Abstract
Background
Although renal denervation significantly reduces blood pressure in patients with resistant hypertension, the role of the renal nerve in hypertension with metabolic syndrome is unknown. We investigated the impact of long‐term renal denervation on SHR/NDmcr‐cp(+/+) (SHRcp) rats, a useful rat model of metabolic syndrome, to determine the role of the renal nerve in hypertension with metabolic syndrome.
Methods and Results
SHRcp rats were divided into (1) a renal denervation (RD) group and (2) a sham operation group (control) to examine the effects of long‐term RD on blood pressure circadian rhythm, renal sodium retention‐related molecules, the renin‐angiotensin‐aldosterone system, metabolic disorders, and organ injury. RD in SHRcp rats not only significantly reduced blood pressure but also normalized blood pressure circadian rhythm from the nondipper to the dipper type, and this improvement was associated with an increase in urinary sodium excretion and the suppression of renal Na+‐Cl− cotransporter upregulation. RD significantly reduced plasma renin activity. RD significantly prevented cardiovascular remodeling and impairment of vascular endothelial function and attenuated cardiovascular oxidative stress. However, RD failed to ameliorate obesity, metabolic disorders, and renal injury and failed to reduce systemic sympathetic activity in SHRcp rats.
Conclusions
By including the upregulation of the Na+‐Cl− cotransporter, the renal sympathetic nerve is involved in the disruption of blood pressure circadian rhythm as well as hypertension in metabolic syndrome. Thus, RD seems to be a useful therapeutic strategy for hypertension with metabolic syndrome.
Keywords: circadian rhythm, hypertension, obesity, renin, sodium
Introduction
Convincing evidence indicates a major role for the sympathetic nervous system in the development of hypertension and cardiovascular and renal diseases.1–3 In particular, renal nerves are involved in renal function, body fluid homeostasis, blood pressure (BP) control, and the pathophysiology of heart failure and chronic kidney disease.4–5 Renal efferent sympathetic nerve activation enhances volume retention and sodium reabsorption, reduces renal blood flow, and activates the renin‐angiotensin‐aldosterone system.4–5 Moreover, renal afferent sensory nerves transmit important sensory information to the central nervous system and consequently modulate central sympathetic activity, thereby potentially influencing sympathetic outflow to the kidneys and other organs such as the heart and systemic blood vessels,4–5 although much less is known about the role of renal afferent sensory nerves than of renal efferent sympathetic nerves.
A proof‐of‐concept study6 and a subsequent randomized controlled trial7 have applied a novel catheter‐based ablation technique to selectively denervate the kidneys in patients with treatment‐resistant hypertension. They have shown that bilateral renal denervation (RD) leads to a significant and sustained reduction of blood pressure in patients with resistant hypertension. Recent reports indicate that RD significantly reduces 24‐hour BP and daytime and nighttime blood pressure in patients with treatment‐resistant hypertension8 and significantly reduces central BP.9 Catheter‐based renal nerve ablation decreases renal norepinephrine spillover,6 muscle sympathetic nerve activity, systemic norepinephrine spillover,10 and single sympathetic nerve firing11 in some patients with resistant hypertension, suggesting that RD may potentially inhibit of systemic sympathetic activity in addition to renal sympathetic activity. Furthermore, RD had a beneficial effect on arterial stiffness12 and provided ventricular rate control in a patient with permanent atrial fibrillation.13 On the other hand, there is substantial variability in the BP‐lowering effects of RD in patients with resistant hypertension. A more recent clinical trial14 reported the controversial finding that RD did not significantly reduce BP and did not decrease sympathetic activity in unselected patients with resistant hypertension. Therefore, reductions in centrally generated sympathetic activity observed in previous reports6,10 may be the exception rather than the rule after renal nerve ablation. Furthermore, it remains to be defined whether RD with this novel approach is also useful in less severe forms of hypertension or in other conditions characterized by enhanced renal sympathetic nerve activity, such as metabolic syndrome.15 Patients can be responders or nonresponders to RD. Thus, further experimental studies are required to define the appropriate indication of RD in clinical practice.
Hypertension is frequently associated with aspects of metabolic syndrome, such as obesity, impaired glucose tolerance, and insulin resistance.16–17 Importantly, sympathetic nervous system activation can also be involved in impairment of insulin sensitivity and glucose metabolism, and enhanced sympathetic nerve activity is generally observed in patients with metabolic syndrome or diabetes.16–17
SHR/NDmcr‐cp(+/+) (SHRcp) rats are a congenic SHR rat strain with a nonsense mutation introduced in the leptin receptor.18 SHRcp rats exhibit obesity, hypertension, insulin resistance, glucose intolerance, and hyperlipidemia and are regarded as a useful rat model of human metabolic syndrome.19–22 Moreover, we reported that SHRcp rats exhibit non‐dipper‐type hypertension, in contrast to SHR rats, which have dipper‐type hypertension, indicating that SHRcp rats are also a useful model for investigations of the mechanism and treatment of disrupted BP circadian rhythm.23 In this study, to determine the effect of RD on metabolic syndrome with hypertension, we examined the potential beneficial effect of chronic RD on SHRcp rats.
Materials and Methods
Ethics Statement
All procedures were performed in accordance with institutional guidelines for animal research approved by the Animal Care and Use Committee of Kumamoto University.
Experimental Animals
Male Wistar‐Kyoto (WKY) and SHR/NDmcr‐cp(+/+) rats (SHRcp) rats,19–22 metabolic syndrome model rats, were purchased from Japan SLC (Shizuoka, Japan). All rats were housed in an animal facility with a 12‐hour light–dark cycle and were given standard chow and water ad libitum.
Experimental Protocol
To test the effects of long‐term RD on SHRcp rats, they were followed for 19 weeks after RD. SHRcp rats (n=22) were divided into 2 groups: one received bilateral RD (RD group, n=11), and the other received a sham operation (control group, n=11). At 7 weeks of age, RD or sham operation was performed, as described below. Age‐matched WKY rats (n=11) were subjected to sham operation. Body weight was measured every week. At 13 weeks after RD or sham operation, SHRcp and age‐matched WKY rats (n=6 each) were housed in metabolic cages to collect urinary samples for 24 hours, as described below. An intraperitoneal glucose tolerance test and intraperitoneal insulin tolerance test (IITT) were performed 14 and 15 weeks, respectively, after RD or sham operation. Sixteen weeks after RD or sham operation, RD‐operated SHRcp (n=5), sham‐operated SHRcp (n=5), and sham‐operated WKY (n=5) rats were subjected to implantation of miniaturized telemetry devices to measure the circadian rhythm of BP, as described below. Nineteen weeks after RD or sham operation, SHRcp and WKY rats were anesthetized with isoflurane, arterial blood was immediately collected by cardiac puncture, and plasma was collected by centrifugation and stored at −80°C until use. After perfusion with phosphate‐buffered saline, the carotid artery, thoracic aorta, heart, and kidney were immediately excised to measure various parameters, as described below.
In another experiment, the effects of short‐term RD on BP, cumulative sodium balance, plasma renin activity, and aldosterone were examined in SHRcp rats. For measurement of BP, at 6 weeks of age, SHRcp rats (n=14) were subjected to implantation of miniaturized telemetry devices to measure direct BP. At 7 weeks of age, they were divided into 2 groups and were subjected to RD (n=7) or sham operation (n=7). Mean arterial pressure was measured over 4 weeks after RD. The cumulative sodium balance of SHRcp rats was estimated with metabolic cages from 5 days before to 29 days after RD or sham operation, as described below. Twenty‐nine days after the operation, plasma renin activity and plasma aldosterone were measured by collecting blood samples from the tail vein of SHRcp rats, and 24‐hour urinary aldosterone excretion was measured by using urinary samples collected in metabolic cages.
Collection of Urine Samples in Metabolic Cages
In long‐term RD experiments, 13 weeks after RD or sham operation, SHRcp rats and WKY rats were acclimatized to the metabolic cages (Techniplast 3701M001, Buguggiate, Italy) for 48 hours, then 24‐hour urine was collected with metabolic cages to measure urinary sodium excretion.
