Abstract
Background
A high consumption of omega‐3 long‐chain polyunsaturated fatty acids, and particularly docosahexaenoic acid (DHA), has been suggested to reduce the risk of cardiovascular disease (CVD). However, while DHA supplementation may have benefits for secondary prevention, few studies have investigated the role of DHA in the primary prevention of CVD. Here, we tested the hypothesis that DHA supplementation improves endothelial function and risk factors for CVD.
Methods and Results
Healthy volunteers (n=328), aged 18 to 37 years, were randomly assigned to 1.6 g DHA/day (from a microalgae source) together with 2.4 g/day carrier oil (index group) or to 4.0 g/day olive oil (control) (both given in eight 500‐mg capsules/day for 16 weeks). Flow‐mediated endothelium‐dependent vasodilation (FMD) of the brachial artery (primary outcome) was measured before and after the intervention (n=268) using high‐resolution vascular ultrasound. FMD was the same in both groups at randomization (mean, SD; 0.27, 0.1 mm), but postintervention was higher in the control group (0.29, 0.1 mm) compared with the DHA‐supplemented group (0.26, 0.1 mm; mean difference −0.03 mm; 95% CI −0.005 to −0.06 mm; P=0.02). Of other outcomes, only triglyceride (mean difference −28%, 95% CI −40% to −15%; P<0.0001) and very low‐density lipoprotein concentrations were significant lower in DHA‐supplemented individuals compared with controls.
Conclusions
DHA supplementation did not improve endothelial function in healthy, young adults. Nevertheless, lower triglyceride concentrations with DHA supplementation was consistent with previous reports and could have benefits for the prevention of CVD.
Clinical Trial Registration Information
URL: http://www.controlled-trials.com/ Unique identifier: ISRCTN no: 19987575.
Keywords: atherosclerosis, docosahexaenoic acid, endothelial function
Introduction
A high intake of long‐chain omega‐3 polyunsaturated fatty acids (n‐3 LC‐PUFAs) ecosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) has been shown to reduce the risk of cardiovascular disease (CVD) in both epidemiological studies1–2 and randomized controlled trials (RCTs).2–3 However, previous RCTs have largely focused on secondary prevention in high‐risk patients,3 and relatively few studies have investigated the role of n‐3 LC‐PUFAs in the primary prevention of CVD.2
Beneficial effects of n‐3 LC‐PUFA supplementation on inflammatory makers,4–5 serum risk factors for CVD,4–5 blood pressure,6 lipid concentrations,4–5,4–8, and particularly endothelial function,9–24 a key early stage in the vascular biology of atherosclerosis,25 support their potential role in the prevention of CVD. Supplementation with n‐3 LC‐PUFAs has been shown to improve endothelial function in healthy middle‐aged adults17 and in patients with hypercholesterolemia,11–12,15 hyperlipidemia,14,18,20 type 2 diabetes,10 coronary heart disease,13,22 or following heart transplants.9 However, relatively few studies have investigated the effects of n‐3 LC‐PUFAs on endothelial function in healthy young populations,16,19,21,24 early in the clinical development of atherosclerotic disease.
Previously, red cell DHA concentration was associated with greater flow‐mediated endothelial‐dependent vasodilation (FMD) of the brachial artery in healthy adults who smoked, who were in the top thirds of cardiovascular risk factors such as fasting insulin, glucose, or plasma triglyceride concentration,16 and plasma n‐3 LC‐PUFA levels were associated with greater FMD in carriers for the Glu298ASP polymorphism (an allele in the gene that encodes for vascular endothelial nitric oxide synthase, which is postulated to affect adversely endothelial function and CVD risk26). Furthermore, epidemiological associations between dietary intakes of n‐3 LC‐PUFA and lower levels of inflammation and endothelial activation27 suggest that high n‐3 LC‐PUFA consumption may have a role in the prevention of early vascular disease. Nevertheless, previous n‐3 LC‐PUFA supplementation studies in healthy populations have been small17,19 and shown inconsistent findings21,23–24 and few have been conducted in healthy young adults who may take n‐3 LC‐PUFA supplements to reduce CVD risk.
In the present study, we investigated the impact of n‐3 LC‐PUFA supplementation on brachial artery FMD (primary outcome), surrogate vascular markers for atherosclerosis,28–29 and conventional biochemical risk factors for CVD in healthy young adults. We focused only on the effects of DHA supplementation, which has been suggested to have greater benefits for endothelial function14,16–17 and CVD risk factors30 than EPA, and used a dose within the normal dietary range (equivalent to 1 portion of oily fish per day for total n‐3 LC‐PUFA).
Methods
Study Population
Healthy volunteers (N=328) were recruited from participants in a previous study16 and from adverts placed in local newspapers and around University College London. Individuals who were aged 18 to 37 years, clinically well at the clinic visit, and free from chronic disease likely to affect endothelial function (eg, insulin‐dependent diabetes) were eligible. Those who were pregnant, on unusual diets, or taking regular medication or n‐3 LC‐PUFA supplements were excluded. All participants gave written consent, and the study was approved by a National Research Ethics Committee. The trial was registered on Current Controlled Trials (ISRCTN No. 19987575) (http://www.controlled-trials.com/ISRCTN19987575/singhal).
Study Design
The study was a double‐blind, parallel‐group, placebo‐controlled randomized trial conducted at the Childhood Nutrition Research Centre, UCL, London, between 2003 and 2008. Participants were randomly assigned to DHA supplementation or control groups using a randomization list generated by an independent statistician and held at a collaborating center (Martek, Biosciences Corporation, Columbia, MD, now DSM Nutritional Lipids). To ensure that the control and index groups were similar with respect to smoking status, or the Glu298Asp polymorphism for the eNOS gene (as suggested previously26) randomization was stratified by eNOS polymorphism (Glu298 homozygotes, Asp298 heterozygotes, and Asp298 homozygotes) and smoking status (current smoker or not). The assigned dietary group was allocated using numbered, sealed, and opaque envelopes, and all participants and research staff were blind to the dietary assignment. Endothelial function and other study outcomes were measured during a 1‐day visit to the research center before and after 4 months of dietary intervention. All subjects were asked to participate in postintervention measurements regardless of whether they complied with the study protocol (intention to treat).
Supplementation
Participants were randomized to 1.6 g DHA/day with 2.4 g/day carrier oil (predominantly palmitic acid [16:0], myristic acid [14:0], and the omega‐6 fatty acid, docosapentaenoic [22:5n‐6]) or to 4.0 g/day olive oil (control). Supplements were given as eight 500‐mg gelatin capsules each day with each capsule providing 200 mg DHA in the index group. DHA was from a microalgae source (Schizochytrium sp), and all capsules were provided by Martek Biosciences Corporation. The proposed level of supplementation was chosen because it is approximately equivalent to the total n‐3 LC‐PUFA intake from a portion of oily fish (eg, herring or salmon) per day (and therefore within the normal dietary range for the population).31 Supplementation of less than 3 g of n‐3 LC‐PUFA per day has been categorized as generally regarded as safe (GRAS) by the US Food and Drug Administration.31 Olive oil was chosen as the control supplement because saturated fatty acids have been suggested to have a detrimental effect on vascular function, while oils rich in n‐6 fatty acids were likely to compete for Δ5‐desaturase needed to form DHA.
Compliance with dietary supplements was encouraged by weekly phone calls and text messages and monitored by counting the number of capsules consumed by the end of the intervention. Information on any serious adverse events was also recorded during these calls. Data on adverse effects and tolerance were recorded for the preceding 7 days during monthly phone calls. Red cell concentrations of n‐3 LC‐PUFAs before and after the intervention were used as an objective measure of compliance.
