Abstract
Background
Extracorporeal membrane oxygenation (ECMO) unloads the heart, providing a bridge to recovery in children after myocardial stunning. ECMO also induces stress which can adversely affect the ability to reload or wean the heart from the circuit. Metabolic impairments induced by altered loading and/or stress conditions may impact weaning. However, cardiac substrate and amino acid requirements upon weaning are unknown. We assessed the hypothesis that ventricular reloading with ECMO modulates both substrate entry into the citric acid cycle (CAC) and myocardial protein synthesis.
Methods and Results
Sixteen immature piglets (7.8 to 15.6 kg) were separated into 2 groups based on ventricular loading status: 8‐hour ECMO (UNLOAD) and postwean from ECMO (RELOAD). We infused into the coronary artery [2‐13C]‐pyruvate as an oxidative substrate and [13C6]‐L‐leucine as an indicator for amino acid oxidation and protein synthesis. Upon RELOAD, each functional parameter, which were decreased substantially by ECMO, recovered to near‐baseline level with the exclusion of minimum dP/dt. Accordingly, myocardial oxygen consumption was also increased, indicating that overall mitochondrial metabolism was reestablished. At the metabolic level, when compared to UNLOAD, RELOAD altered the contribution of various substrates/pathways to tissue pyruvate formation, favoring exogenous pyruvate versus glycolysis, and acetyl‐CoA formation, shifting away from pyruvate decarboxylation to endogenous substrate, presumably fatty acids. Furthermore, there was also a significant increase of tissue concentrations for all CAC intermediates (≈80%), suggesting enhanced anaplerosis, and of fractional protein synthesis rates (>70%).
Conclusions
RELOAD alters both cytosolic and mitochondrial energy substrate metabolism, while favoring leucine incorporation into protein synthesis rather than oxidation in the CAC. Improved understanding of factors governing these metabolic perturbations may serve as a basis for interventions and thereby improve success rate from weaning from ECMO.
Keywords: amino acids, congenital heart disease, extracorporeal circulation, metabolism, pediatrics
Introduction
Extracorporeal membrane oxygenation (ECMO) offers a route to recovery after myocardial stunning and damage sustained during cardiovascular surgery in infants and children.1 Approximately 2% to 5% of children undergoing surgery for congenital heart defects with cardiopulmonary bypass require subsequent support by ECMO.2 Additional indications for cardiac support by ECMO include ventricular arrest and myocarditis. The majority of these patients undergoing ECMO for cardiac indications are infants and young children older than neonates and the Extracorporeal Life Support Organization (ELSO) database shows that these numbers are rising.1 Veno‐arterial ECMO for cardiac support unloads the ventricles, supports the systemic blood pressure, and maintains blood oxygenation.
Adversely, ventricular volume unloading by the ECMO circuit promotes an inflammatory response, which disrupts hormonal homeostasis and induces metabolic stress.3–4 Despite these stresses, the immature heart maintains metabolic flexibility and protein synthesis under ECMO conditions.5 However, weaning from ECMO reintroduces cardiac work requirements. Though many patients tolerate initial weaning, mortality in the subsequent 30 days remains high in part due to inability to adequately sustain cardiac output.6 The myocardial energy requirements and metabolic shifts associated with weaning are unknown but may play an important role in determining cardiac functional recovery. Furthermore, appropriate nutritional support may be critical in maintaining cardiac function after weaning particularly in the immature heart, which must also sustain normal growth. Carbohydrates and fatty acids (FAs) represent the primary energy substrates used by the heart. However, to a lesser extent amino acids, which can be shuttled into protein synthesis, are also used as sources for ATP production in the citric acid cycle (CAC).7 In fact, amino acids contribute carbons CAC intermediates both through oxidative pathways and anaplerosis. Thus, amino acids provide integration between oxidative metabolism and protein synthesis. The branched chain amino acid, leucine, in particular regulates protein turnover in the heart,8 and also behaves as a ketogenic species broken down to acetyl‐coenzyme A (CoA) and acetoacetyl‐CoA.
We tested the hypothesis that ventricular reloading after ECMO increases energy requirements and modulates both substrate entry into the CAC and myocardial protein synthesis. Elucidation of these modifications could identify potential therapeutic targets for sustaining adequate cardiac function after weaning. In this study we used an established immature piglet model, which emulates ECMO and weaning in infants and children supported for cardiac indications.5 We used 13‐Carbon (13C)–labeled substrates as well as nuclear magnetic resonance (NMR) and gas chromatography/mass spectroscopy (GC‐MS) methods to track metabolism and protein synthesis.
Materials and Methods
Animal Models
Sixteen male Yorkshire piglets (body weight 7.8 to 15.6 kg, age 27 to 42 days) were used for the experiments and were divided into 2 groups based on ventricular loading status: 8 hours of ECMO (UNLOAD, n=6, 2 cases were excluded for coronary artery anomaly and severe anemia) and postwean from ECMO (RELOAD, n=8). They were fasted overnight with free access to water and were premedicated with an intramuscular injection of ketamine (33 mg/kg) and xylazine (2 mg/kg). After intubation through surgical tracheostomy, the piglets were mechanically ventilated with an oxygen and isoflurane (1% to 2%) mixture. All experimental procedures were approved by Seattle Children's Institutional Animal Use and Care Committee.
After median sternotomy, a flow probe was placed around the ascending aorta to measure cardiac output (TS420, Transonic Systems Inc). A 5Fr high‐fidelity micromanometer was used to measure pressure within the LV body via apex. Coronary venous flow and myocardial oxygen consumption (MVO2) were measured via a shunt created between the coronary sinus and the superior vena cava.5,9–10 We used a miniaturized extracorporeal circuit consisting of a roller peristaltic pump console (Sarn8000 Terumo) and a hollow fiber membrane oxygenator (CX‐RX05RW, Terumo). The circuit was primed with about 80 mL containing dextran 40 in 0.9% sodium chloride, 5% dextrose and 2000 units of heparin. The ascending aorta and right atrium were cannulated to create a veno‐arterial ECMO circuit. Management during ECMO kept the pump flow rates of 80 to 100 mL/kg per minute. We maintained a pH of 7.35 to 7.45, an arterial Pco2 of 35 to 45 mm Hg, an arterial Po2 of >120 mm Hg, and a rectal temperature of 36 to 37.5°C. ECMO duration time was 8 hours. Perfusion flow of ECMO was decreased gradually for 30 minutes and then ECMO was weaned. After 1.5 hours of ECMO wean, all parameters were measured.