To estimate cumulative sodium balance, SHRcp rats were housed in metabolic cages for 24 hours every other day from 5 days before to 29 days after RD to collect 24‐hour urine samples and measure 24‐hour food intake, water intake, and urinary volume. Twenty‐four‐hour sodium intake was calculated by multiplying food intake (g) by the sodium content of the diet (0.31% NaCl=0.053041 mmol Na+/g food). Twenty‐four‐hour sodium balance was calculated as 24‐hour sodium intake minus 24‐hour sodium excretion. Cumulative sodium balance was calculated from sequential summation of the daily balances over the duration of the experiment.
Renal Denervation and Sham Surgery
The rats were placed in the prone position under general anesthesia with isoflurane. To minimize the invasive approach to the kidney, an incision was made in the dorsal midline. The traction of the erector spinae muscles exposed the kidney. Thereby, the renal arteries and veins were identified. After isolating the vessels from connective tissue, all visible nerves along the vessels were cut. Furthermore, the vessels were painted with a solution of 10% phenol in absolute ethanol.24 Next, the vessels were washed with saline, and the skin was closed. In the sham groups, we approached the kidney in the same way as in the RD group. The kidney was exposed, but the vessels were not stripped or coated with phenol. The achievement of renal denervation was confirmed by evaluating whether the renal tissue content of norepinephrine was <10% of the mean value in the sham‐operated group.
Direct Measurement of BP With Telemetry
Sixteen weeks after the operations, SHRcp rats subjected to RD (n=5), sham‐operated control SHRcp rats (n=5), and sham‐operated WKY rats (n=5) were anesthetized with isoflurane and were surgically implanted with miniature telemetry devices (TA11PA‐C40; Data Science International, St Paul, MN) in the abdominal cavity.25 The BP catheter was placed into the abdominal aorta. BP signals and heart rate (HR) derived from pressure waves from abdominal aorta were measured in conscious, unrestrained animals. After 2 weeks of recovery, 24‐hour online recordings were digitized (1 kHz) and stored for further analysis. BP and HR data were obtained from each implanted animal, and BP and HR data were recorded as 5‐minute averages every 60 minutes using a computer system (DATAQUEST ART4.2 Acquisition; Data Sciences International, St Paul, MN).
In a short‐term RD experiment, BP was directly measured with telemetry in the same manner as in the above long‐term RD experiment.
Intraperitoneal Glucose Tolerance Test and Intraperitoneal Insulin Tolerance Test
For the intraperitoneal glucose tolerance test, SHRcp, and WKY rats were intraperitoneally injected with glucose (1 g/kg body weight) after overnight fasting. Blood samples were collected from the tail vein 0, 30, 60, and 120 minutes after glucose administration to measure blood glucose.
For the IITT, SHRcp, and WKY rats were intraperitoneally injected with human regular insulin (2 units/kg body weight) after overnight fasting. Blood samples were collected from the tail vein 0, 20, 40, and 60 minutes after insulin administration to measure blood glucose.
Analysis of Biochemistry
Blood and urine biochemistry were performed at SRL Inc (Tokyo, Japan). Urine aldosterone was measured with a kit (Enzo Life Science, New York). Renal tissue norepinephrine was quantified using a commercial ELISA kit (LDN, Nordhorn, Germany).
Vessel Ring Preparation and Organ Chamber Experiments
Isometric tension was measured as previously described.26 In brief, carotid aortas from rats were cut into 5‐mm rings with special care to preserve the endothelium, and they were then mounted in organ baths filled with modified Tyrode buffer (pH 7.4; NaCl, 121 mmol/L; KCl, 5.9 mmol/L; CaCl2, 2.5 mmol/L; MgCl2, 1.2 mmol/L; NaH2PO4, 1.2 mmol/L; NaHCO3, 15.5 mmol/L; and d‐glucose, 11.5 mmol/L) aerated with 95% O2 and 5% CO2 at 37°C. The preparations were attached to a force transducer, and isometric tension was recorded on a polygraph. A resting tension of 1 g was maintained throughout the experiment. Vessel rings were primed with KCl (50 mmol/L) and then precontracted with l‐phenylephrine (10−7 mol/L). After the plateau of tension was attained, the rings were exposed to increasing concentrations of acetylcholine (10−9 to 10−4 mol/L) or sodium nitroprusside (10−9 to 10−4 mol/L) to obtain cumulative concentration–response curves.
Histological Examination and Immunohistochemistry
Hearts were fixed in 4% (wt/vol) paraformaldehyde, embedded in paraffin, sectioned at 5 μm, and stained with Sirius Red F3BA (0.5% wt/vol in saturated aqueous picric acid; Aldrich Chemical Company, St Louis, MO) for the measurement of collagen volume fraction. For cardiac sections, coronary arterial thickness and interstitial fibrosis were quantified as described previously.27 The positive area of fibrosis per field area was assessed by examining at least 10 fields per rat using Lumina Vision version 2.2 analysis software.
For ED‐1 immunohistochemistry, frozen cardiac sections were incubated overnight with rat anti‐mouse primary antibody (×500; Serotec, Raleigh, NC) followed by anti‐rat secondary antibody (BioSource, Camarillo, CA), as described previously.27 The number of cardiac ED‐1‐positive cells per square millimeter was counted in a blinded manner by examining >10 fields per section using a microscope with ×200 magnification; the average ED‐1‐positive cell number was obtained in each rat.
Renal sections (5 μm thick) were stained with periodic acid–Schiff and were analyzed for the degree of glomerulosclerosis, defined as the disappearance of cellular elements from the tuft, capillary loop collapse, and folding of the glomerular basement membrane with accumulation of amorphous material, as described.28 Glomerulosclerosis was graded as follows: score 0, 0%; I, 1% to 25%; II, 26% to 50%; III, 51% to 75%; and IV, 76% to 100% of glomeruli involved. The glomerulosclerosis score was calculated as (1× grade I/100)+(2× grade II/100)+(3× grade III/100)+(4× grade IV/100) in each rat. Fifty glomeruli were examined in each rat.
Measurement of Tissue Superoxide
Hearts and aortas removed from rats were immediately frozen in Tissue‐Tek OCT embedding medium (Sakura Finetek, Tokyo, Japan). Dihydroethidium (DHE) was used to evaluate tissue superoxide levels in situ, as described in detail previously.26 In brief, DHE fluorescence was visualized by fluorescence microscopy using an excitation wavelength of 520 to 540 nm and a rhodamine emission filter. DHE fluorescence of tissue was captured with the same exposure time (1.0 seconds), and it was quantified using Lumina Vision. The mean fluorescence was quantified and expressed relative to values obtained from control rats.
To verify whether DHE fluorescence in cardiovascular tissues was derived from superoxide, cardiac and vascular sections from control SHRcp rats were preincubated with 250 U/mL polyethylene‐glycol superoxide dismutase (Sigma‐Aldrich) for 30 minutes followed by DHE staining. This fluorescence was compared with DHE fluorescence from WKY and control SHRcp rats without preincubation with polyethylene‐glycol superoxide dismutase, as described previously.29
Preparation of Cardiac Protein Extracts and Western Blot Analysis
The detailed method was previously described.27 Briefly, after renal protein extracts were subjected to SDS‐PAGE and transferred to polyvinylidene difluoride membranes, the membranes were probed with specific antibodies. The antibodies used were anti‐WNK4 (×10 000 dilution; Millipore), anti‐Na+‐Cl− cotransporter (NCC, ×5000; Millipore), anti‐epithelial Na+ channel (×10 000; ABR), anti‐SGK1 (×2000; Abcam), anti‐angiotensin 1 receptor (×2000; Santa Cruz Biotechnology), and β‐actin (×2000; Cell Signaling Technology). The intensity of each band was quantified using NIH ImageJ analysis software v1.61 (National Institutes of Health, Bethesda, MD). In individual samples, each value was normalized to β‐actin.
