Abstract
Background
Childhood obesity is a significant risk factor for cardiovascular disease in adulthood. Although ventricular remodeling has been reported in obese youth, early tissue‐level markers within the myocardium that precede organ‐level alterations have not been described.
Methods and Results
We studied 21 obese adolescents (mean age, 17.7±2.6 years; mean body mass index [BMI], 41.9±9.5 kg/m2, including 11 patients with type 2 diabetes [T2D]) and 12 healthy volunteers (age, 15.1±4.5 years; BMI, 20.1±3.5 kg/m2) using biomarkers of cardiometabolic risk and cardiac magnetic resonance imaging (CMR) to phenotype cardiac structure, function, and interstitial matrix remodeling by standard techniques. Although left ventricular ejection fraction and left atrial volumes were similar in healthy volunteers and obese patients (and within normal body size‐adjusted limits), interstitial matrix expansion by CMR extracellular volume fraction (ECV) was significantly different between healthy volunteers (median, 0.264; interquartile range [IQR], 0.253 to 0.271), obese adolescents without T2D (median, 0.328; IQR, 0.278 to 0.345), and obese adolescents with T2D (median, 0.376; IQR, 0.336 to 0.407; P=0.0001). ECV was associated with BMI for the entire population (r=0.58, P<0.001) and with high‐sensitivity C‐reactive protein (r=0.47, P<0.05), serum triglycerides (r=0.51, P<0.05), and hemoglobin A1c (r=0.76, P<0.0001) in the obese stratum.
Conclusions
Obese adolescents (particularly those with T2D) have subclinical alterations in myocardial tissue architecture associated with inflammation and insulin resistance. These alterations precede significant left ventricular hypertrophy or decreased cardiac function.
Keywords: CT or MRI, obesity, type 2 diabetes
Introduction
Over the last decade, adolescent obesity has taken on epidemic proportions, with nearly 17% of children aged 2 to 19 years classified as obese.1 Childhood obesity is strongly associated with future cardiovascular risk,2 with elevated body mass index (BMI) and type 2 diabetes in adolescence associated with metabolic syndrome,3–4 and with reduced survival in adulthood.5 Obese children exhibit a cardiometabolic phenotype similar to that seen in adult metabolic syndrome, including left ventricular hypertrophy (LVH), diastolic dysfunction, and increased vascular stiffness,6–7 precursors of future heart failure. These structural and functional changes likely represent an advanced, established stage of disease, in which adverse prognosis and permanent alterations may not be amenable to reversal. Indeed, data from animal models of obesity suggest that early, subtle cardiomyocyte hypertrophy and interstitial matrix expansion (with collagen or advanced glycation end products) may predate organ‐level pathology.8–11 Novel noninvasive imaging techniques to address myocardial tissue‐level phenotypes would afford unique insights into myocardial tissue remodeling not easily accessible by traditional cardiac imaging.
Recently, our group and others have developed and histologically validated T1‐based cardiac magnetic resonance imaging (CMR) techniques to quantify myocardial interstitial matrix expansion (extracellular volume fraction [ECV]).12–13 Postcontrast myocardial T1 is abnormal in adults with diabetes, and correlates with the degree of diastolic dysfunction, inflammation, and insulin resistance.14–15 However, whether these preclinical alterations in myocardial tissue structure exist earlier in the life course of diabetic heart disease (eg, obese pediatric subjects) remains unknown.
To investigate whether obesity is associated with such abnormalities in a pediatric population, we compared cardiac structural, functional, and tissue phenotypes in obese adolescents with and without type 2 diabetes (T2D) with those in normal‐weight, healthy volunteers. We specifically hypothesized that measurements of interstitial matrix expansion would be abnormal in obese adolescents, specifically those with T2D. We also hypothesized that adverse tissue remodeling would be associated with circulating biomarkers of inflammation and insulin resistance, postulated to play central roles in cardiometabolic pathways implicated in subclinical myocardial tissue remodeling.
Methods
Study Population
We prospectively enrolled 21 obese adolescents (age, 15 to 19) from the Massachusetts General Hospital for Children (Boston, MA) and the Boston Children's Hospital (Boston, MA). Obesity was defined by BMI ≥30 kg/m2 or ≥95th percentile for age and sex.16 Patients with a history of hypertension and type 2 diabetes (based on American Diabetes Association criteria17) were included in the study, given their association with adolescent obesity. We excluded patients with (1) contraindications to MRI (eg, metallic hazards or allergy to gadolinium), (2) prior bariatric surgery, (3) creatinine clearance ≤30 mL/min, (4) active pregnancy, (5) body weight >250 kg or waist size >70 cm (limits for our cardiac MRI system), and (6) prior heart disease (eg, repaired/unrepaired congenital, cardiomyopathy, myocardial infarction). We selected adolescents who were postpubertal to limit the effects of physiologic insulin resistance of puberty. Before CMR imaging, patient height, weight, systolic and diastolic blood pressure, and heart rate were collected. A group of 12 healthy, normal‐weight adolescent and young adult volunteers free of any cardiovascular risk factors or clinical disease were collected from Kiel, Germany, and Boston, Massachusetts, and imaged using similar protocols for comparison (age, 15.1±4.5). The institutional review boards of each participating institution approved the study protocol, and all subjects and their parents or guardians (for age <18 years) gave assent and/or informed consent for the study.
CMR Protocol and Analysis
For obese adolescents, CMR imaging was performed on a 3.0‐Tesla scanner (16‐element coil, 70‐cm bore size; Siemens Verio, Siemens, Erlangen, Germany) with vector‐cardiographic gating. No premedication was used. For healthy volunteers, a 3.0‐Tesla scanner (Achieva 3.0T, Philips Medical Systems, the Netherlands) was used with a phased‐array coil for cardiac imaging. In patients younger than 7 years old, sedation with propofol and midazolam was necessary. Cine steady‐state free precession (SSFP) was performed for a stack of short‐axis slices to cover the left and right ventricles (25 phases; TR/TE/flip angle=3.2 ms/1.8 ms/45°; slice thickness=6 mm; 192×170 matrix; FOV ≈340×320 mm; iPAT=2; temporal resolution=35 to 50 ms) and in radial views for left atrial volume assessment.18 Single‐shot SSFP late‐enhancement imaging in the short‐axis orientation with full ventricular coverage was performed 10 minutes after administration of 0.15 mmol/kg intravenous gadolinium‐DPTA (Magnevist; Bayer Healthcare, Wayne, NJ). To assess thoracic aortic arterial stiffness, we measured aortic pulse wave velocity (PWV) via free‐breathing phase‐contrast imaging (gradient echo acquisition; rectangular FOV=27×32 cm; 192×162 matrix; effective temporal resolution ≤12.5 ms; 80 phases per R‐to‐R interval; 3 averages; retrospective ECG gating) of an axial slice intersecting the ascending and descending aorta at the right pulmonary artery level. The distance between the ascending and descending aortic axial slices (for calculation of PWV) was measured on a separate candy‐cane aortic image. All images acquired with breath holding were timed to end‐expiration.
