Abstract
Background
Evidence regarding the role of dairy fat intake in cardiovascular disease (CVD) has been mixed and inconclusive. Most earlier studies have used self‐reported measures of dietary intake and focused on relatively racially homogeneous populations. Circulating biomarkers of dairy fat in a multiethnic cohort provide objective measures of dairy fat intake and facilitate conclusions relevant to populations with different diets and susceptibility to CVD.
Methods and Results
In a multiethnic cohort of 2837 US adults aged 45 to 84 years at baseline (2000–2002), phospholipid fatty acids including 15:0, 14:0, and trans‐16:1n7 were measured using standardized methods, and the incidence of CVD prospectively adjudicated. Self‐reported whole‐fat dairy and butter intakes had strongest associations with 15:0, rather than 14:0 or trans‐16:1n7. In multivariate models including demographics and lifestyle and dietary habits, each SD‐unit of 15:0 was associated with 19% lower CVD risk (hazard ratio [95% CI] 0.81 [0.68 to 0.98]) and 26% lower coronary heart disease (CHD) risk (0.74 [0.60 to 0.92]). Associations were strengthened after mutual adjustment for 14:0 and trans‐16:1n‐7 and were similar after adjustment for potential mediators. Plasma phospholipid 14:0 and trans‐16:1n‐7 were not significantly associated with incident CVD or CHD. All findings were similar in white, black, Hispanic, and Chinese American participants.
Conclusion
Plasma phospholipid 15:0, a biomarker of dairy fat, was inversely associated with incident CVD and CHD, while no association was found with phospholipid 14:0 and trans‐16:1n‐7. These findings support the need for further investigation of CVD effects of dairy fat, dairy‐specific fatty acids, and dairy products in general.
Keywords: cardiovascular diseases, dairy fat, diet, fatty acids
Introduction
The role of dairy products, and especially dairy fat intake, in cardiovascular health is controversial. Some epidemiological studies have suggested beneficial effects of low‐fat dairy consumption on hypertension1–3 and stroke risk,4–5 and low‐fat dairy consumption is a component of the beneficial Dietary Approaches to Stop Hypertension (DASH) diet.6 Conversely, consumption of whole‐fat dairy is discouraged due to potential adverse effects of saturated fat on coronary heart disease (CHD). A recent meta‐analysis that included >250 000 participants in 6 prospective observational studies found no evidence of harmful associations between self‐reported overall milk consumption and incidence of CHD or stroke (pooled relative risk (RR) [95% CI] for each 200 mL/day of 1.00 [0.96 to 1.04] for CHD and 0.87 [0.72 to 1.07] for stroke).7 This pooled meta‐analysis also found no differences in associations of self‐reported total, whole‐fat, or low‐fat/nonfat dairy products and CHD risk, but reported data on these dairy subgroups were restricted to fewer studies. Consequently, based on current evidence, whether dairy fat is associated with cardiovascular disease (CVD) risk remains unclear.
Possible explanations for the inconclusive findings could relate to (1) competing benefits of certain ingredients in nonfat dairy products, such as protein, calcium, and phosphorous, versus harms of saturated fat in dairy fat or (2) offsetting beneficial components of specific factors in dairy fat, such as vitamin D or dairy‐specific fatty acids or (3) potential misclassification or reporting bias of self‐reported dietary assessment, which could attenuate measures of association or cause bias.
Compared with self‐reported dietary estimates, circulating fatty acid biomarkers such as pentadecanoic acid (15:0) and trans‐palmitoleic acid (trans‐16:1n‐7) cannot be synthesized by humans and are useful objective biomarkers of dairy fat consumption.8–11 Dairy products also contain myristic acid (14:0), but endogenous synthesis of this fatty acid makes its circulating levels less useful as a biomarker of dairy fat consumption.12 The evaluation of objective biomarkers of dairy fat may improve accuracy in dietary assessment methods and help elucidate associations between dairy and cardiovascular health.
Relatively few studies have evaluated associations between biomarkers of dairy fat and incidence of CVD.8–9,8–15 In addition, none of these prior studies have investigated multiethnic populations with diverse dietary behaviors and susceptibilities to CVD. To address these gaps in knowledge about potential effects of dairy fat on CVD, we prospectively assessed the associations of plasma phospholipid 15:0, trans‐16:1n‐7, and 14:0 with incident total CVD and CHD in the Multi‐Ethnic Study of Atherosclerosis (MESA). Measures of plasma 17:0, another potential biomarker of dairy fat, were not available in MESA. We hypothesized that circulating biomarkers of dairy fat would be inversely associated with CVD risk.
Methods
Design and Population
Details of MESA, a prospective cohort study designed to identify risk factors associated with subclinical CVD development and progression in multiple US races/ethnicities, have been published.16 Between 2000 and 2002, the study enrolled and performed baseline examinations in 6814 adults (38% whites, 28% blacks, 22% Hispanics, and 12% Chinese Americans) aged 45 to 84 years in 6 US communities: Baltimore (city and county), MD; Chicago, IL; Forsyth County, NC; New York, NY; Los Angeles County, CA; and St Paul, MN. All participants were free of clinical CVD at baseline. Follow‐up cohort examinations were conducted in 2002–2003, 2004–2005, 2005–2007, and 2010–2011. Protocols were approved by local institutional review boards, and all participants gave written informed consent. Plasma phospholipid fatty acids were measured at baseline in a subset of 2856 participants randomly selected from within each race/ethnicity stratum to provide similar proportions of participants. There were no material differences when comparing baseline characteristics of participants with and without biomarker measurement in each race/ethnicity group. After the exclusion of 43 participants with missing data on incident CVD, 2837 participants were included in the present analysis, including equal proportions of black (n=697), Asian (of Chinese descent, n=711), Hispanic (n=705), and white (n=724) participants.
Analysis of Plasma Phospholipid Fatty Acids
Fasting blood samples were collected at the baseline examination and stored at −70°C according to standardized protocols.16–17 Fatty acids were extracted from EDTA plasma using a chloroform/methanol extraction method,18 with phospholipids separated from cholesterol esters, triglycerides, and free fatty acids by using thin layer chromatography. Forty‐one individual phospholipid fatty acids were derivatized to methyl esters and separated by using gas chromatography equipped with flame ionization detection, quantified as a percentage of total fatty acids. In the present analysis, we focused on 14:0, 15:0, and trans‐16:1n‐7. For each fatty acid, the automated (computer software) limit of detection was 0.03%. Lab CVs across a range of control sample concentrations (very low to high) were 11.6% for 14:0, 14.5% for 15:0, and 29.5% for trans‐16:1n‐7. Lab CVs excluding very low control sample concentrations (≤0.05%) were 5.7% for 14:0, 7.7% for 15:0, and 10.9% for trans‐16:1n‐7.
