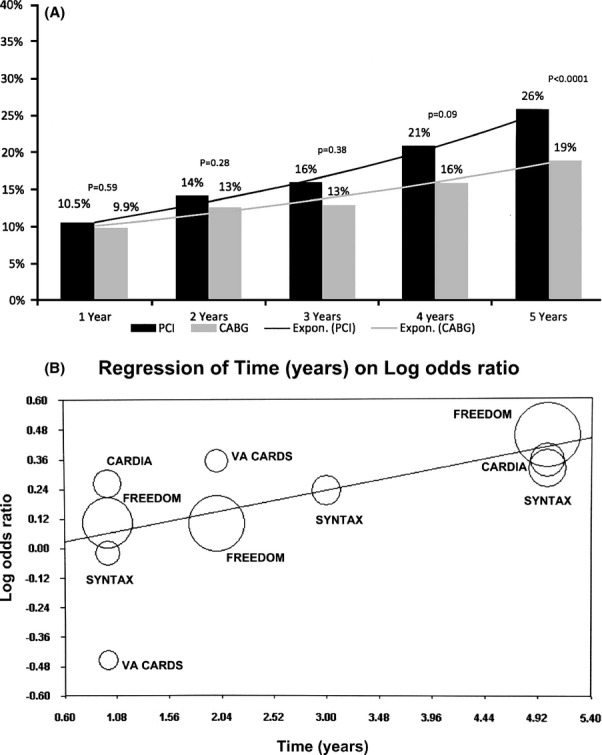
A, Pooled (MH RR) MACE events at follow‐up—Differences in pooled incidence (random‐effects analysis) of MACE at different time points for percutaneous coronary intervention (PCI) vs coronary artery bypass graft surgery (CABG). B, Meta regression of time over log odds ratio for MACE—Meta regression plot for log odds ratio of MACE for PCI vs CABG as a function of time (in years). Markers above the regression line favor CABG and below the line favor PCI. The size of the data markers represents the weight of each trial. MH indicates Mantel–Haenszel; RR, risk ratio; MACE, major adverse cardiac events.
Primary End Point
At a mean follow‐up of 4 years, the primary outcome was 22.5% in the PCI arm and 16.8% in the CABG arm (RR 1.34, 95% CI 1.16 to 1.54, P<0.0001). There was no significant heterogeneity with respect to the composite endpoint (I2=0%, P=0.89) (Figure 2A). There was no evidence of publication bias for the primary end point both on visual estimation of the funnel plot and on Egger's regression analysis (P=0.45) (Figure 2B). Sensitivity analysis demonstrated that the superiority of CABG over PCI was most evident in the group with a high‐risk SYNTAX Score with no statistically significant difference in outcomes in the low‐ and intermediate‐risk SYNTAX Score groups (Table 2).
Table 2.
Sensitivity Analysis
| Variable | PCI | CABG | RR | P Value | Heterogeneity | |
|---|---|---|---|---|---|---|
| MACE based on SYNTAX Score* | <22 (n=805) | 22.2% | 17.5% | 1.27 (0.96 to 1.68) | 0.09 | 0%; P=0.32 |
| 23 to 32 (n=992) | 26.1% | 18.3% | 1.32 (0.86 to 2.02) | 0.21 | 48%; P=0.16 | |
| >33 (n=541) | 24.7% | 14.4% | 1.73 (1.21 to 2.46) | 0.003 | 0%; P=0.81 | |
| After excluding VA CARDS Trial | MACE (n=2854) | 22.4% | 16.7% | 1.34 (1.15 to 1.55) | 0.0002 | 0%; P=0.94 |
| Death (n=2854) | 13.5% | 9.9% | 1.36 (1.11 to 1.66) | 0.003 | 0%; P=0.53 | |
| MI (n=2854) | 10.6% | 5.3% | 2.01 (1.54 to 2.62) | <0.0001 | 0%; P=0.83 | |
| Repeat revascularization (n=2854) | 17.3% | 6.5% | 2.61 (2.09 to 3.2) | <0.00001 | 0%; P=0.89 |
PCI indicates percutaneous coronary intervention; CABG, coronary artery bypass graft surgery; RR, risk ratio; MACE, major adverse cardiac events; MI, myocardial infarction.
