Abstract
Background
A hallmark of aging of the cardiac myocyte is impaired sarcoplasmic reticulum (SR) calcium uptake and relaxation due to decreased SR calcium ATPase (SERCA) activity. We tested the hypothesis that H2O2‐mediated oxidation of SERCA contributes to impaired myocyte relaxation in aging.
Methods and Results
Young (5‐month‐old) and senescent (21‐month‐old) FVB wild‐type (WT) or transgenic mice with myocyte‐specific overexpression of catalase were studied. In senescent mice, myocyte‐specific overexpression of catalase (1) prevented oxidative modification of SERCA as evidenced by sulfonation at Cys674, (2) preserved SERCA activity, (3) corrected impaired calcium handling and relaxation in isolated cardiac myocytes, and (4) prevented impaired left ventricular relaxation and diastolic dysfunction. Nitroxyl, which activates SERCA via S‐glutathiolation at Cys674, failed to activate SERCA in freshly isolated ventricular myocytes from senescent mice. Finally, in adult rat ventricular myocytes in primary culture, adenoviral overexpression of SERCA in which Cys674 is mutated to serine partially preserved SERCA activity during exposure to H2O2.
Conclusion
Oxidative modification of SERCA at Cys674 contributes to decreased SERCA activity and impaired myocyte relaxation in the senescent heart. Strategies to decrease oxidant levels and/or protect target proteins such as SERCA may be of value to preserve diastolic function in the aging heart.
Keywords: aging, catalase, left ventricular diastolic dysfunction, oxidative stress
Introduction
Cardiac aging is characterized by left ventricular (LV) diastolic dysfunction that is due, at least in part, to slowed relaxation of cardiac myocytes.1 A mechanism that may contribute to slowed myocyte relaxation is decreased sarcoplasmic reticulum (SR) calcium cycling due to decreased SR calcium ATPase (SERCA) activity.1 The mechanism responsible for decreased SERCA activity in aging is not known. However, because the expression levels of SERCA protein are not consistently decreased in aging,1–5 a decrease in SERCA protein, per se, does not appear necessary. Another potential mechanism for decreased SERCA activity is oxidative posttranslational modification of the protein. Studies in skeletal and cardiac muscle have shown extensive age‐related oxidation of SERCA.6–7 In this regard, we have shown that the activity of SERCA can be regulated by oxidative posttranslational modification of Cys674: Reversible S‐glutathiolation increases SERCA activity, whereas irreversible oxidative sulfonation is associated with decreased activity.8–9 Furthermore, in cardiac myocytes, we found that redox‐stimulated SERCA activation is mediated by S‐glutathiolation of Cys674,10 and in related experiments we noticed that redox‐stimulated SERCA activation is absent in myocytes from senescent (versus young) mice (Figure S1), thus suggesting that Cys674 may be oxidized in senescent myocytes.
These observations led to our hypothesis that impaired myocyte relaxation in aging is due, at least in part, to oxidation of SERCA leading to decreased SERCA activity. This thesis is consistent with the demonstration that transgenic overexpression of catalase prevents impairments in myocyte calcium handling and myocyte relaxation in senescent mice.11–13 However, prior studies of the effects of catalase overexpression in aging did not measure SERCA activity or assess for oxidative modifications of SERCA. Therefore, we assessed SERCA activity and oxidative posttranslational modification in myocardium from young and senescent mice. Diastolic dysfunction in senescent mice was associated with a decrease in SERCA activity but no change in SERCA protein level. Using a sequence‐specific antibody, we found that aging was associated with oxidation (sulfonation) of cardiac SERCA at Cys674. Transgenic overexpression of catalase prevented SERCA Cys674 oxidation and normalized SERCA activity in senescent mice. Likewise, slowed relaxation and calcium reuptake in myocytes freshly isolated from senescent mouse hearts were corrected by catalase overexpression. Finally, in cardiac myocytes in primary culture, mutation of Cys674 to serine partially protected SERCA from oxidative inactivation, thereby supporting the functional importance of Cys674 oxidation in senescent heart.
Methods
Experimental Animals
Young (5‐month‐old) and senescent (21‐month‐old) transgenic mice having myocyte‐specific overexpression of catalase (Line 742; 60× catalase activity; FVB/N)14 and age‐ and gender‐matched WT (FVB/N) mice were used in this study. The protocol was approved by the Institutional Animal Care and Use Committee at Boston University School of Medicine.
Two‐dimensional, M‐Mode, and Doppler Echocardiography for LV Structure and Systolic Function
LV dimensions and systolic function were measured in nonanesthetized mice using an Acuson Sequoia C‐256 echocardiograph machine equipped with a 15‐MHz linear transducer (model 15L8), as we have described.15 LV diastolic function was assessed by transmitral and tissue Doppler echocardiography using a VisualSonics Vevo 770 high‐resolution imaging system equipped with a 30‐MHz RMV‐707B transducer, as we have described.16 Briefly, mice were anesthetized with isofluorane at a concentration of 2.5% for induction and then 1.5% for maintenance by a facemask during the entire procedure. Pulsed‐wave Doppler images were collected with the apical 4‐chamber view to record the mitral Doppler flow spectra. Peak early and late mitral inflow velocities, ratio of peak early‐to‐late diastolic transmitral velocity, deceleration time of early filing, and isovolumetric relaxation time were measured. Tissue Doppler images were collected with the use of parasternal short‐axis views. Early diastolic myocardial tissue velocity was measured. Doppler spectra were recorded for 12 to 14 cardiac cycles; at least 5 consecutive cardiac cycles were selected for the measurements, and the values were averaged in accordance with the American Society of Echocardiography guidelines.17 All data analysis was performed offline using customized Vevo 770 Analytic software.