Covariates
Information on sociodemographics, medical history, medication use, and smoking status and history was obtained at baseline using interviewer‐administered and self‐completed questionnaires. Blood lipid levels were measured using standardized methods as previously described.19 Anthropometric measurements were performed using standard procedures. Resting seated blood pressure was measured 3 times (Dinamap model Pro 100 automated oscillometric sphygmomanometer, Critikon, Tampa, FL), with mean of the last 2 measurements used for analyses. Physical activity was assessed using the MESA Typical Week Physical Activity Survey,20 a validated semiquantitative questionnaire adapted from the Cross‐Cultural Activity Participation Study21 that captures time and frequency of various physical activities during a typical week in the previous month. Usual dietary intake over the previous year was assessed at baseline using a Block‐type,22 120‐item food frequency questionnaire, modified to include Chinese foods.23 Food questions from the FFQ were categorized into food groups (eg, salty snacks, desserts, processed meats) as previously described.24 Nutrient intakes were estimated by multiplying food frequency and serving size by specific nutrient content in each food. Criterion validity of macronutrient intake estimates has been demonstrated using baseline plasma lipid measurements.25 Intakes of food‐specific saturated fat were estimated by summing saturated fat intake from selected sources (ie, dairy, meat, butter, and plant sources) as previously described.26 Food frequency (servings per day) was standardized to the medium portion size (ie, frequencies from participants selecting small or large serving sizes were multiplied by the ratio of small/medium [0.5] or large/medium [1.5], respectively).
Ascertainment of Incident CVD
Information on potential CVD events was obtained during each cohort examination and by follow‐up calls to each participant, which occurred every 9 to 12 months. Reported events were evaluated and confirmed by a centralized medical end points committee based on medical record abstractions, death certificates, autopsy reports, and/or obituaries.27–28 Medical records were obtained for 98% of reported hospitalized events and 95% of outpatient procedures.29 Total CVD events were defined as the incidence of any of the following: myocardial infarction, resuscitated cardiac arrest, CHD death, other atherosclerotic death, definite and probable angina, stroke, or other CVD death. CHD events comprised myocardial infarction, resuscitated cardiac arrest, CHD death, and definite and probable angina. In sensitivity analyses, we also assessed “hard” CVD events (ie, excluding definite and probable angina).
Statistical Analysis
We evaluated dairy‐specific phospholipid fatty acids continuously as percentage of total fatty acids and as categorical variables in quintiles of each fatty acid. We used multivariate regression models adjusting for demographic, lifestyle and other dietary covariates to estimate differences in phospholipid fatty acids associated with 1 daily serving of potential dietary sources in the MESA population (ie, whole‐ and low‐fat dairy products, processed and unprocessed meats, French fries, salty snacks, bakery desserts). We assessed the adjusted Spearman correlations among 14:0, 15:0, and trans‐16:1n7 fatty acids and their potential dietary sources in the MESA population, adjusting for demographics and lifestyle and dietary factors. We used linear regression to assess associations of dairy fatty acids with CVD risk factors. Multivariable‐adjusted Cox proportional hazards models estimated hazard ratios (HRs) of incident CVD, with time at risk until the first CVD event, death, or last follow‐up in 2009–2010. We found no evidence of violation of the proportional hazard assumption on the basis of Schoenfeld residuals or the Wald test for an interaction between the exposures of interest and follow‐up time. In addition, we found no evidence of nonlinear relationships between each fatty acid and the outcomes of interest based on restricted cubic spline analysis.30 We used serial models to adjust for potential confounding, with covariates selected based on biologic interest, established relationships with CVD risk, associations with exposures/outcomes in the current dataset, and the effects of their inclusion on the β coefficient for the risk estimates of interest. We found no evidence of multicollinearity based on variance inflation factor analyses. Multivariate‐adjusted models included age (years), sex, race/ethnicity (non‐Hispanic whites, blacks, Hispanics, and Chinese Americans), field center (6 sites), education (<high school, high school, >high school), cigarette smoking (never, current, or former; and pack‐years of cigarette smoking), alcohol (g/day), physical activity (eg, walking for exercise, sports/dancing, and conditioning activities in metabolic equivalents per minute/week), whole‐fat dairy (servings/day), processed and unprocessed meat (servings/day), total energy intake (kcal/day), fiber (g/day), and fruits and vegetables (servings/day). Subsequently, we additionally included simultaneous adjustment for each dairy fat biomarker (14:0, 15:0, and trans‐16:1n7).
We separately evaluated the influence of factors that could be potential confounders or mediators of the associations of interest, including body mass index (BMI) (kg/m2), diabetes (no, impaired fasting glucose, diabetes), hypertensive medication use (yes/no), lipid‐lowering medication use (yes/no), and LDL cholesterol (mg/dL).
We imputed missing covariate data (<2% for most lifestyle factors; 8% to 12% for dietary factors) using single imputation (SAS proc MI, SAS Institute) based on age, sex, race/ethnicity, education, physical activity, BMI, smoking status, LDL cholesterol, HDL cholesterol, lipid‐lowering medication use, and diabetes mellitus; results were similar when we used multiple imputation or excluded missing values. All P values were 2 sided, with α=0.05. Analyses were conducted with SAS version 9.3 (SAS Institute).
Results
At baseline, mean (SD) age was 61.5 (10.2) years, mean (SD) BMI was 27.9 (5.5) kg/m2, and 13% of participants had prevalent diabetes (Table 1). The mean levels of 14:0, 15:0, and trans‐16:1n7 were each relatively low (<1% of fatty acids), consistent with reports in other cohorts.8,14 The mean (SD) self‐reported consumption of total dairy was 1.5 (2.0) servings per day, equally distributed between low‐fat and whole‐fat dairy products.
Table 1.