CMR images are analyzed off‐line using specialized postprocessing software (MASS, Medis Research, Leiden, the Netherlands). Standard modified Simpson's methods were used to analyze left ventricular (LV) and right ventricular volumes, mass, and function. Left atrial volumes and function (markers of diastolic dysfunction) were assessed from radial cine views of the left ventricle as described.18 Flow‐versus‐time curves from locations in the ascending and descending thoracic aorta were obtained from phase‐contrast images and analyzed by the cross‐correlation method to determine pulse wave delays. Ventricular volumes and mass were indexed to height.
CMR T1 Measurements
T1 measurements were performed once precontrast and 4 times postcontrast with a breath‐hold Look‐Locker technique.19–20 The segmented gradient‐echo sequence had a temporal resolution of 117 ms for precontrast T1 measurements and 55 ms for postcontrast T1 measurements and included a non‐slice‐selective BIR‐4 adiabatic inversion pulse applied after the detection of an R‐wave, followed by a acquisition of segmented k‐space data for 17 cardiac phases, that is, times after inversion (TR/TE/flip angle=5 ms/2.2 ms/10°; slice thickness=8 mm; 192×100 matrix; FOV ≈340×30 to 340 mm; NEX=1; IPAT=2). The flip angle was low (10°) to maintain high sensitivity to T1 changes. The sequence was validated against inversion‐recovery‐prepared spin‐echo measurements, as described previously.21 For healthy volunteers, an identical sequence was used with a temporal resolution of 90 ms precontrast, and 40 ms postcontrast. T1 measurements were made in a single mid–left ventricular slice. Postcontrast T1 measurements started 5 minutes after the injection of gadolinium contrast, with 4 subsequent T1 measurements performed over a period of 30 minutes postcontrast. Endocardial and epicardial contours were drawn in a midventricular slice, taking care to avoid blood pool, using dedicated software (MASS). A region of interest was also placed in the blood pool. The left ventricle was segmented into 6 sectors for analysis. Segmental T1 was derived by exponential fitting of the inversion recovery and correction for radio‐frequency pulse effects. Segmental myocardial R1 (=1/T1) was plotted against blood R1. We subsequently fitted the data for R1 for each myocardial segment as a function of R1 in blood with a 2‐space water‐exchange model of equilibrium transcytolemmal water exchange, originally developed by Landis et al,22 with the slope of the initially linear relation defining a partition coefficient for gadolinium, λGd, for each segment. Myocardial ECV for each segment was calculated by multiplying λGd by (1−hematocrit), and then the 6 myocardial segments were averaged for a final myocardial ECV. We have established that this technique demonstrates a high degree of reliability, with a mean±standard deviation of the test–retest difference of 0.00±0.012 (95% CI, −0.015 to 0.015) over a period of 1 month, with an intraclass correlation coefficient of 0.902.
Biomarker Collection
Blood was collected in a fasting state for assessment of selected biomarkers of inflammation, adiposity, and insulin resistance. A lipid panel was obtained clinically in all obese patients. We assayed markers of systemic inflammation (eg, high‐sensitivity C‐reactive protein, triglycerides), insulin resistance (insulin, homeostatic model assessment of insulin resistance [HOMA‐IR], hemoglobin A1c), adiponectin, and leptin. In addition, we assayed cardiac‐specific markers of hemodynamic stress and fibrosis (N‐terminal pro‐BNP, galectin‐3). Biomarkers were collected in fasting conditions at the time of CMR. Hemoglobin A1c was obtained via review of medical records (median time between hemoglobin A1c and CMR, 101 days; IQR, 51 to 219 days). HOMA‐IR was calculated as fasting insulin (in μU/mL)×fasting glucose (in mg/dL)/405.23 Biomarker assessment was not performed on healthy volunteers in our control population.
Lipid panel analyses (total cholesterol, triglyceride, low‐density lipoprotein cholesterol, and high‐density lipoprotein cholesterol) were performed using direct, automated, enzymatic, colorimetric methods on a Roche P‐Modular system (Roche Diagnostics, Indianapolis, IN). High‐sensitivity C‐reactive protein was measured using an immunoturbidimetric method on a Roche P‐Modular system. Insulin and NT‐proBNP were measured by the electrochemiluminescent immunoassay using Roche reagents on a Roche Modular E170 system. Adiponectin was measured using a latex particle–enhanced immunoturbidimetric assay from MedTestDx (Arlington, VA) on a Roche P‐Modular system. Leptin was analyzed using an enzyme‐linked immunosorbant assay (ELISA) from Mercodia, (Uppsala, Sweden) on a DSX automated immunoassay analyzer from Inova diagnostics (San Diego, CA). Galectin‐3 was measured using a microtiter plate‐based ELISA method form BG Medicine (Waltham, MA).
Statistical Analysis
We powered the study on the basis of preliminary data in healthy adult volunteers (<40 years; mean ECV, 0.252±0.016). In the absence of previous data on the effect of obesity on ECV, we aimed to detect a difference in means of 0.024 units, which corresponds to the difference in ECV between its mean and 95th percentile in normal healthy volunteers.24 With a standard deviation of 0.016, as observed for ECV in volunteers, the assumed effect size is 1.5. This could be deemed a minimal, clinically significant change in ECV. With an effect size of 1.5, the analysis of 21 patients gives >90% power to detect this difference with a type 1 error probability of 0.01. Baseline clinical and CMR characteristics were compared by the Wilcoxon rank‐sum test (for continuous covariates) and the Fisher exact test (for categorical covariates). For the purpose of identifying the association between obesity and tissue remodeling (myocardial ECV), we performed nonparametric Kruskal–Wallis testing across multiple groups (healthy volunteers, obese adolescents without T2D, obese adolescents with T2D), with adjustment of the P values from pairwise post hoc comparisons (Wilcoxon) by the Hochberg method. To clarify possible mechanistic relationships between inflammation and insulin resistance with myocardial tissue phenotypes, we measured Spearman rank‐order correlation coefficients between serum biomarkers and myocardial ECV.