Pooled analysis (random‐effects model) from SYNTAX (5‐year follow‐up) and FREEDOM (5‐year follow‐up).
Death
The incidence of death was 14% in the PCI group and 9.7% in the CABG group with an RR of 1.51 (95% CI 1.09 to 2.10, P=0.01) in the random‐effects model, translating into a relative risk reduction of 51% for the end point of death with CABG (Figure 3A). There was moderate heterogeneity with respect to the end point of death among the trials (I2=52%, P=0.89). Sensitivity analysis demonstrated that this heterogeneity in the point estimate of death was largely due to the VA CARDS trial. After exclusion of this trial, random‐effects meta‐analysis yielded an RR 1.36 (1.11 to 1.16) for death (P=0.03) with no residual heterogeneity (I2=0%, P=0.53) (Table 2).
Figure 3.
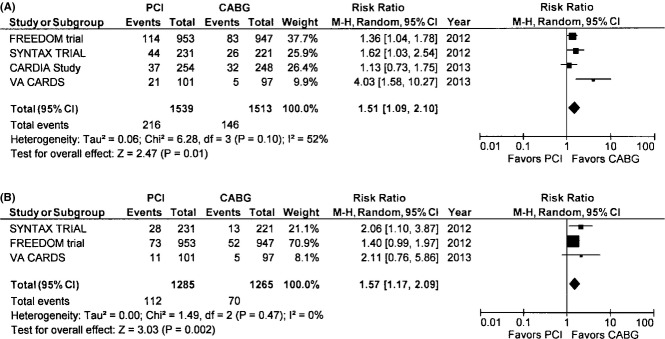
A, Death—The Forest plot depicts the individual trials and subtotal risk ratios and 95% CIs comparing the outcome of death for percutaneous coronary intervention (PCI) vs coronary artery bypass graft surgery (CABG). B, Cardiac death—The Forest plot depicts the individual trials and subtotal risk ratios and 95% CIs comparing the outcome of cardiac death for PCI vs CABG. MH indicates Mantel–Haenszel.
Cardiovascular Death
This end point was reported in 3 of the 4 included trials. The pooled incidence of cardiac mortality was 8.7% in the PCI group and 5.5% in the CABG group (RR 1.57, 95% CI 1.17 to 2.09, P=0.002) with no heterogeneity among the trials for this end point (Figure 3B).
Myocardial Infarction
The pooled MI rate was 10.3% in the PCI group and 5.9% in the CABG arm yielding an RR 1.44, 95% CI 0.79 to 2.6, P=0.23. There was significant heterogeneity (I2=75%, P=0.007) among studies with respect to this end point (Figure 4A). Sensitivity analysis showed that this was related primarily to the inclusion of the VA CARDS trial, where, unlike all other trials, MI incidence was higher in the CABG group (Table 2). The point estimate for MI, after excluding the VA CARDS trial, reached statistical significance (RR 2.01, 95% CI 1.54 to 2.62, P<0.0001) with no residual heterogeneity (I2=0%, P=0.83).
Figure 4.

A, Myocardial infarction—The Forest plot depicts the individual trials and subtotal risk ratios and 95% CIs comparing the outcome of myocardial infarction for percutaneous coronary intervention (PCI) vs coronary artery bypass graft surgery (CABG). B, Stroke—The Forest plot depicts the individual trials and subtotal risk ratios and 95% CIs comparing the outcome of stroke for PCI vs CABG. MH indicates Mantel–Haenszel.
Stroke
Stroke risk was significantly higher in the CABG group (2.3% versus 3.8%, RR 0.59, 95% CI 0.39 to 0.94, P=0.01) with no heterogeneity between trials for this end point (I2=0%, P=0.94) (Figure 4B).