Infusion of Labeled Substrates
[13C6, 15N]‐L‐leucine (3.7 mmol/L), as an indicator of protein synthesis and amino acid oxidation, was infused into coronary artery with [2‐13C]‐pyruvate (7.4 mmol/L) as an alternate substrate (Sigma all 99% enriched) as previously described.5 A 24‐gauge BD Saf‐T‐Infusion catheter (Becton Dickinson) was advanced just distal to the origin of the first branch from the left anterior descending coronary artery (LAD). The 13C‐labeled substrates were infused directly into the LAD for the final 60 minutes of the protocol. Intracoronary concentration of infusing labeled substrates was decided, based on coronary artery flow. Immediately upon completion of the 13C‐labeled substrates infusion, portions of LV tissue perfused by the LAD were quickly freeze‐clamped and stored at −80°C for later extraction.
Metabolic Analyses by Nuclear Magnetic Resonance (NMR)
13C‐NMR was performed on the myocardium for determination of specific carbon glutamate labeling.11 LV extracts were prepared using perchloric acid as previously described.10 13C‐NMR spectra were acquired on a Varian Direct Drive (VNMRS) 600 MHz spectrometer (Varian Inc) equipped with a Dell Precision 390 Linux workstation running VNMRJ 2.2C. The spectrometer system was outfitted with a Varian triple resonance salt‐tolerant cold probe with a cold carbon preamplifier. Protons were decoupled with a Waltz decoupling scheme. Final spectra were obtained using a 45° excitation pulse (7.05 μs at 58 dB), with an acquisition time of 1.3 seconds, a recycle delay of 3 seconds, and a spectral width of 224.1 ppm. Fourier‐transformed spectra were fitted with commercial software (NUTS, Acorn NMR Inc). The labeled carbon resonances (C3 to C5) of glutamate were integrated and values used as starting parameters for the CAC analysis‐fitting algorithm tcaCALC (kindly provided by the Advanced Imaging Research Center at the University of Texas, Southwestern). This algorithm provided the fractional contribution for each substrate to the acetyl‐CoA pool (FC) entering the CAC. Label from exogenously administrated [2‐13C]‐pyruvate gives rise to label in [1‐13C]‐acetyl‐CoA. [13C6, 15N]‐L‐leucine gives [2‐13C] and [1,2‐13C]‐acetyl‐CoA (Figure 1).5 Methanol‐extracted metabolites from tissue samples were also analyzed using 1H‐NMR. A standard 1‐dimensional proton nuclear Overhauser effect spectroscopy (NOESY) with presaturation (TNNOESY) was collected on each sample, using the Chenomx standard data collection protocol12: a nonselective 90° excitation pulse (≈7 μs at 53 dB), a mixing time of 100 ms, acquisition time of 4 seconds, a recycle delay of 1 second, a sweep width of 12 ppm, and temperature control set to 25°C. Collected spectra were analyzed using Chenomx software version 7.1 (Chenomx Inc), with quantifications based on spectral intensities relative to 0.5 mmol/L 2,2‐dimethyl‐2‐silapentane‐5‐sulfonate, which was added as a spike to each sample.
Figure 1.
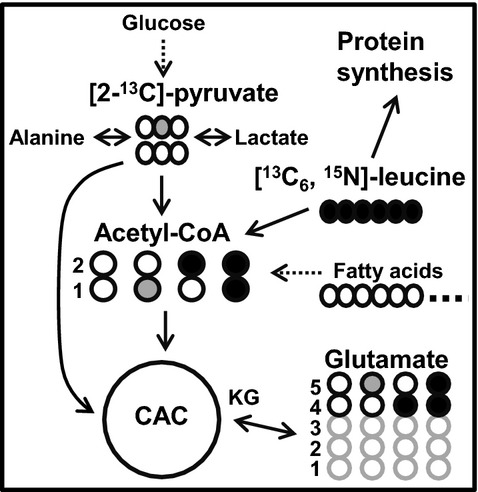
Schematic demonstrating the 13C‐labeling of acetyl‐CoA and glutamate with [13C6, 15N]‐L‐leucine and [2‐13C]‐pyruvate as substrates at the end of first turn of the CAC. Filled black circles=13C from [13C6, 15N]‐L‐leucine; filled gray circles=13C from [2‐13C]‐pyruvate; open circles=12C. FC, fraction of acetyl‐CoA enriched in 13C. The arrow with a dotted line, sourced from endogenous substrates including fatty acids. CAC indicates citric acid cycle; FC, fractional substrate contribution; KG, ketoglutarate.
Metabolic Analyses by Gas Chromatography‐Mass Spectrometry (GC‐MS)
GC‐MS was performed to measure the 13C‐enrichment and concentrations of CAC intermediates, pyruvate, lactate and intracellular free leucine enrichment as described previously.10,13–14 GC‐MS data are reported as the 13C‐molar percent enrichment (MPE) and absolute quantity of each metabolite. A detailed description for the calculations of flux ratios has also been published previously.5,10–11 Mass isotopomers of metabolites containing 1 to n 13C‐labeled atoms were identified as Mi, with i=1, 2, … n. The pathway from pyruvate entering into the CAC via acetyl‐CoA is called pyruvate decarboxylation (PDC). PDC flux rate relative to citrate synthase (CS) was determined using the following formula:
| 1 |
where M1 is the 13C‐MPE in M1 isotopomers.
