Abstract
This study aimed to examine the frequency of different subsets of circulating B and T follicular helper (Tfh) cells in patients with new-onset rheumatoid arthritis (RA) and following standard therapies. Twenty-five RA patients and 15 healthy controls (HC) were recruited for characterizing the frequency of CD27+, immunoglobulin (Ig)D+, CD86+, CD95+, Toll-like receptor (TLR)-9+ B cells and inducible T cell co-stimulator (ICOS) and programmed death 1 (PD-1)-positive Tfh cells and the level of serum interleukin (IL)-21. The potential correlation between the frequency of different subsets of B and Tfh cells and the values of clinical measures in RA patients was analysed. In comparison with HC, significantly higher percentages of circulating IgD+CD27−CD19+ naive B, CD86+CD19+ and CD95+CD19+ activated B, CD3+CD4+CXCR5+, CD3+CD4+CXCR5+ICOS+, CD3+CD4+CXCR5+PD-1+ and CD3+CD4+CXCR5+ICOS+PD-1+ Tfh cells but lower IgD+CD27+CD19+ preswitch memory B cells were detected, accompanied by significantly higher levels of serum IL-21 in the RA patients. Furthermore, the percentages of CD95+ B cells were correlated positively with the frequency of PD-1+ Tfh cells, but negatively with ICOS+ Tfh cells. The percentages of CD86+ B cells and ICOS+ Tfh cells were correlated positively with the values of disease activity score 28 (DAS28). Following the drug therapies for 1 month, the percentages of CD86+ B and PD-1+ Tfh cells were reduced significantly in the drug-responding patients. Our data suggest that activated B and Tfh cells may contribute to the pathogenesis of RA and the frequency of activated B and Tfh cells may be used as biomarkers for evaluating the therapeutic responses of individual patients with RA.
Keywords: B cells, CD86, RA, Tfh cells, therapy
Introduction
Rheumatoid arthritis (RA) is a severe chronic autoimmune inflammatory disease. RA is characterized by symmetric polyarthritis associated with pain and swelling in multiple joints. Importantly, most RA patients eventually develop cartilage lesions and bone destruction, leading to functional incapacity. In addition, RA patients are affected by an increased frequency of other co-morbidities and decreased life expectancy [1]. Currently, the pathogenic process of RA is still unclear. The pathogenesis of RA is attributed to the interaction of many types of immunocompetent cells, such as antigen-specific T and B cells, aberrant activation of antigen-presenting cells (APC) and autoantibodies [2]. Although antigen-specific T cells are crucial for the pathogenesis of RA, recent evidence suggests that B cells play an important role in the development and progression of RA [3].
CD27 is expressed on somatically mutated B cells and the distinct subsets of B cells can be defined as naive immunoglobulin (Ig)D+CD27−, preswitch memory IgD+CD27+, post-switch memory IgD−CD27+ and double-negative IgD−CD27− B cells [4,5]. Activation of B cells up-regulates CD86, CD95 and major histocompatibility complex (MHC) class II expression and some activated B cells differentiate into plasma cells which express CD38 [6], while others become memory B cells which express CD27 [5]. The up-regulated CD95 expression in activated B cells makes them sensitive to ligand-mediated apoptosis [7,8]. However, little is known about the frequency of these different subsets of activated B cells in patients with new-onset RA.
The activation and functional differentiation of B cells are regulated by CD4+ T cells, particularly by T follicular helper (Tfh) cells [9,10]. Tfh cells are characterized by increased levels of CXCR5, inducible co-stimulator (ICOS), programmed death-1 (PD-1), CD40-ligand (CD40L) and transcription factor Bcl-6 expression, and secreting interleukin (IL)-21, which are important for their function [11–14]. Tfh cells can enter the follicle and secrete cytokines and other molecules to help the formation of germinal centre (GC), high-affinity long-living plasma cells and memory B cells [15,16]. A previous study has shown a higher frequency of Tfh cells and increased levels of anti-CCP antibodies in patients with new-onset RA [17].
However, how Tfh cells are associated with different stages of differentiated B cells in the pathogenesis of RA is not fully understood. In addition, how these immunocompetent cells respond to the commonly used therapies of disease-modifying anti-rheumatic drugs (DMARDs), such as methotrexate (MTX) and Tripterygium wilfordii in RA patients has not been clarified. T. wilfordii, a Chinese herb, has potent immunosuppressive activity and has been used for the treatment of RA in the clinic for some time [18,19]. In the current study, we characterized the frequency of Tfh and different stages of differentiated B cells in 25 patients with new-onset RA and 1 month after therapies with T. wilfordii and DMARDs as well as 15 gender- and age-matched healthy controls. Our findings suggest that activated B and Tfh cells may contribute to the pathogenesis of RA and the frequency of activated B and Tfh cells may be used as a biomarker for evaluating the therapeutic responses of individual patients with RA.