Isovolumic Langendorff Measurements
Mice were heparinized (100 U IP) and anesthetized with sodium pentobarbital (150 mg/kg IP). The heart was excised and perfused at a constant pressure of 80 mm Hg at 37°C as previously described.18 The perfusate was equilibrated with 95% O2 and 5% CO2 (pH 7.4) and contained (in mmol/L) NaCl 118, NaHCO3 25, KCl 5.3, CaCl2 1.2, MgSO4 1.2, glucose 10, and pyruvate 0.5. Hearts were paced at 7.5 Hz throughout the protocol. A water‐filled balloon was inserted into the left ventricle to record ventricular pressure. After stabilization, an LV pressure‐volume (P‐V) relationship was obtained by stepwise increases of the balloon volumes, until the maximum LV developed pressure was reached for each heart.
Organ Weight and Histology
The mice were killed at 5 or 21 months of age. The heart, left ventricle with septum, lung, and liver were weighed. LV samples were fixed in 10% buffered formalin, embedded with paraffin, and then sectioned. Sections were stained with hematoxylin and eosin and examined under a light microscopy (BX 40; Olympus). Five random fields from each of 4 sections per animal were analyzed, and 60 myocytes per animal were measured. The quantification of myocyte cross‐sectional area was determined using NIH ImageJ software. To assess fibrosis, sections were stained with Masson's trichrome kit (Sigma) and examined under a light microscope (BX 40).
Myocyte Contraction, Relaxation, and Intracellular Calcium Concentration
Myocytes were isolated as we have described previously.19 Briefly, mice were heparinized and anesthetized with pentobarbital (50 mg/kg), and hearts were rapidly excised, cannulated, and perfused with Ca2+‐free Tyrode solution (3 minutes) followed by a solution containing collagenases B and D (Roche) and protease XIV (Sigma) until digestion was complete. Tissue was dissociated using forceps and filtered through a 250‐μm filter. Myocytes were exposed to solutions of increasing Ca2+ concentration and plated in culture chambers (Cell MicroControls) for contractility studies.
Myocyte contraction, relaxation, and intracellular calcium transients were measured as we have described previously.20 Briefly, freshly isolated myocytes were loaded with 0.5 μmol/L Fura 2‐AM (Invitrogen) diluted in 1.2 mmol/L Ca2+ Tyrode buffer containing 500 μmol/L probenecid for 15 minutes at 37°C. The cells were washed with buffer and then incubated for an additional 30 minutes at 37°C to allow complete deesterification of intracellular acetoxymethyl esters. After rinsing, cells were paced at 5 Hz at 37°C for 3 minutes. Myocyte shortening was measured using a video‐based edge‐detection system (IonOptix). The calcium transient was measured with Fura 2 fluorescence amplitude using the 360/380 nm ratio. Contraction, relaxation, and calcium measurements were acquired simultaneously in 1 cell at a time, in from 15 to 25 cells per heart with 6 hearts in each experimental group.
Immunoblotting for SERCA Protein
Hearts were homogenized in lysis buffer (1% Triton X‐100, 0.5% Nonidet P‐40, Tris 10 mmol/L, EDTA 1 mmol/L, EGTA 1 mmol/L, NaCl 150 mmol/L, PMSF 0.4 mmol/L, sodium orthovanadate 0.2 mmol/L, and leupeptin 1 g/L). Protein concentration was determined using the Bradford assay (Bio‐Rad). Equal amounts of total protein were separated by SDS‐PAGE onto 10% gels and transferred to nitrocellulose membrane. The following primary antibodies were used: mouse monoclonal anti‐SERCA2 (Affinity Bioreagents) and rabbit polyclonal anti‐GAPDH antibody (Abcam). Protein–primary antibody complex was detected by using infrared‐dye conjugated goat polyclonal antibody IRDye 680 or IRDye 800 (LICOR Biosciences) and scanned with use of the LI‐COR Odyssey Infrared Imaging System.
SERCA Activity in Myocardium and Cultured Myocytes
SERCA activity was measured in mouse myocardium using calcium‐stimulated, thapsigargin‐inhabitable 45Ca uptake in a crude homogenate that contains SR vesicles, via a modification of published methods, as we have described previously.10 LV tissues were homogenized on ice in Tris‐sucrose homogenization buffer (8% sucrose [w/v] in Tris‐HCl 3 mmol/L, pH 7.0, PMSF 1 mmol/L, 1% Protease Inhibitor Cocktail I [Calbiochem]). The homogenate was centrifuged for 5 minutes at 1520g. The protein concentration of the supernatant was determined by use of the Bradford assay. Samples were pretreated with and without 10 μmol/L thapsigargin, the SERCA inhibitor. Calcium uptake was initiated by the addition of sample to assay buffer (in mmol/L: KCl 100, NaN3 5, MgCl2 6, EGTA 0.15, CaCl2 0.12, Tris‐HCl 30, pH 7.0, oxalate 10, ATP 2.5, ruthenium red 0.01) containing 1 μCi 45CaCl2 (New England Nuclear) in a 37°C water bath. Aliquots of each sample taken at 30, 60, and 90 seconds were vacuum filtered on glass filters (Whatman GF/C; Fisher Scientific), washed 3 times with wash buffer (in mmol/L: imidazole 30, sucrose 250, EGTA 0.5), and counted with a scintillation counter. SERCA activity is expressed as the initial rate of thapsigargin‐sensitive 45Ca uptake as nmol/mg protein per minute.
SERCA activity was also measured in cultured adult rat ventricular myocytes (ARVMs), as we have described previously.10,21 The cells were homogenized on ice via sonication in Tris‐sucrose homogenization buffer (8% [w/v] sucrose in Tris‐HCl 3 mmol/L, pH 7.0, PMSF 1 mmol/L). The homogenate was centrifuged for 5 minutes at 1520g. The protein concentration of the supernatant was determined by use of the Bradford assay. SERCA activity was then measured exactly as described for myocardium except that, due to limited tissue, calcium content was measured at a single time point (90 seconds).