Absolute PDC flux rate was calculated using the stoichiometric relationship between myocardial oxygen consumption (MVO2) and citrate formation from pyruvate. According to equations by Panchal et al and Vincent et al,13,15 1 μmol of consumed O2 results in the formation of 0.333 μmol of citrate from carbohydrates. Absolute PDC flux for each piglet was calculated using PDC/CS derived from GC‐MS and experimentally determined MVO2 where:
| 2 |
Protein fractional synthesis rate (FSR) was calculated following the methods of Jaleel et al16 Briefly, the isotopic enrichment of [13C6, 15N]‐L‐leucine in the proteins (Epro) and the free tissue fluid (Etf) were used with the following equation:
| 3 |
where t is the time of tracer incorporation, in hours (1 hour for all groups).
Statistical Analyses
Reported values are means±standard error (SE) in figures, text, and tables. The statistical differences between 2 groups were determined using Mann–Whitney U test. Analyses of variance (ANOVA) for repeated measures were used where appropriate (eg, time related changes during ECMO in RELOAD). Significant differences from baseline value in Table 1 and Figure 2 in individual groups were estimated by paired t test if ANOVA indicated significant differences. Criterion for significance was P<0.05 for all comparisons.
Table 1.
Parameters of Cardiac Function
| UNLOAD (n=6) | RELOAD (n=8) | ||||
|---|---|---|---|---|---|
| Baseline | ECMO | Baseline | ECMO | WEAN | |
| Hemoglobin, g/dL | 9.3±1.4 | 7.7±0.1* | 9.6±0.1 | 7.6±0.1* | 7.6±0.4* |
| HR, BPM | 104±11 | 119±33 | 110±3 | 108±5 | 130±6*,† |
| Mean SBP, mm Hg | 65±3 | 51±4 | 62±2 | 53±2 | 51±3 |
| Pulse P, mm Hg | 20±4 | 4±1* | 25±4 | 8±2* | 18±3† |
| LVEDP, mm Hg | 8.5±2.7 | 4.8±2.1* | 6.0±0.6 | 3.7±0.2* | 7.5±0.6† |
| dP/dtmax, mm Hg/s | 954±186 | 567±128* | 911±99 | 632±114 | 906±148† |
| dP/dtmin, mm Hg/s | −1157±140 | −604±174* | −1680±103 | −741±66* | −1044±70*,† |
| MVO2, μmol/min per g | 2.84±0.23 | 2.00±0.19* | 2.67±0.17 | 1.92±0.38* | 3.37±0.25*,† |
Values are means±SE. ECMO indicates extracorporeal membrane oxygenation; HR, heart rate; BPM, beats per minute; Mean SBP, mean systemic blood pressure; Pulse P, pulse pressure; LVEDP, left ventricular end‐diastolic pressure; dP/dt, first derivative of LV pressure; MVO2, myocardial oxygen consumption; SE, standard error.
P<0.05 vs baseline,
P<0.05 vs UNLOAD at ECMO.
Figure 2.
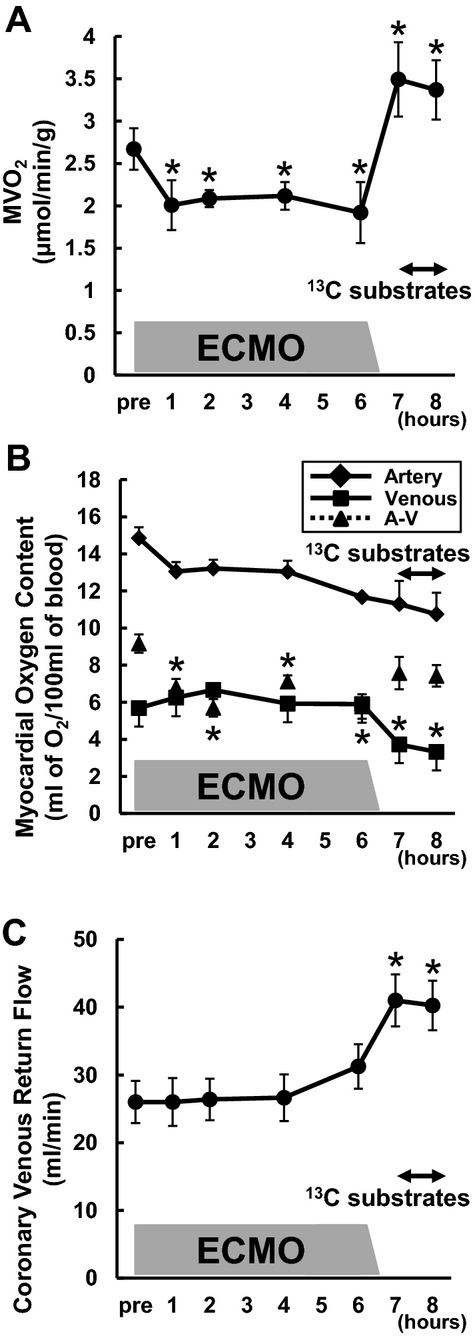
Myocardial oxygen consumption rate (MVO2) in RELOAD. The time‐course of changes in MVO2 (A), oxygen content in the coronary vessels (B), and coronary flow (C). MVO2 was markedly decreased with ventricular unloading (*P<0.05 vs baseline), and increased more than baseline by RELOAD (*P<0.05 vs baseline). Substrate infusion itself did not lead to change MVO2. Moreover difference between arterial and venous oxygen content was significantly decreased during ECMO, whereas coronary flow was significantly increased by RELOAD (*P<0.05 vs baseline). Values are means±SE; n=8. ECMO indicates extracorporeal membrane oxygenation; SE, standard error; A‐V, difference between arterial and venous oxygen content.