Statistical Analysis
All data are presented as mean±SEM. The data on body weight, oral glucose tolerance test, IITT, vascular relaxation, cumulative Na balance, and mean arterial pressure were analyzed by 1‐way ANOVA with repeated measures, followed by Fisher's protected least‐squares difference test using Prism (Graph Pad Software Inc, San Diego, CA). The data on systolic and diastolic arterial pressure, locomotor activity, and heart rate measured by telemetry were analyzed with 2‐way ANOVA followed by Fisher's protected least‐squares difference test. Other data were analyzed with 1‐way ANOVA followed by Fisher's protected least‐squares difference test, in the case of comparison among 3 groups. For comparison between 2 groups, other data were analyzed with the unpaired t test. In all tests, differences were considered statistically significant at a value of P<0.05.
Results
Effects of Long‐Term RD on Obesity, Glucose Intolerance, and Insulin Resistance
Compared with the control group (sham operation), RD did not affect body weight in SHRcp rats throughout the follow‐up period (Figure 1A). Twenty weeks after RD, visceral fat and subcutaneous fat tissue weights (Figure 1B and 1C, respectively) did not significantly differ between control and RD SHRcp rats. RD did not ameliorate the elevation of plasma total cholesterol, triglycerides, or free fatty acids in SHRcp rats (Table 1). RD did not prevent the impairment of glucose tolerance and insulin resistance in SHRcp rats, as shown by the oral glucose tolerance test and IITT (Figure 1D and 1E, respectively).
Figure 1.
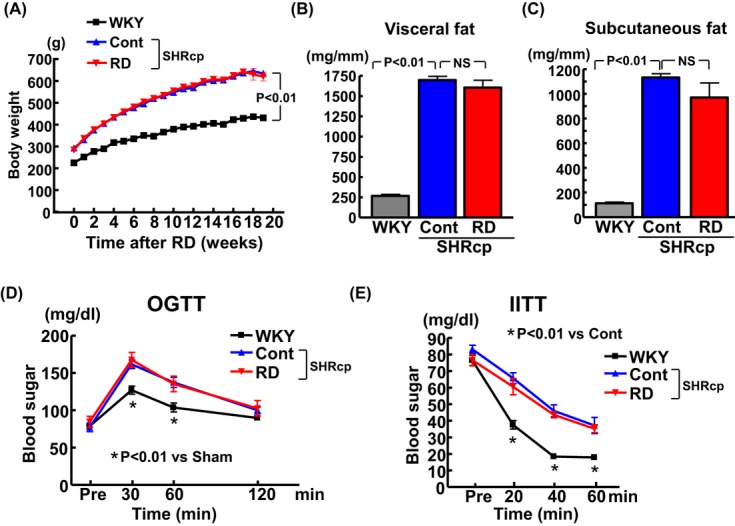
Effects of renal denervation on body weight (A), visceral fat (B), subcutaneous fat (C), glucose tolerance (D), and insulin resistance (E) in SHRcp rats. Values are mean±SEM (n=5 to 6 in each group). Body weight was significantly influenced by strain (P<0.01) and period (P<0.01). Glucose tolerance was significantly influenced by strain (P<0.05) and period (P<0.01). Insulin resistance was significantly influenced by strain (P<0.01) and period (P<0.01). Visceral fat and subcutaneous fat were significantly influenced by strain (P<0.01). SHRcp indicates SHR/NDmcr‐cp(+/+);SEM, standard error of the mean; WKY, sham‐operated Wistar‐Kyoto rats; Cont, sham‐operated SHRcp rats; RD, SHRcp rats subjected to renal denervation; NS, not significant; OGTT, oral glucose tolerance test; IITT, intraperitoneal insulin tolerance test.
Table 1.
Serum Lipids in Each Group of Rats 19 Weeks After RD or Sham Operation
| SHRcp | |||
|---|---|---|---|
| WKY | Cont | RD | |
| Total cholesterol, mg/dL | 113.0±4.7* | 163.3±4.0 | 199.0±10.8* |
| Triglycerides, mg/dL | 35.3±5.1* | 665.8±50.5 | 858.8±182.4 |
| Free fatty acids, μEq/dL | 341.5±39.3* | 1061.5±72.1 | 932.4±87.4 |
Values are mean±SEM (n=6 in WKY and Cont groups, n=5 in RD group). SHRcp indicates SHR/NDmcr‐cp(+/+) rats; WKY, sham‐operated Wistar‐Kyoto rats; Cont, sham‐operated SHR/NDmcr‐cp(+/+) rats; RD, SHR/NDmcr‐cp(+/+) rats subjected to renal denervation; SEM, standard error of the mean.
P<0.01 vs Cont.
Effects of Long‐Term RD on BP
Figures 2 and 3 show the results of continuous direct systolic and diastolic BP measurement with telemetry in SHRcp and WKY rats 19 weeks after RD or sham operation. Both systolic and diastolic BP of WKY rats were significantly lower during the light (inactive) period than during the dark (active) period (P<0.05), indicating that WKY rats showed the dipper‐type BP circadian rhythm, consistent with previous reports.30–31 Direct BP measurement with telemetry demonstrated that BP in the control SHRcp rats was significantly higher than that in WKY rats. BP in control SHRcp rats was comparable between the 12‐hour dark period and the 12‐hour light period, indicating that SHRcp rats displayed the nondipper type of hypertension. Systolic and diastolic BP of SHRcp rats with RD were significantly lower than those of control SHRcp rats during both dark (P<0.01) and light (P<0.01) periods. Furthermore, systolic and diastolic BP in the RD group were significantly lower during the light period than during the dark period (P<0.01), indicating that SHRcp rats subjected to RD exhibited the dipper‐type BP circadian rhythm.
Figure 2.
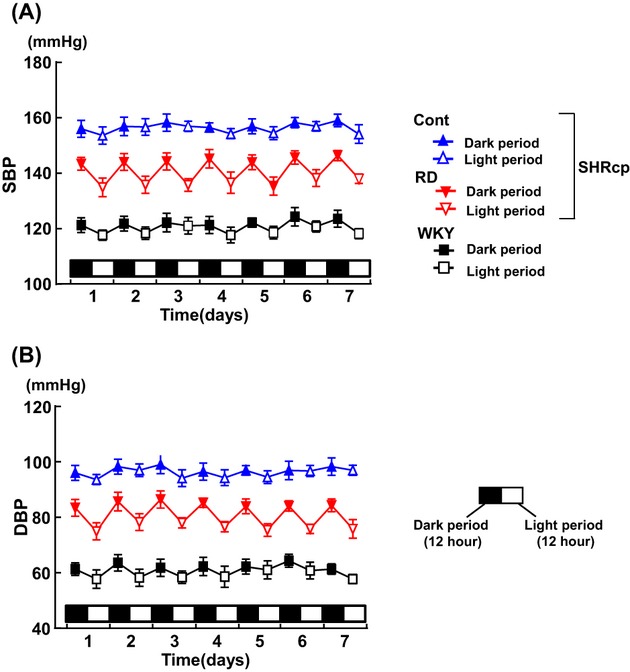
Twelve‐hour averaged systolic BP (A) and diastolic BP (B) during dark and light periods over 7 consecutive days measured by telemetry 19 weeks after renal denervation. Values are mean±SEM (n=5 in each group). BP indicates blood pressure; SEM, standard error of the mean; Cont, sham‐operated SHRcp rats; RD, SHRcp rats subjected to renal denervation; WKY, sham‐operated Wistar‐Kyoto rats; SHRcp, SHR/NDmcr‐cp(+/+); DBP, diastolic blood pressure; SBP, systolic blood pressure.
Figure 3.