Immunohistochemistry for Cys674‐Sulfonated SERCA, 3‐Nitrotyrosine, and 4‐Hydroxy‐2‐Nonenal
LV tissue sections (4 μm) were blocked with 10% goat serum in phosphate‐buffered saline, incubated with (1) rabbit anti–3‐nitrotyrosine (NY) polyclonal antibody, (2) mouse anti–4‐hydroxy‐2‐nonenal (HNE) monoclonal antibody, or (3) rabbit polyclonal anti‐SERCA antibody raised against a peptide containing the sulfonated Cys674 residue.22 Sections were then incubated with goat biotin–conjugated anti‐rabbit IgG or goat biotin–conjugated anti‐mouse IgG (Vector Laboratory). The sections were incubated with avidin and biotinylated horseradish peroxidase macromolecular complex (Vector Laboratory) and stained with 3‐amino‐9‐ethylcarbazole (Vector Laboratory) and hematoxylin (Vector Laboratory). For negative control, the primary antibody was omitted instead of normal rabbit IgG or normal mouse IgG. The samples were examined under a light microscope (BX 40).
SERCA Expression in ARVMs
ARVMs in primary culture were isolated and cultured as previously described.10 WT SERCA and SERCA in which Cys674 was mutated to serine (Cys674Ser) were overexpressed in ARVMs using adenoviral vectors as previously described.8 SERCA adenoviruses were screened for proper constructs through extraction of viral DNA using the RedExtract‐N‐Amp Tissue PCR kit (Sigma). Extracted DNA was subjected to PCR using a forward primer specific to the viral promoter and a reverse primer located beyond the Cys674 codon: 5′‐ACCGTCAGATCCGCTAGAGA‐3′ and 5′‐GCCACAATGGTGGAGAAGTT‐3′. PCR products were cleaned using a Qiagen QiaQuick PCR Purification Kit and then sequenced using the sequencing primer: 5′‐GATCACTGGGGACAACAAGG‐3′. SERCA adenovirus was purified using the double cesium chloride purification technique. Tissue culture infectious dose and total viral particle units were determined. E1A contamination of the purified SERCA adenovirus was excluded as described previously.23 ARVMs were infected with adenoviruses encoding LacZ (multiplicity of infection=10), WT SERCA (multiplicity of infection=10), or Cys674S (multiplicity of infection=10) for 36 hours. Using these conditions, we previously demonstrated equivalent levels of WT and mutant SERCA expression in ARVMs.10 Cells were then treated with H2O2 (100 μmol/L; 20 minutes), washed with PBS, and used for the SERCA activity assay.
Statistical Analysis
Results are presented as mean±SEM. The statistical significance of differences among multiple groups was determined using Kruskal–Wallis ANOVA and Dunn test. A Mann–Whitney test was used to compare 2 groups. P‐V curves were analyzed by use of repeated‐measures 2‐way ANOVA. P<0.05 values were considered significant. All analyses were performed using GraphPad Prism 5.
Results
Myocyte‐Specific Overexpression of Catalase Prevents LV Hypertrophy and Diastolic Dysfunction in Senescent Mice
LV weight, wall thickness, and chamber size were increased in senescent WT mice compared with young WT mice (Table). In mice with myocyte‐specific overexpression of catalase, there was partial amelioration of these age‐related structural changes with decreases in LV wall thickness and mass (Table).
Table 1.
Echocardiographic Data and Organ Weights
| Y‐WT | Y‐CAT | S‐WT | S‐CAT | |
|---|---|---|---|---|
| n | 8 | 8 | 10 | 11 |
| IVSTd, mm | 0.63±0.02 | 0.62±0.03 | 0.83±0.02 | 0.74±0.01 |
| PWTd, mm | 0.91±0.01 | 0.91±0.01 | 1.46±0.04*** | 1.26±0.03*† |
| LV EDD, mm | 2.96±0.03 | 3.00±0.04 | 3.39±0.10** | 3.27±0.05* |
| LV ESD, mm | 1.14±0.02 | 1.15±0.03 | 1.44±0.08** | 1.24±0.04*† |
| LV FS, % | 61.2±0.6 | 62.3±0.8 | 57.7±1.2 | 62.1±0.7 |
| LV EF, % | 91.4±0.2 | 91.3±0.5 | 88.5±1.3 | 91.5±0.5 |
| BW, g | 36.2±0.8 | 38.8±4.2 | 40.1±2.8 | 45.3±2.8 |
| HW, mg | 135.9±3.6 | 143.9±11 | 193.3±17.9* | 175.3±8.8*† |
| HW/BW, mg/g | 3.75±0.02 | 3.76±0.24 | 4.71±0.36* | 3.94±0.12† |
| LV W, mg | 94.1±2.3 | 95.9±6.8 | 131.2±13.8* | 122.1±6.5* |
| LV W/BW, mg/g | 2.60±0.03 | 2.51±0.11 | 3.28±0.11* | 2.74±0.09† |
| Lung W, wet/dry | 4.18±0.19 | 4.53±0.13 | 4.74±0.07 | 4.57±0.07 |
| Liver W, wet/dry | 3.03±0.02 | 3.21±0.05 | 3.33±0.07 | 3.23±0.03 |
Values are mean±SEM. BW indicates body weight; CAT, catalase; EDD, end‐diastolic dimension; EF, ejection fraction; ESD, end‐systolic dimension; FS, fractional shortening; HW, heart weight; IVSTd, interventricular septal thickness at diastole; LV, left ventricular; PWTd, posterior wall thickness at diastole; S, senescent; W, weight; WT, wild‐type; Y, young.
*P<0.05, **P<0.01, ***P<0.001 versus Y‐WT or Y‐CAT group; †P<0.05 versus S‐WT group.