Results
Cardiac Function
This study was designed to determine the metabolic shifts that were promoted with reloading after a period of unloading. The metabolic studies required termination of the animals with tissue extraction. Therefore, substrate oxidation and protein synthesis data from the 2 different conditions, ECMO and reloading, could not be obtained in individual animals. However, full set hemodynamic data at multiple time points were available in individual animals, which completed the protocol through reloading. The serial time data for UNLOAD and RELOAD in Table 1 show that unloading by ECMO markedly reduces the hemodynamic parameters. The systolic parameters in RELOAD are reestablished to approximate baseline values indicating successful weaning (WEAN). In particular the pulse pressure, an index of volume loading, is reduced by more than 70% by ECMO and reestablished with WEAN. Diastolic function or ventricular relaxation as indexed by minimum dP/dt was impaired at ≈60% baseline during reloading. The baseline data are similar for both experimental groups, and the UNLOAD hemodynamic data also illustrate that ECMO reduces all the hemodynamic parameters to a similar extent as occurs in the RELOAD group. Hemoglobin, as expected, is reduced by ECMO in both groups, but remains constant through weaning in the RELOAD as these animals did not receive any blood transfusions. Thus, the data establish that metabolic differences between UNLOAD and RELOAD are not caused by hemodynamic differences between the 2 experimental groups at baseline or during ECMO, but are elicited by the WEAN.
Myocardial Oxygen Consumption
Data in Figure 2 illustrate the oxygen cost for successful reloading and reestablishment of hemodynamic parameters, which are similar to baseline. ECMO induces a substantial reduction in MVO2 largely due to a drop in O2 extraction (A‐V), which then remains constant over the term, as the ECMO circuit maintains coronary flow (Figure 2B and 2C). WEAN at hour 6 induces an abrupt and marked increase in MVO2 in RELOAD secondary to an increase coronary blood flow with the reestablishment of normal circulation. The MVO2 at WEAN then exceeds the baseline values by ≈35%. Substrate infusion over the final hour of the protocol did not lead to further change in MVO2. Thus the metabolic cost in terms of oxygen consumption ≈35% baseline value for successful reestablishment of systolic function, although accompanied by diastolic impairment.
RELOAD Alters Myocardial Energy Metabolism
The principal objective for this study was to determine the substrate preferences and oxidation rates associated with weaning after ECMO. Prior studies in this experimental model showed that ECMO alone altered FC for pyruvate and leucine or 13C‐MPE for the CAC intermediates when compared to control loading conditions under normal circulation.5 The effect of RELOAD after ECMO on 13C‐NMR spectra is illustrated in Figure 3, where the differences are apparent in the C5 complex between UNLOAD and RELOAD. The summarized data (Figure 4) show that RELOAD exhibits a near 50% reduction in FC‐pyruvate along with a comparable increase in FC from endogenous unlabeled substrate. The relatively minor FC‐leucine is similar between the 2 groups.
Figure 3.
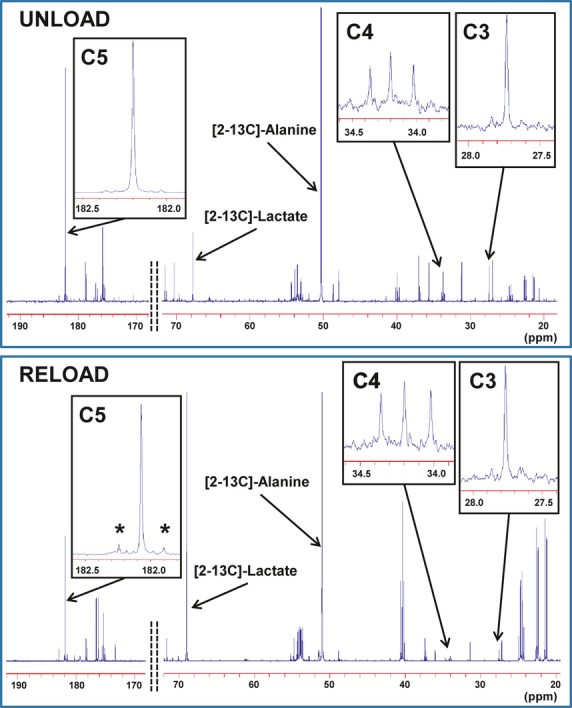
Typical 13C‐NMR spectra obtained from left ventricular extract after a 60‐minute infusion of [2‐13C]‐pyruvate and [U‐13C]‐leucine into the left anterior descending coronary artery under UNLOAD and RELOAD conditions. The spectra show adequate signal to noise ratio for peak integration. Chemical shifts in parts per million (ppm) were as follows: C3, 27.7; C4, 34.2; C5 of glutamate, 182.2; [2‐13C]‐alanine, 51.9 and [2‐13C] lactate, 69.2. Marked differences occur in glutamate peak complexes between the 2 conditions. C5 in RELOAD shows prominently increased doublet peak area (*), indicating increased leucine contribution or decreased pyruvate contribution, compared to UNLOAD. Additionally, the greater labeled lactate to alanine ratio can be observed in RELOAD. NMR inidcates nuclear magnetic resonance.
Figure 4.
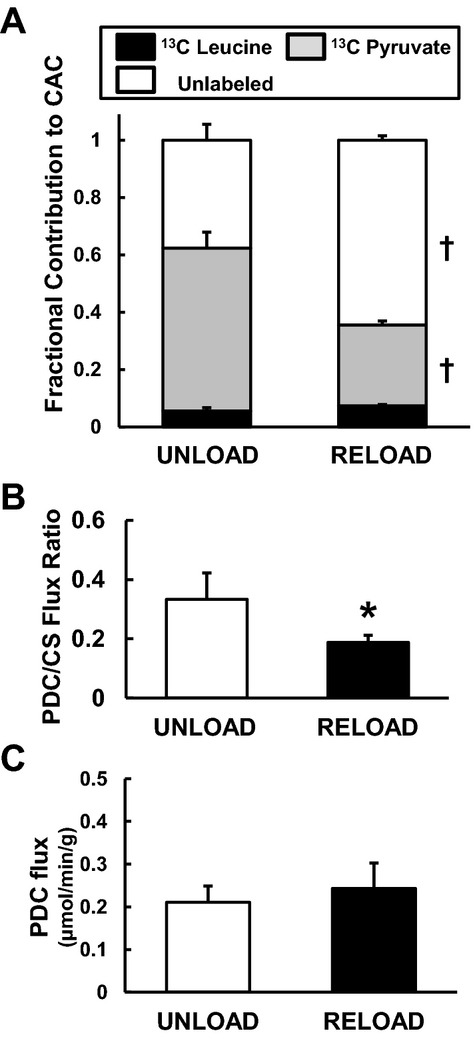
Pyruvate decarboxylation (PDC). A, Fractional substrate contribution (FC) to acetyl‐CoA by 13C‐NMR from left ventricular tissues at the end of the protocol. FC‐pyruvate significantly decreased and FC of unlabeled substrates increased, while FC‐leucine maintained with RELOAD when compared to UNLOAD animals. B, PDC‐to‐citrate synthase (CS) flux ratio by GC‐MS. RELOAD significantly decreased PDC/CS. C, Absolute PDC flux calculated by MVO2 and PDC/CS. Values are means±SE; n=6 to 8 per group. *P<0.05, †P<0.01 compared with UNLOAD. NMR inidcates nuclear magnetic resonance; GC‐MS, gas chromatography/mass spectroscopy; MVO2, myocardial oxygen consumption rate; SE, standard error; CAC, citric acid cycle.