Vascular Study Outcomes
All studies were performed in a temperature‐controlled (22°C to 26°C) vascular laboratory by 1 of 2 trained operators. Subjects had fasted overnight and rested for 10 minutes before measurements were taken.
Brachial artery vasomotor function
The principal trial outcome was FMD of the brachial artery measured as described previously.16,32 Briefly, the brachial artery was imaged in longitudinal section, 5 to 10 cm above the antecubital fossa, using high‐resolution ultrasound (GE Vivid 7; General Electric Healthcare Technologies). A pneu‐matic cuff was inflated around the forearm to 300 mm Hg for 5 minutes followed by rapid deflation causing a large increase in blood flow (reactive hyperemia). Brachial artery diameter was measured with edge detection software (Brachial Tools; MIA) from electrocardiogram (ECG)‐triggered images captured every 3 seconds throughout the 11‐minute recording protocol (for 1 minute resting, 5 minutes cuff inflation, and 5 minutes post cuff deflation). Reactive hyperemia was calculated from the maximal flow (recorded continuously by pulsed‐wave Doppler) within the first 15 seconds after deflation of the pneumatic cuff, relative to baseline flow. FMD was expressed as the absolute maximal change between prehyperemic and posthyperemic brachial artery diameter adjusted for prehyperemic diameter using regression analysis and as a percent change from baseline arterial diameter.32 Absolute change in diameter was chosen as the primary outcome because it is independent of baseline arterial diameter, which contributes to sex differences in FMD expressed as a percent change.32
Brachial artery distensibility
Brachial artery distensibility was assessed on the arterial segment subsequently imaged for FMD measurement (see earlier).32 Real‐time B‐mode images were recorded on video for 20 seconds and used for later offline analysis. The distention of the artery was determined by measuring the luminal diameter excursion from diastole to systole and expressed as a percentage of baseline diameter.32 Blood pressure was measured in the left brachial artery using an automated device (Accutorrsat; Datascope Corp) during distention measurement in the right arm. This provided a representative measure of the pulse pressure in the right brachial artery, which was used to derive distensibility coefficient as the change in cross‐sectional area per unit change in blood pressure as described previously.33 Blood pressure was also determined on 2 occasions before the measurement of arterial distensibility after 10 minutes resting supine. The mean of the 3 measurements was used to assess effects of DHA supplementation on systolic, diastolic, and mean arterial blood pressures. Due to technical difficulties at the start of the trial, brachial artery distensibility was not measured in the first 114 (of 324) participants.
Carotid artery distensibility
The carotid artery was imaged using B‐mode ultrasonography at a region 1 cm proximal to the bifurcation of the right and left common carotid arteries. The transducer was manipulated such that the near wall of the carotid artery was parallel to the transducer footprint and the lumen was maximized in the longitudinal plane. Real‐time B‐mode images were recorded on video for 20 seconds and used for later offline analysis with edge detection software (Brachial Tools/Carotid Analyzer; MIA). Brachial blood pressure was recorded at 5‐minute intervals throughout the period of ultrasound scanning. The artery diameter changes for assessing carotid distensibility and the distensibility coefficient were analyzed as described previously.33
Carotid artery intima–media thickness
The common carotid was imaged 1 cm proximal to the bifurcation. Longitudinal images of the far wall intima–media interface was clearly defined and recorded on videotape for later analysis. The distance between the leading edge of the intima and the media–adventitia was measured with ultrasonic calipers. Three measurements were taken in both the right and left common carotid arteries, and mean intima–media thickness (IMT) was calculated as described previously.34
Pulse wave velocity
Pressure‐pulse wave signals were recorded using an application tonometer (SphygmoCor; AtCor Medical) positioned at the base of the right common carotid artery and over the right radial artery. Pressure‐pulse wave signals were recorded with an ECG signal, which provided an R‐timing reference. Integral software processed each set of pulse pressures using ECG waveform data to calculate the mean time difference between the R waves and the pressure waves on a beat‐to‐beat basis over 10 seconds. Pulse wave velocity was calculated using the mean time difference and arterial path length between the 2 recording points.32
Anthropometry, Biochemistry, and Fatty Acid Analysis
Height and weight were measured using standard equipment and protocols. Socioeconomic class was based on the participant's occupation according to the Registrar General's Classification. Smoking and alcohol consumption were recorded in a brief lifestyle questionnaire and by salivary cotinine measurements. Blood was obtained via venipuncture between 9:00 am and 11:00 am after an overnight fast, and plasma concentrations of cardiovascular risk factors were determined in the laboratories of Great Ormond Street Hospital using standard laboratory methods. Red cell and plasma concentrations of the n‐3 LC‐PUFAs were determined before and after the intervention using standardized methods in the laboratories of Professor von Schacky35–36 and expressed as a percentage of total fatty acids.
Sample Size
The minimum projected sample size was calculated as 256 subjects (128 each in n‐3 LC‐PUFA–supplemented and control groups). This was sufficient to detect a 0.35‐SD difference in FMD (primary outcome) between randomized groups at 5% significance and with 80% power, an effect considerably smaller than the ≈1‐SD increase in FMD with fish oil supplementation shown previously in healthy volunteers.19 Successful recruiting meant that 324 subjects were randomized and 274 completed the intervention.
Statistical Analysis
Student t test was used compare randomized groups for normally distributed variables, and χ2 test was used for dichotomous variables. Initial analyses were on an intention‐to‐treat basis. Linear regression was used to investigate the effect of randomized diets on absolute change in FMD adjusted for baseline arterial diameter. The distributions of fasting very low‐density lipoprotein (VLDL), triglyceride, insulin, C‐reactive protein (CRP), and cotinine concentrations and sum of skinfold thickness were right skewed and so were loge transformed and then multiplied by 100 before analysis.37 The SD for 100 loge transformed data represents the coefficient of variation of the original data, while regression coefficients represent the percentage difference in dependent variable per unit change in the independent variable.37
In secondary analyses, multiple linear regression was used to adjust differences in absolute FMD between randomized groups for potential confounding factors (age, sex, room temperature, skin temperature, and fasting concentrations of LDL and triglycerides). A priori planned analyses included investigating the effects of DHA supplementation on FMD according to sex, smoking status, presence of Asp298 allele, and CVD risk factors (ie, in individuals in the highest third of fasting concentrations of insulin, glucose, or triglyceride16). All analyses were conducted using SPSS for Windows (version 18.0; SPSS Inc), and statistical significance was taken as P<0.05.
Results
Demographic factors, measures of vascular structure and function, and risk factors for CVD (eg, body mass index [BMI] and lipid concentration) were similar to those for the general young adult population in the United Kingdom at the time of the study16 (Table 1). Of the 328 subjects recruited, 4 dropped out before randomization, 324 were randomized to DHA or control supplements, and 274 were reviewed 4 months after the start of the study (Figure 1).
Table 1.