Histological analysis showed increases in myocyte cross‐sectional area and interstitial fibrosis in senescent WT mice (Figure S2). In transgenic mice with cardiac‐specific overexpression of catalase, myocyte hypertrophy was prevented and interstitial fibrosis was decreased, indicating amelioration of the aging phenotype. In senescent WT mice, immunohistochemical analysis showed marked increases in 2 markers of oxidation in the myocardium: HNE and NY (Figure S3). Catalase overexpression decreased the levels of HNE and NY to those observed in young mice, confirming that oxidative damage in the aging heart is sensitive to a remarkable extent to catalase.
Mitral inflow and tissue Doppler echocardiography in senescent WT mice showed prolongation of LV isovolumetric relaxation time and deceleration time of early filing in association with decreases in the ratio of peak early‐to‐late diastolic transmitral velocity (Figure 1A through 1C) and early diastolic myocardial tissue velocity (Figure 1D), a pattern typical of abnormal relaxation.17 The abnormalities in diastolic function were prevented by catalase overexpression in senescent mice (Figure 1A through 1D).
Figure 1.
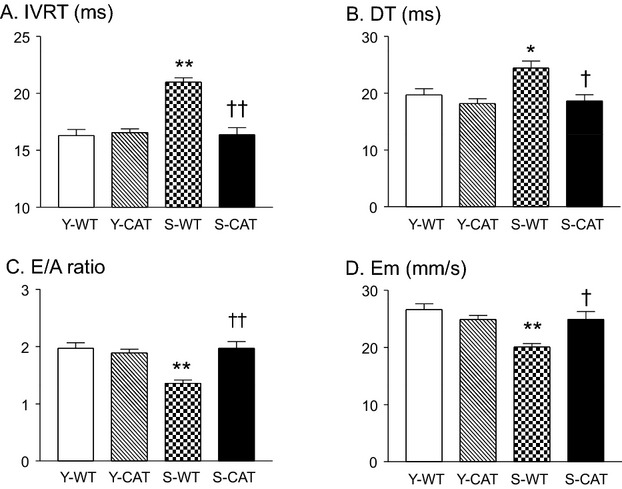
Doppler echocardiographic measures of left ventricular (LV) diastolic function in young (5 months; Y) and senescent (21 months; S) wild‐type (WT) and transgenic catalase‐overexpressing (CAT) mice. In senescent WT mice, there were increases in isovolumic relaxation time (IVRT; A) and deceleration time (DT; B); and decreases in the ratio of early‐to‐late diastolic mitral inflow velocity (E/A; C) and myocardial peak early diastolic velocity (Em; D) indicative of diastolic dysfunction. Diastolic dysfunction was not present in senescent mice overexpressing catalase. Values are mean±SEM; n=8‐11; *P<0.05, **P<0.01 versus Y‐WT or Y‐CAT mice. †P<0.05, ††P<0.01 versus S‐WT mice.
Consistent with the Doppler findings, isovolumic balloon–in–left ventricle Langendorff studies showed a marked leftward/upward shift in the end‐diastolic P‐V relationship and a rightward/downward shift in LV peak developed P‐V relationship in senescent WT mice (versus young WT mice) (Figure 2), indicating LV diastolic and systolic dysfunction in senescent WT mice. Catalase overexpression prevented both diastolic and systolic dysfunction in the senescent hearts (Figure 2).
Figure 2.
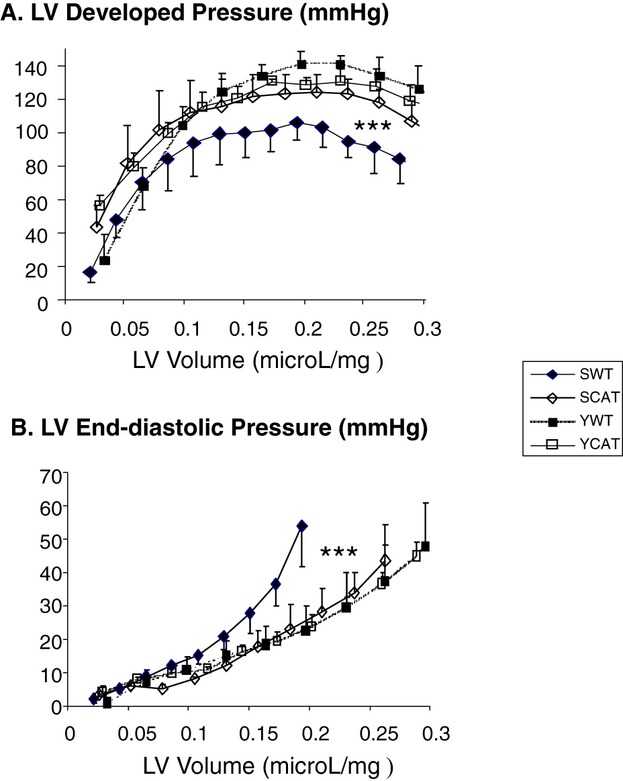
Isovolumic balloon–in–left ventricle Langendorff measurements of left ventricular (LV) developed pressure (A) and LV end‐diastolic pressure (B) in young wild‐type (Y‐WT), young catalase‐overexpressing (Y‐CAT), senescent WT (S‐WT), and senescent catalase‐overexpressing (S‐CAT) mice. The x‐axis depicts LV balloon volume in μL/mg of LV weight. Values are mean±SEM; n=4; ***P<0.001 versus Y‐WT or Y‐CAT mice by repeated‐measures 2‐way ANOVA.
Catalase Preserves SERCA Activity and Prevents SERCA Oxidation in Senescent Myocardium
Maximal SERCA activity was decreased by 38% in senescent (versus young) WT mice (Figure 3A). Overexpression of catalase prevented the age‐related decrease in SERCA activity (Figure 3A). The expression of SERCA protein was not decreased in senescent (versus young) WT myocardium, and was not affected by the overexpression of catalase in young or senescent mice (Figure S4). We previously developed an antibody that is specific for SERCA sulfonated at Cys674.22 Immunohistochemistry using this antibody revealed a marked increase in staining of Cys674‐sulfonated SERCA in senescent (versus young) WT myocardium that was prevented by catalase overexpression (Figure 3B and 3C).