Material and methods
Patients and controls
A total of 25 patients with new-onset RA (<6 months of disease duration) were recruited sequentially at the in-patient service of the First Hospital and China–Japan Union Hospital of Jilin University from February 2013 to May 2013. Another 15 gender-, age- and ethnicity-matched HC were recruited during the same period and they had no history of any chronic inflammatory disease. Individual patients with RA were diagnosed according to the diagnosis criteria established by the American College of Rheumatology [20] and the disease severity of individual patients was evaluated using the disease activity score 28 (DAS28) [21]. Individual RA patients were excluded if she/he received treatment with DMARDs, corticosteroids or immunosuppressive for any reason during the past 6 months or had other chronic inflammatory and autoimmune diseases, such as diabetes, multiple sclerosis, inflammatory bowel disease, metabolic syndrome, hypertension, cardiovascular diseases, cancer or recent infection. Written informed consent was obtained from individual subjects and the experimental protocol was approved by the Ethical Committee of the First Hospital of Jilin University.
Demographic and clinical characteristics, including age and gender, were recoded by physicians and are shown in Table 1. Venous blood samples were taken immediately after enrolment and the patients were treated orally with 10 mg methotrexate weekly (MTX; Shanghai Xinyi Pharmacy, Shanghai, China), with 20 mg Leflunomide daily (Fujian Huitian Pharmacy, Fujian, China) and 60 mg T. wilfordii (Guizhou Han Prescription Pharmacy, Guizhou, China). One month after the beginning of the treatment, their blood samples were collected again for subsequently laboratory examination.
Table 1.
Demographic and clinical characteristics of participants
| Parameters | Healthy controls | RA (0M) | RA (1M-responders) | RA (1M-non-responders) |
|---|---|---|---|---|
| Number of subjects | 15 | 25 | 9 | 4 |
| Age (year) | 48 (36–60) | 54 (37–68) | 54 (39–63) | 57 (48–68) |
| Female n (%) | 11 (73) | 20 (80) | 8 (89) | 3 (75) |
| RF (IU/ml) | 14 (0·3–27) | 164 (0·45–1740)* | 45 (12·4–567)*# | 98 (1·56–1000)* |
| RF (+/−) | n.a. | 21/4 | 6/3 | 2/2 |
| ESR (mm/h) | 10 (3–20) | 35 (5–138)* | 18 (5–37)*# | 25 (5–89)* |
| CRP (mg/dl) | 3·2 (0·4–7·2) | 24 (0·59–221)* | 5·3 (0·77–60·5)*# | 19·4 (4·2–88·5)* |
| Anti-CCP (IU/ml) | 4·21 (0·32–5·92) | 478 (7·67–3180)* | 74·8 (0·67–768)* | 98·92 (14·5–1805)* |
| Anti-CCP (+/−) | n.a. | 23/2 | 7/2 | 3/1 |
| DAS28 | n.a. | 5·45 (3·30–7·84)* | 2·7 (2·67–3·18)*# | 5·21 (3·9–6·21)* |
| WBC (×109/l) | 6·0 (3·9–9·2) | 5·70 (3·82–10·6) | 6·98 (3·9–10·3) | 4·7 (4·1–9·1) |
| Lymphocytes (×109/l) | 38 (30·1–51·4) | 36·3 (24·1–45·3) | 33·5 (28·3–39·6) | 32·7 (26·3–42·7) |
| B cells (×109/l) | 8·21 (5·12–17·8) | 9·56 (3·21–20·8) | 8·78 (2·92–20·6) | 7·98 (4·3–21·5) |
*P < 0·05 versus healthy controls (HC); #P < 0·05 versus baseline values. Data shown are median (range) of each group of subjects. 0M: baseline; 1M: month after therapies; CCP: cyclic citrullinated peptide; CRP: C-reactive protein; DAS28: disease activity score in 28 joints; ESR: erythrocyte sedimentation rate; n.a.: not applicable; RF: rheumatoid factor; WBC: white blood cell.
Laboratory tests
The full blood counts and erythrocyte sedimentation rates (ESR) of individual subjects were examined. The levels of serum C-reactive protein (CRP), rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) were determined by scatter turbidimetry using a Siemens special protein analyser (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany).
Flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) were isolated from individual patients by density-gradient centrifugation using Ficoll-Paque Plus (Amersham Biosciences, Little Chalfont, UK). PBMCs at 5 × 105/tube were stained in duplicate with APC-cyanin 7 (Cy7)-anti-CD3 (BD Bioscience, San Diego, CA, USA), peridinin chlorophyll (PerCP)-anti-CD19, phycoerythrin (PE)-anti-CD38, APC-anti-CD86 or APC-Cy7-anti-CD3, PerCP-anti-CD19, fluorescein isothiocyanate (FITC)-anti-IgD, PE-anti-CD27 and APC-anti-CD95 (BD PharMingen, San Diego, CA, USA) for 30 min, and APC-Cy7-anti-IgG (BD Bioscience), PerCP-anti-IgG1, PE-anti-IgG1 APC-anti-IgG1 and FITC-anti-IgG (BD PharMingen) as the isotype controls. Furthermore, PBMCs (5 × 105/tube) were stained in duplicate with PerCP-anti-CXCR5 (Biolegend, San Diego, CA, USA), APC-anti-CD4, PE-anti-ICOS, FITC-anti-PD-1, APC-Cy7-anti-CD3 or isotype-matched controls (BD Bioscience) for 30 min. After being washed with phosphate-buffered saline (PBS), the cells were characterized on a BD fluorescence activated cell sorter (FACS)Aria II.
Stimulation of PBMC
PBMCs at 4 × 106/ml were stimulated in duplicate with or without 3 μg/ml of CpGB (cytosine-phosphate-guanine class B) (R&D Systems, Minneapolis, MN, USA) in the presence of 10 ng/ml of recombinant IL-2 (R&D Systems) in RPMI-1640 supplemented with 10% fetal calf serum (FCS) (Hyclone, Logan, UT, USA) in 5% CO2 at 37°C for 3 days [22]. The cells were harvested and then stained in duplicate with PerCP-anti-CD19 and APC-Cy7-anti-CD3 for 30 min, fixed, permeabilized with permeabilization solution (BD Bioscience) and stained with APC-anti-Toll-like receptor (TLR)-9 or the isotype control, followed by flow cytometry analysis of TLR-9 expression.
Measurement of serum IL-21 by enzyme-linked immunosorbent assay (ELISA)
The concentrations of serum IL-21 in individual patients and HC were determined by ELISA using the human IL-21 ELISA kit, according to the manufacturer's instructions (R&D Systems). Briefly, individual sera at 1:4 dilutions were subjected to ELISA analysis, and the concentrations of serum IL-21 in individual samples were calculated according to the standard curve established by using the recombinant IL-21 provided. The limitation of detection for the level of IL-21 was 10 ng/l.
Statistical analysis
Data are expressed as median and range or individual mean values. The difference between the groups was analysed by Mann–Whitney U non-parametric test using spss version 19·0 software. The relationship between variables was evaluated using Spearman's rank correlation test. A two-sided P-value of <0·05 was considered statistically significant.
Results
A high frequency of circulating activated B cells in patients with new-onset RA
To determine the role of different differentiation stages of B cells and Tfh cells in the pathogenesis of RA, a total of 25 patients with new-onset RA and 15 gender- and age-matched HC were recruited. There was no significant difference in the distribution of age and gender and the numbers of white blood cells (WBC) and lymphocytes between the patients and HC (Table 1). As expected, the levels of serum RF, CRP and anti-CCP and the values of ESR in the patients were significantly higher than that in the HC.
We characterized the frequency of different differentiation stages of B cells by flow cytometry analysis. As shown in Fig. 1, the percentages of IgD+CD27−CD19+ (naive B), CD86+CD19+, CD95+CD19+ B cells in those patients were significantly higher than that in the HC. In contrast, the frequency of IgD+CD27+CD19+ preswitch memory B cells was significantly lower in the patients than that in the HC. There was no significant difference in the frequency of IgD−CD27+CD19+ post-switch memory B cells, IgD−CD27−CD19+ double-negative B cells, CD38+CD19+ and TLR-9+CD19+ B cells between the RA patients and HC. Interestingly, the percentages of CD86+CD19+ B cells were correlated positively with the values of DAS28 in those patients (Fig. 1c). However, there was no significant correlation between the values of DAS28 and the frequency of other B cell subsets in this population (data not shown). Given that CD86 and CD95 were up-regulated in B cells, our data indicated that the higher frequency of activated B cells contributed to the pathogenesis of RA in Chinese patients with new-onset RA.
Fig. 1.