Despite the lower FC‐pyruvate in RELOAD, the GC‐MS data show that the abundance of pyruvate within the myocardium is dramatically higher in RELOAD compared to UNLOAD along with the elevated pyruvate MPE (Figure 5). Absolute lactate levels determined by both GC‐MS (Figure 5) and 1H‐NMR (Figure 6) are lower in RELOAD, while the concentration for alanine, another potential product of pyruvate metabolism, is similar between the 2 groups. The PDC/CS ratio, an index of overall pyruvate flux through pyruvate dehydrogenase including labeled and unlabeled substrate and estimated by GC‐MS, was substantially lower in RELOAD (18.8±0.2%) compared to UNLOAD (33.3±0.9%) (Figure 4B). Estimation of absolute PDC flux, calculated by MVO2 and PDC/CS, showed no significant difference in RELOAD (0.24±0.06 μmol/minute) compared to UNLOAD (0.21± 0.04 μmol/minute) (Figure 4C).
Figure 5.
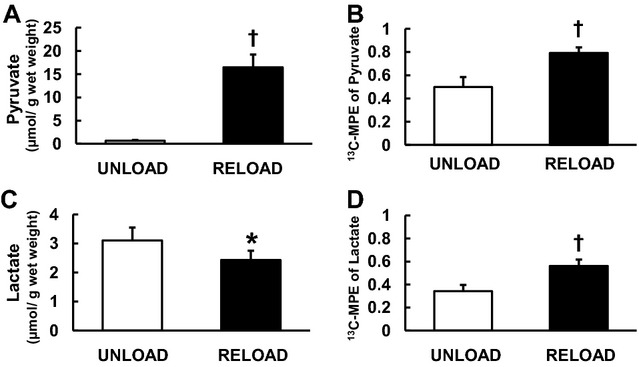
Absolute quantity and 13C‐molar percent enrichment (MPE) of pyruvate and lactate by GC‐MS. Absolute pyruvate concentration (A) markedly increased in RELOAD. Moreover RELOAD increased 13C‐MPE of pyruvate (B) and lactate (D), and significantly decreased absolute lactate concentration (C) in comparison to UNLOAD. Values are means±SE; n=6 to 8 per group. *P<0.05, †P<0.01 compared with UNLOAD. GC‐MS indicates gas chromatography/mass spectroscopy; SE, standard error.
Figure 6.
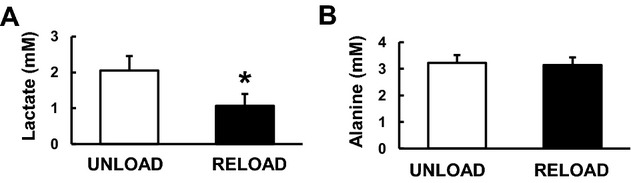
Left ventricular tissue concentration of pyruvate metabolites by 1H‐NMR at the end of the protocol. Lactate concentration (A) decreased with RELOAD when compared to UNLOAD animals, while alanine concentration (B) was not different. Values are means±SE; n=6 to 8 per group. *P<0.05 compared with UNLOAD. NMR indicates nuclear magnetic resonance; SE, standard error.
GC‐MS demonstrated that RELOAD increased absolute levels of all measured CAC intermediates including citrate, α‐ketoglutarate (α‐KG), fumarate, and malate (Figure 7A) by ≈80%. 13C‐MPE of CAC intermediate for the expanded pool by GC‐MS did not change significantly with the exception of α‐KG (55.1±5.6 in UNLOAD, 71.2±2.9 in RELOAD, P<0.05), which showed a significant increase with RELOAD (Figure 7B).
Figure 7.
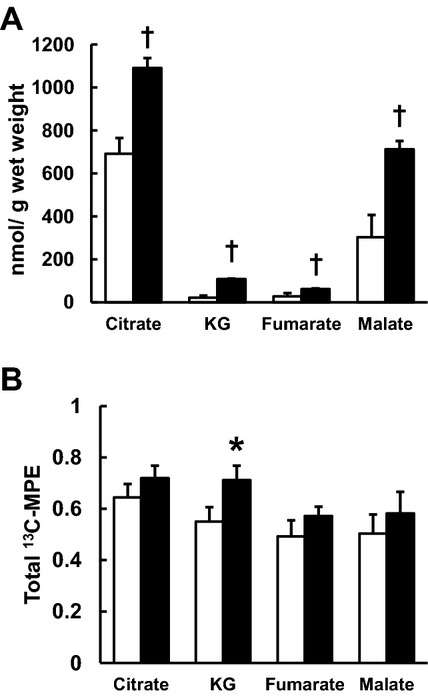
Concentrations and their 13C‐molar percent enrichment (MPE) of CAC intermediates by GC‐MS. A, absolute CAC intermediates concentrations. B, total 13C‐MPE of CAC intermediates. RELOAD increased all absolute CAC intermediates. Moreover RELOAD significantly elevated 13C‐MPE of KG when compared to UNLOAD. Clear column: UNLOAD; black column: RELOAD. Values are means±SE; n=6 to 8 per group. *P<0.05, †P<0.01 compared with UNLOAD. CAC indicates citric acid cycle; GC‐MS, gas chromatography/mass spectroscopy; KG, ketoglutarate; SE, standard error.