Baseline Characteristics of Multi‐Ethnic Study of Atherosclerosis Participants (N=2837)
| Characteristics | |
|---|---|
| Mean age (SD), y | 61.5 (10.2) |
| Woman, % | 53 |
| Race/ethnicity, % | |
| Whites | 25.5 |
| Hispanic | 24.8 |
| Chinese American | 25.1 |
| Blacks | 24.6 |
| Education, % | |
| Less than high school | 21.6 |
| High school | 18.1 |
| Some college/college degree | 60.3 |
| Current smokers, % | 13.7 |
| Physical activity (MET: min/week) | |
| Sedentary leisure | 1632 (1110) |
| Active leisure | 2316 (2786) |
| BMI, kg/m2 | 27.9 (5.5) |
| Diabetes at baseline, % | 13.1 |
| Circulating fatty acids, % of total FA | |
| Myristic acid 14:0 | 0.26 (0.08) |
| Pentadecanoic acid 15:0 | 0.17 (0.05) |
| trans‐Palmitoleic acid (trans‐16:1n‐7) | 0.05 (0.03) |
| Mean (SD) food intakes, serving/day | |
| Total dairy | 1.5 (2.0) |
| Low‐fat dairy | 0.8 (1.4) |
| Whole‐fat dairy | 0.7 (1.2) |
| Processed meat | 0.2 (0.3) |
| Unprocessed meat | 0.4 (0.5) |
| Fried potato | 0.1 (0.2) |
| Salty snacks | 0.2 (0.4) |
| Desserts | 0.2 (0.5) |
| Margarine | 0.2 (0.4) |
| Butter | 0.1 (0.3) |
| Fruits and vegetables | 3.3 (2.6) |
| Fiber, g/day | 17.5 (6.1) |
Values are mean (SD) for continuous variables and percent for categorical variables. Whole‐fat dairy included whole milk, cheese, whole‐fat yogurt, and ice cream. Low‐fat dairy included 2% milk, 1%/skim milk, and cottage or ricotta cheese. Processed meats included ham, hot dogs, bologna, salami, lunchmeats, liver, sausage, chorizo, scrapple, and bacon. Unprocessed red meat included hamburger; cheeseburger; meatloaf; hash; beef, pork, or lamb steaks; roasts; barbeque or ribs; and red meat in stir‐fried and other mixed dishes. Salty snacks included potato, corn, or tortilla chips; crackers; pretzels; and popcorn. Desserts included white and chocolate doughnuts, cookies, cakes, brownies, candy, pastries, Pop‐Tarts, Chinese desserts, Mexican desserts, pies, pudding, custard, and flan. Fruits and vegetables included peaches, apricots, nectarines, plums, cantaloupe, mango, papaya, strawberries, blueberries, other berries, apples, applesauce, pears, bananas, plantains, oranges, grapefruit, tangerines, kiwi, dried fruits, tossed salad (with spinach, Romaine, or dark greens), cooked spinach, turnip greens, collards, broccoli, cabbage, cauliflower, Brussels sprouts, sauerkraut, kimchi, carrots, winter squash, acorn squash, sweet potatoes, yams, green beans, peas, snow peas, squash, zucchini, asparagus, and tomatoes. MET indicates metabolic equivalent; BMI, body mass index; FA, fatty acid.
In multivariate analyses adjusting for sociodemographics, lifestyle, and dietary factors, higher concentrations of plasma phospholipid 15:0 were associated with younger age, white race, lower BMI, and nonsmoking status at baseline (Table S2). Among self‐reported dietary factors, plasma phospholipid 15:0 was most strongly associated with consumption of high‐fat dairy products (Figure). For example, each additional daily serving of regular cheese, whole‐fat milk, and butter was associated with a 44%, 11%, and 23% SD increase in 15:0 concentrations, respectively. A generally similar pattern was observed for 14:0, but with much smaller differences in circulating levels for a similar self‐reported intake of dairy products. In contrast, greater consumption of margarine, French fries, and bakery desserts was associated with higher concentrations of trans‐16:1n7, suggesting that circulating phospholipid concentrations of this fatty acid in the MESA cohort were mostly derived from food sources of industrialized trans‐fat. When we evaluated overall estimated dairy fat consumption, each additional g/day was associated with 3% SD increase in 15:0, 2% SD increase in 14:0, and no increase in trans‐16:1n7. Consistently, we observed stronger partial correlations between high‐fat dairy products and 15:0 compared with other dairy subgroups (Table S1). We found no major differences in correlations with dairy foods across race/ethnicity groups, except for a higher correlation between dairy and trans‐16:1n7 in Chinese Americans and a higher correlation between butter intake and 15:0 in whites (data not shown).
Figure 1.
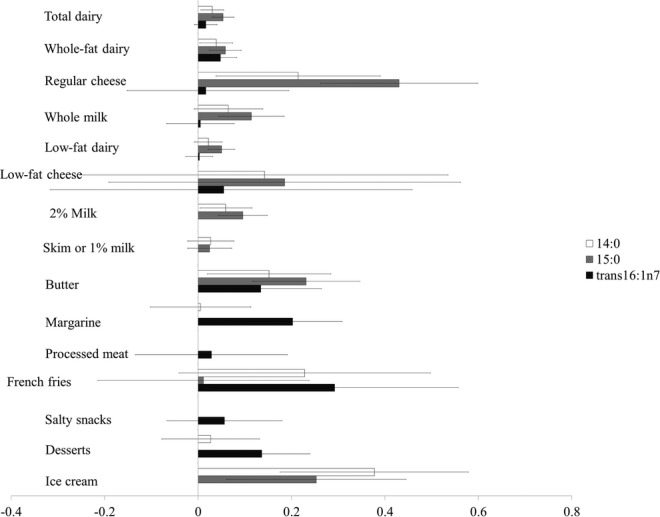
Multiadjusted differences in circulating markers of dairy according to 1 serving per day of selected food intakes in the Multi‐Ethnic Study of Atherosclerosis. Bars represent multivariate‐adjusted differences and 95% CI in plasma phospholipid fatty acid levels associated with each higher serving per day intake for selected foods, expressed as SD‐units (1 SD=0.08%, 0.05%, and 0.03% difference in 14:0, 15:0, and trans‐16:1n7 fatty acid levels, respectively). Error bars represent 95% CI. Multivariate‐adjusted models included field center, age (years), sex, race/ethnicity (non‐Hispanic whites, blacks, Hispanics, and Chinese Americans), education (<high school, high school, >high school), cigarette smoking (never, current, or former smokers and pack‐years of cigarette smoking), alcohol (g/day), physical activity (active and inactive leisure in metabolic equivalents per minute/week), body mass index (kg/m2), diabetes at baseline (no, impaired fasting glucose, yes), weekly use of dietary supplement (yes, no), energy intake (kcal/day), fiber intake (g/day), unprocessed meat (serving/day), fruits and vegetables, and mutual adjustment for all groups presented. Whole‐fat dairy included whole milk, cheese, and whole‐fat yogurt. Low‐fat dairy included 2% milk, 1%/skim milk, cottage or ricotta cheese, and low‐fat yogurt. Processed meats included ham, hot dogs, bologna, salami, lunchmeats, liver, sausage, chorizo, scrapple, and bacon. Unprocessed red included meat hamburger; cheeseburger; meatloaf; hash; beef, pork, or lamb steaks; roasts; barbeque or ribs; and red meat in stir‐fried and other mixed dishes. Salty snacks included potato, corn, or tortilla chips; crackers; pretzels; and popcorn. Desserts included white and chocolate doughnuts, cookies, cakes, brownies, candy, pastries, PopTarts, Chinese desserts, Mexican desserts, pies, pudding, custard, and flan. Fruits and vegetables included peaches, apricots, nectarines, plums, cantaloupe, mango, papaya, strawberries, blueberries, other berries, apples, applesauce, pears, bananas, plantains, oranges, grapefruit, tangerines, kiwi, dried fruits, tossed salad (with spinach, Romaine, or dark greens), cooked spinach, turnip greens, collards, broccoli, cabbage, cauliflower, Brussels sprouts, sauerkraut, kimchi, carrots, winter squash, acorn squash, sweet potatoes, yams, green beans, peas, snow peas, squash, zucchini, asparagus, and tomatoes.