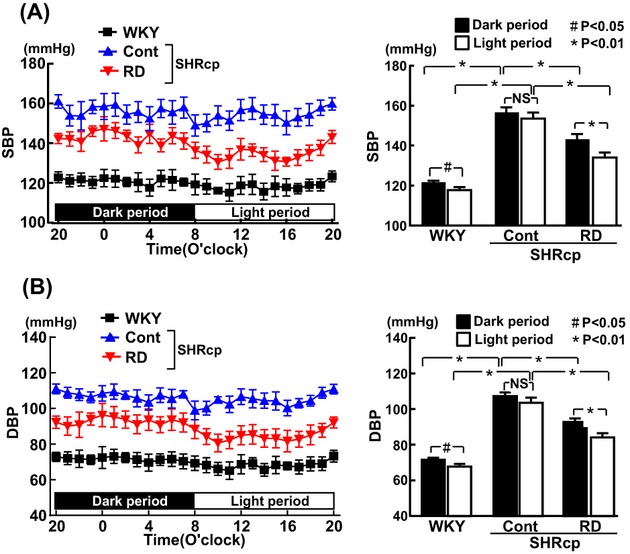
Hourly averaged systolic BP (A) and diastolic BP (B) during 24 hours (12‐hour dark period and 12‐hour light period) measured by telemetry 19 weeks after renal denervation. Values are mean±SEM (n=5 in each group. Both SBP and DBP were significantly influenced by strain (P<0.01) and period (P<0.01). BP indicates blood pressure; SEM, standard error of the mean; SBP, systolic blood pressure; DBP, diastolic blood pressure; WKY, sham‐operated Wistar‐Kyoto rats; Cont, sham‐operated SHRcp rats; RD, SHRcp rats subjected to renal denervation; SHRcp, SHR/NDmcr‐cp(+/+) NS, not significant.
Locomotor activity of SHRcp rats was significantly less than that of WKY rats during dark (P<0.01) and light (P<0.01) periods. RD did not alter locomotor activity in SHRcp rats during the dark or light period (Figure 4A). HR in SHRcp rats was lower than that in WKY rats during both periods. RD did not affect HR in SHRcp rats during the dark or light period (Figure 4B).
Figure 4.

Effects of renal denervation on locomotor activity (A) and heart rate (B) in SHRcp rats. A, Left panel indicates hourly locomotor activity in each group of rats, and right panel indicates 12‐hour averaged locomotor activity during dark and light periods. B, Left and right panels indicate hourly HR and 12‐hour averaged HR, respectively, in each group of rats. Values are mean±SEM (n=5 in each group). *P<0.01; #P<0.05. Locomotor activity and heart rate were significantly influenced by strain (P<0.01) and period (P<0.01). SHRcp indicates SHR/NDmcr‐cp(+/+);HR, heart rate; SEM, standard error of the mean; WKY, sham‐operated Wistar‐Kyoto rats; Cont, sham‐operated SHRcp rats; RD, SHRcp rats subjected to renal denervation; NS, not significant.
Effects of Long‐Term RD on Urinary Sodium Excretion
As shown in Figure 5A and 5B, RD resulted in a significant increase in urinary sodium excretion (P<0.05) and urinary sodium‐to‐creatinine ratio (P<0.01) in SHRcp rats. There was no significant difference in food intake, water intake, or urine volume between the control and RD SHRcp groups (Figure 5C through 5E).
Figure 5.

Effects of renal denervation on 24‐hour urinary sodium excretion (A), urinary sodium excretion–to‐creatinine ratio (B), food intake (C), water intake (D), and urine volume (E) in SHRcp rats. Values are mean±SEM (n=6 in each group). Twenty‐four‐hour urinary sodium excretion, urinary sodium excretion–to‐creatinine ratio, food intake, water intake, and urine volume were all significantly influenced by strain (P<0.05). SHRcp indicates SHR/NDmcr‐cp(+/+);SEM, standard error of the mean; WKY, sham‐operated Wistar‐Kyoto rats; Cont, sham‐operated SHRcp rats; RD, SHRcp rats subjected to renal denervation; NS, not significant.
Effects of Long‐Term RD on Renal NCC, WNK4, SGK‐1, ENaC, and AT1 Receptor
As shown in Figure 6A, renal NCC protein level in control SHRcp rats was significantly greater than in WKY rats (P<0.01). RD significantly ameliorated the upregulation of renal NCC protein in SHRcp rats (P<0.05). There was no significant difference in WNK4, SGK‐1, ENaC, and AT1 receptor levels among WKY, control SHRcp, and RD SHRcp rats (Figure 6B through 6E, respectively).
Figure 6.
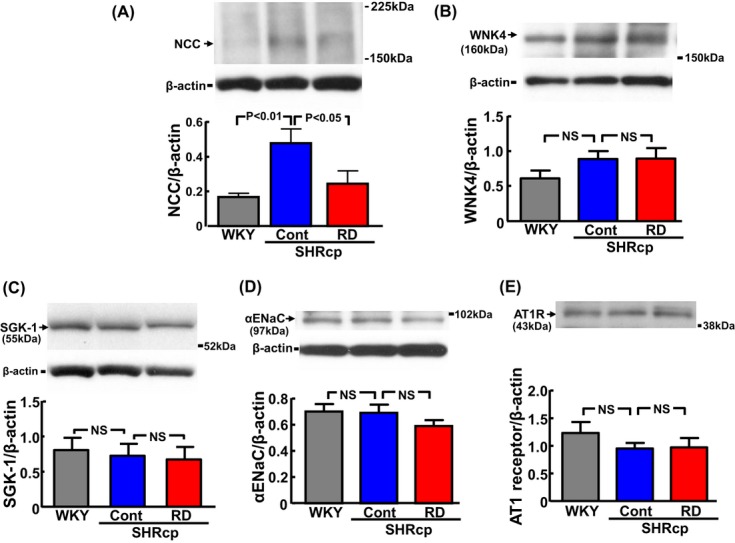
Effects of renal denervation on renal NCC (A), WNK4 (B), SGK‐1 (C), ENaC (D), and AT1 receptor (E) in SHRcp rats. Upper panels show representative Western blots. Each protein value in individual rats was normalized to β‐actin. The same membranes were consecutively reprobed with WNK4 (B), AT1 receptor (E), and β‐actin. Therefore, representative Western blot bands of β‐actin were the same in (B) and (E), and representative β‐actin bands are not shown in (E). Values are mean±SEM (n=5 to 6 in each group). NCC/β‐actin was significantly influenced by strain (P<0.05). However, WNK4, SGK‐1, αENaC, and AT1 receptor were not influenced by strain. NCC indicates Na+‐Cl− cotransporter; WNK‐4, with‐no‐lysine‐kinase 4; SGK, serum and glucocorticoid‐induced protein kinase; ENaC, epithelial sodium channel; AT1, angiotensin type I; SHRcp, SHR/NDmcr‐cp(+/+); SEM, standard error of the mean; WKY, sham‐operated Wistar‐Kyoto rats; Cont, sham‐operated SHRcp rats; RD, SHRcp rats subjected to renal denervation; NS, not significant.
Effects of Long‐Term RD on Cardiac Injury, Vascular Endothelial Dysfunction, and Oxidative Stress
As shown in Figure 7, compared with in control SHRcp rats, RD significantly reduced the increase in cardiac weight (P<0.01), cardiac fibrosis (P<0.01), cardiac ED‐1‐positive cell (macrophage) numbers (P<0.01), coronary arterial thickening (P<0.05), and coronary perivascular fibrosis (P<0.01).
Figure 7.