Repeat Revascularization
PCI was associated with several‐fold higher risk of subsequent revascularization compared with CABG (17.4% versus 8.0%, RR 1.85, 95% CI 1.0 to 3.4, P=0.05) (Figure 5). Repeat revascularization was consistently higher in the PCI group in all studies except for the VA CARDS trial, which contributed to the significant heterogeneity (I2=88%, P<0.0001). Excluding this trial, the point estimate for repeat revascularization was RR 2.61(2.09 to 3.2, P<0.0001) with no residual heterogeneity (I2=0%, P=0.89) (Table 2).
Figure 5.
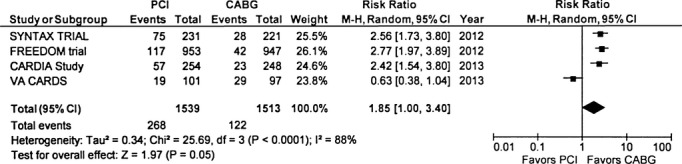
Repeat revascularization—The Forest plot depicts the individual trials and subtotal risk ratios and 95% CIs comparing the outcome of repeat revascularization for percutaneous coronary intervention (PCI) vs coronary artery bypass graft surgery (CABG). MH indicates Mantel–Haenszel.
Comparative Effectiveness of Revascularization Strategies Over Time
Using data from publications of the included trials at varying follow‐up times,19–25 we were able to estimate the influence of time on the comparative advantage of one revascularization strategy versus the other. As seen in Figure 6A for the MACE end point, the advantage of CABG became statistically significant over PCI after 4 years of revascularization (pooled ARR 6%). Meta regression analysis confirmed the advantage of CABG over PCI in reducing MACE as a function of time (P=0.04) (Figure 6B). Similar trends were observed for end points of death, MI, and repeat revascularization (data not shown).
Discussion
This study is the first comprehensive systematic review and meta‐analysis of RCTs comparing PCI with DES versus CABG for revascularization of multivessel CAD in diabetic patients. In this meta‐analysis comprising over 3000 randomized diabetic patients with multivessel disease (with the majority being 3‐vessel disease), CABG improved survival, decreased MI, and repeat revascularization compared with multivessel PCI with first‐generation DES. After 5 years of follow‐up, patients treated with CABG had a 6% ARR in the primary end point, which was a composite of death, MI, and stroke (MACE), 4.4% ARR in total mortality, 3.3% ARR in cardiovascular mortality, and 11% ARR in repeat revascularization, corresponding to an NNT of 18 for prevention of 1 MACE event, NNT of 23 to prevent 1 death, NNT of 30 to prevent 1 cardiovascular mortality, and NNT of 11 to prevent 1 repeat revascularization. CABG was associated with increased risk of stroke with an NNH of 58 treated patients for 1 additional stroke.
The observed advantage of CABG was consistent among all trials for the primary end point of MACE with absolutely no heterogeneity observed (I2=0%, P=0.83). Sensitivity analysis suggested that the CABG advantage was most pronounced in the high‐risk SYNTAX Score group with no statistical difference in outcomes for the intermediate‐ and low‐risk SYNTAX Score patients. Sensitivity analysis found that heterogeneity for the end points of death, MI, and repeat revascularization was mainly due to the inclusion of the VA CARDS trial.24 This trial had major issues with enrollment, with only 25% of the intended sample size being ultimately recruited and early termination at a mean follow‐up of 2 years instead of the planned 3.7 years, leaving it severely underpowered (9.7%) for the primary end point, seriously putting into question the validity of its results (observed results could all have been due to chance alone).24 Furthermore, the VA CARDS trial constitutes only 6.5% of the total weight to our meta‐analysis. Repeating the analysis after excluding the VA CARDS trial demonstrates that CABG is superior at reducing nonfatal MI (ARR 5.4%, NNT 18.5).