Cross‐sectional Associations of Dairy Fatty Acid Biomarkers With CVD Risk Factors
In multivariable‐adjusted analyses, higher plasma phospholipid 15:0 and trans‐16:1n7 levels were significantly associated with lower plasma triglycerides and lower measures of both systolic and diastolic blood pressures (Table 2). In contrast, higher 14:0 was positively associated with plasma triglycerides, blood pressure, and total:HDL cholesterol, which has been suggested to be an useful predictor of CHD events.31 Positive association between 14:0 and triglycerides suggests this fatty acid originated at least in part from endogenous synthesis, which is influenced by excessive intake of dietary carbohydrates. Higher plasma phospholipid trans‐16:1n7 was also associated with higher LDL cholesterol levels in MESA. No cross‐sectional associations were observed between dairy‐fat markers and HDL cholesterol. Additional adjustment for potential dietary confounders did not materially change cross‐sectional associations of dairy fatty acids and CVD risk factors.
Table 2.
Multivariable‐Adjusted Cross‐sectional Associations of Dairy Plasma Phospholipid With CVD Risk Factors in the Multi‐Ethnic Study of Atherosclerosis (N=2837)
| Quintiles of Plasma Phospholipid Fatty Acids | P Trend* | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 14:0 | ||||||
| Median, % of total fatty acids | 0.17 | 0.21 | 0.25 | 0.3 | 0.38 | |
| Systolic BP, mm Hg* | 125.4 (0.8) | 126.2 (0.8) | 124.2 (0.7) | 126.2 (0.9) | 128.5 (0.9) | 0.02 |
| Diastolic BP, mm Hg* | 71.7 (0.4) | 71.6 (0.4) | 71.3 (0.4) | 72.4 (0.4) | 72.7 (0.4) | 0.03 |
| Triglycerides, mg/dL* | 117.9 (2.9) | 130.2 (4) | 134.0 (2.9) | 149 (3.8) | 152.7 (4.9) | <0.0001 |
| HDL‐C, mg/dL* | 50.8 (0.5) | 50.9 (0.5) | 50.1 (0.5) | 50.5 (0.5) | 50.7 (0.6) | 0.77 |
| LDL‐C, mg/dL* | 115.5 (1.2) | 118.5 (1.4) | 116.8 (1.3) | 116.6 (1.3) | 117.7 (1.4) | 0.58 |
| Total:HDL‐C* | 4.0 (0.1) | 4.1 (0.1) | 4.1 (0.1) | 4.2 (0.1) | 4.2 (0.1) | <0.01 |
| CRP, mg/L* | 3.6 (0.3) | 3.9 (0.3) | 3.4 (0.2) | 3.5 (0.2) | 3.6 (0.2) | 0.54 |
| 15:0 | ||||||
| Median, % of total fatty acids | 0.12 | 0.14 | 0.16 | 0.19 | 0.24 | |
| Systolic BP, mm Hg* | 129.1 (0.8) | 126.5 (0.8) | 125.3 (0.7) | 124.6 (0.8) | 123.9 (0.9) | <0.0001 |
| Diastolic BP, mm Hg* | 73.3 (0.4) | 71.9 (0.4) | 71.7 (0.4) | 71.6 (0.4) | 70.7 (0.4) | <0.0001 |
| Triglycerides, mg/dL* | 146.4 (3.7) | 137.9 (4.3) | 135.9 (3.6) | 130.3 (3.5) | 124.5 (3.6) | <0.01 |
| HDL‐C, mg/dL* | 50.6 (0.5) | 51.1 (0.5) | 49.8 (0.5) | 50.9 (0.5) | 50.7 (0.6) | 0.89 |
| LDL‐C, mg/dL* | 117.2 (1.3) | 116.6 (1.3) | 117.4 (1.2) | 117.8 (1.4) | 115.9 (1.4) | 0.64 |
| Total:HDL‐C* | 4.14 (0.1) | 4.1 (0.1) | 4.2 (0.1) | 4.1 (0.1) | 4 (0.1) | 0.22 |
| CRP, mg/L* | 3.5 (0.2) | 4.1 (0.3) | 3.4 (0.2) | 3.5 (0.2) | 70.9 (0.3) | 0.66 |
| trans‐16:1n7 | ||||||
| Median, % of total fatty acids | 0.03 | 0.04 | 0.06 | 0.07 | 0.1 | |
| Systolic BP, mm Hg* | 126.7 (0.8) | 127.3 (0.8) | 126.2 (0.8) | 123.7 (0.9) | 125.2 (0.9) | 0.03 |
| Diastolic BP, mm Hg* | 72.7 (0.4) | 72.6 (0.4) | 71.5 (0.4) | 71 (0.5) | 71.2 (0.4) | <0.01 |
| Triglycerides, mg/dL* | 147.8 (3.5) | 139.9 (3.7) | 133.5 (3.3) | 128.3 (4) | 121.7 (4.1) | <0.0001 |
| HDL‐C, mg/dL* | 50.5 (0.5) | 51.5 (0.5) | 49.9 (0.5) | 50.7 (0.7) | 50.2 (0.6) | 0.35 |
| LDL‐C, mg/dL* | 113 (1.2) | 118 (1.2) | 118.9 (1.4) | 118.6 (1.5) | 118.0 (1.5) | 0.04 |
| Total:HDL‐C* | 4.06 (0.1) | 4.1 (0.1) | 4.2 (0.1) | 4.1 (0.1) | 4.1 (0.1) | 0.91 |
| CRP, mg/L* | 3.6 (0.2) | 3.4 (0.2) | 3.7 (0.3) | 3.4 (0.3) | 3.7 (0.3) | 0.67 |
Values are mean (SE) determined using linear regression with robust variance estimators. Means are adjusted for field center, age (years), sex, race/ethnicity (non‐Hispanic whites, blacks, Hispanics and Chinese Americans), education (<high school, high school, >high school), cigarette smoking (never, current or former smokers, and pack‐years of cigarette smoking), alcohol (g/day), physical activity (metabolic equivalents per minute/week), prevalent diabetes (yes, no) alcohol intake (g/day) and BMI (kg/m2). CVD indicates cardiovascular disease; BP, blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; BMI, body mass index; CRP, C‐reactive protein.
Tests for trend across quintiles were performed by assigning each participant the median fatty acid value in their quintile and modeling this variable as a continuous term.
Included adjustment for antihypertensive medication use at baseline (yes/no).
Included adjustment for lipid‐lowering medication use at baseline (yes/no).
Included adjustment for anti‐inflammatory medication at baseline (yes/no).