Fluorescence activated cell sorter (FACS) analysis of the frequency of different subsets of B cells in new-onset rheumatoid arthritis (RA) patients and healthy controls (HC). Peripheral blood mononuclear cells (PBMCs) were isolated from individual subjects and PBMCs (5 × 105/tube) were stained in duplicate with antigen-presenting cells–cyanin 7 (APC-Cy7)-anti-CD3, peridinin chlorophyll (PerCP)-anti-CD19, phycoerythrin (PE)-anti-CD38, APC-anti-CD86 or APC-Cy7-anti-CD3, PerCP-anti-CD19, fluorescein isothiocyanate (FITC)-anti-immunoglobulin (Ig)D, PE-anti-CD27, APC-anti-CD95 or isotype controls. The cells were characterized by flow cytometry by gating on living lymphocyte cells and then on CD3−CD19+ B cells for further analysis of different subsets of B cells. At least 30 000 events were analysed for each sample. (a) Flow cytometry analysis. Data shown are representative charts from each group of subjects. (b) Quantitative analysis. Data shown are individual mean values of the percentages of specific subset of B cells in the total CD19+ B cells. The horizontal lines show the medians. (c) Correlation analysis. The percentages of CD86+ B cells were plotted against the values of disease activity score 28 (DAS28) in the RA patients.
Higher percentages of circulating Tfh cells in patients with new-onset RA
Tfh cells can promote B cell activation, expansion and differentiation. To investigate the potential role of Tfh cells in the development of RA, we characterized the percentages of peripheral blood CD3+CD4+CXCR5+ cells in total CD3+CD4+ T cells in patients and HC by flow cytometry analysis (Fig. 2a). We found that the percentages of CD3+CD4+CXCR5+cells, CD3+CD4+ICOS+CXCR5+, CD3+CD4+PD-1+CXCR5+ and CD3+CD4+ICOS+PD-1+CXCR5+ Tfh cells in CD3+CD4+CXCR5+ cells in the patients were significantly higher than those in the HC (Fig. 2b). Given that Tfh cells can secrete IL-21, which has been shown to regulate B cell differentiation and proliferation [23–25], we examined the concentrations of serum IL-21 in those patients and HC by ELISA (Fig. 2c). We found that the levels of serum IL-21 in the patients were significantly higher than that in the HC. These data clearly indicated a higher frequency of activated Tfh cells and higher levels of serum IL-21 in patients with new-onset RA, and may contribute to the development of RA.
Fig. 2.

Fluorescence activated cell sorter (FACS) analysis of the frequency of different subsets of T follicular helper (Tfh) cells and enzyme-linked immunosorbent assay (ELISA) analysis of the levels of serum interleukin (IL)-21 in new-onset rheumatoid arthritis (RA) patients and healthy controls (HC). (a) Flow cytometry analysis of different subsets of Tfh cells. Peripheral blood mononuclear cells (PBMCs) were isolated from individual subjects and were stained in duplicate with anti-CD3, anti-CD4, anti-CXCR5, anti-inducible T cell co-stimulator (ICOS) and anti-programmed death 1 (PD-1) or isotype-matched immunoglobulin (Ig)G, respectively. The cells were characterized by flow cytometry analysis by gating initially on living lymphocytes and then on CD3+CD4+ T cells for the analysis of different subsets of CD4+CXCR5+ cells. Subsequently, the percentages of ICOS+ and PD-1+ Tfh in CD3+CD4+CXCR5+ cells were analysed, and at least 30 000 events were analysed for each sample. Data are representatives of different groups of samples from at least two independent experiments. (b) Quantitative analysis. Data shown are individual values of the mean percentages of different subsets of Tfh cells in individual subjects (n = 25 for RA patients; n = 15 for HC). (c) ELISA analysis of the concentrations of serum IL-21 in individuals. Data are expressed as the mean values of individual participants from two separate experiments. The horizontal lines indicate the median values of different groups.
The relationship between Tfh and B cells in the patients with new-onset RA
Next, we examined the relationship between Tfh and B cells in RA patients and found that the percentages of CD3+CD4+CXCR5+ cells were correlated positively with the frequency of CD19+ B cells in those patients (Fig. 3a). Similarly, the percentages of CD3+CD4+PD-1+CXCR5+ Tfh cells were also correlated positively with the frequency of CD95+CD19+ B cells (Fig. 3b). Interestingly, the percentages of CD3+CD4+ICOS+CXCR5+ Tfh cells were correlated negatively with the frequency of CD95+CD19+ B cells in those patients (Fig. 3c). However, there was no significant association between the percentages of other types of Tfh cells and B cells tested in those patients (data not shown). These data suggest that different types of Tfh cells may have variable functions in regulating the differentiation of B cells during the development of RA in humans.