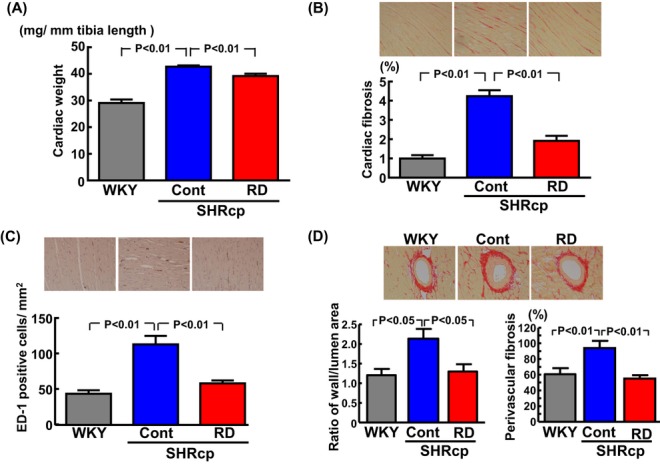
Effects of renal denervation on cardiac weight (A), cardiac fibrosis (B), cardiac macrophage infiltration (C), and coronary arterial thickening and perivascular fibrosis (D) in SHRcp rats. B through D, Upper panels show representative photomicrographs of a Sirius red–stained cardiac section, an ED‐1‐immunostained cardiac section, and a Sirius red–stained cardiac section, respectively. Values are mean±SEM (n=5 to 6 in each group). Cardiac weight, cardiac fibrosis, cardiac ED‐1‐positive cells/mm2, coronary arterial thickening, and perivascular fibrosis were significantly influenced by strain (P<0.05). SHRcp indicates SHR/NDmcr‐cp(+/+);SEM, standard error of the mean; WKY, sham‐operated Wistar‐Kyoto rats; Cont, sham‐operated SHRcp rats; RD, SHRcp rats subjected to renal denervation.
RD significantly ameliorated the impairment of vascular endothelium‐dependent relaxation with acetylcholine (Figure 8A). L‐NAME pretreatment abolished vascular relaxation with acetylcholine in all 3 groups (Figure 8B). There was no significant difference in sodium nitroprusside–induced vascular relaxation among the 3 groups (Figure 8C).
Figure 8.
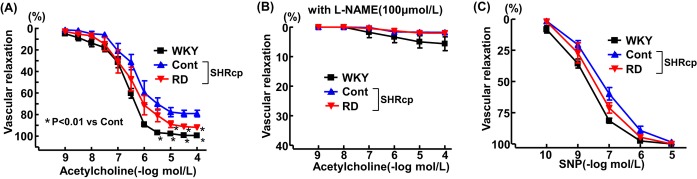
Effects of renal denervation on vascular endothelium‐dependent relaxation with acetylcholine but without pretreatment with L‐NAME (A) or with acetylcholine and pretreatment with L‐NAME (B) and on vascular endothelium‐independent relaxation with SNP (C) in SHRcp rats. Values are mean±SEM (n=6 in each group). Vascular endothelium‐dependent relaxation with acetylcholine was significantly influenced by strain (P<0.01) and concentration (P<0.01). Vascular endothelium‐dependent relaxation with acetylcholine with L‐NAME pretreatment and vascular endothelium‐independent relaxation with SNP were not influenced by strain or concentration. L‐NAME indicates N omega‐nitro‐L‐arginine methyl ester; SNP, sodium nitroprusside; SHRcp, SHR/NDmcr‐cp(+/+); SEM, standard error of the mean; WKY, sham‐operated Wistar‐Kyoto rats; Cont, sham‐operated SHRcp rats; RD, SHRcp rats subjected to renal denervation.
As shown in Figure 9A and 9B, compared with sham operation, RD significantly attenuated the increase in cardiac (P<0.01) and vascular superoxide (P<0.05) levels in SHRcp rats. As shown in Figure 9C and 9D, preincubation of cardiac and vascular sections from control SHRcp rats with SOD abolished the increase in DHE fluorescence.
Figure 9.
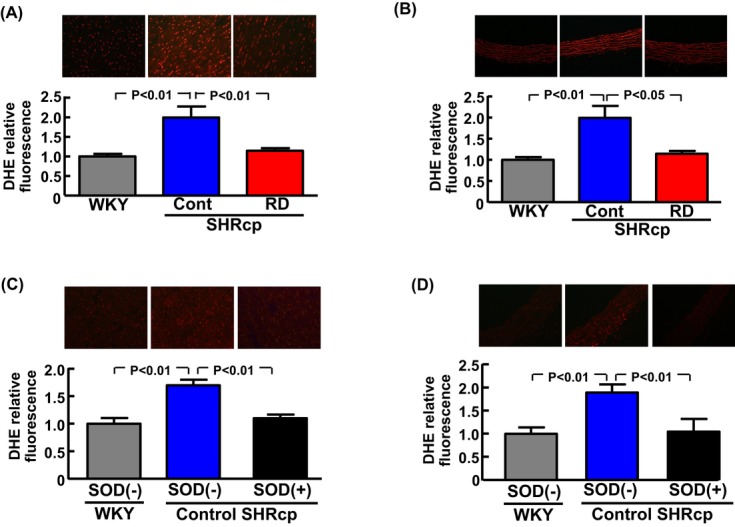
Effects of renal denervation on cardiac oxidative stress and vascular oxidative stress in SHRcp rats. A and B, Upper panels show representative photomicrographs of DHE‐stained cardiac and carotid arterial sections from each group. Values are mean±SEM (n=5 to 6 in each group). C and D, Cardiac (C) and vascular (D) sections from control SHRcp rats (sham operation group) were preincubated with superoxide dismutase (SOD) and stained with DHE, and their DHE signals were compared with those without SOD preincubation. Values are mean±SEM (n=5 in each group). SOD(−), DHE fluorescence without SOD preincubation; SOD(+), DHE fluorescence with SOD preincubation. Cardiac and vascular DHE fluorescence were significantly influenced by strain (P<0.01). SHRcp indicates SHR/NDmcr‐cp(+/+);DHE, dihydroethidium; SEM, standard error of the mean; Cont, sham‐operated SHRcp rats; WKY, sham‐operated Wistar‐Kyoto rats; RD, SHRcp rats subjected to renal denervation.
Effects of Long‐Term RD on Renal Injury, Plasma Renin Activity, and Urinary and Plasma Aldosterone
As shown in Figure 10A through 10C, 24‐hour urinary protein excretion, 24‐hour urinary albumin excretion, and the glomerular sclerosis index in control SHRcp rats were significantly greater than those in WKY rats. There was no significant difference in these parameters between the control and RD SHRcp groups.
Figure 10.

Effects of renal denervation on urinary protein excretion (A), urinary albumin excretion (B), glomerular sclerosis index (C), plasma renin activity (D), 24‐hour urinary aldosterone excretion (E), and plasma aldosterone (F) in SHRcp rats. Values are mean±SEM (n=6 in each group). Urinary protein excretion, urinary albumin excretion, glomerular sclerosis index, and 24‐hour urinary aldosterone excretion were significantly influenced by strain (P<0.01). Plasma renin activity and plasma aldosterone were not influenced by strain. SHRcp indicates SHR/NDmcr‐cp(+/+);SEM, standard error of the mean; NS, not significant; WKY, sham‐operated Wistar‐Kyoto rats; Cont, sham‐operated SHRcp rats; RD, SHRcp rats subjected to renal denervation.
There was no significant difference in plasma renin activity among WKY, control SHRcp, and RD SHRcp rats, although plasma renin activity tended to be lower in RD than in control SHRcp rats (P=0.072; Figure 10D). Twenty‐four‐hour urinary aldosterone excretion in both SHRcp groups was significantly greater than in the WKY group (P<0.01), and RD did not reduce urinary aldosterone excretion in SHRcp rats (Figure 10E). RD did not significantly alter plasma aldosterone level in SHRcp rats (Figure 10F).
As shown in Table 2, there was no significant difference in plasma sodium, potassium, or chloride concentration between the control and RD SHRcp groups.
Table 2.
Plasma Electrolytes in Each Group of Rats 19 Weeks After RD or Sham Operation
| SHRcp | |||
|---|---|---|---|
| WKY | Cont | RD | |
| Sodium, mEq/L | 145±1 | 147±0.7 | 145±0.9 |
| Potassium, mEq/L | 6.00±0.28 | 5.37±0.29 | 5.58±0.32 |
| Chloride, mEq/L | 101.7±0.76* | 98.3±0.42 | 97.2±1.02 |
Values are mean±SEM (n=6 in each group). SHRcp indicates SHR/NDmcr‐cp(+/+) rats; WKY, Wistar‐Kyoto rats; Cont, sham‐operated SHR/NDmcr‐cp(+/+) rats; RD, SHR/NDmcr‐cp(+/+) rats subjected to renal denervation; SEM, standard error of the mean.