Previous meta‐analyses of RCTs comparing CABG versus PCI with BMS or balloon angioplasty have demonstrated improved outcomes with CABG (Table S3). Recent observational studies and meta‐analysis of studies comparing multivessel PCI with DES versus CABG have shown comparable outcomes with the exception of higher revascularization rates in the PCI arm.5,7–8,26 However, observational studies are inherently flawed with numerous biases, primarily selection bias (patients who are sicker and who have more severe disease receive CABG). Hence, RCTs are the benchmark for establishing clinical efficacy and safety.9 We observed a temporal association in the advantage conferred by CABG over PCI. The primary end point of MACE did not reach statistical significance until after 4 years of follow‐up. However, except for the first 30 days at no point was the event rate lower in the PCI compared with the CABG arm with the exception of stroke. At least until 3 years of follow‐up, no statistically significant difference was observed in the hard end points of MACE. The durability of CABG became more manifest with the passage of time.
Caveats
This meta‐analysis of RCTs clearly demonstrates superior long‐term outcomes for diabetic patients with multivessel CAD with CABG compared with PCI. These findings, nonetheless, must be interpreted in the context of the following important caveats. All trials included in this meta‐analysis included a small fraction of the overall population screened (FREEDOM trial 5%, VA CARDS Trial 3%, SYNTAX Trial 41% overall, and diabetic subgroup 10.4%). Thus, the included trials enrolled highly selected patient populations, not necessarily typical of those encountered in everyday clinical practice. There were key exclusions common to all trials, including significant heart failure, cardiogenic shock, recent or acute ST‐segment elevation MI, and previous revascularization (refer to Table S2). Furthermore, the mean EUROSCORE (European System for Cardiac Operative Risk Evaluation) was 3.5, which signifies a relatively low to intermediate surgical risk. Thus, results of these trials should be applied only to the included diabetic subgroups with caution warranted in extrapolating results to the excluded diabetic subgroups. In addition, these trials mostly used first‐generation drug‐eluting stents (sirolimus‐ and paclitaxel‐coated stents). Whether CABG will continue to show superiority over PCI with newer‐generation drug‐eluting stents in diabetic patients is unknown. Recent data from a Swedish registry of >4700 patients showed a lower event rate in diabetic patients with the use of second‐generation stents (everolimus) compared with first‐generation stents.27 These results were mainly driven by a lower incidence of stent thrombosis and mortality with no significant differences in restenosis. Another important aspect is the duration of dual antiplatelet therapy (DAPT) in diabetic patients who undergo multivessel stenting. In the RCTs included in this meta‐analysis, the duration was variable, ranging at 1 year from 50% in CARDia to 90% in the FREEDOM Trial. The suboptimal DAPT duration in some of these trials may have contributed to a higher MI rate (stent thrombosis) or death, but it is unclear from our analysis as stent thrombosis was not an adjudicated end point in the trials. Furthermore, newer thienopyridines, including prasugrel and ticagrelor, were not used in these trials. It has been well established that prasugrel (and perhaps ticagrelor) has a particular advantage in the diabetic subgroup (52% relative risk reduction in ST compared with clopidogrel in the TRITON TIMI 38 [Trials to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction‐38] trial).28–30
The major reason for long‐term durability and improved clinical efficacy of CABG may be related in part to the “completeness of revascularization.” Several studies have credibly demonstrated that incomplete revascularization (as reflected by the “residual SYNTAX Score” after revascularization) is an independent predictor of MACE and repeat revascularization.31–35 None of the trials included in this meta‐analysis evaluated the impact of degree of revascularization (and comparison between CABG and PCI) on outcomes. A recent post‐hoc analysis from the 4‐year outcomes of the SYNTAX Trial and Registry showed that angiographic complete revascularization was achieved in only 52.8% of the PCI arm and 66.9% of the CABG arm.33 Patients with incomplete revascularization had a significantly higher risk of death, MI, and repeat revascularization in both the PCI and CABG groups; however, the magnitude of risk was much higher in the incompletely revascularized PCI group compared with the CABG group for all these end points. Interestingly, MACE rate was 14% in the completely revascularized CABG group compared with 17.2% in the completely revascularized PCI group at 4 years.33 Will complete revascularization with new‐generation drug‐eluting stents with newer P2Y12 inhibitors yield comparable results to CABG? This important question will need to be confirmed in randomized trials.