RELOAD compared to UNLOAD significantly elevated [NADH]/[NAD+] obtained by 1H‐NMR spectra from LV tissues at the end of the protocol (Table 2). Phosphocreatine [PCr])/[ATP] ratio also tended upwards in RELOAD but did not achieve significance.
Table 2.
Energy Metabolite Ratios by 1H‐NMR Spectra From Left Ventricular Tissues
| UNLOAD | RELOAD | |
|---|---|---|
| [PCr]/[ATP] | 1.36±0.04 | 1.53±0.12 |
| [ADP]/[ATP] | 0.12±0.02 | 0.15±0.02 |
| [NADH]/[NAD+] | 0.09±0.01 | 0.12±0.02* |
Values are means±SE; n=6 to 8 per group. NMR indicates nuclear magnetic resonance; PCr, Phosphocreatine; SE, standard error.
P<0.05 compared with UNLOAD.
RELOAD Alters Myocardial Protein Synthesis
Compared with UNLOAD, RELOAD significantly increased the 13C‐MPE of leucine (74.3±5.8% versus 92.7±1.4%, P<0.01) (Figure 8A). FSR substantially increased ≈2‐fold with RELOAD (Figure 8B). As FC‐leucine did not change with RELOAD (Figure 4), the data suggest that leucine is redirected into protein synthesis rather than oxidative metabolism. Further 1H‐NMR data supporting a shift in amino acid metabolism during RELOAD is shown in Figure 8C. Although the aspartate pool did not change and glutamate decreased by RELOAD, glutamine increased.
Figure 8.
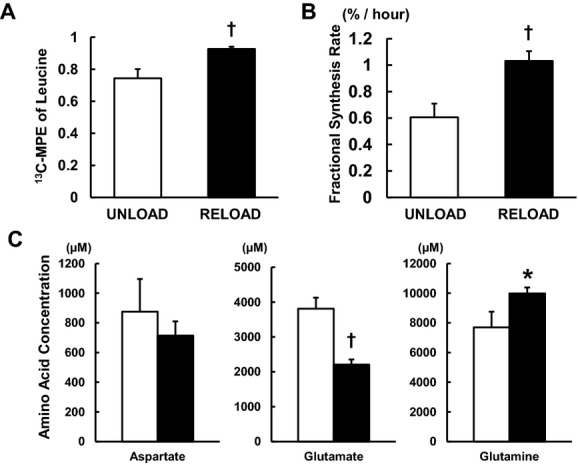
Protein fractional synthesis rates (FSRs) by GC‐MS and amino acid concentration by 1H‐NMR from left ventricular tissues. A, 13C‐MPE of intracellular free leucine. B, FSRs. RELOAD significantly increased 13C‐MPE of leucine, and cardiac FSRs by >75% over UNLOAD. C, Amino acid concentration. Glutamate concentration was decreased, while glutamine was increased in RELOAD. Clear column: UNLOAD; black column: RELOAD. Values are means±SE; n=6 to 8 per group. *P<0.05, †P<0.01 compared with UNLOAD. GC‐MS indicates gas chromatography/mass spectroscopy; NMR, nuclear magnetic resonance; MPE, 13C‐molar percent enrichment; SE, standard error.
Discussion
This study describes the metabolic response promoted by weaning from the circuit in a model emulating clinical ECMO in infants and young children. In a previous study, we showed that ECMO lowered myocardial consumption, as expected, indicating a global decrease in overall oxidative metabolism, including amino acids, while metabolic flexibility was maintained.5 In this study, we demonstrate that weaning from ECMO produces, as expected, an abrupt increase in cardiac work and MVO2, which was associated with shifts in substrate or pathways contribution to tissue pyruvate and acetyl‐CoA formation, as well as to expansion in the CAC intermediate pool. These shifts occur in conjunction with maintained or marginally elevated myocardial energy state, assessed by [PCr]/[ATP] and [NADH]/[NAD+] ratios, and increased protein synthesis. MVO2 with reloading was higher than occurs prior to initiation of ECMO. The systolic hemodynamic parameters with reloading do not generally exceed those observed in the baseline condition and likely do not contribute to the elevation in MVO2 through higher contractile energy requirements. In part, the elevation in MVO2 may be due to altered loading conditions and reduced LV compliance compared to baseline as illustrated by our diastolic parameters. The observed shifts in substrate preference during the reloading phase might also be responsible for these changes in MVO2, particularly as we observed decreased reliance on oxygen‐efficient carbohydrate metabolism at the expense of endogenous sources, presumably FAs. The maintenance of [PCr]/[ATP] during this phase suggests that increased uncoupling of oxidative phosphorylation does not occur during reloading and is not responsible for the difference in MVO2 between baseline and reloading.
Indeed, the labeling strategy used in our experiments produced complementary NMR and GC‐MS data. 13C‐NMR data showed decreased FC‐pyruvate, while GC‐MS showed lower overall PDC/CS ratio and no elevation in the estimated absolute PDC flux during the abrupt work increase with RELOAD. These 2 complementary methods indicate that the substrate preference for acetyl‐CoA formation shifts away from pyruvate dehydrogenase. Additionally, the large increases in total cardiac pyruvate level in conjunction with elevation in its 13C‐MPE suggest that glycolysis or other pathways contribute relatively less to pyruvate formation and PDC during reloading. Combined, these data indicate that alternative oxidative pathways provide the increase in acetyl‐CoA supply to the CAC necessary for oxidation and the increase in cardiac work requirements during reloading. The labeling strategy for leucine also eliminated branched‐chain amino acids as a potential acetyl‐CoA source of energy for the increased work. Thus, through a process of elimination, our data suggest that FAs or ketones provide the oxidative source for meeting the increased energy requirements. This contention is supported by the elevation of [NADH]/[NAD+], previously associated with increased FA or ketone oxidation in the heart in vivo,17–18 as well as prior studies in swine, which showed that abrupt increases in cardiac energy expenditure caused by inotropic dobutamine stimulation are matched by ATP produced via free FA oxidation.17 Further studies using 13C‐labeled free FAs are, however, required to prove this contention, and are currently being performed in our laboratory.