P<0.01 vs Cont.
Effects of Long‐Term RD on Renal and Urinary Norepinephrine
As shown in Figure 11A, renal norepinephrine content in control SHRcp rats was significantly higher than in WKY rats (P<0.05). Renal norepinephrine content in RD SHRcp rats was considerably lower than that in control SHRcp rats (10.0±3.5 versus 114.9±26.7 ng/g per tissue; P<0.01).
Figure 11.

Effects of renal denervation on renal norepinephrine content (A), 24‐hour urinary norepinephrine excretion (B), and urinary norepinephrine excretion–to‐creatinine ratio (C) in SHRcp rats. Values are mean±SEM (n=6 in each group). Renal norepinephrine content, 24‐hour urinary norepinephrine excretion, and urinary norepinephrine excretion–to‐creatinine ratio were significantly influenced by strain (P<0.01). SHRcp indicates SHR/NDmcr‐cp(+/+); SEM, standard error of the mean; WKY, sham‐operated Wistar‐Kyoto rats; Cont, sham‐operated SHRcp rats; RD, SHRcp rats subjected to renal denervation; NS, not significant; OGTT, oral glucose tolerance test; IITT, intraperitoneal insulin tolerance test.
Twenty‐four‐hour urinary norepinephrine excretion was significantly higher in SHRcp than in WKY rats (P<0.01; Figure 11B and 11C). However, there was no significant difference in 24‐hour urinary norepinephrine excretion between the control and RD SHRcp groups.
Effects of Short‐Term RD on BP, Cumulative Na Balance, Plasma Renin Activity, and Plasma and Urinary Aldosterone
Mean arterial pressure continuously measured over 28 days by telemetry after RD (Figure 12A) confirmed that RD rapidly caused a significant and continuous reduction in BP in SHRcp rats. The data on cumulative Na± balance in Figure 12B confirmed that RD significantly increased urinary sodium excretion in SHRcp rats. Plasma renin activity was significantly lowered in the RD group compared with the control group of SHRcp rats (P<0.05; Figure 12C). On the other hand, no significant difference was found between RD and control groups regarding plasma aldosterone or 24‐hour urinary aldosterone excretion (Figure 12D and 12E).
Figure 12.
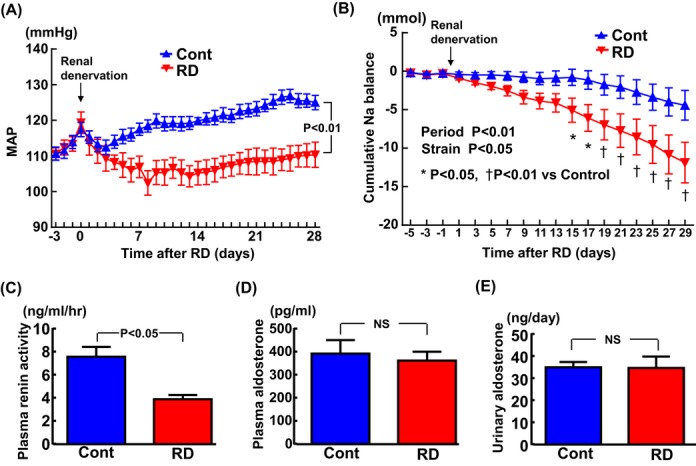
Effects of short‐term renal denervation on mean arterial pressure (A), cumulative Na balance (B), plasma renin activity (C), plasma aldosterone (D), and 24‐hour urinary aldosterone (E). Twenty‐four‐hour averaged mean arterial pressure (MAP) of SHRcp from 3 days before to 28 days after RD or sham operation. Values are mean±SEM (n=7 in each group). B, Cumulative sodium balance of SHRcp rats from 5 days before to 29 days after RD or sham operation. Values are mean±SEM (n=6 in each group). C and D, n=10 in the control and n=7 in the RD group. E, n=6 in each group. MAP was significantly influenced by strain (P<0.01) and time (P<0.01). Cumulative Na+ balance was influenced by strain (P<0.05) and time (P<0.01). SHRcp indicates SHR/NDmcr‐cp(+/+); RD, SHRcp rats subjected to renal denervation; SEM, standard error of the mean; Cont, sham‐operated SHRcp rats.
Discussion
Impaired circadian BP rhythm, such as non‐dipper‐type hypertension, is frequently observed in hypertensive patients with metabolic syndrome and is significantly associated with an increased risk of cardiovascular events.32–33 Using SHRcp rats,19–23 a useful rat model of metabolic syndrome with nondipper hypertension, we investigated the impact of long‐term RD on BP circadian rhythm, metabolic disorders, and cardiovascular and renal injury in metabolic syndrome. The major findings of our present work are: (1) RD not only significantly lowered BP in SHRcp rats but also normalized disrupted BP circadian rhythm in SHRcp rats from the nondipper to the dipper type; (2) these beneficial effects on BP were accompanied by the suppression of cardiac hypertrophy and remodeling and the amelioration of vascular endothelial dysfunction; and (3) RD significantly increased sodium excretion, ameliorated renal NCC overexpression, and reduced plasma renin activity in SHRcp rats. These findings suggest that normalization of disrupted BP circadian rhythm in SHRcp rats by RD might be partially mediated by natriuresis attributable to amelioration of renal NCC overexpression and suppression of renin production. Our work provides the first evidence for the causative role of the renal nerve in non‐dipper‐type hypertension in metabolic syndrome.
Accumulating evidence33–35 indicates that the impairment of renal sodium excretion plays a causative role in the pathogenesis of non‐dipper‐type hypertension or salt‐sensitive hypertension. Of note, in the present study, long‐term RD significantly increased urinary sodium excretion in SHRcp rats. Moreover, the findings on cumulative sodium balance in SHRcp rats after RD confirmed the significant increase in urinary sodium excretion caused by RD. Therefore, our findings suggest that normalization of BP circadian rhythm in SHRcp rats by RD might be attributed to natriuresis. Therefore, to elucidate the mechanism underlying the increase in urinary sodium excretion by RD, we examined the effects of RD on various molecules involved in renal sodium retention such as NCC,36–37 WNK4,36 ENaC,38 SGK1,39 and AT1 receptor.40 It is noteworthy that renal NCC, but not WNK4, ENaC, SGK1, or AT1 receptor, was significantly greater in control SHRcp than in WKY rats. Interestingly, RD significantly attenuated the upregulation of renal NCC in SHRcp rats, whereas it did not affect renal WNK4, ENaC, SGK1, or AT1 receptor. Given the critical role of NCC in sodium reabsorption and salt‐sensitive hypertension,36–37 our results support the notion that normalization of BP circadian rhythm by RD in SHRcp rats is in part mediated by the suppression of NCC overexpression and subsequent natriuresis. Therefore, RD may be a promising therapeutic strategy for hypertension with disrupted BP circadian rhythm such as non‐dipper‐type hypertension.
The renin‐angiotensin‐aldosterone system plays a key role in the regulation of BP and sodium and body fluid balance.41 Therefore, we examined the effects of RD on plasma renin activity and on plasma and urinary aldosterone in SHRcp rats. Interestingly, short‐term RD significantly reduced plasma renin activity. Therefore, the beneficial effects of RD on BP and urinary sodium excretion seemed to be partially mediated by the reduction of plasma renin activity. Despite the reduction of plasma renin activity by RD, 24‐hour urinary aldosterone excretion and plasma aldosterone levels were not significantly altered by short‐term or long‐term RD, suggesting that aldosterone regulation in SHRcp rats subjected to RD is independent of renin. Plasma aldosterone in SHRcp rats increases with aging compared with SHR and WKY rats, independently of plasma renin.20 Collectively, our present observations provide no evidence for the involvement of aldosterone in the beneficial effects of RD in SHRcp. However, it cannot be completely ruled out that aldosterone‐mediated ENaC activation was partially involved in the beneficial effects of RD that we observed. Further study is needed to elucidate the exact role of aldosterone in the effects of RD on BP and natriuresis.