Figure 3.
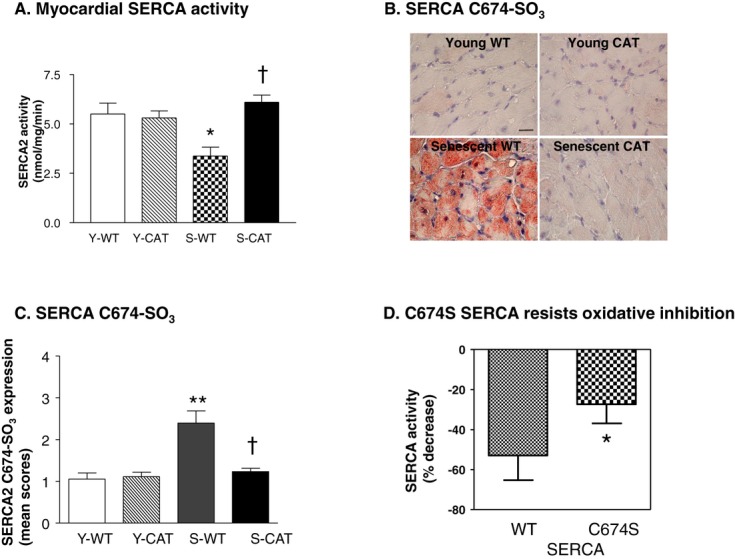
A, Maximal calcium‐stimulated SERCA activity in myocardial SR vesicles from young wild‐type (Y‐WT), young catalase overexpressing (Y‐CAT), senescent WT (S‐WT), and senescent catalase overexpressing (S‐CAT) mice. Values are mean±SEM; n=3. *P<0.05 versus Y‐WT or Y‐CAT mice. †P<0.05 versus S‐WT mice. B, Representative photomicrographs of myocardium subjected to immunohistochemical staining with a site‐specific antibody that recognizes SERCA that is sulfonated on Cys674 (SERCA C674‐SO3), and (C) shows the quantification of myocardial SERCA Cys674‐SO3 as per (B). Values are mean±SEM; n=3‐4; **P<0.01 versus Y‐WT or Y‐CAT mice. †P<0.05 versus S‐WT mice. D, The H2O2‐mediated decrease in SERCA activity in adult rat ventricular myocytes overexpressing wild‐type (WT) SERCA or SERCA in which Cys674 was mutated to serine (Cys674Ser). Values are mean±SEM; n=3; *P<0.05 versus WT SERCA. SERCA indicates sarcoplasmic reticulum calcium ATPase.
Cys674Ser‐Mutated SERCA Resists H2O2‐Mediated Oxidation and Inhibition In Vitro in Cardiac Myocytes
We previously showed that Cys674 accounts for the majority of physiologically reactive cysteines in SERCA and is required for glutathiolation‐mediated enzyme activation.8,10 We further showed that brief exposure (20 minutes) of ARVMs to H2O2 (100 μmol/L) in vitro inhibited SERCA activity and caused oxidation of SERCA cysteines.21 Therefore, the observation that decreased SERCA activity in senescent myocardium is associated with oxidation (sulfonation) of SERCA at Cys674 suggests that oxidative modification of Cys674 may be of pathophysiological relevance. To test the relevance of Cys674 oxidation, in cultured ARVMs we overexpressed WT SERCA or SERCA in which Cys674 was mutated to serine (Cys674Ser), as we have previously described.10,24 In myocytes overexpressing WT SERCA, exposure to H2O2 (100 μmol/L, 20 minutes) inhibited SERCA activity by ≈60% (Figure 3D). In cells overexpressing Cys674Ser SERCA, the ability of H2O2 to inhibit SERCA activity was diminished by approximately half (Figure 3D), indicating increased resistance of Cys674Ser SERCA to oxidative inactivation.
Myocyte‐Specific Overexpression of Catalase Prevents Age‐Related Dysfunction and Calcium Dysregulation in Isolated Myocytes
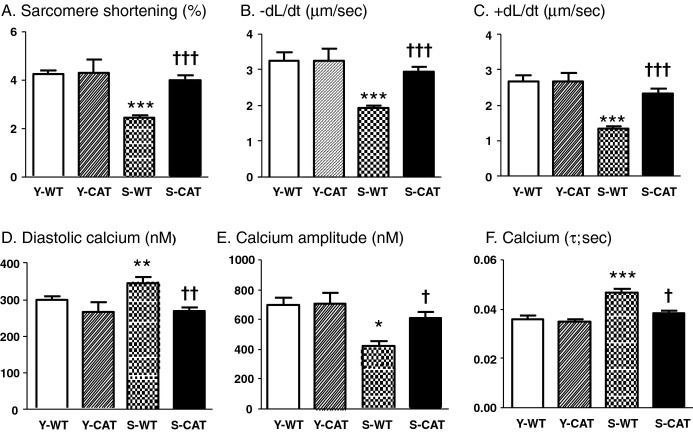
To determine whether catalase improves relaxation at the level of the cardiac myocyte, we assessed myocyte function and intracellular calcium concentration in isolated ventricular myocytes. In myocytes from senescent WT mice, sarcomere shortening, maximal rate of shortening (−dL/dt), and maximal rate of relengthening (+dL/dt) were decreased by 42%, 41%, and 50%, respectively (Figures 4 and S5). Likewise, in myocytes from senescent WT mice, intracellular diastolic calcium was increased by 18%, the calcium transient amplitude was decreased by 40%, and the time constant of cytosolic calcium decline (τ), an index of SERCA activity, was prolonged by 25%. In senescent mice, overexpression of catalase improved all of these measures of myocyte function and calcium handling (Figures 4 and S5).