Results
Patient Characteristics
Baseline characteristics of patients stratified by obesity and diabetic status (as defined above) are summarized in Table 1. The mean body mass index was 20.1±3.5 kg/m2 in healthy volunteers, 41.4±6.0 kg/m2 in adolescents with obesity alone, and 42.4±12.1 kg/m2 in adolescents with obesity and diabetes (P<0.0001). The age and sex distributions were similar in all 3 groups. However, obese individuals had a higher median systolic blood pressure (139 mm Hg) than obese diabetics (127 mm Hg), and healthy volunteers (108 mm Hg); Kruskal–Wallis P=0.0005 between all 3 groups. Of the 11 diabetic adolescents, 5 (45%) were on insulin therapy (versus none of the obese nondiabetic patients; P=0.04). Nine of the 11 obese diabetic adolescents (82%) used oral antihyperglycemic therapy (versus 1 nondiabetic; P=0.002). Obese adolescents (particularly those with T2D) had an adverse cardiometabolic and inflammatory profile (Table 2), with abnormalities in high‐sensitivity C‐reactive protein, proatherogenic dyslipidemia, and markers of insulin resistance, dysglycemia, and adiposity. When comparing obese individuals with and without diabetes, there were no significant differences in BMI (Table 1). Obese individuals with T2D had a higher fasting glucose (P=0.0098), a higher serum triglyceride level (P=0.022), a higher galectin‐3 level (P=0.042), and a lower adiponectin level (P=0.027) compared with obese adolescents without T2D.
Table 1.
Baseline Characteristics Stratified by Obesity and Diabetic Status
| Variable | Healthy Volunteers (n=12) | Obesity Alone (n=10) | Obesity+T2D (n=11) | P Value All Patients | P Value Obesity+T2D vs Obesity Alone |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Age, y | 15.1±4.5 | 18.1±3.6 | 17.4±1.4 | 0.2 | 0.7 |
| Female, n (%) | 5 (42) | 5 (50) | 7 (64) | 0.6 | 0.7 |
| Weight, kg | 53.6±15.4 | 120±18.7 | 122±29.8 | <0.0001 | 0.9 |
| Body mass index, kg/m2 | 20.1±3.49 | 41.4±5.99 | 42.4±12.1 | <0.0001 | 0.8 |
| Body surface area, m2 | 1.56±0.31 | 2.28±0.199 | 2.21±0.282 | <0.0001 | 0.7 |
| Systolic blood pressure, mm Hg | 108 [97.8 to 110] | 139 [120 to 150] | 127 [117 to 136] | 0.0005 | 0.003 |
| Hypertension, % | 0 (0%) | 3 (30%) | 3 (27%) | 0.09 | 1 |
| CMR characteristics | |||||
| LV end‐diastolic volume (indexed), mL/m | 77.4 [67.4 to 84.8] | 111 [93.7 to 117] | 87 [81 to 111] | 0.004 | 0.001 |
| LV end‐systolic volume (indexed), mL/m | 30.3 [26.5 to 35.3] | 41.6 [36.7 to 50.2] | 34.8 [32.5 to 38.6] | 0.01 | 0.01 |
| LV ejection fraction, % | 58.7 [56.4 to 60] | 58.7 [57.1 to 60.9] | 58.8 [58.2 to 61.5] | 0.7 | 0.8 |
| LV mass (indexed), g/m | 46.9 [38.6 to 53.3] | 74.6 [64.5 to 80.5] | 66.7 [55.4 to 69.7] | 0.001 | 0.0006 |
| LV mass‐to‐volume ratio | 0.59 [0.537 to 0.711] | 0.689 [0.658 to 0.723] | 0.604 [0.559 to 0.769] | 0.3 | 0.4 |
| RV end‐diastolic volume (indexed), mL/m | 76.9 [71 to 80.4] | 97 [94.1 to 106] | 91 [76.1 to 98.5] | 0.04 | 0.02 |
| RV end‐systolic volume (indexed), mL/m | 37.8 [36 to 41.7] | 48.2 [43.2 to 50.7] | 43.4 [34.6 to 48.6] | 0.1 | 0.09 |
| RV ejection fraction, % | 49 [46.4 to 52] | 49.4 [47.5 to 51.8] | 51.4 [48.6 to 56.7] | 0.5 | 0.8 |
| Maximum left atrial volume, mL | 45.7 [38.3 to 51.6] | 54 [44.4 to 70.9] | 51 [44.5 to 66.8] | 0.4 | 0.9 |
| Myocardial extracellular volume | 0.264 [0.253 to 0.271] | 0.328 [0.278 to 0.345] | 0.376 [0.336 to 0.407] | 0.0001 | 0.03 |
| Aortic pulse wave velocity, m/s | 3.4 [3.21 to 3.58] | 3.01 [2.87 to 3.07] | 3.23 [3.06 to 3.86] | 0.1 | 0.2 |
| Biomarkers | |||||
| Glucose, mg/dL | — | 83 [83 to 88] | 122 [94 to 312] | 0.01 | 0.01 |
| Hemoglobin A1c, % | — | 5.55 [5.30 to 5.60] | 8.0 [6.70 to 10.75] | 0.0004 | 0.0005 |
| HOMA‐IR | — | 3.2 [2.5 to 6.8] | 7.7 [3.85 to 15.5] | 0.1 | 0.1 |
| Triglycerides, mg/dL | — | 96 [80 to 106] | 206 [104 to 322] | 0.02 | 0.02 |
| High‐sensitivity CRP, mg/L | — | 1.7 [1.1 to 6.0] | 8.25 [4.47 to 10.5] | 0.09 | 0.1 |
| Galectin‐3, ng/mL | — | 11.8 [11.0 to 14.6] | 15.5 [14 to 16] | 0.04 | 0.05 |
| Adiponectin, μg/mL | — | 8 [8 to 9] | 5.5 [4.25 to 7.75] | 0.03 | 0.03 |
All volumes are indexed to height. Values are expressed as mean±standard deviation, or median and interquartile range (in brackets) depending on data normality. T2D indicates type 2 diabetes; CMR, cardiac magnetic resonance imaging; LV, left ventricular; RV, right ventricular; HOMA‐IR, homeostatic model assessment of insulin resistance; CRP, C‐reactive protein.
Table 2.