Subject Characteristics at Baseline
| Control | DHA Supplemented | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | |||||||
| N | 162 | 162 | ||||||||||
| Sex,* %, n | 40 | 65 | 60 | 97 | 34 | 55 | 66 | 107 | ||||
| Age, y | 27.6 | 4.7 | 29.0 | 4.6 | 26.6 | 4.5 | 28.2 | 4.8 | 28.8 | 4.8 | 27.9 | 4.7 |
| Social class* | ||||||||||||
| Manual, %, n | 42 | 68 | 35 | 23 | 46 | 45 | 44 | 71 | 54 | 30 | 38 | 41 |
| Nonmanual, %, n | 58 | 94 | 65 | 42 | 54 | 52 | 56 | 91 | 45 | 25 | 62 | 66 |
| Current smoker,* %, n | 14 | 23 | 12 | 8 | 15 | 15 | 13 | 20 | 9 | 5 | 14 | 15 |
| Weight, kg | 68.9 | 13.4 | 76.7 | 11.7 | 63.7 | 11.9 | 67.4 | 14.1 | 75.1 | 9.8 | 63.5 | 14.4 |
| Height, cm | 170.5 | 9.3 | 177.9 | 8.0 | 165.6 | 6.5 | 168.9 | 9.0 | 176.7 | 6.6 | 165.0 | 7.4 |
| Body mass index, kg/m2 | 23.6 | 3.5 | 24.2 | 2.8 | 23.2 | 3.9 | 23.6 | 4.3 | 24.1 | 3.2 | 23.3 | 4.8 |
| Sum skinfold,†‡ mm | 48.4 | 39 | 42.3 | 39 | 53.0 | 36 | 46.4 | 43 | 38.7 | 43 | 51.0 | 41 |
| Waist circumference, cm | 77.5 | 9.7 | 83.6 | 8.0 | 73.4 | 8.5 | 77.1 | 10.6 | 83.0 | 8.4 | 74.0 | 10.3 |
| Waist:hip ratio | 0.8 | 0.07 | 0.8 | 0.05 | 0.7 | 0.05 | 0.8 | 0.07 | 0.8 | 0.06 | 0.8 | 0.05 |
| Blood pressure, mm Hg | ||||||||||||
| Systolic | 113 | 11 | 118 | 10 | 109 | 10 | 111 | 11 | 114 | 8 | 109 | 11 |
| Diastolic | 67 | 8 | 69 | 8 | 65 | 7 | 67 | 8 | 68 | 7 | 66 | 8 |
| Mean arterial | 84 | 9 | 88 | 9 | 81 | 8 | 83 | 8 | 86 | 6 | 82 | 9 |
| Pulse pressure | 46 | 6 | 49 | 6 | 44 | 6 | 44 | 6 | 47 | 6 | 44 | 6 |
| Resting heart rate, beats/min | 66 | 9 | 65 | 10 | 67 | 8 | 66 | 10 | 63 | 8 | 67 | 10 |
| Total cholesterol, mmol/L | 4.2 | 0.9 | 4.3 | 0.9 | 4.1 | 0.9 | 4.3 | 0.8 | 4.4 | 0.8 | 4.3 | 0.8 |
| LDL cholesterol, mmol/L | 2.3 | 0.8 | 2.5 | 0.8 | 2.2 | 0.8 | 2.4 | 0.7 | 2.6 | 0.7 | 2.3 | 0.7 |
| HDL cholesterol, mmol/L | 1.4 | 0.3 | 1.3 | 0.3 | 1.5 | 0.3 | 1.5 | 0.4 | 1.3 | 0.3 | 1.5 | 0.4 |
| VLDL cholesterol,† mmol/L | 0.4 | 50 | 0.4 | 56 | 0.4 | 46 | 0.4 | 50 | 0.4 | 55 | 0.4 | 46 |
| Total:HDL cholesterol ratio | 3.1 | 1.0 | 3.6 | 1.0 | 2.8 | 0.9 | 3.1 | 1.0 | 3.5 | 1.0 | 2.9 | 0.9 |
| LDL:HDL cholesterol ratio | 1.8 | 0.8 | 2.1 | 0.9 | 1.5 | 0.7 | 1.8 | 0.8 | 2.1 | 0.7 | 1.6 | 0.8 |
| Triglyceride,† mmol/L | 0.8 | 50 | 0.9 | 55 | 0.8 | 46 | 0.9 | 50 | 0.9 | 55 | 0.8 | 46 |
| Glucose, mmol/L | 4.8 | 0.5 | 5.0 | 0.5 | 4.7 | 0.4 | 4.8 | 0.5 | 4.9 | 0.5 | 4.7 | 0.5 |
| Insulin,† mU/L | 25.5 | 58 | 24.3 | 58 | 26.4 | 58 | 25.9 | 61 | 23.8 | 58 | 27.0 | 62 |
| Insulin resistance† (HOMA), μm/L | 0.8 | 61 | 0.7 | 63 | 0.8 | 61 | 0.8 | 66 | 0.7 | 64 | 0.8 | 67 |
| CRP,† mg/L | 0.9 | 120 | 0.8 | 108 | 1.0 | 128 | 1.0 | 122 | 0.8 | 126 | 1.2 | 120 |
| Cotinine,† ng/mL | 1.8 | 261 | 2.0 | 280 | 1.6 | 250 | 1.5 | 249 | 1.2 | 266 | 1.6 | 242 |
Data are mean, SD except *% (n) and †geometric mean (coefficient of variation). There is <5% loss of n for some variables. DHA indicates docosahexaenoic acid; LDL, low‐density lipoprotein; HDL, high‐density lipoprotein; VLDL, very low‐density lipoprotein; HOMA, homeostatic model assessment; CRP, C‐reactive protein.
‡Measured at 4 sites: triceps, biceps, subscapular, and suprailiac.
Figure 1.
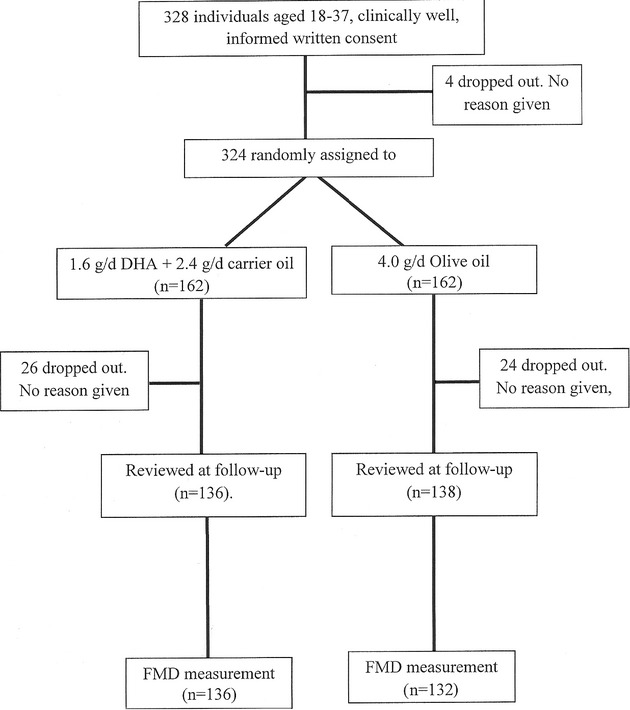
Flowchart of participants through trial. DHA indicates docosahexaenoic acid; FMD, flow‐mediated endothelium‐dependent vasodilation.
Control and DHA‐supplemented groups were closely matched for demographic, anthropometric, and socioeconomic variables; biochemical cardiovascular risk factors; and vascular measures at baseline (Tables 1 and 2). There was evidence to suggest high compliance with DHA supplementation. DHA‐supplemented individuals had higher red cell DHA concentration at follow‐up than did control subjects (Table 3), and more individuals in the DHA‐supplemented group (87%, n=114/130) had an increase in their red cell omega‐3 index35 ([DHA+EPA]/total fatty acids) compared with controls (53%, n=70/130) (P<0.0001).
Table 2.