Fig. 3.
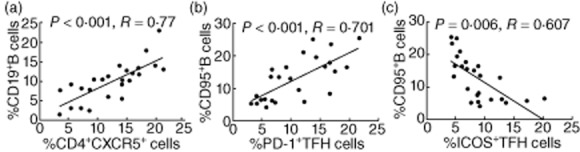
The relationship between the percentages of different subsets of T follicular helper (Tfh) and B cells in the rheumatoid arthritis (RA) patients. (a) The percentages of CD4+CXCR5+ T cells were correlated positively with the frequency of B cells in the RA patients. (b) The percentages of CD95+ B cells were correlated positively with the frequency of programmed death 1 (PD-1)+ Tfh cells in the RA patients. (c) The percentages of CD95+ B cells were correlated negatively with the frequency of ICOS+ Tfh cells in the RA patients.
The relationship between the percentages of Tfh cells and disease severity in patients with new-onset RA
To understand the importance of Tfh cells, we analysed the potential association of the percentages of different types of Tfh cells with the values of clinical parameters in those patients. We found that the percentages of CD3+CD4+ICOS+ CXCR5+ Tfh cells were correlated positively with the concentrations of serum anti-CCP and the values of DAS28, while the percentages of CD3+CD4+PD-1+CXCR5+ Tfh cells were correlated negatively with the concentrations of serum RF in those patients (Fig. 4). There was no significant association between other subsets of Tfh and B cells with the values of clinical measures tested. These data suggest that different types of Tfh cells may have different functions in the pathogenesis of RA in humans.
Fig. 4.
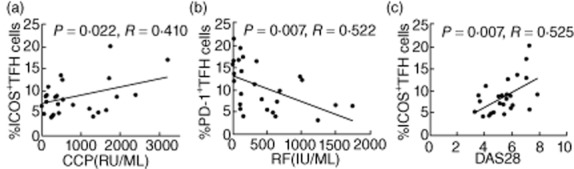
The percentages of T follicular helper (Tfh) cells are correlated with the values of clinical measures in the rheumatoid arthritis (RA) patients. (a) The percentages of ICOS+ Tfh cells were correlated positively with the levels of serum anti-CCP in the RA patients. (b) The percentages of programmed death 1 (PD-1)+ Tfh cells were correlated negatively with the levels of serum RF in the RA patients. (c) The percentages of inducible T cell co-stimulator (ICOS)+ Tfh cells were correlated positively with the values of disease activity score 28 (DAS28) in the RA patients (n = 25).
Treatment with drugs changes the frequency of B and Tfh cells significantly in RA patients
Finally, we tested how treatment with DMARDs and T. wilfordii affected the percentages of different types of B and Tfh cells in those patients. Following treatment with the drugs for 1 month, we found that nine of 13 patients responded to the treatment by dramatically reducing the values of DAS28 (<3·2) and others did not respond to the treatment (DAS28 > 3·2). Interestingly, we found that the percentages of CD86+CD19+ B cells and CD3+CD4+PD-1+CXCR5+ Tfh cells were reduced significantly in the drug-responding patients compared with the baseline values, accompanied by significantly reduced levels of serum IL-21 in those patients (Fig. 5). However, there was no significant difference in the percentages of CD86+CD19+ B cells and CD3+CD4+PD-1+CXCR5+ Tfh cells and in the levels of serum IL-21 between before and after treatment with drugs in those drug non-responding patients (data not shown). Similarly, there was no significant correlation between the percentages of CD3+CD4+ICOS+CXCR5+ and CD3+CD4+PD-1+CXCR5+ Tfh cells and the concentrations of serum anti-CCP as well as the values of DAS28 in those drug-responding patients after treatment for 1 month (data not shown). Collectively, treatment with DMARDs and T. wilfordii improved clinical symptoms dramatically, which was associated with a reduction in the frequency of CD86+CD19+ B cells and PD-1+ Tfh cells in those patients.
Fig. 5.

Treatment with the drugs modulates the frequency B and T follicular helper (Tfh) cells in the responding rheumatoid arthritis (RA) patients. Thirteen RA patients were treated with medicines for 1 month, and nine patients responded to the treatment. The frequency of CD86+ B and programmed death 1 (PD-1)+ Tfh cells in those patients was determined by flow cytometry analysis and was compared with that before treatment (a,b). The levels of serum interleukin (IL)-21 in those patients were determined by enzyme-linked immunosorbent assay (ELISA) and were compared with that before treatment (c).