In the present study, long‐term RD prevented cardiovascular injury in SHRcp rats, as shown by amelioration of cardiac hypertrophy, fibrosis and inflammation, coronary arterial remodeling, and vascular endothelial dysfunction. These beneficial cardiovascular effects of RD were associated with the attenuation of cardiac and vascular oxidative stress. Therefore, RD may be a useful therapeutic strategy for cardiovascular diseases in hypertension with metabolic syndrome. In contrast to the significant improvement of cardiovascular injury by RD, unexpectedly, RD failed to ameliorate renal injury in SHRcp rats, as shown by the lack of attenuation of urinary protein and albumin excretion and the lack of amelioration of glomerulosclerosis by RD. Thus, our results show that renal nerves play a minor role in the pathogenesis of renal injury in SHRcp rats. However, it is unclear whether our findings can be applied to hypertensive patients with metabolic syndrome.
The method of RD24 employed in the present work causes denervation of afferent renal sensory nerves as well as efferent renal sympathetic nerves. The afferent renal nerve plays a key role in the regulation of systemic sympathetic activity by modulating the central nervous system.4–5 Therefore, there is an emerging clinical hypothesis that RD in patients with resistant hypertension may lead to both the attenuation of renal sympathetic activity and, potentially, the attenuation of systemic sympathetic activity, thereby improving glucose metabolism, insulin sensitivity, or obesity.16,42 In the present study, despite the marked reduction of renal norepinephrine contents by long‐term RD, urinary norepinephrine excretion, the most popular indicator of systemic sympathetic activity, was not reduced by RD in SHRcp rats. Continuous recording of HR with telemetry showed that RD did not alter 24‐hour HR or HR circadian rhythm in SHRcp rats. Furthermore, metabolic parameters such as body weight, fat tissue weight, serum lipids, glucose intolerance, and insulin resistance were not affected by long‐term RD in SHRcp rats. All these findings suggest that the afferent renal nerve plays a minor role in the increased systemic sympathetic activity and metabolic disorders in SHRcp rats.
Study Limitations
The mechanism underlying disrupted BP circadian rhythm is multifactorial and includes not only renal sodium retention and the renin‐angiotensin‐aldosterone system but also the reduction of endothelium‐derived nitric oxide,43 the impairment of daily sleep rhythm induced by sympathetic activation,44 and the impaired regulation of the neuroendocrine system.45 However, we did not address potential mechanisms other than renal sodium handling and the renin‐angiotensin‐aldosterone system. Therefore, further study is needed to define the precise mechanism responsible for normalization of disrupted BP circadian rhythm by RD in SHRcp rats.
In conclusion, the present work provides experimental evidence that RD normalized abnormal BP circadian rhythm from the nondipper to the dipper type, significantly reduced BP, and ameliorated cardiovascular injury in metabolic syndrome with hypertension. These beneficial effects of RD on BP seem to be mediated at least in part by the suppression of renal NCC overexpression and subsequent natriuresis and by the reduction of plasma renin activity. However, our work provides no evidence for the usefulness of RD in preventing renal injury and metabolic disorders. Our results provide novel insight into the potential clinical value of RD in the treatment of hypertension with metabolic syndrome.
Sources of Funding
This work was supported by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (23390058) and the Japan Cardiovascular Research Foundation.
Disclosures
None.
Acknowledgments
We thank Miho Kataoka, Michie Uchikawa, Keiko Morozumi, Yuriko Shimamura, Kazuko Noda, and Tomoko Moriyama for their kind support during the study.
References
- 1.Esler M. The 2009 Carl Ludwig Lecture: pathophysiology of the human sympathetic nervous system in cardiovascular diseases: the transition from mechanisms to medical management. J Appl Physiol. 2010; 108:227-237 [DOI] [PubMed] [Google Scholar]
- 2.Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012; 33:1058-1066 [DOI] [PubMed] [Google Scholar]
- 3.Schlaich MP, Krum H, Sobotka PA, Esler MD. Renal denervation and hypertension. Am J Hypertens. 2011; 24:635-642 [DOI] [PubMed] [Google Scholar]
- 4.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997; 77:75-197 [DOI] [PubMed] [Google Scholar]
- 5.Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension. 2009; 54:1195-1201 [DOI] [PubMed] [Google Scholar]
- 6.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter‐based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof‐of‐principle cohort study. Lancet. 2009; 373:1275-1281 [DOI] [PubMed] [Google Scholar]
- 7.Symplicity HTNI Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment‐resistant hypertension (the Symplicity HTN‐2 Trial): a randomised controlled trial. Lancet. 2010; 376:1903-1909 [DOI] [PubMed] [Google Scholar]
- 8.Mahfoud F, Ukena C, Schmieder RE, Cremers B, Rump LC, Vonend O, Weil J, Schmidt M, Hoppe UC, Zeller T, Bauer A, Ott C, Blessing E, Sobotka PA, Krum H, Schlaich M, Esler M, Bohm M. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013; 128:132-140 [DOI] [PubMed] [Google Scholar]
- 9.Ott C, Janka R, Schmid A, Titze S, Ditting T, Sobotka PA, Veelken R, Uder M, Schmieder RE. Vascular and renal hemodynamic changes after renal denervation. Clin J Am Soc Nephrol. 2013; 8:1195-1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic‐nerve ablation for uncontrolled hypertension. N Engl J Med. 2009; 361:932-934 [DOI] [PubMed] [Google Scholar]
- 11.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013; 61:457-464 [DOI] [PubMed] [Google Scholar]
- 12.Hering D, Lambert EA, Marusic P, Ika‐Sari C, Walton AS, Krum H, Sobotka PA, Mahfoud F, Bohm M, Lambert GW, Esler MD, Schlaich MP. Renal nerve ablation reduces augmentation index in patients with resistant hypertension. J Hypertens. 2013; 31:1983-1900 [DOI] [PubMed] [Google Scholar]
- 13.Linz D, Mahfoud F, Schotten U, Ukena C, Hohl M, Neuberger HR, Wirth K, Bohm M. Renal sympathetic denervation provides ventricular rate control but does not prevent atrial electrical remodeling during atrial fibrillation. Hypertension. 2013; 61:225-231 [DOI] [PubMed] [Google Scholar]
- 14.Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, Haller H, Sweep FC, Diedrich A, Jordan J, Tank J. Catheter‐based renal nerve ablation and centrally generated sympathetic activity in difficult‐to‐control hypertensive patients: prospective case series. Hypertension. 2012; 60:1485-1490 [DOI] [PubMed] [Google Scholar]
- 15.Schmieder RE, Redon J, Grassi G, Kjeldsen SE, Mancia G, Narkiewicz K, Parati G, Ruilope L, van de Borne P, Tsioufis C. ESH position paper: renal denervation—an interventional therapy of resistant hypertension. J Hypertens. 2012; 30:837-841 [DOI] [PubMed] [Google Scholar]
- 16.Lambert GW, Straznicky NE, Lambert EA, Dixon JB, Schlaich MP. Sympathetic nervous activation in obesity and the metabolic syndrome—causes, consequences and therapeutic implications. Pharmacol Ther. 2010; 126:159-172 [DOI] [PubMed] [Google Scholar]