Figure 4.
Sarcomere shortening (A), the maximal rate of shortening (−dL/dt; B), the maximal rate of relengthening (+dL/dt; C), diastolic calcium (D), calcium amplitude (E), and the time constant of diastolic calcium decline (τ; F) in young wild‐type (Y‐WT, n=4), young catalase (Y‐CAT, n=3), senescent WT (S‐WT, n=6) and senescent catalase (S‐CAT, n=6) mice. Values are mean±SEM; *P<0.05, **P<0.01, ***P<0.001 versus Y‐WT or Y‐CAT mice. †P<0.05, ††P<0.01, †††P<0.001 versus S‐WT mice.
Discussion
In this study we demonstrate that (1) decreased SERCA activity in senescent myocardium is associated with oxidative sulfonation of SERCA at Cys674, a cysteine known to be involved in the regulation of SERCA activity, and (2) both Cys674 oxidation and SERCA inhibition are prevented by overexpression of catalase. We further show in cardiac myocytes in vitro that (1) redox activation of SERCA is decreased in myocytes from senescent mice and (2) Cys674 is oxidized by H2O2 and participates in H2O2‐mediated SERCA inhibition, thus supporting the functional relevance of Cys674 sulfonation observed in the senescent heart. While it is likely that other oxidations on SERCA also contribute to decreased activity in the senescent heart, our observations support the thesis that impaired myocyte relaxation in the aging heart is due, in part, to oxidation of SERCA at Cys674.
SERCA in the Senescent Heart
Our finding of a decrease in SERCA activity in senescent hearts is consistent with most prior studies in rats and mice,1,12,25–26 although it should be noted that at least 1 study in senescent sheep did not find a decrease in SERCA activity, raising questions about species‐related differences.27 The functional relevance of this decrease in SERCA activity is supported by the finding of impaired calcium reuptake and relaxation in myocytes isolated from the senescent heart, as observed previously by ourselves19 and others.12 While some studies have observed decreased levels of SERCA protein in the senescent heart,1,11–12 our current data and those from several laboratories,1–5 demonstrate that a decrease in SERCA protein expression does not occur consistently in senescent hearts. Thus, a decrease in protein expression does not appear necessary for decreased SERCA activity in the senescent heart.
Another possibility for decreased SERCA activity is an alteration in phospholamban (PLN) expression or activity. Changes in PLN expression or activity regulate SERCA activity by shifting the EC50 for calcium but without affecting maximal calcium‐stimulated activity, as measured in this study.28 Conversely, for the same EC50, changes in maximal activity reflect directionally similar changes in activity within the physiological range.28 Therefore, (1) a change in PLN activity would not explain the changes in myocardial SERCA activity that we observed and (2) the observed changes in maximal SERCA activity would be predicted to reflect directionally similar changes in activity in the physiological range. Thus, it is likely that the observed changes in maximal activity reflect similar changes in activity within the physiological calcium range. This premise is supported by the observation that the rate of calcium reuptake, which is a reasonable reflection of SERCA activity, changed in parallel with maximal SERCA activity. Finally, while we cannot comment on PLN activity, prior studies in mouse heart have not observed an increase in PLN expression or activity with aging.11,29
Oxidative Posttranslational Modification of SERCA
The finding of decreased SERCA activity in the presence of normal protein expression is highly suggestive of a posttranslational event that modifies enzyme activity. While SERCA can be regulated by phosphorylation,26,30 the ability of catalase overexpression to preserve SERCA activity, as well as relaxation and calcium reuptake in isolated myocytes, strongly suggests that in senescent heart the posttranslational modification of SERCA is mediated by reactive oxygen species and, in particular, H2O2. Increased oxidative stress is well recognized in the senescent heart and has been implicated in the pathophysiology of cardiac aging.11–12,11–33 Markers of protein and lipid oxidation (NY and HNE, respectively) were markedly increased in senescent hearts, and the increases were prevented by catalase overexpression, indicating that H2O2 is an important oxidant in the senescent heart and confirming the antioxidant effectiveness of catalase overexpression in our mice.
SERCA Cys674 Sulfonation
We previously developed and validated a site‐specific antibody that recognizes SERCA sulfonated at Cys674.22 Using this antibody for immunohistochemistry, we demonstrate that cardiac aging is associated with oxidation (sulfonation) of SERCA on Cys674. While several oxidative modifications of SERCA have been observed in senescent myocardium,6–7 the functional significance of these modifications is not known. Cys674 is located in the hinge region of SERCA, which is thought to be involved in regulating the passage of calcium and is accessible from the cytosolic compartment.34 Recent work has indicated that the Cys674 thiol plays an important role in regulating SERCA activity: In arterial smooth muscle nitric oxide–induced relaxation is mediated by S‐glutathiolation of SERCA Cys674, whereas decreased S‐glutathiolation and decreased activation of SERCA by nitric oxide in atherosclerotic aorta are associated with sulfonation at Cys674.8 Likewise, in mice with heart failure, we found that decreased SERCA activity in the myocardium is associated with SERCA sulfonation at Cys674 in the absence of a decrease in protein expression.20
Recently, we used a SERCA mutant in which Cys674 was replaced by serine (Cys674Ser) to show that glutathiolation of Cys674 is necessary for the redox activation of SERCA by nitroxyl anion.10 In studies leading to the current project, oxidation of Cys674 was implicated by the finding that SERCA activity could not be stimulated by nitroxyl in myocytes from senescent mice (Figure S1). This observation is consistent with the thesis that SERCA oxidation at Cys674 contributes to decreased SERCA activity and calcium handling in the senescent heart. We previously found that exposure to H2O2 causes rapid inhibition of SERCA activity that is associated with oxidative modification of cysteines.21 Therefore, to test the role of Cys674 oxidation in inhibiting myocyte SERCA activity, we overexpressed WT SERCA and the Cys674Ser SERCA mutant in cultured ARVMs. Because the Cys674S SERCA mutant cannot be oxidized at Cys674, it provides an opportunity to test the functional consequence of Cys674 oxidation. As we previously observed in native cardiac myocytes,21 H2O2 exposure markedly inhibited SERCA activity in myocytes overexpressing WT SERCA. By comparison, the ability of H2O2 to inhibit SERCA activity was attenuated by approximately half in myocytes overexpressing the Cys674Ser mutant, suggesting that oxidation of Cys674 accounts for approximately half of the decrease in activity caused by exposure to H2O2. Thus, the functional importance of Cys674 in mediating the effects of H2O2 on SERCA, together with the identification of catalase‐sensitive Cys674 sulfonation in the senescent heart, supports our thesis that SERCA oxidation at Cys674 contributes to decreased SERCA activity and impaired myocyte relaxation in the aging heart.