Spearman Correlations Between Biomarkers of Inflammation, Insulin Resistance, Adiposity, Cardiac Stress, and CMR Indices for the Entire Population (Obese and Obese/T2D Pooled)
| Variable | Inflammation | Dysglycemia and Insulin Resistance | Adiposity | Cardiac Stress and Fibrosis | |||||
|---|---|---|---|---|---|---|---|---|---|
| hsCRP | Serum Triglycerides | HOMA‐IR | Fasting Glucose | Hemoglobin A1c | Adiponectin | Leptin | NT‐Pro‐BNP | Gal‐3 | |
| LVEF | 0.18 | 0.28 | 0.15 | −0.03 | 0.22 | −0.39 | −0.08 | −0.11 | 0.08 |
| LVESV index | −0.17 | −0.08 | 0.15 | −0.29 | −0.47* | 0.19 | 0.19 | −0.09 | −0.04 |
| LVEDV index | −0.14 | −0.07 | 0.18 | −0.36 | −0.45* | 0.09 | 0.25 | −0.15 | −0.12 |
| LV mass index | −0.42 | −0.24 | 0.05 | −0.23 | −0.34 | −0.11 | −0.17 | −0.11 | −0.42 |
| LV mass/volume | −0.09 | −0.13 | −0.04 | 0.20 | 0.12 | −0.17 | −0.25 | 0.01 | −0.26 |
| LA volume index | 0.01 | 0.15 | 0.36 | 0.01 | −0.03 | −0.05 | 0.29 | −0.13 | −0.25 |
| Aortic PWV | −0.11 | 0.26 | −0.10 | 0.03 | 0.21 | −0.39 | −0.09 | −0.14 | 0.16 |
| Myocardial ECV | 0.47* | 0.51* | 0.29 | 0.29 | 0.76*** | −0.24 | −0.16 | 0.09 | 0.36 |
Each cell contains the Spearman correlation coefficient between the given CMR index (row) and the biomarker (column). LVESV, LVEDV, LV mass, and LA volume are all indexed to height. CMR indicates cardiac magnetic resonance imaging; T2D, type 2 diabetes; hsCRP, high‐sensitivity C‐reactive protein; HOMA‐IR, homeostatic model assessment of insulin resistance; NT‐pro‐BNP, N‐terminal probrain natriuretic peptide; Gal‐3, galectin‐3; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVEDV, left ventricular end‐diastolic volume; LA, left atrial; PWV, pulse wave velocity; ECV, extracellular volume fraction.
P<0.05,
**P<0.01,
P<0.005.
Standard Markers of Cardiac Structure and Function by CMR
Left and right ventricular function was similar in healthy volunteers and obese patients (Table 1, Figure 1). Left ventricular ejection fraction (LVEF) was indistinguishable between the healthy volunteers (58.7%), obese adolescents without T2D (58.7%), and obese adolescents with T2D (58.8%); P=0.74. When indexed to height, the left ventricular end‐diastolic volume (LVEDVI) was larger in adolescents with obesity alone (111 mL/m) than in adolescents with obesity and T2D (87 mL/m) or healthy volunteers (77.4 mL/m); P=0.004. Height‐indexed left ventricular mass was also larger in obese adolescents (74.6 g/m) compared with obese adolescents with T2D (66.7 g/m) and healthy volunteers (46.9 g/m); P=0.001. Based on threshold echocardiographic values (>95th percentile of normal) to define LVH in children and adolescents (male LV mass >99.8 g/m, female LV mass >81.0 g/m),25 only 1 female (81.9 g/m) and 1 male (103 g/m) had indexed LV mass barely higher than the threshold value. Given differences in systolic blood pressure among the groups and potential confounding by degree of hypertension, we adjusted volumes and mass by systolic blood pressure to compare LV parameters between groups. With adjustment for systolic blood pressure, left ventricular volumes did not differ among the 3 groups. Height‐indexed left ventricular mass corrected for systolic blood pressure was highest in the group with obesity alone. Left ventricular mass‐to‐volume ratio (a marker of concentric LV remodeling) among the 3 groups was not significantly different with and without simultaneous adjustment by blood pressure. Finally, left atrial volume and aortic pulse wave velocity (markers of left atrial pressure overload and vascular stiffness in obese cardiovascular remodeling, respectively) did not significantly differ among healthy volunteers, obese adolescents with T2D, and obese adolescents without T2D.
Figure 1.
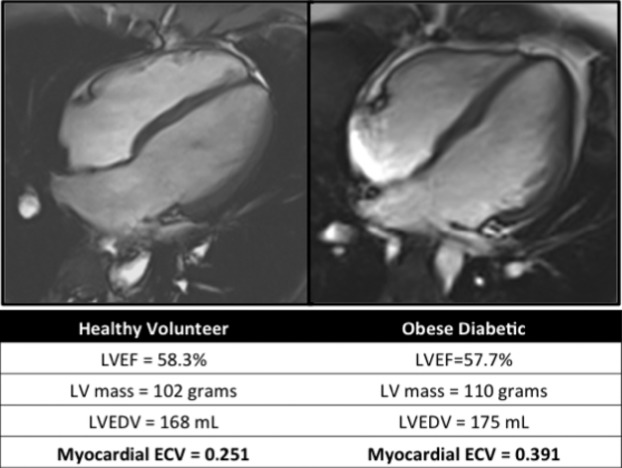
Healthy volunteer versus obese diabetic individual. Four chamber cine‐SSFP image in end‐diastole and representative left‐ventricular measurements. This obese diabetic adolescent has left ventricular measures of function and volume similar to the healthy volunteer; however, the myocardial extracellular volume fraction is significantly higher in the obese diabetic adolescent. SSFP indicates steady‐state free precession; LVEF, left ventricular ejection fraction; LV mass, left ventricular mass; LVEDV, left ventricular end‐diastolic volume; ECV, extracellular volume fraction.
Myocardial Tissue Remodeling in Pediatric Obesity
Myocardial ECV differed significantly by obesity and diabetic status. Median myocardial ECV was 0.264 (0.253 to 0.271) in healthy volunteers versus 0.328 (0.278 to 0.345) in obese adolescents without T2D. Obese adolescents with T2D had the highest degree of interstitial matrix expansion, at 0.376 (0.336 to 0.407; Figure 2A). Of note, ECV was still higher in the obese with and without T2D after adjustment by systolic blood pressure in linear regression models adjusted for blood pressure. Myocardial ECV was significantly associated with body mass index in the entire population (r=0.58, P<0.001; Figure 2B). A higher myocardial ECV was also associated with smaller height‐indexed LV end‐systolic volume index (in obese, r=‐0.46, P=0.03) and higher LVEF (in obese, r=0.45, P=0.04), but not with height‐indexed LV mass, LV mass‐to‐volume ratio, aortic pulse wave velocity, or LA volumes in the obese and in the overall pooled (healthy volunteers and obese) population.
Figure 2.