We also identified expansion of the CAC intermediate pool during ventricular reloading. Our prior studies using a similar immature pig model showed that under fully aerobic conditions pyruvate contributed to the CAC primarily through decarboxylation to acetyl‐CoA versus anaplerotic carboxylation to oxaloacetate.9 In this study, we did not assess flux through anaplerotic pyruvate carboxylation, but additional data provide evidence—albeit indirect— indicating that this pathway contributes carbon for the CAC expansion. Our previous study using our pig model showed that ≈20% to 25% of the pyruvate contribution to the CAC occurred through carboxylation during normal load conditions for LV.9 This 4 carbon anaplerotic entry into the CAC occurs through reactions catalyzed by pyruvate carboxylase and/or malic enzyme.19 Elevations in tissue concentration for pyruvate, as noted in our study, favor flux in these reactions relative to pyruvate dehydrogenase.20 Prior studies performed in isolated rat hearts showed that FAs supplied in high concentration in perfusate inhibited pyruvate dehydrogenase and redirected pyruvate through carboxylation, and this was associated with an increase in tissue CAC intermediate level, principally citrate and malate as is observed in this study.13 In addition, increased cardiac work by dobutamine stimulation in mature swine increased FA oxidation and also promoted anaplerotic flux from pyruvate.17 Consistent with our findings, these perturbations, which increase FA oxidation and pyruvate anaplerosis, all promoted expansion of the CAC intermediate pool. Nevertheless, we cannot eliminate a reduction in metabolism of CAC intermediates through nonoxidative pathways as a potential mechanism for the pool expansion.
Limitations
Many of the advantages and limitations of this ECMO model and the 13C isotopomer methods have been previously described in detail.5,9–10 Our principal goal was to define the specific metabolic shifts which are associated with ventricular reloading in a clinical model. Of note, though cardiac systolic function successfully recovered during reloading, diastolic function exemplified by minimum dP/dt remains slightly impaired. It has been suggested that specific limbs of the Frank‐Starling curve are more dependent on glucose metabolism than others.21 Our current piglet model was subjected to metabolic and inflammatory stress induced by ECMO,5 which can cause cardiac stunning and emulate some clinical scenarios such as failure to wean. We did not perform these experiments in a cardiac injury model, although damage caused by ischemia/reperfusion is a primary ECMO indication in the pediatric age group.1 We also delivered supraphysiological intracoronary doses of 13C‐labeled substrates in order to assure acquisition of high‐quality NMR and GC‐MS data. Our prior studies indicated that these doses did not substantially elevate tissue levels for pyruvate and leucine.5,9 Myocardial pyruvate levels did rise substantially during reloading, probably due to the excess availability, and may have affected glucose utilization. Finally, leucine itself promotes protein synthesis in cardiomyocytes in vitro,22 and at high‐dose could potentially stimulate protein synthesis. Our data, reflecting the marked increase in protein synthesis with reloading, demonstrate the myocardial capacity to achieve even higher rates.
Our studies show that the increased workload associated with weaning promoted a near doubling in the FSR. Although, the studies were not designed to determine the mechanism, the data indicate that reloading under these conditions promotes protein synthesis, which should be necessary for physiological growth in the immature heart. The clinical implications for expansion of the CAC intermediate pool remain unclear. Unlike our prior studies,9 the labeling strategy was not specifically designed to determine the source of carbons for this expansion or whether these CAC changes related directly or indirectly to amino acid availability for protein synthesis. We did observe shifts in the glutamate/glutamine pools. Glutamine, which was increased by reloading, is generally considered a promoter of protein synthesis. Interestingly, recent experiments in isolated perfused hearts show that glutamine also stimulates oxidation of FAs,23 which we assume as the major alternate substrate in our model.
Summary and Clinical Implications
Our study identifies metabolic perturbations as potential therapeutic targets for maintaining cardiac contractile function and protein synthesis after ECMO weaning. Specifically, our data show that substrate flux through PDC was not increased during reloading. Successful reestablishment of systolic cardiac function, as occurred in our study, relies on the heart's ability to optimally select substrates for oxidation and limitations in carbohydrate oxidation could adversely affect the ability to reload. Our prior studies showed that stimulating PDC flux through either pyruvate supplementation or with thyroid hormone improves cardiac function after ischemia‐reperfusion injury in these immature pigs.9–10 Future studies are currently ongoing to determine whether these interventions would also be beneficial after ECMO weaning in an injury model.
Sources of Funding
Research reported in this publication was supported the National Heart Lung and Blood Institute of the National Institutes of Health under award number R01HL60666. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A portion of the research was performed using Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory.
Disclosures
None.