Limitations
As with any meta‐analysis, the conclusions drawn from such data are subject to the limitations of the original studies. Patient‐level data were not available precluding subgroup analysis. Similarly, due to the lack of patient‐level data, we could not account for different follow‐up times and for censoring or drop‐out by performing any meaningful survival analysis. While we did perform appropriate statistical analysis to evaluate sources of bias and heterogeneity, there is no way to eliminate bias caused by the influence of unmeasured confounders or the presence of patients deemed to be ineligible for one of the procedures.
Clinical Implications
While the ease and minimal morbidity of PCI have a strong appeal to patients, families, and some physicians, current clinical approaches to the treatment of the diabetic patient with multivessel disease may exceed scientific evidence. The 2011 American Heart Association/American College of Cardiology PCI guideline update recommends a “Heart Team” approach, with the implicit understanding that each patient's case takes into account surgical and interventional risks, long‐term outcomes, and patient preferences. Based on the current study, CABG is preferred for the most favorable long‐term outcomes in high‐risk SYNTAX Score patients without high surgical risk. For patients with low‐risk and possibly intermediate‐risk SYNTAX Scores, PCI may be a reasonable primary strategy, given the early increased morbidity from surgery and the higher risk of stroke with comparable long‐term MACE. The preferred strategy in diabetic patients with high surgical risk requires further study.
Disclosures
None.
References
- 1.Nicholls SJ, Tuzcu EM, Kalidindi S, Wolski K, Moon KW, Sipahi I, Schoenhagen P, Nissen SE. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol. 2008; 52:255-262 [DOI] [PubMed] [Google Scholar]
- 2.Smith SC, Jr, Faxon D, Cascio W, Schaff H, Gardner T, Jacobs A, Nissen S, Stouffer R. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group VI: revascularization in diabetic patients. Circulation. 2002; 105:e165-e169 [DOI] [PubMed] [Google Scholar]
- 3.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FWSYNTAX Investigators Percutaneous coronary intervention versus coronary‐artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009; 360:961-972 [DOI] [PubMed] [Google Scholar]
- 4.The Bypass Angioplasty Revascularization Investigation (BARI) Investigators Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med. 1996; 335:217-225 [DOI] [PubMed] [Google Scholar]
- 5.Hlatky MA, Boothroyd DB, Bravata DM, Boersma E, Booth J, Brooks MM, Carrié D, Clayton TC, Danchin N, Flather M, Hamm CW, Hueb WA, Kähler J, Kelsey SF, King SB, Kosinski AS, Lopes N, McDonald KM, Rodriguez A, Serruys P, Sigwart U, Stables RH, Owens DK, Pocock SJ. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009; 373:1190-1197 [DOI] [PubMed] [Google Scholar]
- 6.Stettler C, Allemann S, Wandel S, Kastrati A, Morice MC, Schomig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabate M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, De Carlo M, Erglis A, Chechi T, Ortolani P, Schalij MJ, Diem P, Meier B, Windecker S, Juni P. Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta‐analysis. BMJ. 2008; 337:a1331-a1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MS, Yang T, Dhoot J, Iqbal Z, Liao H. Meta‐analysis of studies comparing coronary artery bypass grafting with drug‐eluting stenting in patients with diabetes mellitus and multivessel coronary artery disease. Am J Cardiol. 2010; 105:1540-1544 [DOI] [PubMed] [Google Scholar]