Implications
The ability of cardiac‐specific catalase to correct age‐related abnormalities in SERCA activity and calcium handling and relaxation in the cardiac myocyte support the thesis that oxidation of SERCA contributes to slowed relaxation of cardiac myocytes in senescent heart. However, it is important to recognize that there are multiple other abnormalities in the aging heart that may contribute to impaired relaxation (eg, increased interstitial fibrosis or altered of titan function). This study does not allow assignment of the relative importance of decreased SERCA activity versus other potential mechanisms in causing diastolic dysfunction. However, because overexpression of SERCA has been shown to improve LV diastolic function in aging hearts,35 it is likely that the ability of catalase to prevent the oxidative inactivation of SERCA contributed to improvement in myocyte relaxation and, at least in part, to improvement in diastolic function in our senescent mice. These observations therefore suggest that strategies to decrease oxidant levels and/or protect SERCA from oxidation at particular sites (eg, Cys674) may be of value in the amelioration of cardiac aging. Because impaired SERCA activity and SERCA oxidation are present in other pathophysiological settings such as heart failure,36 it is also possible that oxidative modification of SERCA will be relevant to other conditions that are associated with impaired SERCA activity.
Sources of Funding
This study was supported by National Institutes of Health grants HL‐061639 and HL‐064750 (to Dr. Colucci), HL031607 (to Drs Cohen and Tong), and PO1 HL 068758 (to Dr Cohen) and the National Heart, Lung, and Blood Institute–sponsored Boston University Cardiovascular Proteomics Center (Contract N01‐HV‐28178, to Drs Cohen and Colucci).
Disclosures
None.
References
- 1.Janczewski AM, Lakatta EG. Modulation of sarcoplasmic reticulum Ca(2+) cycling in systolic and diastolic heart failure associated with aging. Heart Fail Rev. 2010; 15:431-445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim CC, Liao R, Varma N, Apstein CS. Impaired lusitropy‐frequency in the aging mouse: role of Ca(2+)‐handling proteins and effects of isoproterenol. Am J Physiol. 1999; 277:H2083-H2090 [DOI] [PubMed] [Google Scholar]
- 3.Slack JP, Grupp IL, Dash R, Holder D, Schmidt A, Gerst MJ, Tamura T, Tilgmann C, James PF, Johnson R, Gerdes AM, Kranias EG. The enhanced contractility of the phospholamban‐deficient mouse heart persists with aging. J Mol Cell Cardiol. 2001; 33:1031-1040 [DOI] [PubMed] [Google Scholar]
- 4.Isenberg G, Borschke B, Rueckschloss U. Ca2+ transients of cardiomyocytes from senescent mice peak late and decay slowly. Cell Calcium. 2003; 34:271-280 [DOI] [PubMed] [Google Scholar]
- 5.Thomas MM, Vigna C, Betik AC, Tupling AR, Hepple RT. Cardiac calcium pump inactivation and nitrosylation in senescent rat myocardium are not attenuated by long‐term treadmill training. Exp Gerontol. 2011; 46:803-810 [DOI] [PubMed] [Google Scholar]
- 6.Sharov VS, Dremina ES, Galeva NA, Williams TD, Schoneich C. Quantitative mapping of oxidation‐sensitive cysteine residues in SERCA in vivo and in vitro by HPLC‐electrospray‐tandem MS: selective protein oxidation during biological aging. Biochem J. 2006; 394:605-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knyushko TV, Sharov VS, Williams TD, Schoneich C, Bigelow DJ. 3‐Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005; 44:13071-13081 [DOI] [PubMed] [Google Scholar]
- 8.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S‐Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004; 10:1200-1207 [DOI] [PubMed] [Google Scholar]
- 9.Tong X, Ying J, Pimentel DR, Trucillo M, Adachi T, Cohen RA. High glucose oxidizes SERCA cysteine‐674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J Mol Cell Cardiol. 2008; 44:361-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lancel S, Zhang J, Evangelista A, Trucillo MP, Tong X, Siwik DA, Cohen RA, Colucci WS. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ Res. 2009; 104:720-723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009; 119:2789-2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren J, Li Q, Wu S, Li SY, Babcock SA. Cardiac overexpression of antioxidant catalase attenuates aging‐induced cardiomyocyte relaxation dysfunction. Mech Ageing Dev. 2007; 128:276-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, Li Q, Du M, Li SY, Ren J. Cardiac‐specific overexpression of catalase prolongs lifespan and attenuates ageing‐induced cardiomyocyte contractile dysfunction and protein damage. Clin Exp Pharmacol Physiol. 2007; 34:81-87 [DOI] [PubMed] [Google Scholar]
- 14.Kang YJ, Chen Y, Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem. 1996; 271:12610-12616 [DOI] [PubMed] [Google Scholar]