References
- 1.Haines NM, Rycus PT, Zwischenberger JB, Bartlett RH, Undar A. Extracorporeal life support registry report 2008: neonatal and pediatric cardiac cases. ASAIO J. 2009; 55:111-116 [DOI] [PubMed] [Google Scholar]
- 2.Salvin JW, Laussen PC, Thiagarajan RR. Extracorporeal membrane oxygenation for postcardiotomy mechanical cardiovascular support in children with congenital heart disease. Paediatr Anaesth. 2008; 18:1157-1162 [DOI] [PubMed] [Google Scholar]
- 3.Liakopoulos OJ, Teucher N, Muhlfeld C, Middel P, Heusch G, Schoendube FA, Dorge H. Prevention of TNFalpha‐associated myocardial dysfunction resulting from cardiopulmonary bypass and cardioplegic arrest by glucocorticoid treatment. Eur J Cardiothorac Surg. 2006; 30:263-270 [DOI] [PubMed] [Google Scholar]
- 4.Portman MA, Slee A, Olson AK, Cohen G, Karl T, Tong E, Hastings L, Patel H, Reinhartz O, Mott AR, Mainwaring R, Linam J, Danzi S. Triiodothyronine supplementation in infants and children undergoing cardiopulmonary bypass (TRICC): a multicenter placebo‐controlled randomized trial: age analysis. Circulation. 2010; 122:S224-S233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Priddy CM, Kajimoto M, Ledee DR, Bouchard B, Isern N, Olson AK, Des Rosiers C, Portman MA. Myocardial oxidative metabolism and protein synthesis during mechanical circulatory support by extracorporeal membrane oxygenation. Am J Physiol Heart Circ Physiol. 2013; 304:H406-H414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shuhaiber J, Thiagarajan RR, Laussen PC, Fynn‐Thompson F, del Nido P, Pigula F. Survival of children requiring repeat extracorporeal membrane oxygenation after congenital heart surgery. Ann Thorac Surg. 2011; 91:1949-1955 [DOI] [PubMed] [Google Scholar]
- 7.Kodde IF, van der Stok J, Smolenski RT, de Jong JW. Metabolic and genetic regulation of cardiac energy substrate preference. Comp Biochem Physiol A Mol Integr Physiol. 2007; 146:26-39 [DOI] [PubMed] [Google Scholar]
- 8.Chua BH. Specificity of leucine effect on protein degradation in perfused rat heart. J Mol Cell Cardiol. 1994; 26:743-751 [DOI] [PubMed] [Google Scholar]
- 9.Olson AK, Hyyti OM, Cohen GA, Ning XH, Sadilek M, Isern N, Portman MA. Superior cardiac function via anaplerotic pyruvate in the immature swine heart after cardiopulmonary bypass and reperfusion. Am J Physiol Heart Circ Physiol. 2008; 295:H2315-H2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson AK, Bouchard B, Ning XH, Isern N, Des Rosiers C, Portman MA. Triiodothyronine increases myocardial function and pyruvate entry into the citric acid cycle after reperfusion in a model of infant cardiopulmonary bypass. Am J Physiol Heart Circ Physiol. 2012; 302:H1086-H1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Des Rosiers C, Lloyd S, Comte B, Chatham JC. A critical perspective of the use of (13)C‐isotopomer analysis by GCMS and NMR as applied to cardiac metabolism. Metab Eng. 2004; 6:44-58 [DOI] [PubMed] [Google Scholar]
- 12.Weljie AM, Newton J, Mercier P, Carlson E, Slupsky CM. Targeted profiling: quantitative analysis of 1H NMR metabolomics data. Anal Chem. 2006; 78:4430-4442 [DOI] [PubMed] [Google Scholar]
- 13.Vincent G, Bouchard B, Khairallah M, Des Rosiers C. Differential modulation of citrate synthesis and release by fatty acids in perfused working rat hearts. Am J Physiol Heart Circ Physiol. 2004; 286:H257-H266 [DOI] [PubMed] [Google Scholar]
- 14.Khairallah M, Labarthe F, Bouchard B, Danialou G, Petrof BJ, Des Rosiers C. Profiling substrate fluxes in the isolated working mouse heart using 13C‐labeled substrates: focusing on the origin and fate of pyruvate and citrate carbons. Am J Physiol Heart Circ Physiol. 2004; 286:H1461-H1470 [DOI] [PubMed] [Google Scholar]
- 15.Panchal AR, Comte B, Huang H, Kerwin T, Darvish A, Des Rosiers C, Brunengraber H, Stanley WC. Partitioning of pyruvate between oxidation and anaplerosis in swine hearts. Am J Physiol Heart Circ Physiol. 2000; 279:H2390-H2398 [DOI] [PubMed] [Google Scholar]
- 16.Jaleel A, Short KR, Asmann YW, Klaus KA, Morse DM, Ford GC, Nair KS. In vivo measurement of synthesis rate of individual skeletal muscle mitochondrial proteins. Am J Physiol Endocrinol Metab. 2008; 295:E1255-E1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma N, Okere IC, Brunengraber DZ, McElfresh TA, King KL, Sterk JP, Huang H, Chandler MP, Stanley WC. Regulation of pyruvate dehydrogenase activity and citric acid cycle intermediates during high cardiac power generation. J Physiol. 2005; 562:593-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DK, Heineman FW, Balaban RS. Effects of beta‐hydroxybutyrate on oxidative metabolism and phosphorylation potential in canine heart in vivo. Am J Physiol. 1991; 260:H1767-H1773 [DOI] [PubMed] [Google Scholar]
- 19.Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, Taegtmeyer H, O'Donnell JM, Lewandowski ED. Substrate‐enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res. 2009; 104:805-812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lancha AH, Jr, Recco MB, Curi R. Pyruvate carboxylase activity in the heart and skeletal muscles of the rat. Evidence for a stimulating effect of exercise. Biochem Mol Biol Int. 1994; 32:483-489 [PubMed] [Google Scholar]
- 21.Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T. Cardiac system bioenergetics: metabolic basis of the Frank‐Starling law. J Physiol. 2006; 571:253-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King N, Suleiman MS. L‐leucine transport in rat heart under normal conditions and effects of a simulated hypoxia. Mol Cell Biochem. 2001; 221:99-108 [DOI] [PubMed] [Google Scholar]
- 23.Lauzier B, Vaillant F, Merlen C, Gelinas R, Bouchard B, Rivard ME, Labarthe F, Dolinsky VW, Dyck JR, Allen BG, Chatham JC, Des Rosiers C. Metabolic effects of glutamine on the heart: anaplerosis versus the hexosamine biosynthetic pathway. J Mol Cell Cardiol. 2013; 55:92-100 [DOI] [PMC free article] [PubMed] [Google Scholar]