Vascular Variables at Baseline
| Control | DHA Supplemented | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | |||||||
| N | 162 | 65 | 97 | 162 | 55 | 107 | ||||||
| Brachial artery | ||||||||||||
| Diameter, mm | 3.2 | 0.6 | 3.7 | 0.4 | 2.8 | 0.3 | 3.2 | 0.6 | 3.8 | 0.4 | 2.9 | 0.4 |
| Reactive hyperemia % | 713 | 249 | 613 | 232 | 778 | 240 | 759 | 276 | 665 | 216 | 808 | 292 |
| Flow‐mediated dilation, mm | 0.27 | 0.1 | 0.27 | 0.1 | 0.27 | 0.1 | 0.27 | 0.1 | 0.26 | 0.1 | 0.28 | 0.1 |
| Flow‐mediated dilation, % | 8.3 | 3.7 | 7.1 | 3.3 | 9.1 | 3.7 | 8.5 | 3.7 | 6.8 | 3.2 | 9.3 | 3.7 |
| Distention,* mm | 0.08 | 0.04 | 0.09 | 0.04 | 0.08 | 0.04 | 0.09 | 0.04 | 0.1 | 0.04 | 0.09 | 0.04 |
| Distention,* % | 11.4 | 5.4 | 10.0 | 3.8 | 12.5 | 6.1 | 13.2 | 5.8 | 11.1 | 4.3 | 14.0 | 6.1 |
| Distensibilty coefficient,* ×10−3 kPa−1 | 8.4 | 3.9 | 7.3 | 2.7 | 9.2 | 4.4 | 9.8 | 4.7 | 8.8 | 5.5 | 10.3 | 4.2 |
| Right common carotid | ||||||||||||
| Diameter, mm | 6.5 | 0.5 | 6.8 | 0.5 | 6.3 | 0.5 | 6.5 | 0.5 | 6.8 | 0.5 | 6.3 | 0.4 |
| Distention, mm | 0.7 | 0.1 | 0.7 | 0.2 | 0.7 | 0.1 | 0.7 | 0.2 | 0.7 | 0.2 | 0.7 | 0.1 |
| Distention, % | 11.6 | 2.4 | 11.0 | 2.5 | 12.0 | 2.4 | 11.1 | 2.7 | 11.1 | 3.4 | 11.2 | 2.4 |
| Distensibilty coefficient, % (×10−3 kPa−1) | 33.8 | 7.3 | 30.3 | 6.2 | 36.3 | 7.0 | 34.5 | 10.3 | 33.0 | 14.4 | 35.3 | 7.5 |
| Carotid intima–media thickness, mm | 0.54 | 0.08 | 0.56 | 0.1 | 0.53 | 0.07 | 0.54 | 0.07 | 0.56 | 0.08 | 0.54 | 0.07 |
| Carotid–radial PWV, m/s | 8.6 | 1.2 | 8.9 | 1.1 | 8.3 | 1.2 | 8.6 | 1.4 | 8.6 | 1.2 | 8.6 | 1.4 |
Data are mean, SD except: mean, SD, and range. There is <5% loss of n for some variables. DHA indicates docosahexaenoic acid; PWV, pulse wave velocity.
N=110 in control and DHA‐supplemented groups.
Table 3.
Red Blood Cell Fatty Acid Concentrations Preintervention and Postintervention
| Control | DHA Supplemented | Comparison of Randomized Groups (P Value) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | All | Male | Female | |||||||
| N* | |||||||||||||||
| Preintervention | 156 | 65 | 91 | 160 | 54 | 106 | |||||||||
| Postintervention | 135 | 56 | 79 | 131 | 50 | 81 | |||||||||
| Docosohexenoic acid,† % | |||||||||||||||
| Preintervention | 5.3 | 1.7 | 5.2 | 1.8 | 5.3 | 1.6 | 5.5 | 1.7 | 5.3 | 1.8 | 5.6 | 1.6 | 0.3 | 0.8 | 0.3 |
| Postintervention | 5.3 | 1.8 | 5.1 | 1.8 | 5.4 | 1.7 | 8.9 | 2.6 | 8.8 | 2.7 | 8.9 | 2.5 | <0.0001 | <0.0001 | <0.0001 |
| Eicosapentenoic acid,† % | |||||||||||||||
| Preintervention | 1.0 | 0.6 | 1.1 | 0.6 | 0.9 | 0.5 | 1.0 | 0.5 | 1.0 | 0.6 | 1.0 | 0.5 | 0.7 | 0.5 | 0.2 |
| Postintervention | 0.9 | 0.5 | 1.0 | 0.5 | 0.9 | 0.5 | 1.3 | 0.6 | 1.2 | 0.6 | 1.4 | 0.6 | <0.0001 | 0.01 | <0.0001 |
| Omega‐3 index† | |||||||||||||||
| Preintervention | 6.2 | 2.1 | 6.2 | 2.3 | 6.3 | 2.0 | 6.5 | 2.1 | 6.3 | 2.3 | 6.6 | 2.0 | 0.3 | 0.9 | 0.2 |
| Postintervention | 6.2 | 2.2 | 6.1 | 2.2 | 6.3 | 2.2 | 10.2 | 3.0 | 10.0 | 3.1 | 10.3 | 2.9 | <0.0001 | <0.0001 | <0.0001 |
| Omega 3 index ≥8%, ‡%, n | |||||||||||||||
| Preintervention | 19 | 30 | 17 | 11 | 21 | 19 | 22 | 35 | 22 | 12 | 22 | 23 | 0.3 | 0.3 | 0.5 |
| Postintervention | 16 | 22 | 16 | 9 | 16 | 13 | 76 | 100 | 70 | 35 | 80 | 65 | <0.0001 | <0.0001 | <0.0001 |
DHA indicates docosahexaenoic acid; omega‐3 index, docosahexenoic+eicosapentenoic/total fatty acids.
Red cell fatty acids concentrations were successfully obtained in 98% (316/324) of participants at randomization and 97% (266/274) postintervention.
†Data are mean, SD % of total fatty acids except ‡percent and number of individuals.
The study completion rates were high (>80%) for both randomized groups and sexes (Figure 1 and Table 3). Individuals who did not complete the study did not significantly differ from those who did in age, sex, anthropometry, socioeconomic status, vascular measures such as blood pressure or FMD, baseline biochemistry, or red cell DHA concentrations (data not presented).
There were no serious adverse events in either dietary group and both diets were well tolerated. No participant dropped out of the study due to adverse effects associated with either dietary supplement (the most common reason for dropping out was social). Tolerance data were obtained in 133 and 129 participants from control and DHA‐supplemented groups, respectively. Most study participants did not experience any adverse events (86/133 and 84/129 in control and DHA‐supplemented groups, respectively). The most common problems were gastrointestinal, but the incidence of these was similar in both randomized groups (23% [n=31/133] and 25% [n=32/129] in control and DHA‐supplemented groups, respectively) (for specific symptoms in control and DHA‐supplemented groups, respectively: abdominal pain, n=3 and 5; nausea, n=2 and 5; bloating, n=6 and 7; flatulence, n=12 and 9; diarrhea, n=4 and 4; and constipation, n=4 and 2).
Compared with controls, absolute FMD of the brachial artery adjusted for baseline diameter was significantly lower in the DHA‐supplemented group (mean difference −0.03 mm, 95% CI −0.005 to −0.06 mm, P=0.02) (Table 4). This difference remained after further adjustment for potential confounding factors (age, sex, skin temperature, room temperature. and fasting concentrations of LDL cholesterol and triglycerides) (mean difference −0.03 mm, 95% CI −0.005 to −0.06 mm, P=0.02). Randomized groups did not differ in other vascular outcomes, blood pressure, or fasting concentrations of insulin, glucose, or CRP (Table 4). However, fasting concentrations of VLDL and triglycerides were lower in the DHA‐supplemented compared with the control group (mean difference for VLDL: −28%, 95% CI −40% to −15%, P<0.0001; mean difference for triglycerides: −28%, 95% CI −40% to −15%, P<0.0001). Similar findings were obtained using nonparametric statistics for both VLDL (median, interquartile range: 0.4, 0.3 to 0.5 mmol/L in controls versus 0.3, 0.2 to 0.4 mmol/L in DHA supplemented; Mann–Whitney U test: P<0.0001) and triglyceride concentration (median, interquartile range: 0.8, 0.6 to 1.2 mmol/L in controls versus 0.6, 0.4 to 0.9 mmol/L in DHA‐supplemented; Mann–Whitney U test: P<0.0001).