Discussion
The pathological progression of RA was characterized by various immunological abnormalities, including dysregulated activation of both T and B cells and subsequent polyclonal activation of B cells. The activated B cells differentiate into plasma cells that produce RF, anti-CCP and other autoreactive antibodies. Activated B cells also infiltrate into the rheumatoid synovium [26]. In this study, we found that the frequency of CD19+IgD+CD27− naive B cells in RA patients was significantly higher than that in the HC, while the percentages of preswitch CD19+IgD+CD27+ B memory cells in RA patients were significantly lower than that in the HC. Our findings were consistent with a previous report that showed a higher frequency of naive B cells, but lower percentages of memory B cells in patients with new-onset RA [27]. This suggests that antigen stimulation may promote the redistribution of naive B cells from lymph tissues to circulation. Souto-Carneiro et al. [28] found that the percentages of circulating preswitch CD19+IgD+CD27+ memory B cells decreased in RA patients, while the frequency of preswitch CD19+IgD+CD27+ memory B and post-switch CD19+IgD−CD27+ memory B cells increased in the synovial membrane. It is possible that circulating CD19+IgD+CD27+ B cells could migrate and accumulate in the synovium of RA patients. However, a previous study has suggested that there may be an accumulation of post-switch CD19+IgD−CD27+ memory B cells, whereas the CD19+IgD+CD27+ memory B cells are reported in RA patients with long-standing disease [29]. The disparities between our data and the results of previous studies may be due to a number of factors, including varying genetic backgrounds, disease duration, cohort size and therapy.
Activated B cells increased the expression levels of certain activation markers, such as CD86 and CD95 [30,31]. CD86 is a critical co-stimulatory molecule for B cell activation and CD95 is associated with apoptosis. To assess activated B cells further in RA patients, we analysed the frequency of CD86+ or CD95+ B cells and found that the percentages of CD86+CD19+ and CD95+CD19+ B cells were significantly higher in the RA patients than that in the HC, consistent with a previous report [32,33]. These data indicated more activated B cells in RA patients. Given that CD95 is a death receptor, the higher frequency of CD95+ B cells in RA patients suggests that those activated B cells may be susceptible to spontaneous apoptosis, diminishing the total number of activated B cells in RA patients. Moreover, it is possible that the relatively higher frequency of naive B cells may stem from high differentiation of bone marrow stem cells due to the continuous loss of memory B cells, and this feedback regulation will help in maintaining B cell homeostasis in RA patients. O'Neill et al. [34] found that the expression of CD80/CD86 co-stimulatory molecules on B cells was critical for inducing autoreactive T cell activation and autoimmunity during the development of arthritis. In our study the percentages of CD86+CD19+ B cells in the RA patients were correlated positively with the DAS28 scores, suggesting that activated B cells might be major players in the pathogenesis of RA. Interestingly, treatment with DMARDs and T. wilfordii reduced significantly the frequency of CD86+CD19+ B cells in the drug-responding patients, further indicating the importance of activated B cells in the pathogenesis of RA.
Tfh cells are important for helping B cell activation and differentiation. Previous studies have suggested the importance of Tfh in the pathogenesis of systemic lupus erythematosus (SLE) and RA [17,35,36]. CXCR5, ICOS and PD-1 are expressed by Tfh cells and IL-21 is crucial for the development and function of Tfh. In this study, we found that the percentages of circulating CD3+CD4+ICOS+CXCR5+ and CD3+CD4+PD-1+CXCR5+ Tfh cells were significantly higher in the RA patients than that in the HC. Our findings extend a previous observation of a higher frequency of circulating CD3+CD4+ICOS+CXCR5+ Tfh cells in SLE patients [36]. Because the number of circulating Tfh cells increased in proportion to their GC counterparts [36], our data suggest an increased number of activated Tfh cells in the GCs of second lymphoid organs.
ICOS-mediated co-stimulation is crucial for Tfh differentiation. We also found that the percentages of ICOS+ Tfh cells were correlated positively with the levels of serum anti-CCP and the values of DAS28 in RA patients, consistent with a previous observation [17]. It is conceivable that the frequency of ICOS+ Tfh cells can be used as a biomarker for the evaluation of disease severity in the RA patients. PD-1 is expressed on activated T cells, particularly on Tfh cells. PD-1 promotes cognate T–B interactions and provides an inhibitory signal to Tfh cells [37]. Zhu et al. [38] showed that the percentages of CD3+CD4+ICOS+CXCR5+ and CD3+CD4+PD-1+CXCR5+ T cells were significantly higher in patients with autoimmune thyroid disease (AITD) than that in HC and were correlated positively with the levels of serum autoantibodies [38]. We found that the percentages of CD3+CD4+PD-1+CXCR5+ Tfh cells were correlated negatively with the levels of serum RF and treatment with DMARDs and T. wilfordii reduced significantly the frequency of CD3+CD4+PD-1+CXCR5+ Tfh cells in the drug-responding patients. Our data suggest that PD-1+ Tfh may serve as negative regulators to limit the number of functional Tfh cells and to minimize RF production.