- 15.Qin F, Lennon‐Edwards S, Lancel S, Biolo A, Siwik DA, Pimentel DR, Dorn GW, Kang YJ, Colucci WS. Cardiac‐specific overexpression of catalase identifies hydrogen peroxide‐dependent and ‐independent phases of myocardial remodeling and prevents the progression to overt heart failure in G(alpha)q‐overexpressing transgenic mice. Circ Heart Fail. 2010; 3:306-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liesa M, Luptak I, Qin FZ, Hyde BB, Sahin E, Siwik DA, Zhu ZK, Pimentel DR, Xu XJ, Ruderman NB, Huffman KD, Doctrow SR, Richey L, Colucci WS, Shirihai OS. Mitochondrial transporter ATP binding cassette mitochondrial erythroid is a novel gene required for cardiac recovery after ischemia/reperfusion. Circulation. 2011; 124:806-813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009; 10:165-193 [DOI] [PubMed] [Google Scholar]
- 18.Luptak I, Yan J, Cui L, Jain M, Liao RL, Tian R. Long‐term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation. 2007; 116:901-909 [DOI] [PubMed] [Google Scholar]
- 19.Lim CC, Apstein CS, Colucci WS, Liao RL. Impaired cell shortening and relengthening with increased pacing frequency are intrinsic to the senescent mouse cardiomyocyte. J Mol Cell Cardiol. 2000; 32:075-082 [DOI] [PubMed] [Google Scholar]
- 20.Lancel S, Qin FZ, Lennon SL, Zhang JM, Tong XY, Mazzini MJ, Kang YJ, Siwik DA, Cohen RA, Colucci WS. Oxidative posttranslational modifications mediate decreased SERCA activity and myocyte dysfunction in Galphaq‐overexpressing mice. Circ Res. 2010; 107:228-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuster GM, Lancel S, Zhang JM, Communal C, Trucillo MP, Lim CC, Pfister O, Weinberg EO, Cohen RA, Liao RL, Siwik DA, Colucci WS. Redox‐mediated reciprocal regulation of SERCA and Na(+)‐Ca(2+) exchanger contributes to sarcoplasmic reticulum Ca(2+) depletion in cardiac myocytes. Free Radic Biol Med. 2010; 48:1182-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying J, Sharov V, Xu S, Jiang B, Gerrity R, Schoneich C, Cohen RA. Cysteine‐674 oxidation and degradation of sarcoplasmic reticulum Ca(2+) ATPase in diabetic pig aorta. Free Radic Biol Med. 2008; 45:756-762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haeussler DJ, Evangelista AM, Burgoyne JR, Cohen RA, Bachschmid MM, Pimental DR. Checkpoints in adenoviral production: cross‐contamination and E1A. PLoS One. 2011; 6:e23160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying J, Tong X, Pimentel DR, Weisbrod RM, Trucillo MP, Adachi T, Cohen RA. Cysteine‐674 of the sarco/endoplasmic reticulum calcium ATPase is required for the inhibition of cell migration by nitric oxide. Arterioscler Thromb Vasc Biol. 2007; 27:783-790 [DOI] [PubMed] [Google Scholar]
- 25.Froehlich JP, Lakatta EG, Beard E, Spurgeon HA, Weisfeldt ML, Gerstenblith G. Studies of sarcoplasmic reticulum function and contraction duration in young adult and aged rat myocardium. J Mol Cell Cardiol. 1978; 10:427-438 [DOI] [PubMed] [Google Scholar]
- 26.Xu A, Narayanan N. Effects of aging on sarcoplasmic reticulum Ca2+‐cycling proteins and their phosphorylation in rat myocardium. Am J Physiol. 1998; 275:H2087-H2094 [DOI] [PubMed] [Google Scholar]
- 27.Dibb KM, Rueckschloss U, Eisner DA, Isenberg G, Trafford AW. Mechanisms underlying enhanced cardiac excitation contraction coupling observed in the senescent sheep myocardium. J Mol Cell Cardiol. 2004; 37:1171-1181 [DOI] [PubMed] [Google Scholar]
- 28.Frank K, Tilgmann C, Shannon TR, Bers DM, Kranias EG. Regulatory role of phospholamban in the efficiency of cardiac sarcoplasmic reticulum Ca2+ transport. Biochemistry. 2000; 39:14176-14182 [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Wu S, Li SY, Lopez FL, Du M, Kajstura J, Anversa P, Ren J. Cardiac‐specific overexpression of insulin‐like growth factor 1 attenuates aging‐associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol Heart Circ Physiol. 2007; 292:H1398-H1403 [DOI] [PubMed] [Google Scholar]
- 30.Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008; 77:265-273 [DOI] [PubMed] [Google Scholar]
- 31.Li SY, Du M, Dolence EK, Fang CX, Mayer GE, Ceylan‐Isik AF, Lacour KH, Yang XP, Wilbert CJ, Sreejayan N, Ren J. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell. 2005; 4:57-64 [DOI] [PubMed] [Google Scholar]
- 32.Rueckschloss U, Villmow M, Klockner U. NADPH oxidase‐derived superoxide impairs calcium transients and contraction in aged murine ventricular myocytes. Exp Gerontol. 2010; 45:788-796 [DOI] [PubMed] [Google Scholar]
- 33.Wang MY, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age‐associated cardiac remodeling. J Mol Cell Cardiol. 2010; 48:765-772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bishop JE, Squier TC, Bigelow DJ, Inesi G. (Iodoacetamido)fluorescein labels a pair of proximal cysteines on the Ca2+‐ATPase of sarcoplasmic reticulum. Biochemistry. 1988; 27:5233-5240 [DOI] [PubMed] [Google Scholar]
- 35.Schmidt U, del MF, Miyamoto MI, Matsui T, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca(2+)‐ATPase. Circulation. 2000; 101:790-796 [DOI] [PubMed] [Google Scholar]
- 36.Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, Colucci WS. Role of oxidative stress in myocardial hypertrophy and failure. J Mol Cell Cardiol. 2002; 34:379-388 [DOI] [PubMed] [Google Scholar]