Prospective Associations of Dairy Fatty Acid Biomarkers With Incident CVD
During 19 778 person‐years of follow‐up between 2000 and 2010, 189 new CVD cases were diagnosed among participants included in our analyses. In multivariate models adjusting for sociodemographics, lifestyle, and dietary factors, each higher SD‐unit of plasma phospholipid 15:0 (SD=0.05% difference in total fatty acid concentration) was associated with a 19% lower risk of CVD (HR 0.81, 95% CI 0.68 to 0.98) (Table 3). This association was similar after further simultaneous adjustment for plasma 14:0 and trans‐16:1n7 (HR 0.76, 95% CI 0.61 to 0.93). This association was also not appreciably altered by further adjustment for estimated dietary intakes of calcium, phosphorous, vitamin D, carbohydrate, and protein or for total plasma phospholipid trans‐fatty acids (data not shown). When circulating 15:0 was evaluated continuously, associations were similar to those of quintile analyses, especially HRs for extreme quintiles, which are less likely to be influenced by exposure misclassification. Associations were similar across the 4 race/ethnicity groups (HR [95% CI] of CVD for each SD‐unit increase of 15:0 [model 2]: 0.78 [0.58 to 1.05] in whites, 0.69 [0.45 to 1.07] in blacks, 0.89 [0.63 to 1.26] in Hispanics, and 0.85 [0.45 to 1.61] in Chinese Americans). Similarly, associations did not differ between women (HR 0.80, 95% CI 0.60 to 1.08) and men (HR 0.81, 95% CI 0.64 to 1.02). In contrast, plasma phospholipid 14:0 and trans‐16:1n7 were not associated with incident CVD. Associations of trans‐16:1n7 were similar after further adjustment for other sources of trans‐fat in this cohort, such as bakery desserts, salty snacks, and French fries (HR [95% CI] across quintiles of trans‐16:1n7 were 1.04 [0.63 to 1.72], 1.30 [0.80 to 2.14], 1.35 [0.80 to 2.27], and 0.99 [0.58 to 1.70]; P‐trend=0.86; HR [95% CI] for 1‐SD increase in trans‐16:1n7: 0.97 [0.82 to 1.13]).
Table 3.
Incidence of CVD According to Plasma Phospholipid FA Biomarkers of Dairy Fat Consumption in the Multi‐Ethnic Study of Atherosclerosis (N=2837; 189 New Cases)
| HR (95% CI) According to Sex‐Specific Quintiles of Each FA Biomarker | P Trend | HR (95% CI) for 1–SD‐Unit Difference in FA Concentrations | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| 14:0 | |||||||
| Median, % total FA | 0.17 | 0.21 | 0.26 | 0.30 | 0.38 | ||
| Person‐years of follow‐up | 4272 | 4089 | 4249 | 3390 | 3571 | ||
| Incident cases, n | 38 | 35 | 42 | 38 | 36 | ||
| Multivariate adjusted | 1.0 | 0.98 (0.62 to 1.57) | 1.26 (0.80 to 1.98) | 1.18 (0.73 to 1.89) | 1.06 (0.65 to 1.74) | 0.67 | 1.02 (0.87 to 1.20) |
| Multivariate and plasma FA adjusted | 1.0 | 1.06 (0.66 to 1.70) | 1.44 (0.90 to 2.29) | 1.40 (0.86 to 2.28) | 1.44 (0.84 to 2.46) | 0.12 | 1.14 (0.96 to 1.36) |
| 15:0 | |||||||
| Median, % total FA | 0.11 | 0.14 | 0.16 | 0.19 | 0.24 | ||
| Person‐years of follow‐up | 4460 | 3738 | 4196 | 3637 | 3540 | ||
| Incident cases, n | 45 | 31 | 48 | 38 | 27 | ||
| Multivariate adjusted | 1.0 | 0.71 (0.45 to 1.14) | 0.93 (0.60 to 1.44) | 0.85 (0.53 to 1.37) | 0.55 (0.32 to 0.95) | 0.06 | 0.81 (0.68 to 0.98) |
| Multivariate and plasma FA adjusted | 1.0 | 0.69 (0.43 to 1.11) | 0.86 (0.55 to 1.37) | 0.78 (0.47 to 1.28) | 0.47 (0.25 to 0.86) | 0.03 | 0.76 (0.61 to 0.93) |
| trans‐16:1n7 | |||||||
| Median, % total FA | 0.03 | 0.04 | 0.06 | 0.07 | 0.09 | ||
| Person‐years of follow‐up | 4970 | 4404 | 3833 | 2801 | 3563 | ||
| Incident cases, n | 34 | 35 | 46 | 39 | 35 | ||
| Multivariate adjusted | 1.00 | 1.05 (0.64 to 1.74) | 1.27 (0.78 to 2.08) | 1.36 (0.81 to 2.28) | 0.99 (0.58 to 1.69) | 0.88 | 0.97 (0.82 to 1.13) |
| Multivariate and plasma FA adjusted | 1.00 | 1.13 (0.68 to 1.87) | 1.47 (0.89 to 2.42) | 1.61 (0.95 to 2.74) | 1.22 (0.70 to 2.11) | 0.35 | 1.03 (0.88 to 1.22) |
CVD events consist of myocardial infarction (n=69), resuscitated cardiac arrest (n=5), definite (n=51) and probable (n=20) angina (if followed by revascularization), stroke (n=43), and other atherosclerotic death (n=1); 1 SD=0.08%, 0.05%, and 0.03% difference in 14:0, 15:0, and trans‐16:1n7 FA levels, respectively. Multivariate‐adjusted model included field center, age (years), sex, race/ethnicity (non‐Hispanic whites, blacks, Hispanics, and Chinese Americans), education (<high school, high school, >high school), cigarette smoking (never, current, or former smokers and pack‐years of cigarette smoking), alcohol (g/day), physical activity (active and inactive leisure in metabolic equivalents per minute/week), low‐ and whole‐fat dairy (servings/day), processed and unprocessed meat (servings/day), total energy intake (kcal/day), fiber (g/day), and fruits and vegetables (servings/day). Multivariate‐ and plasma FA–adjusted model included all variables listed plus mutual adjustment for 14:0, 15:0, and trans‐16:1n7. CVD indicates cardiovascular disease; HR, hazard ratio; FA, fatty acid.
Prospective Associations of Dairy Fatty Acid Biomarkers With Incident CHD
A total of 146 new CHD cases were diagnosed during follow‐up. Plasma phospholipid 15:0 was inversely associated with risk of CHD (Table 4). For example, each SD‐unit of 15:0 was associated with a 26% lower CHD risk (HR 0.74, 95% CI 0.60 to 0.92). No associations were seen between plasma phospholipid 14:0 or trans‐16:1n7 and incident CHD.
Table 4.