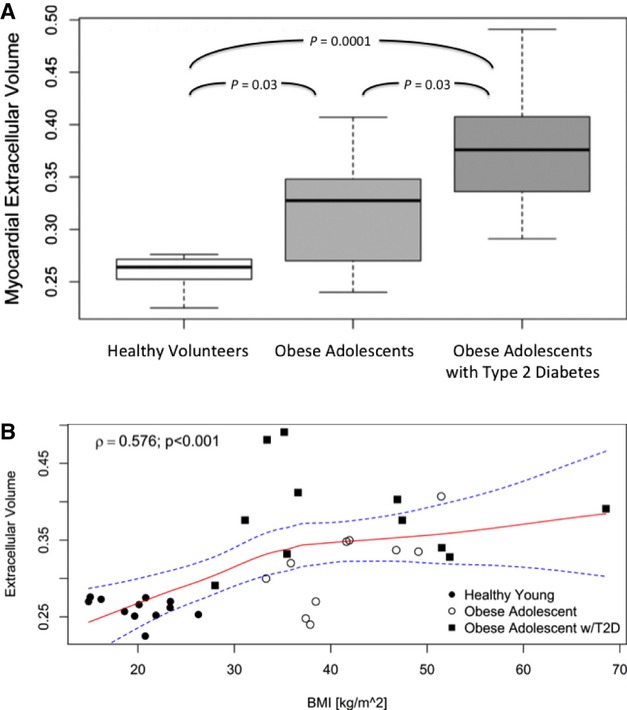
A, Myocardial extracellular volume fraction assessment by CMR stratified by obesity and diabetic status. The central line represents the median, and the whiskers represent 1.5 times the interquartile range. Healthy volunteers had the lowest ECV by CMR, followed by obese adolescents without T2D. Obese adolescents with T2D had the highest ECV by CMR. P values for Kruskal–Wallis comparisons between groups are adjusted for multiple comparisons. B, Relationship of ECV to body mass index across healthy volunteers and obese individuals (fitted using a Loess spline), demonstrating a significant association of ECV with BMI. CMR indicates cardiac magnetic resonance imaging; ECV, extracellular volume fraction; BMI, body mass index; T2D, type 2 diabetes.
Myocardial Tissue Remodeling Is Associated With Biomarkers of Systemic Inflammation and Dysglycemia
Spearman correlations between biomarkers of inflammation, insulin resistance, adiposity, cardiac stress, and CMR indices for obese adolescents are shown in Table 2. Myocardial ECV was associated with high‐sensitivity C‐reactive protein (r=0.47, P<0.05) and serum triglycerides (r=0.51, P<0.05; Figure 3), both markers of cardiometabolic disease. In addition, myocardial ECV demonstrated a strong relationship with dysglycemia (hemoglobin A1c, r=0.76, P<0.0001). More severe dysglycemia was associated with smaller ventricular volumes (correlated with hemoglobin A1c). Importantly, interstitial matrix expansion by myocardial ECV was not associated with biomarkers of cardiovascular stress (N‐terminal pro‐BNP or galectin‐3), adiponectin, or leptin.
Figure 3.
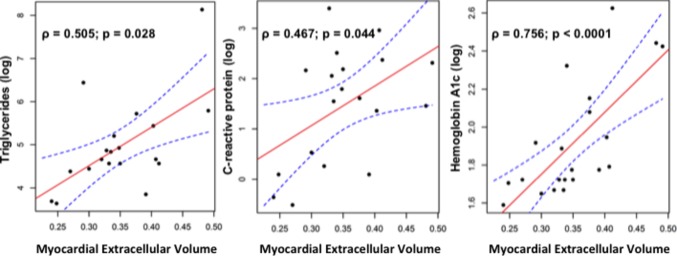
Inflammation and dysglycemia are associated with myocardial tissue remodeling in obese adolescents. Myocardial ECV is associated with high‐sensitivity C‐reactive protein and triglycerides (markers of systemic inflammation and visceral adiposity), as well as hemoglobin A1c (marker of dysglycemia). A linear fit using a least‐squares (Spearman) correlation was used, utilizing a log‐transformed dependent variable (biomarker). A Loess spline was added to the figure to illustrate the nonuniform increase of ECV with BMI. The symbol ρ refers to Spearman's rank correlation. ECV indicates extracellular volume fraction; BMI, body mass index.
Discussion
The novel finding of our study is that significant alterations in myocardial tissue phenotypes as measured by cardiac magnetic resonance imaging occur in obese adolescents (particularly those with T2D) before the onset of frank LV hypertrophy or systolic dysfunction. Obese adolescents demonstrated a significantly greater expansion of the myocardial interstitial matrix relative to healthy volunteers (especially pronounced in those with T2D), and BMI was associated with myocardial ECV. Furthermore, markers of inflammation and dysglycemia—key components of cardiometabolic disease in general—were associated with myocardial interstitial matrix expansion, and T2D and BMI were strongly and independently associated with extracellular matrix remodeling. Together, these results constitute the first observation in a pediatric obese population of altered myocardial tissue phenotypes. This shows that adverse tissue remodeling can manifest early in the course of obesity and diabetes before overt LV hypertrophy or dysfunction, which may be reinforced by cardiometabolic mechanisms central to obesity, namely, inflammation and dysglycemia.
Adolescent obesity remains the most important determinant of risk of metabolic syndrome and its attendant cardiovascular disease in adulthood. Accordingly, there is significant evidence that obesity eventually strongly affects cardiac structure, function, and vascular stiffness in adolescents. LVH is more frequent in obese adolescents (age, 13 to 19) after adjustment for blood pressure6 and particularly in the presence of metabolic syndrome.26 Obesity is accompanied by increased arterial stiffness,27 diastolic dysfunction,28–29 and impaired exercise capacity.30 The degree of diastolic impairment exists on a spectrum from lean to obese to obese diabetic adolescents, associated with hypertension, BMI, and dysglycemia.31 Subclinical systolic dysfunction (by echocardiographic strain) is associated with insulin resistance and BMI.32 Furthermore, these abnormalities may be reversible (even in the morbidly obese) with surgical weight loss.33 Current imaging modalities identify established dysfunction at the organ level (eg, diastolic dysfunction, LVH) in obese youth, when adverse remodeling may be irreversible and unresponsive to potential therapies. Therefore, earlier detection of myocardial tissue phenotypes that may exist before cardiac dysfunction or frank LV hypertrophy are critical to disease prevention and earlier targeted therapy.