Table 4.
Vascular Variables and Cardiovascular Risk Factors Postintervention
| Control | DHA Supplemented | Comparison of Randomized Groups (P Value) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | All | Male | Female | |||||||
| Completed study,* %, n | 85 | 138 | 88 | 57 | 84 | 81 | 84 | 136 | 91 | 50 | 80 | 86 | 0.4 | 0.4 | 0.4 |
| Brachial artery | |||||||||||||||
| Diameter, mm | 3.2 | 0.5 | 3.7 | 0.3 | 2.9 | 0.3 | 3.2 | 0.6 | 3.8 | 0.4 | 2.9 | 0.3 | 0.9 | 0.3 | 0.3 |
| Reactive hyperemia, % | 713 | 232 | 632 | 253 | 770 | 200 | 735 | 243 | 672 | 228 | 773 | 246 | 0.5 | 0.4 | 0.9 |
| Flow‐mediated dilation, mm | 0.29 | 0.1 | 0.32 | 0.1 | 0.27 | 0.1 | 0.26 | 0.1 | 0.25 | 0.1 | 0.27 | 0.1 | 0.02 | 0.002 | 0.8 |
| Flow‐mediated dilation, % | 8.6 | 3.5 | 7.9 | 3.6 | 9.1 | 3.3 | 8.0 | 4.0 | 6.3 | 3.1 | 9.0 | 4.2 | 0.2 | 0.01 | 0.9 |
| Distention,ߤ mm | 0.09 | 0.04 | 0.1 | 0.03 | 0.08 | 0.04 | 0.09 | 0.04 | 0.1 | 0.04 | 0.08 | 0.04 | 0.5 | 0.2 | 0.9 |
| Distention,ߤ % | 12.6 | 6.9 | 11.1 | 6.9 | 13.8 | 6.7 | 12.6 | 5.4 | 11.5 | 4.5 | 13.3 | 5.7 | 0.9 | 0.8 | 0.6 |
| Distensibilty coefficientߤ (×10−3 kPa−1) | 8.9 | 4.0 | 7.5 | 2.5 | 10.0 | 4.7 | 9.2 | 3.7 | 8.3 | 3.2 | 9.7 | 3.9 | 0.6 | 0.2 | 0.7 |
| Right common carotid | |||||||||||||||
| Diameter, mm | 6.5 | 0.5 | 6.7 | 0.5 | 6.3 | 0.4 | 6.4 | 0.5 | 6.8 | 0.4 | 6.2 | 0.4 | 0.4 | 0.4 | 0.2 |
| Distention, mm | 0.7 | 0.1 | 0.7 | 0.2 | 0.7 | 0.1 | 0.7 | 0.2 | 0.8 | 0.2 | 0.7 | 0.1 | 0.4 | 0.5 | 0.07 |
| Distention, % | 11.4 | 2.4 | 11.0 | 2.8 | 11.7 | 2.1 | 11.2 | 2.5 | 11.2 | 3.0 | 11.3 | 2.2 | 0.6 | 0.7 | 0.2 |
| Distensibilty coefficient (×10−3 kPa−1) | 34.1 | 7.8 | 30.2 | 6.6 | 36.9 | 7.4 | 34.0 | 7.5 | 31.3 | 7.0 | 35.6 | 7.3 | 0.9 | 0.4 | 0.3 |
| Intima–media thickness, mm | 0.55 | 0.09 | 0.56 | 0.1 | 0.5 | 0.07 | 0.55 | 0.07 | 0.56 | 0.06 | 0.5 | 0.07 | 0.7 | 0.9 | 0.3 |
| Carotid radial PWV, m/s | 8.6 | 1.3 | 9.0 | 1.3 | 8.3 | 1.2 | 8.5 | 1.5 | 8.8 | 1.3 | 8.4 | 1.6 | 0.8 | 0.3 | 0.6 |
| Blood pressure, mm Hg | |||||||||||||||
| Systolic | 110 | 11 | 116 | 10 | 105 | 9 | 110 | 11 | 113 | 7 | 108 | 12 | 0.9 | 0.07 | 0.2 |
| Diastolic | 65 | 7 | 68 | 6 | 62 | 7 | 65 | 8 | 65 | 7 | 64 | 9 | 0.9 | 0.05 | 0.1 |
| Mean arterial | 82 | 8 | 87 | 7 | 79 | 7 | 82 | 9 | 83 | 7 | 81 | 10 | 0.7 | 0.02 | 0.2 |
| Pulse pressure | 45 | 7 | 49 | 6 | 43 | 6 | 45 | 6 | 48 | 5 | 43 | 6 | 0.5 | 0.3 | 0.7 |
| Resting heart rate, beats/min | 64 | 9 | 63 | 10 | 65 | 8 | 64 | 9 | 61 | 8 | 65 | 10 | 0.7 | 0.2 | 0.7 |
| Total cholesterol, mmol/L | 4.3 | 0.8 | 4.3 | 0.9 | 4.2 | 0.8 | 4.4 | 0.8 | 4.5 | 0.9 | 4.4 | 0.8 | 0.1 | 0.2 | 0.3 |
| LDL cholesterol, mmol/L | 2.4 | 0.8 | 2.6 | 0.8 | 2.3 | 0.7 | 2.6 | 0.8 | 2.8 | 0.8 | 2.5 | 0.7 | 0.06 | 0.2 | 0.1 |
| HDL cholesterol, mmol/L | 1.4 | 0.3 | 1.3 | 0.3 | 1.5 | 0.3 | 1.5 | 0.4 | 1.4 | 0.3 | 1.6 | 0.4 | 0.08 | 0.04 | 0.6 |
| VLDL cholesterol,ߥ mmol/L | 0.4 | 49 | 0.4 | 50 | 0.4 | 50 | 0.3 | 54 | 0.3 | 54 | 0.3 | 54 | <0.0001 | 0.01 | 0.001 |
| Total:HDL cholesterol ratio | 3.2 | 1.0 | 3.5 | 1.0 | 2.9 | 0.9 | 3.1 | 1.0 | 3.4 | 1.0 | 3.0 | 1.0 | 0.8 | 0.6 | 0.7 |
| LDL:HDL cholesterol ratio | 1.8 | 0.8 | 2.1 | 0.9 | 1.6 | 0.7 | 1.9 | 0.9 | 2.1 | 0.9 | 1.7 | 0.8 | 0.6 | 0.9 | 0.3 |
| Triglyceride,ߥ mmol/L | 0.8 | 49 | 0.9 | 49 | 0.8 | 49 | 0.6 | 54 | 0.7 | 54 | 0.6 | 54 | <0.0001 | 0.009 | 0.001 |
| Glucose, mmol/L | 4.8 | 0.5 | 4.9 | 0.4 | 4.6 | 0.5 | 4.7 | 0.6 | 4.9 | 0.7 | 4.6 | 0.5 | 0.7 | 0.7 | 0.9 |
| Insulin,ߥ mU/L | 27.7 | 55 | 28.5 | 58 | 27.0 | 54 | 29.3 | 61 | 29.0 | 67 | 29.5 | 58 | 0.4 | 0.9 | 0.3 |
| Insulin resistance (HOMA),ߥ μm/L | 0.8 | 58 | 0.8 | 58 | 0.8 | 59 | 0.9 | 68 | 0.9 | 74 | 0.8 | 64 | 0.4 | 0.8 | 0.4 |
| CRP,ߥ mg/L | 1.0 | 114 | 1.0 | 110 | 1.1 | 118 | 1.0 | 123 | 0.6 | 111 | 1.2 | 123 | 0.7 | 0.06 | 0.6 |
Data are mean, SD except *% (n) and ‡geometric mean (coefficient of variation). There is <10% loss of n for some variables except †n=105. DHA indicates docosahexaenoic acid; PWV, pulse wave velocity; LDL, low‐density lipoprotein; HDL, high‐density lipoprotein; VLDL, very low‐density lipoprotein; HOMA, homeostatic model assessment; CRP, C‐reactive protein.