Incidence of CHD According to Plasma Phospholipid FA Biomarkers of Dairy Fat Consumption in the Multi‐Ethnic Study of Atherosclerosis (N=2837; 146 New Cases)
| HR (95% CI) According to Sex‐Specific Quintiles of Each FA Biomarker | P Trend | HR (95% CI) for 1–SD‐Unit Difference in FA Concentrations | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| 14:0 | |||||||
| Median, % total FA | 0.17 | 0.21 | 0.26 | 0.30 | 0.38 | ||
| Person‐years of follow‐up | 4309 | 4113 | 4271 | 3416 | 3602 | ||
| Incident cases, n | 25 | 27 | 33 | 30 | 26 | ||
| Multivariate adjusted | 1.0 | 1.16 (0.67 to 2.02) | 1.48 (0.86 to 2.53) | 1.36 (0.78 to 2.37) | 1.13 (0.63 to 2.03) | 0.68 | 1.01 (0.84 to 1.21) |
| Multivariate and plasma FA adjusted | 1.0 | 1.29 (0.74 to 2.25) | 1.76 (1.02 to 3.06) | 1.70 (0.95 to 3.02) | 1.66 (0.87 to 3.14) | 0.10 | 1.15 (0.94 to 1.41) |
| 15:0 | |||||||
| Median, % total FA | 0.11 | 0.14 | 0.16 | 0.19 | 0.24 | ||
| Person‐years of follow‐up | 4493 | 3748 | 4232 | 3680 | 3557 | ||
| Incident cases, n | 36 | 25 | 32 | 29 | 19 | ||
| Multivariate adjusted | 1.0 | 0.68 (0.40 to 1.15) | 0.68 (0.40 to 1.14) | 0.70 (0.41 to 1.20) | 0.41 (0.22 to 0.78) | 0.01 | 0.74 (0.60 to 0.92) |
| Multivariate and plasma FA adjusted | 1.0 | 0.66 (0.39 to 1.12) | 0.63 (0.37 to 1.09) | 0.64 (0.36 to 1.14) | 0.35 (0.17 to 0.72) | 0.01 | 0.70 (0.54 to 0.89) |
| trans‐16:1n7 | |||||||
| Median, % total FA | 0.03 | 0.04 | 0.06 | 0.07 | 0.09 | ||
| Person‐years of follow‐up | 4987 | 4451 | 3859 | 2836 | 3577 | ||
| Incident cases, n | 29 | 24 | 38 | 23 | 27 | ||
| Multivariate adjusted | 1.0 | 0.82 (0.46 to 1.45) | 1.12 (0.65 to 1.92) | 0.83 (0.45 to 1.52) | 0.82 (0.45 to 1.49) | 0.63 | 0.91 (0.75 to 1.10) |
| Multivariate and plasma FA adjusted | 1.0 | 0.90 (0.51 to 1.59) | 1.34 (0.77 to 2.33) | 1.04 (0.56 to 1.94) | 1.06 (0.57 to 1.97) | 0.67 | 0.99 (0.82 to 1.20) |
CHD events consist of myocardial infarction (n=69), resuscitated cardiac arrest (n=5), definite (n=51) and probable (n=20) angina (if followed by coronary revascularization), and CHD death (n=1); 1 SD=0.08%, 0.05%, and 0.03% difference in 14:0, 15:0, and trans‐16:1n7 FA levels, respectively. Model 1: field center, age (years), sex, race/ethnicity (non‐Hispanic whites, blacks, Hispanics, and Chinese Americans), education (<high school, high school, >high school), cigarette smoking (never, current or former smokers, and pack‐years of cigarette smoking), alcohol (g/day), physical activity (active and inactive leisure in metabolic equivalents per minute/week), low‐ and whole‐fat dairy (servings/day), processed and unprocessed meat (servings/day), total energy intake (kcal/day), fiber (g/day), and fruits and vegetables (servings/day). Model 2: Model 1+mutual adjustment for 14:0, 15:0, and trans‐16:1n7. CHD indicates coronary heart disease; HR, hazard ratio; FA, fatty acid.
Sensitivity Analyses
Findings were generally similar in sensitivity analyses excluding probable and definite angina (Table S3). For example, in the multivariable model, plasma 15:0 was inversely associated with incident CVD (HR [95% CI] for each SD of 15:0 were 0.80 [0.64 to 0.99]) and CHD (0.77 [0.59 to 1.01]). These associations were slightly attenuated and no longer statistically significant after further simultaneous adjustment for 14:0 and trans‐16:1n7 (HR [95% CI] for each SD of 15:0 was 0.82 [0.64 to 1.05] for CVD and 0.82 [0.61 to 1.12] for CHD).
Influence of Potential Mediators
Additional adjustment for factors that could be potential mediators or confounders, including BMI, prevalent diabetes, lipid‐lowering medication use, LDL cholesterol levels, and antihypertensive medication use, did not materially change the findings. For example, each 1–SD‐unit difference in 15:0 was associated with 24% lower risk of CVD (HR 0.76, 95% CI 0.61 to 0.95) after adjusting for these mediators. Associations also remained unchanged in sensitivity analysis excluding participants with diabetes at baseline (data not shown).
Discussion
In this large prospective study among multiethnic US adults, we found that higher plasma phospholipid 15:0 was cross‐sectionally associated with lower blood pressure and lower plasma triglycerides and prospectively associated with lower incidence of CVD and CHD events. Conversely, plasma phospholipid 14:0 and trans‐16:1n7 were not associated with CVD risk. These findings were similar among men and women and among whites, Hispanics, blacks, and Asians. Associations were robust to adjustment for a variety of sociodemographic, lifestyle, and dietary factors, in addition to other dairy fatty acid biomarkers.
Among the biomarkers evaluated, 15:0 appeared to be the better biomarker of dairy fat in MESA, which is consistent with prior studies.8,32 We observed largest differences in plasma 15:0 in association with consumption of whole‐fat dairy foods such as butter, cheese, and whole‐fat milk; more modest differences with low‐fat cheese; and no differences with consumption of low‐fat or skim milk, suggesting that plasma 15:0 proportions are specific to dairy fat intake rather than overall dairy consumption. In contrast, plasma phospholipid 14:0 and trans‐16:1n7 were weakly associated with dairy fat intake in our cohort. And 14:0 can be endogenously synthesized, so plasma phospholipid concentrations of this fatty acid may be influenced by metabolic and genetic factors in addition to dairy intake.12 In MESA, trans‐16:1n7 was associated with consumption of foods likely to contain partially hydrogenated oils, suggesting it was more a biomarker of trans‐fat rather than of dairy fat intake.