Hyperglycemia and insulin resistance have been associated with subtle alterations in the myocardial interstitium (myocardial fibrosis, deposition of advanced glycation end‐products) that contribute to diastolic dysfunction.8–11 Studies in animal models of obesity along the spectrum from insulin resistance to T2D suggest the presence of interstitial myocardial fibrosis and cardiomyocyte hypertrophy as early hallmarks of obesity/diabetic cardiomyopathy34–36 before ventricular dysfunction.34,37 Circulating markers of myocardial fibrosis and echocardiographic integrated backscatter have been associated with insulin resistance and diastolic dysfunction in obese adults.38–39
In this work, we provide a comprehensive cardiac phenotype, including tissue‐level alterations in myocardial extracellular matrix, in obese adolescents. There is a significant stepwise increase in myocardial ECV from normal‐weight healthy volunteers to obese individuals without and with T2D, which is in agreement with studies demonstrating progressive worsening of diastolic function with obesity and dysglycemia status.31 Importantly, these increases in extracellular volume fraction occur in parallel with changes in LV mass and volumes but without changes in LV function between obese adolescents and healthy volunteers, suggesting that ECV may be a sensitive parameter for detecting early remodeling before LV hypertrophy or dysfunction. Furthermore, height‐indexed LV mass (a sensitive marker accessible by echocardiography in adolescents) did not meet published criteria for left ventricular hypertrophy in any group,25 suggesting that myocardial remodeling may occur at lesser degrees of hypertrophy in adolescents. Interestingly, higher ECV was associated with smaller LV volumes and higher LVEF, even within a relatively normal range, a phenotype seen in older individuals with heart failure and preserved systolic function. Indeed, the presence of T2D and BMI were strong independent correlates of ECV, suggesting a central role for dysglycemia in extracellular matrix expansion. These findings are congruent with observations in animal models supporting a primary role for dysglycemia and insulin resistance in extracellular matrix expansion in the heart.8–11 Furthermore, given the clear association between obesity/T2D, ventricular remodeling, and heart failure in adults,40–41 these findings provide a rationale for aggressive preventive strategies earlier in the life course of obesity (eg, in adolescence) to prevent incident heart failure.
In addition, we observed important associations between serum biomarkers relevant to cardiometabolic disease and ECV. Although gross cardiac structural and functional parameters in the obese population were similar to healthy volunteers, markers of inflammation and glycemic control were abnormal and associated with extracellular matrix expansion by CMR. Interestingly, although hemoglobin A1c, triglycerides, and high‐sensitivity C‐reactive protein (CRP) were associated with ECV, selected adipokine implicated in adult obesity (including leptin and adiponectin) were not associated with ECV in our population. The role of adiponectin in myocardial remodeling remains complex, with demonstration of antifibrotic and inflammatory effects in animal models and conflicting relationships between adiponectin, hypertrophy, and diastolic function in human heart failure.42–44 In addition, leptin appears to promote myocardial fibrosis.45 Our demonstration of an elevated myocardial ECV reflecting extracellular matrix remodeling in adolescents provides the rationale for future work examining the role of the adipokine response to obesity. Ultimately, these results suggest that obesity‐mediated cardiometabolic pathways (specifically dysglycemia and inflammation) may mediate subclinical myocardial remodeling early in the adolescent obese before overt LV hypertrophy or dysfunction.
Despite an association between interstitial fibrosis and myocardial ECV in pressure‐overload states,13,46 prior work using similar T1‐based CMR measures in diabetes and cardiometabolic disease has been limited. Work by Jellis and colleagues in adults with diabetes without clinical cardiovascular disease demonstrated that a single measurement of postcontrast T1 was modestly associated with echocardiographic diastolic dysfunction, blood pressure, and insulin sensitivity, but not with LV mass or circulating markers of collagen turnover.15. In large community‐based populations without cardiovascular disease, obesity‐mediated increases in LV mass may be mediated by inflammation,47 and markers of proinflammatory visceral adiposity and inflammation are associated with obesity‐mediated concentric LV remodeling and heart failure.40–41
Our finding that myocardial matrix expansion already exists in obese adolescents (particularly those with T2D) extends prior observations to a potentially earlier stage of obesity‐mediated heart disease. Extracellular matrix expansion is prevalent in obese adolescents, suggesting obese adolescents harbor a wide range of subclinical myocardial damage not detected by standard markers of ventricular structure and function. Furthermore, the association with markers of inflammation and dysglycemia highlights the interplay between metabolism and cardiac remodeling in the very early stages of obesity‐related cardiomyopathy. Given the recently reported association between myocardial ECV and mortality in adulthood,47 these results ultimately provide the impetus to larger studies of the effect of early detection and intervention (with a wider array of antiremodeling, weight loss, and dysglycemia therapies) in this population to prevent cardiac remodeling and heart failure.
The results of our study should be viewed in the context of its design and potential limitations. Despite the limited number of individuals in our study, we were adequately powered to detect the differences we observed in myocardial ECV, and the associations between myocardial ECV, weight, and cardiometabolic risk markers (eg, CRP and dysglycemia) remain strong and biologically plausible. Endomyocardial biopsy to confirm collagen as the etiology of ECV in this population is impossible to obtain in this preclinical, young population; however, the prognostic relevance of ECV elevation in an older cohort has been established, regardless of cause.47 Indeed, the relationships observed between inflammation/insulin resistance and ECV—and the young age of the individuals studied here—suggest that alternative mechanisms (eg, advanced glycation end‐products) and not necessarily myocardial fibrosis may be the origin of elevated ECV. Although we did not perform echocardiography in these patients to provide canonical measures of diastolic function, left atrial volumes (a sensitive marker of chronicity of diastolic dysfunction) were similar in the obese and healthy population and were not associated with ECV, suggesting that the obese adolescents studied here may harbor a very early, preclinical phenotype before even the onset of significant diastolic dysfunction. Finally, although our population of healthy young controls (not necessarily postpubertal) was derived from patients without clinical cardiovascular disease imaged at a different site from the obese adolescents, the range of myocardial ECV in this young healthy population was consistent with previous reports of healthy volunteers.48
In conclusion, obesity in adolescents is associated with extracellular matrix expansion before significant ventricular hypertrophy or dysfunction, particularly in obese adolescents with T2D. These changes are associated with dysglycemia and inflammation, suggesting mechanistic links between cardiometabolic risk and early myocardial tissue phenotypes in pediatric obesity. Given the public health impact of pediatric obesity, these results suggest that myocardial tissue‐level remodeling may be present even earlier than previously described in the obese adolescent and motivate more aggressive detection and therapy to improve disease prevention. Larger studies to define the physiologic and biochemical relevance of novel, tissue‐level remodeling and its reversibility are warranted in this emerging at‐risk population.
Sources of Funding
This work was conducted with support from Harvard Catalyst, the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170‐05, and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health. Dr Shah is supported by an American Heart Association Post‐Doctoral Fellowship Award (11POST000002) and a training grant from the Heart Failure NIH Clinical Research Network (U01‐HL084877). Dr Neilan is supported by a grant from the American Heart Association Fellow‐to‐Faculty Award (12FTF12060588). Dr Jerosch‐Herold is supported by NIH grant R01‐HL091157. Studies were, in part, donated by Siemens Healthcare.