- 8.Park DW, Kim YH, Song HG, Ahn JM, Kim WJ, Lee JY, Kang SJ, Lee SW, Lee CW, Park SW, Yun SC, Chung SH, Choo SJ, Chung CH, Lee JW, Park SJ. Long‐term outcome of stents versus bypass surgery in diabetic and nondiabetic patients with multivessel or left main coronary artery disease: a pooled analysis of 5775 individual patient data. Circ Cardiovasc Interv. 2012; 5:467-475 [DOI] [PubMed] [Google Scholar]
- 9.Hannan EL. Randomized clinical trials and observational studies: guidelines for assessing respective strengths and limitations. JACC Cardiovasc Interv. 2008; 1:211-217 [DOI] [PubMed] [Google Scholar]
- 10.Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, Stewart L. PROSPERO at one year: an evaluation of its utility. Syst Rev. 2013; 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth A, Clarke M, Ghersi D, Moher D, Petticrew M, Stewart L. An international registry of systematic‐review protocols. Lancet. 2011; 377:108-109 [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DGPRISMA Group Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264-269 [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [Updated March 2011]. 2008The Cochrane Collaboration; www.cochrane-handbook.org [Google Scholar]
- 14.DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986; 7:177-188 [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002; 21:1539-1558 [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG, Deeks J, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003; 327:557-560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997; 315:629-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted? Stat Med. 2002; 21:1559-1573 [DOI] [PubMed] [Google Scholar]
- 19.Banning AP, Westaby S, Morice MC, Kappetein AP, Mohr FW, Berti S, Glauber M, Kellett MA, Kramer RS, Leadley K, Dawkins KD, Serruys PW. Diabetic and nondiabetic patients with left main and/or 3‐vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel‐eluting stents. J Am Coll Cardiol. 2010; 55:1067-1075 [DOI] [PubMed] [Google Scholar]
- 20.Kapur A, Hall RJ, Malik IS, Qureshi AC, Butts J, de Belder M, Baumbach A, Angelini G, de Belder A, Oldroyd KG, Flather M, Roughton M, Nihoyannopoulos P, Bagger JP, Morgan K, Beatt KJ. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients: 1‐year results of the CARDia (Coronary Artery Revascularization in Diabetes) Trial. J Am Coll Cardiol. 2010; 55:432-440 [DOI] [PubMed] [Google Scholar]
- 21.Mack MJ, Banning AP, Serruys PW, Morice MC, Taeymans Y, Van Nooten G, Possati G, Crea F, Hood KL, Leadley K, Dawkins KD, Kappetein AP. Bypass versus drug‐eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg. 2011; 92:2140-2146 [DOI] [PubMed] [Google Scholar]
- 22.Kapur A, Baumbach A, Beatt K, Crean P, De Belder A, Fath‐Ordoubadi F, Flather M, Hall R, Malik I. Five year results of CARDia (Coronary Artery Revascularization in Diabetes) Trial. Eur Heart J. 2012; 33suppl 1:339-653http://www.escardio.org/congresses/esc-2012/congress-reports/Pages/710-5-CARDia.aspx#presenter [Google Scholar]
- 23.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S, III, Bertrand M, Fuster VFREEDOM Trial Investigators Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012; 367:2375-2384 [DOI] [PubMed] [Google Scholar]
- 24.Kamalesh M, Sharp TG, Tang XC, Shunk K, Ward HB, Walsh J, King S, III, Colling C, Moritz T, Stroupe K, Reda DVA CARDS Investigators Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol. 2013; 61:808-816 [DOI] [PubMed] [Google Scholar]
- 25.Kappetein AP, Head SJ, Morice MC, Banning AP, Serruys PW, Mohr FW, Dawkins KD, Mack MJSYNTAX Investigators Treatment of complex coronary artery disease in patients with diabetes: 5‐year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg. 2013; 43:1006-1013 [DOI] [PubMed] [Google Scholar]