Our findings do not support an adverse association of dairy fat consumption, as reflected by plasma phospholipid 15:0, with incident CVD or CHD. In MESA, dairy fat consumption could be a marker of other factors that benefit cardiovascular health, such as lower BMI, lower meat consumption, less smoking, and lower prevalence of diabetes. On the other hand, 15:0 remained independently associated with lower cardiovascular risk after multivariable adjustment for these factors. The relatively modest correlations between circulating 15:0 and dairy foods may reflect less‐than‐optimal dietary assessment using self‐reported measures. On the other hand, plasma phospholipid concentrations are not influenced by dietary intakes alone but rather by biologic processes related to fatty acid absorption and incorporation into lipid fractions. Thus, it is possible that plasma phospholipid 15:0 is a biomarker of other, unknown metabolic processes, rather than diet, that favorably influence CVD risk. However, this fatty acid is not known to be synthesized by humans.
Could 15:0 itself have protective effects on CVD risk? Besides being substrates for energy production and storage, fatty acids are major components of membrane and organelle structure and function, are natural ligands of nuclear receptors that regulate gene expression, and serve as precursors for an array of bioactive lipid metabolites. Although relative concentrations of this fatty acid in phospholipids are less that 1% of total fatty acids measures, we are increasingly aware that very small amounts of molecules that have regulatory functions can be important to physiology. For example, small increases in n‐3 polyunsaturated fatty acid concentrations have been shown to alter cellular and organelle membrane fluidity, improving protein function and signaling events.33 The mechanisms through which circulating 15:0 may influence CVD have not been established; however, it is possible that small changes in circulating levels of this fatty may be biologically relevant. On the other hand, the observed associations with 15:0 could also represent benefits of other components present in dairy fat or dairy foods in general. Phospholipid 15:0 was most strongly associated with intakes of whole‐fat dairy foods rather than with dairy foods in general. Associations were also unchanged after adjustment for estimated dietary calcium, vitamin D, and phosphorous. Nonetheless, given the complex correlations and interactions among diverse bioactive components present in foods, the associations observed may reflect beneficial effects of dairy fats or dairy foods as a whole, rather than its components in isolation. Our findings strongly support the need to further investigate the biological effects of dairy fat, dairy foods, and circulating 15:0 in the development of CVD.
The inverse cross‐sectional associations we found between plasma phospholipid 15:0 and blood pressure are consistent with previous observational studies of self‐reported total dairy and low‐fat dairy intake,1,3,34 as well as blood pressure–lowering effects of diet patterns rich in low‐fat dairy.6 Investigators in previous studies have emphasized the role of nonfat dairy components such as protein, lactopeptides, and minerals, but the mechanisms underlying these associations remain unclear. The cross‐sectional associations observed in our study support a potential blood pressure–lowering effect of dairy fat.
Our prospective findings using objective biomarkers are consistent with findings for self‐reported saturated fat intake in MESA.26 Saturated fat from dairy, but not from other sources, was associated with lower CVD incidence (per 5 g/day of saturated fat from dairy; HR 0.79, 95% CI 0.68 to 0.92).26 To date, only 3 prospective and 1 retrospective study have evaluated associations between dairy fat biomarkers and CVD events. A recent nested case‐control study including 2424 cases and 4930 controls found no association of plasma 15:0 or 14:0 with CHD risk (OR [95% CI] 0.98 [0.89 to 1.09] and 0.97 [0.87 to 1.08] for 1–SD‐unit increase in plasma 15:0 and 14:0, respectively); however, this study did not include adjustment for dietary confounders.14 Another nested case‐control study including 444 cases and 556 controls in Sweden found no significant association between serum 15:0 and risk of myocardial infarction (OR [95% CI] for 1–SD‐unit: 0.94 [0.72 to 1.24] and 0.56 [0.30 to 1.04] in men and women, respectively).9 Similar findings were seen in a retrospective case‐control study evaluating 15:0 proportions in adipose tissue and risk of nonfatal MI in Costa Rica (OR [95% CI] for extreme quintiles: 0.95 [0.72 to 1.25]).13 A nested case‐control analysis in the Nurses’ Health Study showed a positive association between concentrations of plasma phospholipid 15:0 and risk of CHD (HR [95% CI] for extreme tertiles: 2.36 [1.16 to 4.78]).8 In contrast, plasma 15:0 was associated with a lower risk of incident heart failure in the Atherosclerosis Risk in Communities Study (HR [95% CI] for extreme quintiles: 0.62 [0.38 to 1.02]; P‐trend=0.04); no significant associations were seen for plasma phospholipid 14:0.15 Overall, these prior studies demonstrate mixed results, with most finding no harmful associations of dairy fat biomarkers. Our work builds upon and extends these previous observations by providing prospective evidence from a population‐based study using objective measures of dairy fat in a multiethnic US population.
Our study has several strengths. The use of plasma phospholipid fatty acids provided objective biomarkers of dairy fat intake. The prospective design of our study established temporality and minimized the possibility of reverse causation. The use of a cohort minimized potential selection bias. The ethnic diversity of MESA allowed for study of a greater range of dietary behaviors and susceptibility to disease outcomes than more homogeneous cohorts. Dairy intake in our cohort was similar to that of the US population.35 Consistency in findings for men and women and across race/ethnicity groups increased confidence in the validity and generalizability of our findings. Finally, multivariate adjustment for demographic, lifestyle, and dietary factors minimized the influence of confounding.
Our study also had potential limitations. Associations with CVD risk factors were cross‐sectional, limiting inference on cause‐and‐effect relationships; however, these associations were consistent with prospective analysis of CVD events in our study. Both laboratory and biologic (ie, related to changes over time) imprecision in measurement of dairy biomarkers would result in misclassification of exposure. Laboratory imprecision was largest at low concentrations (eg, quintiles 1 and 2) but would not materially affect comparisons of participants across extreme quintiles. It was not possible to evaluate potential influence of changes in dairy intake over time in our cohort, which would be likely to be random in respect to CVD incidence and could attenuate associations with circulating fatty acids. Our study may be statistically underpowered to assess moderate differences in associations, especially by race/ethnicity. Finally, although we carefully adjusted for several confounders, residual confounding, in particular by healthy lifestyle factors associated with dairy intake, may have biased associations with 15:0 away from the null.
In conclusion, our results show an inverse association between plasma phospholipid 15:0, a biomarker of dairy fat, and CVD risk factors and CVD incidence. Our data support the need for further investigation of CVD effects of dairy fat, dairy‐specific fatty acids, and dairy products in general.
Sources of Funding
Dr. Otto was supported by the Harvard‐Bunge Fellowship Program in Nutrition & Health. Dr. Mozaffarian was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (R21 HL109924).
Disclosures
Dr. Otto was supported by an unrestricted educational grant from Bunge LLC. Dr Mozaffarian reported receiving research grants from GlaxoSmithKline, Sigma Tau, Pronova, and the National Institutes of Health for an investigator‐initiated, not‐for‐profit clinical trial of fish oil and postsurgical complications; ad hoc travel reimbursement and/or honoraria from the International Life Sciences Institute, Aramark, Unilever, SPRIM, and Nutrition Impact for research presentations on diet and cardiometabolic diseases; ad hoc consulting fees from Foodminds; and royalties from UpToDate for an online chapter on fish oil. The other authors report no conflicts.