Disclosures
Dr Ravi Teja Seethamraju is employed by Siemens Healthcare. Drs William S. Harris, Daniel M. Hoefner, and Joseph P. McConnell are employed by Health Diagnostics Laboratory. Siemens and Health Diagnostics Laboratory did not participate in the design of this study. Dr Rhodes is supported by the New Balance Foundation Obesity Prevention Center Boston Children's Hospital. Dr Rhodes is engaged in research with Merck related to youth with type 2 diabetes and has received in‐kind support from Roche. All other authors have no financial or conflict‐of‐interest disclosures relevant to the content of this article.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006; 295:1549-1555 [DOI] [PubMed] [Google Scholar]
- 2.Tirosh A, Shai I, Afek A, Dubnov‐Raz G, Ayalon N, Gordon B, Derazne E, Tzur D, Shamis A, Vinker S, Rudich A. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011; 364:1315-1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker JL, Olsen LW, Sorensen TI. Childhood body‐mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007; 357:2329-2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberger J, Moran A, Hong CP, Jacobs DR, Jr, Sinaiko AR. Adiposity in childhood predicts obesity and insulin resistance in young adulthood. J Pediatr. 2001; 138:469-473 [DOI] [PubMed] [Google Scholar]
- 5.Reilly JJ, Kelly J. Long‐term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond). 2011; 35:891-898 [DOI] [PubMed] [Google Scholar]
- 6.Movahed MR, Bates S, Strootman D, Sattur S. Obesity in adolescence is associated with left ventricular hypertrophy and hypertension. Echocardiography. 2011; 28:150-153 [DOI] [PubMed] [Google Scholar]
- 7.Tounian P, Aggoun Y, Dubern B, Varille V, Guy‐Grand B, Sidi D, Girardet JP, Bonnet D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001; 358:1400-1404 [DOI] [PubMed] [Google Scholar]
- 8.Das A, Das J, Chandrasekar S. Specific heart muscle disease in diabetes mellitus—a functional structural correlation. Int J Cardiol. 1987; 17:299-302 [DOI] [PubMed] [Google Scholar]
- 9.Fang Z, Prins J, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004; 25:543-567 [DOI] [PubMed] [Google Scholar]
- 10.Murarka S, Movahed MR. Diabetic cardiomyopathy. J Card Fail. 2010; 16:971-979 [DOI] [PubMed] [Google Scholar]
- 11.Nunoda S, Genda A, Sugihara N, Nakayama A, Mizuno S, Takeda R. Quantitative approach to the histopathology of the biopsied right ventricular myocardium in patients with diabetes mellitus. Heart Vessels. 1985; 1:43-47 [DOI] [PubMed] [Google Scholar]
- 12.Schelbert EB, Testa SM, Meier CG, Ceyrolles WJ, Levenson JE, Blair AJ, Kellman P, Jones BL, Ludwig DR, Schwartzman D, Shroff SG, Wong TC. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: slow infusion versus bolus. J Cardiovasc Magn Reson. 2011; 13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coelho‐Filho OR, Mongeon FP, Mitchell R, Moreno H, Jr, Nadruz W, Jr, Kwong R, Jerosch‐Herold M. The role of transcytolemmal water exchange in magnetic resonance measurements of diffuse myocardial fibrosis in hypertensive heart disease. Circ Cardiovasc Imaging. 2013; 6:134-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jellis C, Wright J, Kennedy D, Sacre J, Jenkins C, Haluska B, Martin J, Fenwick J, Marwick TH. Association of imaging markers of myocardial fibrosis with metabolic and functional disturbances in early diabetic cardiomyopathy. Circ Cardiovasc Imaging. 2011; 4:693-702 [DOI] [PubMed] [Google Scholar]
- 15.Hollingworth W, Hawkins J, Lawlor DA, Brown M, Marsh T, Kipping RR. Economic evaluation of lifestyle interventions to treat overweight or obesity in children. Int J Obes (Lond). 2012; 36:559-566 [DOI] [PubMed] [Google Scholar]
- 16.Barlow SE, Dietz WH. Obesity evaluation and treatment: expert committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics. 1998; 102:E29. [DOI] [PubMed] [Google Scholar]
- 17. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003; 26suppl 1:S5-S20 [DOI] [PubMed] [Google Scholar]
- 18.Kaminski M, Steel K, Jerosch‐Herold M, Khin M, Tsang S, Hauser T, Kwong RY. Strong cardiovascular prognostic implication of quantitative left atrial contractile function assessed by cardiac magnetic resonance imaging in patients with chronic hypertension. J Cardiovasc Magn Reson. 2011; 13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neilan TG, Coelho‐Filho OR, Shah RV, Feng JH, Pena‐Herrera D, Mandry D, Pierre‐Mongeon F, Heydari B, Francis SA, Moslehi J, Kwong RY, Jerosch‐Herold M. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline‐based chemotherapy. Am J Cardiol. 2013; 111:717-722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mongeon FP, Jerosch‐Herold M, Coelho‐Filho OR, Blankstein R, Falk RH, Kwong RY. Quantification of extracellular matrix expansion by CMR in infiltrative heart disease. JACC Cardiovasc Imaging. 2012; 5:897-907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho CY, Abbasi SA, Neilan TG, Shah RV, Chen Y, Heydari B, Cirino AL, Lakdawala NK, Orav EJ, Gonzalez A, Lopez B, Diez J, Jerosch‐Herold M, Kwong RY. T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013; 6:415-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landis CS, Li X, Telang FW, Molina PE, Palyka I, Vetek G, Springer CS., Jr Equilibrium transcytolemmal water‐exchange kinetics in skeletal muscle in vivo. Magn Reson Med. 1999; 42:467-478 [DOI] [PubMed] [Google Scholar]
- 23.Tison GH, Blaha MJ, Budoff MJ, Katz R, Rivera JJ, Bertoni AG, Wong ND, Blumenthal RS, Szklo M, Eng J, Tracy R, Nasir K. The relationship of insulin resistance and extracoronary calcification in the multi‐ethnic study of atherosclerosis. Atherosclerosis. 2011; 218:507-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neilan TG, Coelho‐Filho OR, Shah RV, Abbasi SA, Heydari B, Watanabe E, Chen Y, Mandry D, Pierre‐Mongeon F, Blankstein R, Kwong RY, Jerosch‐Herold M. Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging. 2013; 6:672-683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniels SR, Meyer RA, Liang YC, Bove KE. Echocardiographically determined left ventricular mass index in normal children, adolescents and young adults. J Am Coll Cardiol. 1988; 12:703-708 [DOI] [PubMed] [Google Scholar]