In addition, we found that the percentages of ICOS+ Tfh cells were correlated positively with the frequency of total B cells and negatively with the frequency of CD95+ B cells in the RA patients. Furthermore, the percentages of PD-1+ Tfh cells were correlated positively with the frequency of CD95+ B cells in those patients. Of note, the ICOS-mediated T and B cell interaction usually promotes B cell activation, while the CD95-mediated T and B cell interaction commonly triggers B cell apoptosis [39]. We found that treatment with DMARDs and T. wilfordii reduced the frequency of PD-1+ Tfh and CD95+ B cells significantly in the drug-responding patients. These data indicate that the activated Tfh cells participate in the pathological progression of RA and the percentage of circulating Tfh cells is a valuable biomarker of disease activity. These findings reveal that active Tfh cells regulate B cell activation in the process of RA.
IL-21 is produced mainly by T lymphocytes including CD3+CD4+CXCR5+ Tfh cells. IL-21 is a key regulator of the differentiation of activated B lymphocytes into plasma and promotes IgM, IgG and IgA production [23,24,40]. We found that the levels of serum IL-21 were significantly higher in the RA patients than that in the HC. These results were in agreement with a previous observation showing that IL-21 regulates Tfh and B cell function [41]. We are interested in investigating further how IL-21 regulates B and Tfh cell activation and differentiation in RA patients.
In conclusion, our data showed that the percentages of activated B and Tfh cells increased significantly in the RA patients, compared with that in the HC, and were correlated with the disease severities in RA patients. Further studies are warranted to explore the roles of different subsets of B and Tfh cells in the pathogenesis of RA and to understand the mechanisms underlying B and Tfh activation in the process of RA.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (no. 30972610 and 81273240), Jilin Province Science and Technology Agency (no. 20110716), The Health Department Research Projects in Jilin Province (2009Z054) and Bethune B plan of Jilin University. The authors thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript. We also thank Professor Guangyu Zhou at the China–Japan Union Hospital of Jilin University for her help in collecting blood samples.
Disclosures
All the authors declare no conflicts of interest.
References
- 1.Sweeney SE, Firestein GS. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol. 2004;36:372–378. doi: 10.1016/s1357-2725(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS. Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol. 2005;11:S39–44. doi: 10.1097/01.rhu.0000166673.34461.33. [DOI] [PubMed] [Google Scholar]
- 3.Chaiamnuay S, Bridges SL., Jr The role of B cells and autoantibodies in rheumatoid arthritis. Pathophysiology. 2005;12:203–216. doi: 10.1016/j.pathophys.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Agematsu K, Ochs HD, Sugane K. Functional analysis of human memory B-cell subpopulations: IgD+CD27+ B cells are crucial in secondary immune response by producing high affinity IgM. Clin Immunol. 2003;108:128–137. doi: 10.1016/s1521-6616(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 5.Agematsu K. Memory B cells and CD27. Histol Histopathol. 2000;15:573–576. doi: 10.14670/HH-15.573. [DOI] [PubMed] [Google Scholar]
- 6.Arpin C, Déchanet J, Van Kooten C, et al. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 7.Hasunuma T, Kayagaki N, Asahara H, et al. Accumulation of soluble Fas in inflamed joints of patients with rheumatoid arthritis. Arthritis Rheum. 1997;40:80–86. doi: 10.1002/art.1780400112. [DOI] [PubMed] [Google Scholar]
- 8.Asahara H, Hasunuma T, Kobata T, et al. In situ expression of protooncogenes and Fas/Fas ligand in rheumatoid arthritis synovium. J Rheumatol. 1997;24:430–435. [PubMed] [Google Scholar]
- 9.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–325. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bentebibel SE, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+T-cell subset specialized for B-cell help outside germinal centers. Proc Natl Acad Sci USA. 2011;108:E488–497. doi: 10.1073/pnas.1100898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–746. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 12.Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng X, Wang D, Chen J, et al. Inhibition of aberrant circulating Tfh cell proportions by corticosteroids in patients with systemic lupus erythematosus. PLoS ONE. 2012;7:e51982. doi: 10.1371/journal.pone.0051982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo C, Li Y, Liu W, et al. Expansion of circulating counterparts of follicular helper T cells in patients with myasthenia gravis. J Neuroimmunol. 