Acknowledgments
Authors are thankful to other investigators, staff, and participants of the MESA for their important contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
References
- 1.Alonso A, Beunza JJ, Delgado‐Rodriguez M, Martinez JA, Martinez‐Gonzalez MA. Low‐fat dairy consumption and reduced risk of hypertension: the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2005; 82:972-979 [DOI] [PubMed] [Google Scholar]
- 2.Alonso A, Nettleton JA, Ix JH, de Boer IH, Folsom AR, Bidulescu A, Kestenbaum BR, Chambless LE, Jacobs DR., Jr Dietary phosphorus, blood pressure, and incidence of hypertension in the Atherosclerosis Risk in Communities Study and the Multi‐Ethnic Study of Atherosclerosis. Hypertension. 2010; 55:776-784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engberink MF, Hendriksen MA, Schouten EG, van Rooij FJ, Hofman A, Witteman JC, Geleijnse JM. Inverse association between dairy intake and hypertension: the Rotterdam Study. Am J Clin Nutr. 2009; 89:1877-1883 [DOI] [PubMed] [Google Scholar]
- 4.Iso H, Stampfer MJ, Manson JE, Rexrode K, Hennekens CH, Colditz GABS, Speizer FE, Willett WC. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke. 1999; 30:1772-1779 [DOI] [PubMed] [Google Scholar]
- 5.Sauvaget C, Nagano J, Allen N, Grant EJ, Beral V. Intake of animal products and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Int J Epidemiol. 2003; 32:536-543 [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health, National Heart, Lung, and Blood Institute Your guide to lowering your blood pressure with DASH NIH Publication No 06‐4082. 1998
- 7.Soedamah‐Muthu SS, Ding EL, Al‐Delaimy WK, Hu FB, Engberink MF, Willett WC, Geleijnse JM. Milk and dairy consumption and incidence of cardiovascular diseases and all‐cause mortality: dose‐response meta‐analysis of prospective cohort studies. Am J Clin Nutr. 2011; 93:158-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Q, Ma J, Campos H, Hu FB. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr. 2007; 86:929-937 [DOI] [PubMed] [Google Scholar]
- 9.Warensjo E, Jansson JH, Cederholm T, Boman K, Eliasson M, Hallmans G, Johansson I, Sjogren P. Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case‐control study. Am J Clin Nutr. 2010; 92:194-202 [DOI] [PubMed] [Google Scholar]
- 10.Wolk A, Vessby B, Ljung H, Barrefors P. Evaluation of a biological marker of dairy fat intake. Am J Clin Nutr. 1998; 68:291-295 [DOI] [PubMed] [Google Scholar]
- 11.Micha R, King IB, Lemaitre RN, Rimm EB, Sacks F, Song X, Siscovick DS, Mozaffarian D. Food sources of individual plasma phospholipid trans fatty acid isomers: the Cardiovascular Health Study. Am J Clin Nutr. 2010; 91:883-893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratnayake WM, Galli C. Fat and fatty acid terminology, methods of analysis and fat digestion and metabolism: a background review paper. Ann Nutr Metab. 2009; 55:8-43 [DOI] [PubMed] [Google Scholar]
- 13.Aslibekyan S, Campos H, Baylin A. Biomarkers of dairy intake and the risk of heart disease. Nutr Metab Cardiovasc Dis. 2012; 22:1039-1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khaw KT, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC‐Norfolk prospective study. PLoS Med. 2012; 9:e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamagishi K, Nettleton JA, Folsom AR. Plasma fatty acid composition and incident heart failure in middle‐aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008; 156:965-974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002; 156:871-881 [DOI] [PubMed] [Google Scholar]
- 17.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995; 41:264-270 [PubMed] [Google Scholar]
- 18.Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega‐3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006; 52:2265-2272 [DOI] [PubMed] [Google Scholar]
- 19.Lamprea‐Montealegre JA, Astor BC, McClelland RL, de Boer IH, Burke GL, Sibley CT, O'Leary D, Sharrett AR, Szklo M. CKD, plasma lipids, and common carotid intima‐media thickness: results from the Multi‐Ethnic Study of Atherosclerosis. Clin J Am Soc Nephrol. 2012; 7:1777-1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertoni AG, Whitt‐Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi‐Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009; 169:444-454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross‐Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999; 8:805-813 [DOI] [PubMed] [Google Scholar]
- 22.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data‐based approach to diet questionnaire design and testing. Am J Epidemiol. 1986; 124:453-469 [DOI] [PubMed] [Google Scholar]
- 23.Mayer‐Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S. Validity and reproducibility of a food frequency interview in a Multi‐Cultural Epidemiology Study. Ann Epidemiol. 1999; 9:314-324 [DOI] [PubMed] [Google Scholar]
- 24.Nettleton JA, Steffen LM, Mayer‐Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DR., Jr Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2006; 83:1369-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nettleton JA, Rock CL, Wang Y, Jenny NS, Jacobs DR., Jr Associations between dietary macronutrient intake and plasma lipids demonstrate criterion performance of the Multi‐Ethnic Study of Atherosclerosis (MESA) food frequency questionnaire. Br J Nutr. 2009; 102:1220-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Oliveira Otto MC, Mozaffarian D, Kromhout D, Bertoni AG, Sibley CT, Jacobs DR, Jr, Nettleton JA. Dietary intakes of saturated fat by food source and incident cardiovascular disease: the Multi‐Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2012; 96:397-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nettleton JA, Polak JF, Tracy R, Burke GL, Jacobs DR., Jr Dietary patterns & incident cardiovascular disease in the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009; 90:647-654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008; 358:1336-1345 [DOI] [PubMed] [Google Scholar]
- 29.Folsom AR, Kronmal RA, Detrano RC, O'Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima‐media thickness in the prediction of cardiovascular disease incidence: the Multi‐Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008; 168:1333-1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989; 8:551-561 [DOI] [PubMed] [Google Scholar]
- 31.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta‐analysis of 60 controlled trials. Am J Clin Nutr. 2003; 77:1146-1155 [DOI] [PubMed] [Google Scholar]
- 32.Smedman AE, Gustafsson IB, Berglund LG, Vessby BO. Pentadecanoic acid in serum as a marker for intake of milk fat: relations between intake of milk fat and metabolic risk factors. Am J Clin Nutr. 1999; 69:22-29 [DOI] [PubMed] [Google Scholar]
- 33.Mozaffarian D, Wu JH. Omega‐3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011; 58:2047-2067 [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle‐aged and older women. Hypertension. 2008; 51:1073-1079 [DOI] [PubMed] [Google Scholar]
- 35.Beydoun MA, Gary TL, Caballero BH, Lawrence RS, Cheskin LJ, Wang Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am J Clin Nutr. 2008; 87:1914-1925 [DOI] [PMC free article] [PubMed] [Google Scholar]