In secondary analyses, the lower FMD with DHA supplementation was shown to be confined to men (Table 4) (randomized dietary group×sex interaction: P=0.01). Similarly, compared with controls, blood pressure was lower in DHA‐supplemented men but not women (Table 4) (P values for randomized group×sex interaction for all measures of blood pressure were <0.04). However, there was no evidence to suggest that FMD varied according to smoking status, being an Asp298 carrier, or according to risk factors for CVD at baseline (randomization) (data not presented). The effect of DHA supplementation on fasting triglyceride concentration was independent of sex (randomized dietary group×sex interaction: P=0.9) but was greater in those with a higher triglyceride concentration at baseline (randomized dietary group×triglyceride concentration at baseline interaction: P=0.04).
Analysis of intraindividual change in FMD between visit 2 (postintervention) and visit 1 (baseline), showed that FMD fell in the DHA supplemented group (mean, SD: −0.34, 3.5%) but increased in the control group (0.47, 3.4%) (mean difference between groups for intraindividual change in FMD%: −0.80%; 95% CI: −1.6% to 0.02%; P=0.05). Similar data were obtained when confining the analysis to men (intraindividual change in FMD% in DHA supplemented group: mean, SD: −0.6, 2.6% compared with controls: 0.8, 3.1%; mean difference between groups: −1.5%; 95% CI −2.6% to −0.4%, P=0.01). There were no significant differences between randomized groups for the intraindividual change in FMD in women (data not presented).
In nonrandomized, epidemiological analyses, for the study population as a whole, there was a dose–response association between red cell DHA concentration and fasting triglyceride concentration (using regression analyses with loge×100 transformed data,37 there was a −4.1% change in triglyceride concentration, per 1% increase in red cell DHA concentration; 95% CI −1.6% to −6.6%, P=0.002) but not for FMD (data not presented).
Discussion
A high dietary intake of n‐3 LC‐PUFA is thought to reduce the risk of CVD, but evidence to support its advantages in healthy populations is relatively scarce.2 In the present study, we investigated the impact of 4 months' DHA supplementation on endothelial function and surrogate vascular measures of atherosclerosis and found no evidence to support a beneficial effect on these key cardiovascular outcomes. In fact, unexpectedly, DHA supplementation appeared to have a small but significant negative effect on endothelial function in men. However, DHA supplementation had favorable effects on blood pressure in men and triglyceride concentration in both sexes. These data therefore raise the possibility that the advantages of a high DHA intake for the primary prevention of CVD1–2 are not a consequence of its effects on endothelial function, but act via benefits on other risk factors for CVD.
Enhancement of endothelial function has been suggested as a key mechanism for the protective effect of n‐3 LC‐PUFAs on the development of atherosclerosis.9–24 However, most previous RCTs have been conducted in patients at high CVD risk and relatively few studies have investigated healthy individuals. Previously, supplementation of 5 g/day of tuna oil for 8 months was shown to improve endothelial function, as measured in forearm skin using laser Doppler, by 17%,17 whereas supplementation with 1 g/day fish oil for 14 days improved both endothelium‐dependent and endothelium‐independent vasodilation of the brachial artery in healthy adults.19 Our data are inconsistent with these reports possibly because these studies were small,17,19 did not have a parallel RCT design,17 investigated endothelial function in resistance vessels (which may correlate poorly with FMD of conduit vessels such as the brachial artery),17 or were conducted in older adults, who are more likely to have subclinical atherosclerosis and impairment of endothelial function.17 However, our findings are consistent with a recent RCT that found no effect of up to 1.8 g/day n‐3 LC‐PUFA supplementation on FMD in middle‐aged adults at moderately increased CVD risk.23 Similarly, in a cross‐sectional analysis in 3045 adults aged 45 to 84 years, the highest quartile of nonfried fish consumption was associated with a 1‐SD lower brachial artery diameter in men and 0.27% smaller FMD in women, compared with the lowest quartlie.38 Collectively, therefore, together with these previous reports,23,38 our data support the hypothesis that although n‐3 LC‐PUFA supplementation may improve endothelial function in individuals with clinical CVD disease13,22 or risk factors such as dyslipidemia and type 2 diabetes,10,21 there is little evidence to suggest a beneficial effect on endothelial function in healthy young adults.
The possible detrimental effect of DHA supplementation on FMD in men was an unexpected finding and requires further investigation. Few previous RCTs have been adequately powered to detect sex differences in effects of n‐3 LC‐PUFAs on endothelial function. However, n‐3 LC‐PUFA supplementation has been shown to increase the concentration of soluble e‐selectin, a marker of endothelial activation, in younger compared with older men.39–40 Moreover, both n‐3 LC‐PUFA metabolism and the association between fish intake and vascular function38 have been shown to vary by sex. Women have greater tissue DHA content than men41 and a higher capacity to metabolize α‐linolenic acid to DHA.42 The clinical implications of these sex differences is unclear, but our finding of 20% lower FMD in DHA‐supplemented men, together with earlier data showing detrimental effects of n‐3 LC‐PUFA on endothelial activation in men,39–40 suggests that future trials of n‐3 LC‐PUFA need to be powered to detect possible sex differences in cardiovascular outcomes.
As in previous reports, we found no effect of DHA supplementation on vascular measures such as carotid IMT29 and arterial stiffness,23 or on CVD risk factors such as CRP concentration.5 There was, however, a small benefit of DHA supplementation on mean arterial blood pressure in men but not in women. Although the reasons for the sex difference are uncertain, our data are consistent with earlier studies and meta‐analyses,6 which showed that low to moderate doses of DHA can lower blood pressure in healthy, normotensive individuals without changes in endothelial function or arterial stiffness.43
Consistent with previous reports, DHA supplementation reduced triglyceride concentration.5,7–8 However, importantly, this effect was seen in a healthy, young population with triglyceride levels in the normal range. The size of the effect (27% lower triglyceride concentration) was similar to data from systematic reviews in older adults5,7–8 that investigated higher n‐3 LC‐LCPUFA doses (≈2.6 g/day8). Furthermore, in contrast to the recent opinion from the European Food Safety Authority, which suggested that 3 to 4 g/day DHA supplementation reduced triglyceride concentrations (and >2 g/day DHA was required for a beneficial effect44), we found similar benefits at lower DHA intakes, within the dietary range for n‐3 LC‐PUFA consumption. As in older adults, there was also a dose–response association between red cell DHA and triglyceride concentration, and a greater benefit of DHA supplementation in individuals with higher presupplementation triglyceride levels.5,7–8 Although the clinical implications of lower triglycerides in young adults is uncertain, hypertriglyceridemia has been proposed as an independent risk factor for CVD45–46 and a target for interventions to reduce cardiovascular risk.47 The cumulative effect of higher DHA intake on triglyceride levels may therefore help in the primary prevention of CVD. The mechanisms for this effect are unknown, but lower VLDL concentration with DHA supplementation in the present study was consistent with the hypothesis that n‐3 LC‐PUFAs decrease the hepatic production of triglyceride‐rich particles.5
The main strengths of our study were a parallel‐group, double‐blind, RCT design; large sample size; objective evidence of good compliance with dietary intervention; and objective, validated outcomes such as FMD. However, our study has several potential limitations. First, we studied a young population at low risk of CVD in whom DHA supplementation may not improve further an already largely healthy endothelium. Nonetheless, the study population was similar to our previous cross‐sectional study, which found strong associations between red cell DHA concentration and brachial artery FMD,16 and although mean values were within the normal range, many participants had evidence of endothelial dysfunction and high levels of CVD risk factors. Moreover, the lack of any effect of DHA supplementation even in those in the highest third of the distribution for CVD risk factors, together with previous data from older adults at moderate CVD risk,23 suggests that DHA supplementation is unlikely to improve endothelial function in healthy adults regardless of underlying cardiovascular risk.