2013;256:55–61. doi: 10.1016/j.jneuroim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 15.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 16.Tarlinton D. Germinal centers: form and function. Curr Opin Immunol. 1998;10:245–251. doi: 10.1016/s0952-7915(98)80161-1. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Zhu C, Ma B, et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin Dev Immunol. 2012;2012:827480. doi: 10.1155/2012/827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asano K, Matsuishi J, Yu Y, Kasahara T, Hisamitsu T. Suppressive effects of Tripterygium wilfordii Hook f., a traditional Chinese medicine, on collagen arthritis in mice. Immunopharmacology. 1998;39:117–126. doi: 10.1016/s0162-3109(98)00006-x. [DOI] [PubMed] [Google Scholar]
- 19.Cibere J, Deng Z, Lin Y, et al. A randomized double blind, placebo controlled trial of topical Tripterygium wilfordii in rheumatoid arthritis: reanalysis using logistic regression analysis. J Rheumatol. 2003;30:465–467. [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 22.Oliviero B, Cerino A, Varchetta S, et al. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections. J Hepatol. 2011;55:53–60. doi: 10.1016/j.jhep.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev. 2008;223:60–66. doi: 10.1111/j.1600-065X.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 24.Konforte D, Simard N, Paige CJ. IL-21: an executor of B cell fate. J Immunol. 2009;182:1781–1787. doi: 10.4049/jimmunol.0803009. [DOI] [PubMed] [Google Scholar]
- 25.Bryant VL, Ma CS, Avery DT, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 26.Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 1996;93:221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moura RA, Weinmann P, Pereira PA, et al. Alterations on peripheral blood B-cell subpopulations in very early arthritis patients. Rheumatology (Oxf) 2010;49:1082–1092. doi: 10.1093/rheumatology/keq029. [DOI] [PubMed] [Google Scholar]
- 28.Souto-Carneiro MM, Mahadevan V, Takada K, et al. Alterations in peripheral blood memory B cells in patients with active rheumatoid arthritis are dependent on the action of tumor necrosis factor. Arthritis Res Ther. 2009;11:R84. doi: 10.1186/ar2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fekete A, Soos L, Szekanecz Z, et al. Disturbances in B- and T-cell homeostasis in rheumatoid arthritis: suggested relationships with antigen-driven immune responses. J Autoimmun. 2007;29:154–163. doi: 10.1016/j.jaut.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol. 2009;182:890–901. doi: 10.4049/jimmunol.182.2.890. [DOI] [PubMed] [Google Scholar]
- 31.Huck S, Jamin C, Youinou P, Zouali M. High-density expression of CD95 on B cells and underrepresentation of the B-1 cell subset in human lupus. J Autoimmun. 1998;11:449–455. doi: 10.1006/jaut.1998.0226. [DOI] [PubMed] [Google Scholar]
- 32.Catalán D, Aravena O, Sabugo F, et al. B cells from rheumatoid arthritis patients show important alterations in the expression of CD86 and FcgammaRIIb, which are modulated by anti-tumor necrosis factor therapy. Arthritis Res Ther. 2010;12:R68. doi: 10.1186/ar2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moodley D, Mody GM, Chuturgoon AA. Initiation but no execution – modulation of peripheral blood lymphocyte apoptosis in rheumatoid arthritis – a potential role for heat shock protein 70. J Inflamm (Lond) 2011;8:30. doi: 10.1186/1476-9255-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Neill SK, Cao Y, Hamel KM, Doodes PD, Hutas G, Finnegan A. Expression of CD80/86 on B cells is essential for autoreactive T cell activation and the development of arthritis. J Immunol. 2007;179:5109–5116. doi: 10.4049/jimmunol.179.8.5109. [DOI] [PubMed] [Google Scholar]
- 35.Linterman MA, Rigby RJ, Wong RK, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–566. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 37.Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30:802–810. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu C, Ma J, Liu Y, et al. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 2012;97:943–950. doi: 10.1210/jc.2011-2003. [DOI] [PubMed] [Google Scholar]
- 39.Linterman MA, Vinuesa CG. T follicular helper cells during immunity and tolerance. Prog Mol Biol Transl Sci. 2010;92:207–208. doi: 10.1016/S1877-1173(10)92009-7. [DOI] [PubMed] [Google Scholar]
- 40.Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu R, Wu Q, Su D, et al. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Res Ther. 2012;14:R255. doi: 10.1186/ar4100. [DOI] [PMC free article] [PubMed] [Google Scholar]