- 26.Daemen J, Boersma E, Flather M, Booth J, Rodriguez‐Granillo G, Hueb WA, Lemos PA, Serruys PW. Long‐term safety and efficacy of percutaneous coronary intervention with stenting and coronary artery bypass surgery for multivessel coronary artery disease: a meta‐analysis with 5‐year patient‐level data from the ARTS, ERACI‐II, MASS‐II, and SoS trials. Circulation. 2008; 11:1146-1154 [DOI] [PubMed] [Google Scholar]
- 27.Kedhi E, Gomes ME, Lagerqvist B, Smith JG, Omerovic E, James S, Harnek J, Olivecrona GK. Clinical impact of second‐generation everolimus‐eluting stent compared with first‐generation drug‐eluting stents in diabetes mellitus patients: insights from a nationwide coronary intervention register. JACC Cardiovasc Interv. 2012; 5:1141-1149 [DOI] [PubMed] [Google Scholar]
- 28.Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, Goodman SG, Corbalan R, Purdy DA, Murphy SA, McCabe CH, Antman EMTRITON‐TIMI 38 Investigators Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with Prasugrel—Thrombolysis in Myocardial Infarction 38. Circulation. 2008; 118:1626-1636 [DOI] [PubMed] [Google Scholar]
- 29.Alexopoulos D, Xanthopoulou I, Mavronasiou E, Stavrou K, Siapika A, Tsoni E, Davlouros P. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with diabetes mellitus. Diabetes Care. 2013. 10.2337/dc12‐2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angiolillo DJ, Badimon JJ, Saucedo JF, Frelinger AL, Michelson AD, Jakubowski JA, Zhu B, Ojeh CK, Baker BA, Effron MB. A pharmacodynamic comparison of prasugrel vs. high‐dose clopidogrel in patients with type 2 diabetes mellitus and coronary artery disease: results of the Optimizing anti‐Platelet Therapy In diabetes MellitUS (OPTIMUS)‐3 Trial. Eur Heart J. 2011; 32:838-846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwietz T, Spyridopoulos I, Pfeiffer S, Laskowski R, Palm S, DE Rosa S, Jens K, Zeiher AM, Schächinger V, Fichtlscherer S, Lehmann R. Risk stratification following complex PCI: clinical versus anatomical risk stratification including “post PCI residual SYNTAX‐score” as quantification of incomplete revascularization. J Interv Cardiol. 2013; 26:29-37 [DOI] [PubMed] [Google Scholar]
- 32.Schwartz L, Bertolet M, Feit F, Fuentes F, Sako EY, Toosi MS, Davidson CJ, Ikeno F, King SB., III Impact of completeness of revascularization on long‐term cardiovascular outcomes in patients with type 2 diabetes mellitus: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D). Circ Cardiovasc Interv. 2012; 5:166-173 [DOI] [PubMed] [Google Scholar]
- 33.Farooq V, Serruys PW, Garcia‐Garcia HM, Zhang Y, Bourantas CV, Holmes DR, Mack M, Feldman T, Morice MC, Ståhle E, James S, Colombo A, Diletti R, Papafaklis MI, de Vries T, Morel MA, van Es GA, Mohr FW, Dawkins KD, Kappetein AP, Sianos G, Boersma E. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial. J Am Coll Cardiol. 2013; 61:282-294 [DOI] [PubMed] [Google Scholar]
- 34.Sarno G, Garg S, Onuma Y, Gutiérrez‐Chico JL, van den Brand MJ, Rensing BJ, Morel MA, Serruys PWARTS‐II Investigators Impact of completeness of revascularization on the five‐year outcome in percutaneous coronary intervention and coronary artery bypass graft patients (from the ARTS‐II study). Am J Cardiol. 2010; 106:1369-1375 [DOI] [PubMed] [Google Scholar]
- 35.Généreux P, Palmerini T, Caixeta A, Rosner G, Green P, Dressler O, Xu K, Parise H, Mehran R, Serruys PW, Stone GW. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J Am Coll Cardiol. 2012; 59:2165-2174 [DOI] [PMC free article] [PubMed] [Google Scholar]