Second, to aid compliance, DHA supplements were given for only 4 months. Although longer periods of supplementation may be necessary for effects on some measures of vascular structure such as carotid IMT,29 previously the effects of n‐3 LC‐PUFA supplementation on endothelial function have been suggested in interventions ranging from 2 weeks19 to 8 months.17 Furthermore, for some outcomes such as triglyceride concentration, study duration (between 4 weeks and 2 years) is not associated with the treatment effect, suggesting that maximal effect is achieved after a shorter intervention and then maintained throughout the intervention period.5 Third, because previous studies suggested stronger effects of DHA than EPA on vascular function,14,16–17 we supplemented with only DHA and not also with EPA. However, the independent vascular effects of EPA and DHA are poorly understood and require further investigation.30
Finally, control and index groups differed in both amounts of DHA and olive oil supplemented and therefore the study does not exclude an effect of both olive oil and DHA on study outcomes. For instance, although the effects of different oils on vascular function are currently inconclusive,48 higher olive oil intake in the control group could have improved FMD and contributed to postintervention differences in FMD between randomized groups. Alternatively, the increase in FMD over the 4‐month intervention in the control group may be a consequence of changes to behavior and lifestyle (“Hawthorne effect”) in individuals motivated by participating in a study of cardiovascular health. However, neither of these possibilities invalidates the main observation that DHA supplementation did not improve endothelial function in this population.
To our knowledge, the present study is the largest RCT to test the impact of n‐3 LC‐PUFA supplementation on endothelial function and CVD risk factors in a healthy, young population. Our data support the hypothesis that higher DHA consumption has benefits for CVD risk factors such as blood pressure and triglyceride concentration. However, the effects of DHA are unlikely to include benefits for endothelial function, at least with n‐3 LC‐PUFA intake within the dietary range.
Sources of Funding
The study was funded by the Medical Research Council (UK) and a charitable contribution from Kellogg's PLc. Dietary supplements were supplied by Martek Biosciences Corporation (DE, USA). Professor Singhal is supported by the Great Ormond Street Hospital Children's Charity.
Disclosures
None.
References
- 1.He K, Song Y, Davilgus MI, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta‐analysis of cohort studies. Circulation. 2004; 109:2705-2711 [DOI] [PubMed] [Google Scholar]
- 2.Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega‐3 fatty acids. Lancet. 2010; 376:540-550 [DOI] [PubMed] [Google Scholar]
- 3.Filion KB, Khoury FE, Bielinski M, Schiller I, Dendukuri N, Brophy JM. Omega‐3 fatty acids in high‐risk cardiovascular patients: a meta‐analysis of randomized controlled trials. BMC Cardiovasc Disord. 2010; 10:24-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006; 9:95-104 [DOI] [PubMed] [Google Scholar]
- 5.Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effect of omega‐3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006; 189:19-30 [DOI] [PubMed] [Google Scholar]
- 6.Cicero AFG, Ertek S, Borghi C. Omega‐3 polyunsaturated fatty acids: their potential role in blood pressure prevention and management. Curr Vasc Pharmocol. 2009; 7:330-337 [DOI] [PubMed] [Google Scholar]
- 7.Ryan AS, Keske MA, Hoffman JP, Nelson EB. Clinical overview of algal‐docosahexaenoic acid: effects on triglyceride levels and other cardiovascular risk factors. Am J Ther. 2009; 16:183-192 [DOI] [PubMed] [Google Scholar]
- 8.Musa‐Velosa K, Binns MA, Kocenas AC, Poon T, Elliot JA, Rice H, Oppedal‐Olsen H, Llyod H, Lemke S. Long chain omega‐3 fatty acids eicosapentaenoic acid and docosahexaenoic acid dose‐dependently reduce fasting serum triglycerides. Nutr Rev. 2010; 68:155-167 [DOI] [PubMed] [Google Scholar]
- 9.Fleischhauer FJ, Yan WD, Fischell TA. Fish oil improves endothelial‐dependent coronary vasodilation in heart transplant recipients. J Am Coll Cardiol. 1993; 21:982-989 [DOI] [PubMed] [Google Scholar]
- 10.McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR. Dietary fish oil augments nitric oxide production or release in patients with type 2 (non‐insulin‐dependent) diabetes mellitus. Diabetologia. 1993; 36:33-38 [DOI] [PubMed] [Google Scholar]
- 11.Chin JP, Dart AM. Therapeutic restoration of endothelial function in hypercholesterolaemic subjects: effect of fish oils. Clin Exp Pharmacol Physiol. 1994; 21:749-755 [DOI] [PubMed] [Google Scholar]
- 12.Goode GK, Garcia S, Heagerty AM. Dietary supplementation with marine fish oil improves in vitro small artery endothelial function in hypercholesterolemic patients: a double‐blind placebo‐controlled study. Circulation. 1997; 96:2802-2807 [DOI] [PubMed] [Google Scholar]
- 13.Johansen O, Seljeflot I, Hostmark AT, Arnesen H. The effect of supplementation with omega‐3 fatty acids on soluble markers of endothelial function in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. 1999; 19:1681-1686 [DOI] [PubMed] [Google Scholar]
- 14.Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. 2000; 102:1264-1269 [DOI] [PubMed] [Google Scholar]
- 15.Goodfellow J, Bellamy MF, Ramsey MW, Jones CJH, Lewis MJ. Dietary supplementation with marine omega‐3 fatty acids improves systemic large artery endothelial function in subjects with hypercholesterolemia. J Am Coll Cardiol. 2000; 35:265-270 [DOI] [PubMed] [Google Scholar]
- 16.Leeson CPM, Mann A, Kattenhorn M, Deanfield JE, Lucas A, Muller DPR. Relationship between circulating n‐3 fatty acid concentrations and endothelial function in early adulthood. Eur Heart J. 2002; 23:216-222 [DOI] [PubMed] [Google Scholar]
- 17.Khan F, Elherik K, Bolton‐smith C, Barr R, Hill A, Murrie I, Belch JJF. The effects of dietary fatty acid supplementation on endothelial function and vascular tone in healthy subjects. Cardiovasc Res. 2003; 59:955-962 [DOI] [PubMed] [Google Scholar]
- 18.Engler MM, Engler MB, Malloy M, Chiu F, Besio D, Paul S, Stuehlinger M, Morrow J, Ridker P, Rifai N, Mietus‐Snyder M. Docosahexaenoic acid restores endothelial function in children with hyperlipidemia: results from the EARLY study. Int J Clin Pharmocol Ther. 2004; 42:672-679 [DOI] [PubMed] [Google Scholar]
- 19.Shah AP, Ichiuji AM, Han JK, Traina M, El‐Bialy A, Meymandi SK, Wachsner RY. Cardiovascular and endothelial effects of fish oil supplementation in healthy volunteers. J Cardiovasc Pharmacol Ther. 2007; 12:213-219 [DOI] [PubMed] [Google Scholar]