- 17.Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S, Reid J, Van Zwieten PA. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007; 25:909-920 [DOI] [PubMed] [Google Scholar]
- 18.Takaya K, Ogawa Y, Hiraoka J, Hosoda K, Yamori Y, Nakao K, Koletsky RJ. Nonsense mutation of leptin receptor in the obese spontaneously hypertensive Koletsky rat. Nat Genet. 1996; 14:130-131 [DOI] [PubMed] [Google Scholar]
- 19.Nagase M, Matsui H, Shibata S, Gotoda T, Fujita T. Salt‐induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension. 2007; 50:877-883 [DOI] [PubMed] [Google Scholar]
- 20.Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, Fujita T. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat‐derived factors. J Am Soc Nephrol. 2006; 17:3438-3446 [DOI] [PubMed] [Google Scholar]
- 21.Nangaku M, Miyata T, Sada T, Mizuno M, Inagi R, Ueda Y, Ishikawa N, Yuzawa H, Koike H, van Ypersele de Strihou C, Kurokawa K. Anti‐hypertensive agents inhibit in vivo the formation of advanced glycation end products and improve renal damage in a type 2 diabetic nephropathy rat model. J Am Soc Nephrol. 2003; 14:1212-1222 [DOI] [PubMed] [Google Scholar]
- 22.Sueta D, Nakamura T, Dong YF, Kataoka K, Koibuchi N, Yamamoto E, Toyama K, Yasuda O, Ogawa H, Kim‐Mitsuyama S. Amlodipine enhances amelioration of vascular insulin resistance, oxidative stress, and metabolic disorders by candesartan in metabolic syndrome rats. Am J Hypertens. 2012; 25:704-710 [DOI] [PubMed] [Google Scholar]
- 23.Sueta D, Kataoka K, Koibuchi N, Toyama K, Uekawa K, Katayama T, Mingjie M, Nakagawa T, Waki H, Maeda M, Yasuda O, Matsui K, Ogawa H, Kim‐Mitsuyama S. Novel mechanism for disrupted circadian blood pressure rhythm in a rat model of metabolic syndrome—the critical role of angiotensin II. J Am Heart Assoc. 2013; 2:e000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luippold G, Beilharz M, Muhlbauer B. Chronic renal denervation prevents glomerular hyperfiltration in diabetic rats. Nephrol Dial Transplant. 2004; 19:342-347 [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto E, Kataoka K, Dong YF, Nakamura T, Fukuda M, Nako H, Ogawa H, Kim‐Mitsuyama S. Benidipine, a dihydropyridine L‐type/T‐type calcium channel blocker, affords additive benefits for prevention of cardiorenal injury in hypertensive rats. J Hypertens. 2010; 28:1321-1329 [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto E, Kataoka K, Shintaku H, Yamashita T, Tokutomi Y, Dong YF, Matsuba S, Ichijo H, Ogawa H, Kim‐Mitsuyama S. Novel mechanism and role of angiotensin II induced vascular endothelial injury in hypertensive diastolic heart failure. Arterioscler Thromb Vasc Biol. 2007; 27:2569-2575 [DOI] [PubMed] [Google Scholar]
- 27.Nakamura T, Yamamoto E, Kataoka K, Yamashita T, Tokutomi Y, Dong YF, Matsuba S, Ogawa H, Kim‐Mitsuyama S. Beneficial effects of pioglitazone on hypertensive cardiovascular injury are enhanced by combination with candesartan. Hypertension. 2008; 51:296-301 [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Kataoka K, Tokutomi Y, Nako H, Toyama K, Dong YF, Koibuchi N, Yamamoto E, Yasuda O, Ogawa H, Kim‐Mitsuyama S. Novel mechanism of salt‐induced glomerular injury: critical role of enos and angiotensin II. J Hypertens. 2011; 29:1528-1535 [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto E, Tamamaki N, Nakamura T, Kataoka K, Tokutomi Y, Dong YF, Fukuda M, Matsuba S, Ogawa H, Kim‐Mitsuyama S. Excess salt causes cerebral neuronal apoptosis and inflammation in stroke‐prone hypertensive rats through angiotensin II‐induced NADPH oxidase activation. Stroke. 2008; 39:3049-3056 [DOI] [PubMed] [Google Scholar]
- 30.Basset A, Laude D, Laurent S, Elghozi JL. Contrasting circadian rhythms of blood pressure among inbred rat strains: recognition of dipper and non‐dipper patterns. J Hypertens. 2004; 22:727-737 [DOI] [PubMed] [Google Scholar]
- 31.Calhoun DA, Zhu S, Wyss JM, Oparil S. Diurnal blood pressure variation and dietary salt in spontaneously hypertensive rats. Hypertension. 1994; 24:1-7 [DOI] [PubMed] [Google Scholar]
- 32.Boer‐Martins L, Figueiredo VN, Demacq C, Martins LC, Consolin‐Colombo F, Figueiredo MJ, Cannavan FP, Moreno H., Jr Relationship of autonomic imbalance and circadian disruption with obesity and type 2 diabetes in resistant hypertensive patients. Cardiovasc Diabetol. 2011; 10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de la Sierra A, Redon J, Banegas JR, Segura J, Parati G, Gorostidi M, de la Cruz JJ, Sobrino J, Llisterri JL, Alonso J, Vinyoles E, Pallares V, Sarria A, Aranda P, Ruilope LMSpanish Society of Hypertension Ambulatory Blood Pressure Monitoring Registry I Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009; 53:466-472 [DOI] [PubMed] [Google Scholar]
- 34.Uzu T, Ishikawa K, Fujii T, Nakamura S, Inenaga T, Kimura G. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1997; 96:1859-1862 [DOI] [PubMed] [Google Scholar]
- 35.Uzu T, Kazembe FS, Ishikawa K, Nakamura S, Inenaga T, Kimura G. High sodium sensitivity implicates nocturnal hypertension in essential hypertension. Hypertension. 1996; 28:139-142 [DOI] [PubMed] [Google Scholar]
- 36.Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami‐Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H, Fujita T. Epigenetic modulation of the renal beta‐adrenergic‐WNK4 pathway in salt‐sensitive hypertension. Nat Med. 2011; 17:573-580 [DOI] [PubMed] [Google Scholar]
- 37.Yang CL, Zhu X, Ellison DH. The thiazide‐sensitive Na‐Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest. 2007; 117:3403-3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ring AM, Cheng SX, Leng Q, Kahle KT, Rinehart J, Lalioti MD, Volkman HM, Wilson FH, Hebert SC, Lifton RP. WNK4 regulates activity of the epithelial Na+ channel in vitro and in vivo. Proc Natl Acad Sci USA. 2007; 104:4020-4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasquez MM, Castro R, Seidner SR, Henson BM, Ashton DJ, Mustafa SB. Induction of serum‐ and glucocorticoid‐induced kinase‐1 (SGK1) by cAMP regulates increases in alpha‐ENaC. J Cell Physiol. 2008; 217:632-642 [DOI] [PubMed] [Google Scholar]
- 40.Gurley SB, Riquier‐Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011; 13:469-475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II‐mediated cardiovascular and renal diseases. Pharmacol Rev. 2000; 52:11-34 [PubMed] [Google Scholar]
- 42.Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, Hoppe UC, Vonend O, Rump LC, Sobotka PA, Krum H, Esler M, Bohm M. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011; 123:1940-1946 [DOI] [PubMed] [Google Scholar]
- 43.Kunieda T, Minamino T, Miura K, Katsuno T, Tateno K, Miyauchi H, Kaneko S, Bradfield CA, FitzGerald GA, Komuro I. Reduced nitric oxide causes age‐associated impairment of circadian rhythmicity. Circ Res. 2008; 102:607-614 [DOI] [PubMed] [Google Scholar]
- 44.Suzuki J, Ogawa M, Tamura N, Maejima Y, Takayama K, Maemura K, Honda K, Hirata Y, Nagai R, Isobe M. A critical role of sympathetic nerve regulation for the treatment of impaired daily rhythm in hypertensive Dahl rats. Hypertens Res. 2010; 33:1060-1065 [DOI] [PubMed] [Google Scholar]
- 45.Fabbian F, Smolensky MH, Tiseo R, Pala M, Manfredini R, Portaluppi F. Dipper and non‐dipper blood pressure 24‐hour patterns: circadian rhythm‐dependent physiologic and pathophysiologic mechanisms. Chronobiol Int. 2013; 30:17-30 [DOI] [PubMed] [Google Scholar]