- 26.Di Bonito P, Moio N, Scilla C, Cavuto L, Sibilio G, Forziato C, Sanguigno E, Saitta F, Iardino MR, Capaldo B. Preclinical manifestations of organ damage associated with the metabolic syndrome and its factors in outpatient children. Atherosclerosis. 2010; 213:611-615 [DOI] [PubMed] [Google Scholar]
- 27.Koopman LP, McCrindle BW, Slorach C, Chahal N, Hui W, Sarkola T, Manlhiot C, Jaeggi ET, Bradley TJ, Mertens L. Interaction between myocardial and vascular changes in obese children: a pilot study. J Am Soc Echocardiogr. 2012; 25:401-410.e1 [DOI] [PubMed] [Google Scholar]
- 28.Urbina EM, Khoury PR, McCoy C, Daniels SR, Kimball TR, Dolan LM. Cardiac and vascular consequences of pre‐hypertension in youth. J Clin Hypertens (Greenwich). 2011; 13:332-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirschler V, Acebo HL, Fernandez GB, de Lujan Calcagno M, Gonzalez C, Jadzinsky M. Influence of obesity and insulin resistance on left atrial size in children. Pediatr Diabetes. 2006; 7:39-44 [DOI] [PubMed] [Google Scholar]
- 30.Kelly AS, Wetzsteon RJ, Kaiser DR, Steinberger J, Bank AJ, Dengel DR. Inflammation, insulin, and endothelial function in overweight children and adolescents: the role of exercise. J Pediatr. 2004; 145:731-736 [DOI] [PubMed] [Google Scholar]
- 31.Shah AS, Khoury PR, Dolan LM, Ippisch HM, Urbina EM, Daniels SR, Kimball TR. The effects of obesity and type 2 diabetes mellitus on cardiac structure and function in adolescents and young adults. Diabetologia. 2011; 54:722-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosmala W, Wong C, Kuliczkowska J, Leano R, Przewlocka‐Kosmala M, Marwick TH. Use of body weight and insulin resistance to select obese patients for echocardiographic assessment of subclinical left ventricular dysfunction. Am J Cardiol. 2008; 101:1334-1340 [DOI] [PubMed] [Google Scholar]
- 33.Ippisch HM, Inge TH, Daniels SR, Wang B, Khoury PR, Witt SA, Glascock BJ, Garcia VF, Kimball TR. Reversibility of cardiac abnormalities in morbidly obese adolescents. J Am Coll Cardiol. 2008; 51:1342-1348 [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto E, Dong Y, Kataoka K, Yamashita T, Tokutomi Y, Matsuba S, Ichijo H, Ogawa H, Kim‐Mitsuyama S. Olmesartan prevents cardiovascular injury and hepatic steatosis in obesity and diabetes, accompanied by apoptosis signal regulating kinase‐1 inhibition. Hypertension. 2008; 52:573-580 [DOI] [PubMed] [Google Scholar]
- 35.Mitzushige K, Yao L, Noma T, Kiyomoto H, Yu Y, Hosomi N, Ohmori K, Matsuo H. Alteration in left ventricular diastolic filling and accumulation of myocardial collagen at insulin‐resistant prediabetic stage of a type II diabetic rat model. Circulation. 1999; 101:899-907 [DOI] [PubMed] [Google Scholar]
- 36.Hosomi N, Noma T, Ohyama H, Takahashi T, Kohno M. Vascular proliferation and transforming growth factor‐B expression in pre‐ and early stage of diabetes mellitus in Otsuka Long‐Evans Tokushima fatty rats. Atherosclerosis. 2002; 162:69-76 [DOI] [PubMed] [Google Scholar]
- 37.Dong Y, Liu L, Kataoka K, Nakamura T, Fukuda M, Tokutomi Y, Nako H, Ogawa H, Kim‐Mitsuyama S. Aliskiren prevents cardiovascular complications and pancreatic injury in a mouse model of obesity and type 2 diabetes. Diabetologia. 2010; 53:180-191 [DOI] [PubMed] [Google Scholar]
- 38.Kosmala W, Przewlocka‐Kosmala M, Wojnalowicz A, Mysiak A, Marwick TH. Integrated backscatter as a fibrosis marker in the metabolic syndrome: association with biochemical evidence of fibrosis and left ventricular dysfunction. Eur Heart J Cardiovasc Imaging. 2012; 13:459-467 [DOI] [PubMed] [Google Scholar]
- 39.Quilliot D, Alla F, Bohme P, Bruntz JF, Hammadi M, Dousset B, Ziegler O, Zannad F. Myocardial collagen turnover in normotensive obese patients: relation to insulin resistance. Int J Obes (Lond). 2005; 29:1321-1328 [DOI] [PubMed] [Google Scholar]
- 40.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd‐Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008; 51:1775-1783 [DOI] [PubMed] [Google Scholar]
- 41.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, Arai AE, Lima JA, Bluemke DA. The impact of obesity on the left ventricle: the Multi‐Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging. 2010; 3:266-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bidulescu A, Liu J, Musani SK, Fox ER, Samdarshi TE, Sarpong DF, Vaccarino V, Wilson PW, Arnett DK, Din‐Dzietham R, Taylor HA, Gibbons GH. Association of adiponectin with left ventricular mass in blacks: the Jackson Heart Study. Circ Heart Fail. 2011; 4:747-753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong SJ, Park CG, Seo HS, Oh DJ, Ro YM. Associations among plasma adiponectin, hypertension, left ventricular diastolic function and left ventricular mass index. Blood Press. 2004; 13:236-242 [DOI] [PubMed] [Google Scholar]
- 44.Park M, Sweeney G. Direct effects of adipokines on the heart: focus on adiponectin. Heart Fail Rev. 2012/‐‐‐ [DOI] [PubMed] [Google Scholar]
- 45.Zibadi S, Cordova F, Slack EH, Watson RR, Larson DF. Leptin's regulation of obesity‐induced cardiac extracellular matrix remodeling. Cardiovasc Toxicol. 2011; 11:325-333 [DOI] [PubMed] [Google Scholar]
- 46.Flett AS, Sado DM, Quarta G, Mirabel M, Pellerin D, Herrey AS, Hausenloy DJ, Ariti C, Yap J, Kolvekar S, Taylor AM, Moon JC. Diffuse myocardial fibrosis in severe aortic stenosis: an equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2012; 13:819-826 [DOI] [PubMed] [Google Scholar]
- 47.Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short‐term mortality. Circulation. 2012; 126:1206-1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JJ, Liu S, Nacif MS, Ugander M, Han J, Kawel N, Sibley CT, Kellman P, Arai AE, Bluemke DA. Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson. 2011; 13:75. [DOI] [PMC free article] [PubMed] [Google Scholar]