- 20.Skulas‐Ray AC, Kris‐Etherton PM, Harris WS, Heuvel JPV, Wagner PR, West SG. Dose‐response effects of omega‐3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr. 2011; 93:243-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egert S, Stehle P. Impact of n‐3 fatty acids on endothelial function: results from human intervention studies. Curr Opin Clin Nutr Metab Care. 2011; 14:121-131 [DOI] [PubMed] [Google Scholar]
- 22.Haberka M, Mizia‐Stec K, Mizia M, Janowska J, Gieszczyk K, Chmiel A, Zahorska‐Markiewicz B, Gasior Z. N‐3 polyunsaturated fatty acids early supplementation improves ultrasound indices of endothelial function, but not through NO inhibitors in patients with acute myocardial infarction. Clin Nutr. 2011; 30:79-85 [DOI] [PubMed] [Google Scholar]
- 23.Sanders TAB, Hall WL, Maniou Z, Lewis F, Seed PT, Chowienczyk PJ. Effect of low doses of long‐chian n‐3 PUFAs on endothelial function and arterial stiffness: a randomised controlled trial. Am J Clin Nutr. 2011; 94:973-980 [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Lian X, Wang L, Lu X, Huang J, Cao J, Li H, Gu D. Effect of omega‐3 fatty acid supplementation on endothelial function: a meta‐analysis of randomised controlled trials. Atherosclerosis. 2012; 221:536-543 [DOI] [PubMed] [Google Scholar]
- 25.Charakida M, Deanfield JE, Halcox JP. The role of nitric oxide in early atherosclerosis. Eur J Clin Pharmacol. 2006; 62:69-78 [Google Scholar]
- 26.Leeson CPM, Hingorani AD, Mullen MJ, Jeerooburkhan N, Kattenhorn M, Cole TJ, Muller DPR, Lucas A, Humphries SE, Deanfield JE. Glu298ASP endothelial nitric oxide synthase gene polymorphism interacts with environmental and dietary factors to influence endothelial function. Circ Res. 2002; 90:1153-1158 [DOI] [PubMed] [Google Scholar]
- 27.He K, Liu K, Daviglus ML, Jenny NS, Mayer‐Davis E, Jiang R, Steffen L, Siscovick D, Tsai M, Herrington D. Associations of dietary long‐chain n‐3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi‐Ethnic study of atherosclerosis [MESA]). Am J Cardiol. 2009; 103:1238-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomiyama H, Yamashina A. Non‐invasive vascular function tests: their pathophysiological background and clinical application. Circ J. 2010; 74:24-33 [DOI] [PubMed] [Google Scholar]
- 29.Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega‐3‐fatty acids on coronary restenosis, intima‐media thickness, and exercise tolerance: a systematic review. Atherosclerosis. 2006; 184:237-246 [DOI] [PubMed] [Google Scholar]
- 30.Cottin SC, Sanders TA, Hall WL. The differential effects of EPA and DHA on cardiovascular risk factors. Proc Nutr Soc. 2011; 70:215-231 [DOI] [PubMed] [Google Scholar]
- 31.Harris WS, Kris‐Ertherton PM, Harris KA. Intakes of long‐chain omega‐3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults. Curr Atheroscler Rep. 2008; 10:503-509 [DOI] [PubMed] [Google Scholar]
- 32.Donald AE, Charakida M, Falaschetti E, Lawlor DA, Halcox JP, Golding J, Hingorani AD, Davey Smith G, Deanfield JE. Determinants of vascular phenotype in a large childhood population: the Avon Longitudinal Study of Parents and Children (ALSPAC). Eur Heart J. 2010; 31:1502-1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang YL, Teede H, Kotsopoulos D, Shiel L, Cameron JD, Dart AM, McGrath BP. Non‐invasive measurements of arterial structure and function: repeatability, interrelationships and trial sample size. Clin Sci. 1998; 95:669-679 [DOI] [PubMed] [Google Scholar]
- 34.Halcox JPJ, Donald AE, Ellins E, Witte DR, Shipley MJ, Brunner EJ, Marmot MG, Deanfield JE. Endothelial function predicts progression of carotid intima‐media thickness. Circulation. 2009; 119:1005-1012 [DOI] [PubMed] [Google Scholar]
- 35.Harris WS, Von Schacky C. The omega‐3 index: a new risk factor for death from coronary heart disease. Prev Med. 2004; 39:212-220 [DOI] [PubMed] [Google Scholar]
- 36.Kohler A, Bittner D, Low A, von Schacky C. Effects of n‐3 convenience drink fortified with n‐3 fatty acids on the n‐3 index. Br J Nutr. 2010; 104:729-736 [DOI] [PubMed] [Google Scholar]
- 37.Cole TJ. Sympercents: symmetric percentage differences on the 100 loge scale simplify the presentation of log transformed data. Stat Med. 2000; 19:3109-3125 [DOI] [PubMed] [Google Scholar]
- 38.Anderson JS, Nettleton JA, Herrington DM, Johnson WC, Tsai MY, Siscovick D. Relation of omega‐3 fatty acid and dietary fish intake with brachial artery flow‐mediated vasodilation in the multi‐ethnic study of atherosclerosis. Am J Clin Nutr. 2010; 92:1204-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miles EA, Thies F, Wallace FA, Powell JR, Hurst TL, Newsholme EA, Calder PC. Influence of age and dietary fish oil on plasma soluble adhesion molecule concentrations. Clin Sci. 2001; 100:91-100 [PubMed] [Google Scholar]
- 40.Cazzola R, Russo‐Volpe S, Miles EA, Rees D, Banerjee T, Roynette CE, Wells SJ, Goua M, Wahle KWJ, Calder PC, Cestaro B. Age‐ and dose‐dependent effects of an eicosapentaenoic acid‐rich oil on cardiovascular risk factors in healthy male subjects. Atherosclerosis. 2007; 193:159-167 [DOI] [PubMed] [Google Scholar]
- 41.Crowe FL, Skeaff CM, Green TJ, Gray AR. Serum n‐3 long‐chain PUFA differ by sex and age in a population based survey of New Zealand adolescents and adults. Br J Nutr. 2008; 99:168-174 [DOI] [PubMed] [Google Scholar]
- 42.Burdge GC, Wootton SA. Conversion of alpha‐linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002; 88:411-420 [DOI] [PubMed] [Google Scholar]
- 43.Theobold HE, Goodall AH, Sattar N, Talbot DCS, Chowiencyk PJ, Sanders TAB. Low‐dose docosahexaenoic acid lowers diastolic blood pressure in middle‐aged men and women. J Nutr. 2007; 137:973-978 [DOI] [PubMed] [Google Scholar]
- 44.European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies Scientific opinion on the substantiation of health claims related to docosahexaenoic acid (DHA). EFSA J. 2010; 8:173410.2903/j.efsa.2010.1734 [Google Scholar]
- 45.Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Triglyceride concentration and ischemic heart disease: an eight year follow‐up in the Copenhagen Male Study. Circulation. 1998; 97:1029-1036 [DOI] [PubMed] [Google Scholar]
- 46.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high density lipoprotein cholesterol level: a meta‐analysis of population‐based prospective studies. J Cardiovasc Risk. 1996; 3:213-219 [PubMed] [Google Scholar]
- 47.European Society of Cardiology European guidelines on cardiovascular disease prevention in clinical practice. Eur J Cardiovasc Prev Rehabil. 2007; 14suppl:E1-E40 [DOI] [PubMed] [Google Scholar]
- 48.Vafeiadou K, Weech M, Sharma V, Yaqoob P, Todd S, Williams CM, Jackson KG, Lovegrove JA. A review of the evidence for the effects of total dietary fat, saturated, monounsaturated and n‐6 polyunsaturated fatty acids on vascular function, endothelial progenitor cells and microparticles. Br J Nutr. 2012; 107:303-324 [DOI] [PubMed] [Google Scholar]


